Abstract
Background
Patient preference for the choice of treatment modality for prostate cancer has increasingly gained attention.
Objective
To assess the impact of client‐oriented decision on long‐term mortality, disease progression and biochemical failure compared with standard treatment protocol (TP).
Methods
With data from a Finnish multicentre, randomized controlled trial with two arms [104 in the enhanced patient participation (EPP) arm and 106 in the TP arm], disease‐specific and disease‐free survival, biochemical failure with elevated prostate‐specific antigen (PSA) level and disease progression were compared between the two arms using Wilcoxon test and also Cox proportional hazards regression model.
Results
Patients in the EPP arm had a higher risk of death by 37% [HR, 1.37 (0.87–2.17)] compared with those in the TP arm. Patients in the EPP arm were at increased risk of having biochemical failure by 14% [HR, 1.14 (0.72–1.79)] and for having disease progression by 2% [HR, 1.02 (0.61–1.70)] compared with those in the TP arm. All the differences were non‐significant.
Conclusions
Patients actively involved in the choice of treatment had higher risk of prostate cancer death but only slightly increased risk of biochemical failure and clinical disease progression. These findings would provide a good reference when patient autonomy for the choice of treatment modality is addressed.
Keywords: patient preference, prostate cancer, survival, treatment modality
Introduction
Patient preferences may play a crucial role in the choice of treatment modality for prostate cancer. Several studies have shown that patients are able and willing to take part in clinical decision making, and the choice of treatment based on patient preference varies between patients and may differ from treatment protocols (TPs) primarily based on survival results.1, 2, 3, 4
Notwithstanding patients' involvement in decision making is mandatory on the ground of ethical reasons, it is imperative to assess whether patient‐oriented decision making results in similar prognosis in terms of prostate cancer death and other surrogate endpoints such as disease progression and biochemical failure than standard TP. Using the data from a Finnish randomized trial on the choice of treatment, we assessed whether the survival rates of prostate cancer death and two intermediate surrogate endpoints of intervention by dint of enhanced patient participation (EPP) are different from those of control standard TP.
Patients and methods
Data used in our study are derived from a previous multicentre randomized controlled trial which included all histologically confirmed cases of prostate cancer diagnosed between September 1993 and November 1994 in four Finnish hospitals. The details of study design and procedures have been described in full elsewhere.1, 4 In brief, the enrolled patients played an active role in the choice of treatment in the intervention arm. The treatment in the control arm was chosen in accordance with standardized TPs. A total of 210 among 251 patients fulfilling the eligibility criteria were randomized into two arms, 104 in the EPP arm and 106 in the TP arm. The main outcome of the trial pertains to the quality of life after long‐term follow‐up. There were fourteen drop‐outs with the following reasons: seven refused to participate in the study and seven had incomplete data. In all, 196 patients had completely available information for the following analysis, including 100 in the intervention group and 96 in the control group.
Written consent was obtained after explaining the purpose and procedures of the study. Four board‐certified urologists with at least 10 years of clinical experience at four hospitals were in charge of the patients in both arms of the trial. After the initial visit, all patients were followed up until death or until 5 years after diagnosis. The follow‐up visits were scheduled at 3‐month intervals in the first year after diagnosis, semiannually for the second year and annually thereafter. At each follow‐up visit, clinical data on disease outcome including serum prostate‐specific antigen (PSA) concentration and any signs or symptoms of disease progression were recorded.
The trial profile of recruitment, random allocation and different outcomes of follow‐up is diagrammed in Fig. 1. Note that the follow‐up time for death was 132 months but only 60 months for biochemical failure and disease progression.
Figure 1.
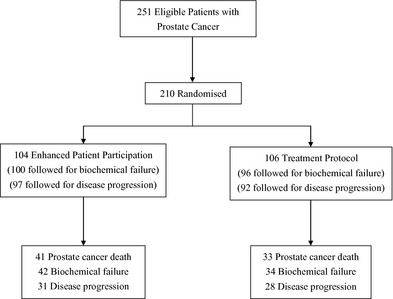
The flowchart of the recruitment and allocation in the randomized controlled trial of the choice of treatment for prostate cancer patients.
Selected endpoints
Three outcomes were compared between the trial arms, including prostate cancer death, disease‐free survival and serum PSA biochemical failure. Prostate cancer deaths were identified from the official cause of death statistics up to the end of 2005. Although fourteen randomized patients did not actually receive the intervention as allocated, they were included in the present mortality analyses according to the intention‐to‐treat principle. For the analyses of the outcomes other than prostate cancer death, these fourteen patients were not included owing to lack of follow‐up data.
The definitions of biochemical failure were subcategorized by initial treatment. For patients who underwent radical prostatectomy, the biochemical failure was defined as serum PSA value equal to or higher than 0.5 ng/ml. For patients treated with radiotherapy or watchful waiting, biochemical failure was deemed as an elevated serum PSA value in two consecutive measurements at least 3 months apart. For patients receiving hormonal therapy, including orchidectomy, the criterion of biochemical failure was an increase in PSA in two consecutive measurements. For disease progression, patients with successful initial treatment were followed from randomization till progression or their last follow‐up visit, whichever came first.
Disease progression was defined as first signs of local recurrence, lymph node involvement, bone change and other evidence of treatment failure. A total of seven patients did not reach remission following initial treatment (three in EPP vs. four in TP arm). These cases were not included in disease progression analyses.
Statistical analyses
Independent t‐test/Chi‐squared test was used for the comparison of continuous/categorical baseline characteristics and the choice of treatment between the two arms. Cumulative survival rates of these outcomes were calculated using the actuarial (life‐table) method. A Wilcoxon test was applied to the comparisons of the survival differences in prostate cancer death, biochemical failure and disease progression between the two arms. Univariate Cox proportional hazards regression models were used to calculate hazard ratios (HR) and their 95% confidence intervals for three outcomes – death, biochemical failure and disease progression. The proportional hazard assumption was assessed by introducing a time‐dependent covariate (i.e. interaction term between time and intervention group) to assess whether the effect of intervention arm was modified by follow‐up time. All analyses were performed using SAS 9.2 software (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics
Of the 210 randomized participants, the median follow‐up time was 27 months (ranging from 2 to 74) in the EPP arm and 28 months (1–70) in the TP arm. Randomization design led to a comparable distribution of the major prognostic factors at baseline between the two trial arms including age, serum PSA, WHO tumour grade and clinical stage (Table 1).
Table 1.
Baseline characteristics by each arm of trial
| Variables | EPP, n (%) | TP, n (%) | P‐value |
|---|---|---|---|
| N = 104 | N = 106 | ||
| Mean (SD) age, years | 70.4 (7.8) | 73.0 (7.5) | 0.27 |
| Age group, years | |||
| ≤59 | 7 (7) | 3 (3) | |
| 65–69 | 44 (42) | 37 (35) | |
| 70–79 | 39 (38) | 45 (42) | |
| ≥80 | 14 (13) | 21 (20) | |
| WHO grade | |||
| I | 27 (26) | 33 (31) | 0.51 |
| II | 58 (56) | 59 (56) | |
| III | 19 (18) | 14 (13) | |
| Clinical stage | |||
| Intracapsular | 39 (38) | 32 (30) | 0.53 |
| Locally advanced | 45 (43) | 51 (48) | |
| Distant metastasis | 20 (19) | 23 (22) | |
| PSA range, ng/ml | |||
| 0–3.9 | 4 (4) | 6 (6) | 0.74 |
| 4–9.9 | 17 (16) | 16 (15) | |
| 10–99 | 72 (69) | 69 (65) | |
| 100–999 | 8 (8) | 8 (8) | |
| ≥1000 | 3 (3) | 7 (7) | |
| Disease progression | |||
| Localized disease | 97 (97) | 92 (96) | 0.90 |
| Lymph node involvement | 1 (1) | 1 (1) | |
| Bone change | 2 (2) | 2 (2) | |
| Other attributes | 0 (0) | 1 (1) | |
| Not known | 4 | 10 | |
EPP, enhanced patient participation; TP, treatment protocol; SD, standard deviation; PSA, prostate‐specific antigen.
Table 2 shows the type of treatment chosen among patients with localized disease (stage cT1b‐T2, N0, M0) in the EPP arm was more frequently administered by radiotherapy than in the TP arm (26% vs. 3%). Radical prostatectomy was the treatment option most commonly chosen in both arms among patients with operable cancer (63% vs. 83%) (data not shown). Of patients with non‐localized disease, the subjects in the EPP arm were more likely to choose treatment with luteinizing hormone‐releasing hormone (LHRH) than those in the TP arm (29% vs. 3%).
Table 2.
Choice of treatment by clinical stage in each arm of trial
| Choice of treatment | EPP, n (%) | TP, n (%) | P‐value |
|---|---|---|---|
| N = 104 | N = 106 | ||
| Localized disease | |||
| Watchful waiting | 10 (26) | 7 (22) | 0.0090 |
| Radical prostatectomy | 17 (44) | 15 (47) | |
| Radiation therapy | 10 (26) | 1 (3) | |
| Orchidectomy | 1 (3) | 7 (22) | |
| LHRH agonist | 1 (3) | 2 (6) | |
| Total | 39 (100) | 32 (100) | |
| Not localized disease | |||
| Watchful waiting | 1 (2) | 3 (4) | <0.0001 |
| Radiation therapy | 2 (3) | – | |
| Orchidectomy | 42 (65) | 69 (93) | |
| LHRH agonist | 19 (29) | 2 (3) | |
| Total androgen blockade | 1 (2) | – | |
| Total | 65 (100) | 74 (100) | |
EPP, enhanced patient participation; TP, treatment protocol; LHRH, luteinizing hormone‐releasing hormone.
Cumulative survival curves by trial arm (EPP vs. TP) on time to prostate cancer death, biochemical failure and signs of disease progression are presented in Figs 2, 3, 4.
Figure 2.
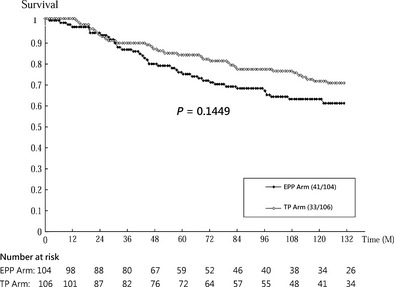
Cumulative disease‐specific survival by trial arm (intervention: EPP, enhanced patient participation; control: TP, treatment protocol).
Figure 3.
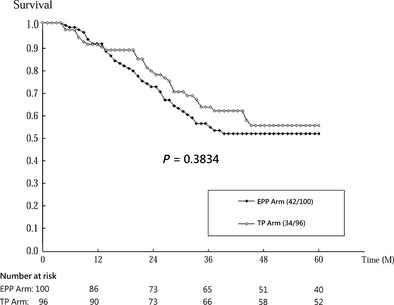
Cumulative survival of being free of biochemical failure by trial arm (intervention: EPP, enhanced patient participation; control: TP, treatment protocol).
Figure 4.
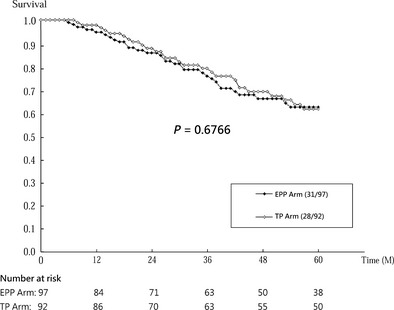
Cumulative progression‐free survival by trial arm (intervention: EPP, enhanced patient participation; control: TP, treatment protocol).
Disease‐specific survival
Overall, there were 41 deaths among the 104 men in the EPP arm and 33 in 106 patients in the TP arm. Survival rate was higher in the EPP arm than in the TP arm (70.8% vs. 62.5% of 10‐year death rate, Wilcoxon test, P = 0.14, Fig. 2). Table 3 shows patients in the EPP arm had a higher risk of death than those in TP arm by 37% [HR 1.37 (0.87–2.17)]. The survival curves of the two arms were identical during the initial follow‐up period and started to diverge after 30 months of follow‐up (Fig. 2). This suggests the effect of intervention on survival may vary with time. By using time‐dependent analysis (adding interaction term between follow‐up time and intervention group into model), the hazard ratio for EPP vs TP changed from 0.93 (0.40–2.21) before 30 months to 1.60 (0.93–2.77) after 30 months of follow‐up. Although such effect modification (different influences of the intervention across follow‐up time) was not statistically significant (P = 0.30), this finding still suggests the risk of prostate cancer death tends to be higher in the EPP arm than in the TP arm with follow‐up time.
Table 3.
Outcomes of prostate cancer death, biochemical failure and disease progression by arms of enhanced patient participation and TP and the estimated hazard ratio of randomization arm using Cox proportional hazards regression analysis
| Outcome/Arm | Subjects | Event | Regression coefficient | SE | HR | 95% CI |
|---|---|---|---|---|---|---|
| Prostate cancer death | ||||||
| EPP | 104 | 41 | 0.31 | 0.23 | 1.37 | 0.87, 2.17 (Reference) |
| TP | 106 | 33 | – | – | 1.00 | |
| Biochemical failure | ||||||
| EPP | 100 | 42 | 0.13 | 0.23 | 1.14 | 0.72, 1.79 (Reference) |
| TP | 96 | 34 | – | – | 1.00 | |
| Disease progression | ||||||
| EPP | 97 | 31 | 0.02 | 0.26 | 1.02 | 0.61, 1.70 (Reference) |
| TP | 92 | 28 | – | – | 1.00 | |
EPP, enhanced patient participation; TP, treatment protocol; SE, standard error; HR, hazard ratio; CI, confidence interval.
Biochemical failure
In all, 76 of 196 patients had biochemical failure, that is, rising serum PSA during the follow‐up, including 42 of the 100 patients from the EPP arm and 34 of the 96 patients from the TP arm. The cumulative survival of being free of biochemical failure was slightly lower in the EPP arm than the TP arm (Wilcoxon test, P = 0.38; see Fig. 3). Patients in the EPP arm were at increased risk of having biochemical failure by 14% (HR, 1.14; 95% CI, 0.72–1.79; P = 0.57; see Table 3) compared with those in the TP arm. However, the survival curves of the two arms started to diverge after 1 year, but the hazard ratios did not vary significantly with follow‐up time (P = 0.63).
Disease progression
Among the 189 patients who were in remission after initial treatment, 59 had disease progressions during the follow‐up: 31 of 97 men in the EPP arm and 28 of 92 in the TP arm. The progression‐free survival rates were slightly lower in the EPP arm than in the TP arm (Wilcoxon test, P = 0.68; see Fig. 4). Patients who were encouraged to participate in the choice of treatment had only slightly higher risk of disease progression than those who recommended treatment in the light of standard protocols (HR, 1.02; 95% CI, 0.61–1.70; P = 0.94; see Table 3).
Discussion
The present study was to evaluate the prognosis in terms of three outcomes, such as prostate cancer death, biochemical failure and disease progression, between trial arms with different approaches to the selection of treatment for prostate cancer in the Finnish randomized trial. Patients who were encouraged to participate in the choice of treatment compared with patients with recommended standard treatment practically tended to have a higher risk of prostate cancer death rate after 3 years of follow‐up whereas there was lacking of substantial differences with respect to the other two intermediate outcomes.
These findings indicate that allowing a more active role for the patient in the choice of treatment may not lead to poor intermediate outcomes like biochemical failure and clinical signs of disease progression, but patients in the EPP arm had poorer survival than in the TP arm. The poor survival in the EPP arm is explained by one possibility that because previous studies reported radical prostatectomy had better survival than watchful waiting in early prostate cancer in one randomized control trial5, 6 and a long‐term follow‐up cohort.7 Therefore, the poorer survival in the EPP arm in our trial might be due to the different treatments chosen in the two arms. The patients with local prostate cancer in the EPP arm were more likely to choose radiotherapy and watchful waiting than in the TP arm. The reason for selecting less‐aggressive therapies is that, in addition to the survival, priorities related to other aspects of treatment may not be consistent between patients and physicians. Patient may be concerned about specific adverse effects (e.g. sexual function) frequently caused by radical prostatectomy affecting quality of life, which is one of the major outcomes in this trial and will be reported, in a separate paper, rather than life years gained.8
The other reason accounting for the disparity between the results of prostate cancer death and biochemical recurrence and clinical disease progression may be because biochemical failure and disease progression are surrogate markers and cannot fully account for the endpoint of prostate cancer (i.e. disease‐specific mortality) death9 owing to measurement error, which has been well recognized to give an underestimation (non‐differential misclassification) of true effect. It has been reported that patients treated with radiotherapy sometimes experience random fluctuation of PSA over 2 years before reaching the nadir,10 and recurrences after radiotherapy cannot be readily detected until at least 3.5 years after treatment.
However, an excess of 37% prostate cancer–specific death for the overall group in enhanced participation may warrant a further long‐term follow‐up of patients in the EPP arm to confirm the poor results of survival.
Our 5‐year relative survival of prostate cancer death was higher than that obtained from the previous studies, 60% in Finland, 59% in Iceland, 55% in Norway and 62% in Sweden. This is plausible as the improvement of survival over the past decade may be due to more early prostate cancers resulting from screening for prostate cancer with PSA test. These studies were during the period between 1983 and 1987.11, 12 Also, the clinical stage distribution in our study subjects, with 35% intracapsular cases (cT1–T2), 45% with locally advanced disease (cT3–T4), and 20% with distant metastases (M1), was consistent with all prostate cancer patients in Finland,13, 14 which suggests that our study subjects were representative of prostate cancer patients in Finland. Most cancers in our study were clinically detected cancers rather than screen‐detected as participants were enrolled prior to the PSA era. Therefore, the generalizability of our results to the screen‐detected cancers after PSA would be taken with great caution.
Methodological consideration
A total of 20 patients changed their treatment decision after the initial choice, 14 in the EPP arm and six in the TP arm. Most of them switched from watchful waiting to active treatment (7 vs. 5). Seven patients did not follow the random allocation because of refusal or of being unable to participate after randomization. In addition, seven patients were not followed up as scheduled: two for death and five lost to follow‐up. These fourteen cases were included in prostate cancer mortality analysis with intention‐to‐treat principle. However, small number of these cases would not seriously jeopardize the validity of the results. Finally, the preferences of patients in the choice of treatment seemed to differ from the priorities of the physicians, as different forms of treatment were chosen in the intervention and control arms. The differences in types of treatment chosen between patients in the two arms of the trial were not adjusted in the Cox regression analyses because it would result in overadjustment leading to the masking effect of the intervention.
It should be noted that although all these differences of three outcomes were not statistically significant, the interpretation of these results on the aspect of statistical power should be taken with great caution as sample sizes or duration of follow‐up may still not be sufficient enough. Small sample size also precludes us from doing post‐stratification analysis by localized and non‐localized stage.
Conclusion
We conclude that patients actively involved in the choice of treatment tended to have higher risk of prostate cancer death but only slightly increased risk of biochemical failure and clinical disease progression although the differences of three outcomes were lacking of statistical significance. These results should be considered when emphasis on patient autonomy in the choice of treatment for prostate cancer is laid.
Conflicts of interest
No conflicts of interest.
Sources of funding
This study was supported financially by the Cancer Society of Finland, Tampere University Hospital Research Fund and the Taiwan National Science Council [NSC95‐2314‐B‐002‐246‐].
References
- 1. Auvinen A, Vornanen T, Tammela TLJ et al A randomized trial of choice of treatment in prostate cancer: design and baseline characteristics. BJU International, 2001; 88: 708–715. [DOI] [PubMed] [Google Scholar]
- 2. Davison BJ, Goldenberg SL, Gleave ME, Degner LF. Provision of individualized information to men and their partners to facilitate treatment decision making in prostate cancer. Oncology Nursing Forum, 2003; 30: 107–114. [DOI] [PubMed] [Google Scholar]
- 3. Davison BJ, Goldenberg SL. Decisional regret and quality of life after participating in medical decision‐making for early‐stage prostate cancer. BJU International, 2003; 91: 14–17. [DOI] [PubMed] [Google Scholar]
- 4. Auvinen A, Hakama M, Ala‐opas M et al A randomized trial of choice of treatment in prostate cancer: the effect of intervention on the treatment chosen. BJU International, 2004; 88: 52–56. [DOI] [PubMed] [Google Scholar]
- 5. Holmberg L, Bill‐Axelson A, Helgesen F et al A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. New England Journal of Medicine, 2002; 347: 781–789. [DOI] [PubMed] [Google Scholar]
- 6. Bill‐Axelson A, Holmberg L, Ruutu M et al Radical prostatectomy versus watchful waiting in early prostate cancer. New England Journal of Medicine, 2005; 352: 1977–1984. [DOI] [PubMed] [Google Scholar]
- 7. Lu‐Yao GL, Yao SL. Population based study of long term survival in patients with clinically localised prostate cancer. Lancet, 1997; 349: 906–910. [DOI] [PubMed] [Google Scholar]
- 8. Singer PA, Tasch ES, Stocking C, Rubin S, Siegler M, Weichselbaum R. Sex or survival. Trade‐offs between quality and quantity of life. Journal of Clinical Oncology, 1991; 9: 328–334. [DOI] [PubMed] [Google Scholar]
- 9. Sandler HM, Pajak TF, Hanks GE, Porter AT, DeSilvio M, Shipley WU. Can biochemical failure (ASTRO definition) be used as a surrogate endpoint for prostate cancer survival in phase III localized prostate cancer clinical trials? Proceeding of American Society of Clinical Oncology, 2003; 22: 381. [Google Scholar]
- 10. Zelefsky MJ. PSA bounce versus biochemical failure following prostate brachytherapy. Nature Clinical Practice. Urology, 2006; 3: 578–579. [DOI] [PubMed] [Google Scholar]
- 11. Tretli S, Engeland A, Haldorsen T et al Prostate cancer: look to Denmark. Journal of the National Cancer Institute, 1996; 88: 128. [DOI] [PubMed] [Google Scholar]
- 12. Johansson JE, Holmberg L, Johansson S, Bergström R, Adami HO. Fifteen‐year survival in prostate cancer. JAMA, 1997; 277: 467–471. [PubMed] [Google Scholar]
- 13. Hakulinen T, Pukkala E, Hakama M, Lehtonen M, Saxen E, Teppo L Survival of cancer patients in Finland in 1953–1974. Annals of Clinical Research, 1981; 13 (Suppl. 31): 59–61. [PubMed] [Google Scholar]
- 14. Dickman PW, Hakulinen T, Pukkala E et al Survival of cancer patients in Finland 1955–1994. Acta Oncologica, 1999; 38 (Suppl. 12): 57–59. [DOI] [PubMed] [Google Scholar]


