Abstract
Simple organic molecules (SOM) based on bis(haloBODIPY) are shown to enable circularly polarized luminescence (CPL) conforming a new structural design for technologically-valuable CPL-SOMs. The established design comprises, all-in-one, synthetic accessibility, labile helicity, possibility of reversing the handedness of the circularly polarized emission and reactive functional groups, making it unique and attractive as advantageous platform for the development of smart CPL-SOMs.
Keywords: Circularly Polarized Luminescence, Small Organic Molecules, BODIPYs, Chirality, Fluorescence
Graphical abstract
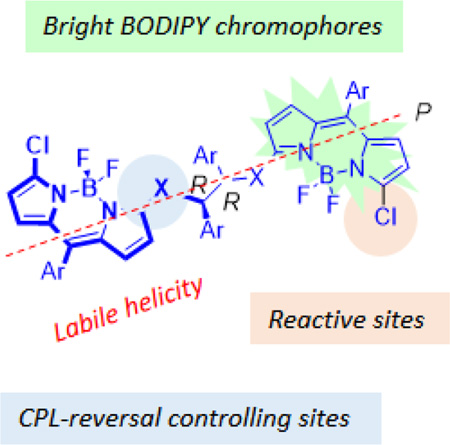
A new structural design for CPL-SOMs comprising, all-in-one, synthetic accessibility, capacity for reversing the polarization handedness, helical lability and reactive functional groups making possible photophysics modulation is established.
Circularly polarized luminescence (CPL) is a chiroptical phenomenon consisting of the differential emission of right and left circularly polarized lights by chiral systems (molecules, ionic pairs, polymers, metal complexes, supramolecular aggregates, etc.).[1] The interest of CPL is due to the resolution provided by the circular polarization of the light, which allows the development of smarter photonic materials for useful technologies, such as 3D displaying,[2] information storage and processing,[3] including high-security chiroptical cryptography,[3c] communication of spin information (spintronics-based devices),[4] or bioimaging (e.g, ellipsometry-based tomography).[5] Moreover, circularly polarized light can be used to promote asymmetric photochemistry,[6] to control the morphology in nanomaterials,[7] or to detect chiral environments (chiral sensing).[8] All these characteristics make CPL valuable for the development of envisaged advanced photonic technologies (e.g., CPL microscopy).[9] Besides, but not less important, spectroscopy based on CPL is an indispensable source of information to know chirality factors in luminescent excited states.[1b,10]
The level of CPL is quantified by the luminescence dissymmetry factor, glum,[1b,11] whose values stand between −2 and +2 (completely right and left circularly polarized emission, respectively). Among the well-established chiral systems enabling CPL, mainly photoluminescent chiral lanthanide complexes with |glum| values typically into the 0.05–0.5 range,[1d,e,9a,10c,12] those based on simple (small, non-polymeric and non-aggregated) organic molecules, CPL-SOMs, have attracted considerable attention during the last five years.[13,14] This is due to the enormous technological potential of these molecules to develop future advanced photonic (chiroptical) materials, mainly due to properties associated to the small size, which makes them interesting for the development of physiological CPL applications, as well as to their excellent organic-solvent solubility, which makes them valuable for the development of CPL-active dye-doped inclusion materials.[13a]
However, CPL-SOMs are rare, exhibit very small levels of circular polarization (|glum| typically into the 10−5–10−3 range), and are restricted to a small number of chiral designs, which usually demand highly inefficient synthetic routes (racemate resolution or complex asymmetric processes are typically involved).[13a] Additionally, in most cases, the best glum values are not coincident with the best emission efficiencies, or with the easiest synthetic procedures.[13] Hence, new structural designs for CPL-SOMs, combining together CPL activity, emission efficiency and synthetic accessibility (allowing future cost-effective materials) are required.
Helicity has been demonstrated to be a privileged structural motive to achieve CPL in SOMs.[13a] In this sense, significant glum values have been reported for several CPL-SOMs having helical chirality (mainly helicenes).[13a,14] However, with the exception of scarce cases (helicene-like molecules),[14b] the distortion of the involved helicene chromophore, which is associated to the enhanced CPL activity, usually spoils the emission efficiency as well.[13a] Furthermore, the enantiopure preparation of these CPL-SOMs is not straightforward in most cases.[13a,14]
Regarding helicity and easily accessible SOMs, we have previously reported the capacity of (R,R)-1 and (S,S)-1 to adopt a self-induced preferential helical conformation in chloroform solution (P for the former, M for the latter; see Figure 1), leading to a strong and clearly bisignated visible (Vis) circular dichroism, which is due to the chirally-perturbed exciton-coupled absorption of the involved identical BODIPY (4,4-difluoro-4-bora-3a,4a-diaza-s-indacene) chromophores.[15] It must be noted here that, despite of the recognized importance of the BODIPY dyes in photonics, the study of chirality features in BODIPYs is still scarce,[13a,15–17] and chirally-perturbed BODIPY chromophores enabling CPL unusual.[13a,17]
Figure 1.
Studied CPL-SOMs (only the R,R enantiomers are shown), and known preferential helical conformation for (R,R)-1 (right).
This chiroptical behaviour of (R,R)-1 and (S,S)-1 at their ground state, joined to their helical character and synthetic accessibility, prompted us to investigate their unknown capacity to enable CPL, directed to the possibility of conforming a new model for CPL-SOMs on the basis of their peculiar structure which combines advantageous properties. The present communication reports the results of such investigation, which has been focused in studying known molecules 1 in comparison with related new molecules 2, demonstrating that these easily-accessible molecules are not only able to display good levels of CPL, but also to exhibit unprecedented capacity for reversing the polarization handedness of the CPL emission by a simple structural change.
Known (R,R)-1 and (S,S)-1 were straightforward obtained in a single synthetic step, by treating accessible 3,5-dichloro-8-(4-methylphenyl)BODIPY[18] with the corresponding commercial enantiopure C2-symmetric 1,2-diphenylethan-1,2-diamine, as described previously by us (isolated yield ca. 50%),[15] and their unknown CPL behaviour investigated in chloroform solution (ca. 10−3 M).
Satisfactorily, circularly polarized emission was detected from (R,R)-1 and (S,S)-1 upon exciting the BODIPY chromophores with Vis light (Figure 2, left plots). This chiroptical activity shows that the inherently achiral BODIPY chromophores are efficiently perturbed by the chiral helical structure in which they are embedded, not only at the ground state, as it was previously shown by CD spectroscopy,[15] but also at the luminescent excited state.
Figure 2.
CPL (upper curves; R,R enantiomers in normal lines, S,S in dotted lines) and total luminescence (lower curves) spectra of 1 (left) and 2 (right) in degassed CHCl3 solution (ca. 10−3 M) at 295 K, upon exciting the BODIPY chromophores (see SI for details).
The recorded CPL spectra for both enantiomers were virtually mirror images, showing maxima matching with the maximum Vis emission of the BODIPY chromophores (λ ca. 570 nm; see Figure 2, left plots). It must be noted that the small values usually found for the CPL activity, mainly for organic chromophores, make the CPL plots highly noisy in most of the cases.[1–10,12,19]. Although the determined |glum| values are small, almost opposite CPL signals (see Figure 2, left) with maximum glum values of ca. +0.001 and −0.001 were measured for each enantiomer, (R,R)-1 and (S,S)-1, respectively. These results also confirm that the constructed helical architecture, despite its lability, is indeed able to produce the searched luminescent phenomenon in solution, since the emitted light is equally polarized in opposite directions by each enantiomer.
In order to test the workability of this new helically labile design for achieving CPL in SOMs, we synthesized (R,R)-2 and (S,S)-2, as oxygenated analogues of (R,R)-1 and (S,S)-1, respectively (Figure 1). For this purpose, we conducted a synthetic procedure similar to that used for enantiomers 1,[15] but employing enantiopure diols instead of diamines (isolated yield ca. 60%; see Figure S1 and experimental details in SI).
The fluorescence signatures of (R,R)-2 and (S,S)-2 in chloroform solution (ca. 5·10−6) were similar to those measured for (R,R)-1 and (S,S)-1 in the same conditions (fluorescence quantum yields ca. 15%; see Table S1 and Figure S2 in SI), displaying also similarly strong Vis CD signals (Figure S3 in SI). Interestingly, the signs of the CD Cotton effects remained unchanged for molecules having the same absolute configuration (e.g., for the strongest Vis CD signal: negative for the R,R enantiomers, positive for the S,S ones; see Figure S3 in SI). Besides, the optical rotations displayed by enantiomers 2 in chloroform solution were huge (|[α]D20| ca. 3000; see SI), as those measured for enantiomers 1 in the same experimental conditions,[15] the rotation signs coinciding, too: the R,R isomers are levogyre in both cases, whereas the S,S ones are dextrogyre. All these results suggest that oxygenated enantiomers 2 adopt an helical conformation in chloroform solution similar to that demonstrated previously for nitrogenated 1 (see Figure 1), and with the same preferential helical configuration, too (i.e., P for the R,R enantiomers, and M for the S,S ones).
Simulation of high level of theory (PCM-B3LYP/6-31+g*; see SI for details) on the structure of both enantiomers of 1 and 2 agrees with our previous computational data of lower level conducted on 1,[15] confirming that both molecules adopt the same helical conformation in chloroform solution, regardless the involved spacer heteroatoms, and also predicting the corresponding CD spectra (TD-PCM-B3LYP/6-31+g*; see Figures S4–S5 in SI). Therefore, it should be expected that enantiomers 2 will also enable Vis CPL in solution upon Vis irradiation.
Indeed, a CPL behaviour similar to that exhibited by enantiomers 1 in chloroform solution was found for enantiomers 2. Thus, upon exciting the BODIPY chromophores of (R,R)-2 and (S,S)-2 with Vis light, almost mirror CPL spectra were recorded, with maxima matching the maximum emission of the said chromophores (λ ca. 550 nm; see Figure 2, right plots), and affording |glum| values almost identical to those measured for enantiomers 1 (ca. 0.001). Strikingly, while nitrogenated (R,R)-1 emits preferentially left circularly polarized light (maximum glum = +0.001), oxygenated (R,R)-2 preferentially emits right circularly polarized light (maximum glum = −0.001), despite the fact that their absolute configuration is the same (see Figure 2). The same behaviour, but sign opposite, was observed for the S,S couple (i.e., negative glum for (S,S)-1, positive for (S,S)-2).
The found CPL reversal is very interesting, since the establishment of ways for the modulation of the circular handedness, by structural changes different to the inversion of the absolute stereochemical configuration, is a hot research objective in CPL-SOMs.[13d,20] In this sense, the achievement of a full inversion in the maximum glum value, from +0.001 to −0.001 and vice versa, by changing only the heteroatoms (nitrogens vs. oxygens) linking the involved BODIPY units to the central chiral spacer, constitutes a new, unexplored, and synthetically simple way to efficiently reverse the circular handedness in BODIPY-based CPL-SOMs.
To get a deeper insight in this intriguing feature, we optimized computationally the first locally excited state (LES) of the studied CPL-SOMs (PCM-CIS/6-31+g*; see SI for details). Despite the conformational flexibility of the structures, no remarkable structural changes were detected in the computed LESs when compared with the corresponding ground states (cf. Figures S4–S5 vs. S6–S7 in SI), as it was suggested by the small Stokes shifts exhibited by these CPL-SOMs (see Table S1 and Figure S2 in SI). Indeed, the theoretically predicted CPL spectra from such optimized LESs (TD-PCM-B3LYP/6-31+g*; see SI for details) did not explain the found CPL reversal. Thus, for oxygenated enantiomers 2 the predicted CPL spectra (see Figure S6 in SI) fit well with the experimental ones (see Figure 2; left plots), but just sign-opposed CPL spectra were predicted for nitrogenated enantiomers 1 (cf. Figure 2, right plots, and Figure S7 in SI).
Nonetheless, it should be kept in mind that the fluorescence signatures of BODIPY chromophores functionalized with both an efficient-enough electron-donating group and an efficient-enough electron-withdrawing group are ruled out by the promotion of an intramolecular charge transfer state (ICTS) upon excitation (push-pull effect).[21] On the other hand, the different ability of 1 and 2 to promote ICTSs (higher for 1, due to the presence of the stronger amino/chlorine push-pull effect) could cause crucial differences in the charge distribution upon excitation, which could also explain the observed CPL reversal, as well as the fail in the prediction of the CPL signalization in the case of 1.
The claimed promotion of ICTSs in 1 and 2 was experimentally confirmed by diminishing its probability by lowering the polarity of the solvent. Thus, changing chloroform by cyclohexane caused an enhancement of the fluorescence quantum yield displayed by these molecules (up to ca. 17% and 30% for enantiomers 1 and 2, respectively; see Table S1 in SI). The lower fluorescence response of the former (nitrogenated) enantiomers also supports that their fluorescence signatures are deeply affected by the promotion of ICTS. However, the change in the solvent polarity did not cause any significant difference in the shapes of the corresponding absorption and emission spectra, neither in the CD and CPL signalizations, nor in the observed glum values. The demonstrated existence of ICTS effects in these CPL-SOMs is very valuable, because it opens the possibility of tuning the emission efficiency by the modulation of the effect through further physical and/or chemical modifications. It must be noted here that possible luminescence from the populated ICTSs could contribute to the final fluorescence emission (superimposed dual emission from the LES and from the ICTS), explaining also the differences found in the shapes of the CPL spectra of 1 and 2 (couplet-like for the latter; see Figure 2). Nonetheless, it must be also taken into account that the shapes of the CPL spectra are highly dependent on small changes in the structure of the chiral luminescent system (including small conformational changes).
In summary, we report on the establishment of new and simple structural design for CPL-SOMs based on haloBODIPY moieties embedded in a helically-labile chiral architecture. In such a design, only two accessible precursors are required: a readily accessible dihaloBODIPY and flexible enantiopure diamine or diol. The goal of the reported design is comprising, all-in-one, synthetic accessibility, capacity for reversing the polarization handedness, helical lability and, what is very important, reactive functional groups making possible photophysics modulation by known chemical transformations.[22] This combination of properties make the new design to be unique as valuable platform for advancing in the development of smarter CPL-SOMs for envisaged photonic tools (e.g., fluorescent dyes for CPL-based microscopy in physiological media).
Supplementary Material
Acknowledgments
Financial support from MINECO (grants MAT2014-51937-C3-2-P, MAT2014-51937-C3-3-P and MAT2015-68837-REDT) is gratefully acknowledged. G.M. thanks the NIH, Minority Bio-medical Research Support (grant 1 SC3 GM089589-07) and the Henry Dreyfus Teacher-Scholar Award for financial support, whereas K.D.C thanks the SJSU RISE program (NIH grant 5R25GM71381) for a research fellowship.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- 1.a) Riehl JP, Richardson FS. Chem. Rev. 1986;86:1–16. [Google Scholar]; b) Brittain HG. Chirality. 1996;8:357–373. [Google Scholar]; c) Berova N, Polavarapu PL, Nakanishi K, Woody RW, editors. Comprehensive Chiroptical Spectroscopy. Vol. 1. Hoboken: Wiley; 2012. Instrumentation, Methodologies, and Theoretical Simulations. [Google Scholar]; d) de Bettencourt-Dias A, editor. Luminescence of Lanthanide Ions in Coordination Compounds and Nanomaterials. Chichester: Wiley; 2014. [Google Scholar]; e) Zinna F, Di Bari L. Chirality. 2015;27:1–13. doi: 10.1002/chir.22382. [DOI] [PubMed] [Google Scholar]
- 2.Schadt M. Annu. Rev. Mater. Sci. 1997;27:305–379. [Google Scholar]
- 3.a) Sherson JF, Krauter H, Olsson RK, Julsgaard B, Hammerer K, Cirac I, Polzik ES. Nature. 2006;443:557–560. doi: 10.1038/nature05136. [DOI] [PubMed] [Google Scholar]; b) Wagenknecht C, Li C-M, Reingruber A, Bao X-H, Goebel A, Chen Y-A, Zhang Q, Chen K, Pan J-W. Nat. Photonics. 2010;4:549–552. [Google Scholar]; c) Amako T, Nakabayashi K, Suzuki N, Guo S, Rahim NAA, Harada T, Fujiki M, Imai Y. Chem. Commun. 2015;51:8237–8240. doi: 10.1039/c5cc01465d. [DOI] [PubMed] [Google Scholar]
- 4.Farshchi R, Ramsteiner M, Herfort J, Tahraoui A, Grahn HT. Appl. Phys. Lett. 2011;98:162508. (3 pages) [Google Scholar]
- 5.a) Yu CJ, Lin CE, Yu LP, Chou C. Appl. Optics. 2009;48:758–764. doi: 10.1364/ao.48.000758. [DOI] [PubMed] [Google Scholar]; b) Jan CM. Opt. Express. 2011;19:5431–5441. doi: 10.1364/OE.19.005431. [DOI] [PubMed] [Google Scholar]
- 6.a) Feringa BL, van Delden RA. Angew. Chem. Int. Ed. 1999;38:3418–3438. doi: 10.1002/(sici)1521-3773(19991203)38:23<3418::aid-anie3418>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]; b) Sato I, Yamashima R, Kadowaki K, Yamamoto J, Shibata T, Soai K. Angew. Chem. Int. Ed. 2001;40:1096–1098. doi: 10.1002/1521-3773(20010316)40:6<1096::aid-anie10960>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]; c) Podlech J, Gehring T. Angew. Chem. Int. Ed. 2005;44:5776–5777. doi: 10.1002/anie.200501742. [DOI] [PubMed] [Google Scholar]; d) Pagni RM, Compton RN. Mini-Rev. Org. Chem. 2005;2:546–564. [Google Scholar]; e) Cave RJ. Science. 2009;323:1435–1436. doi: 10.1126/science.1169338. [DOI] [PubMed] [Google Scholar]
- 7.Yeom J, Yeom B, Chan H, Smith KW, Dominguez-Medina S, Bahng JH, Zhao G, Chang W-S, Chang S-J, Chuvilin A, Melnikau D, Rogach AL, Zhang P, Link S, Král P, Kotov NA. Nat. Mater. 2014;14:66–72. doi: 10.1038/nmat4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song F, Wei G, Jiang X, Li F, Zhu C, Cheng Y. Chem. Commun. 2013;49:5772–5774. doi: 10.1039/c3cc42323a. [DOI] [PubMed] [Google Scholar]
- 9.a) Muller G. Dalton Trans. 2009:9692–9707. doi: 10.1039/b909430j. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Tsumatori H, Harada T, Yuasa J, Hasegawa Y, Kawai T. Appl. Phys. Express. 2011;4:011601. (3 pages) [Google Scholar]
- 10.a) Riehl JP, Muller G. In: Comprehensive Chiroptical Spectroscopy. Berova N, Polavarapu PL, Nakanishi K, Woody RW, editors. Hoboken: Wiley; 2012. pp. 65–90. and references therein. [Google Scholar]; b) Castiglioni E, Abbate S, Lebon F, Longhi G. Methods, Appl. Fluoresc. 2014;2:024006. doi: 10.1088/2050-6120/2/2/024006. (7 pages) [DOI] [PubMed] [Google Scholar]; c) Muller G. In: Luminescence of Lanthanide Ions in Coordination Compounds and Nanomaterials. de Bettencourt-Dias A, editor. Chichester: Wiley; 2014. pp. 77–124. [Google Scholar]
- 11.The level of CPL is usually given by the luminescence dissymmetry ratio, glum(λ) = 2ΔI/I = 2(IL−IR)/(IL+IR), where IL and IR refer, respectively, to the intensity of left and right circularly polarized emissions.
- 12.a) Seitz M, Moore EG, Ingram AJ, Muller G, Raymond KN. J. Am. Chem. Soc. 2007;129:15468–15470. doi: 10.1021/ja076005e. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Okutani K, Nozaki K, Iwamura M. Inorg. Chem. 2014;53:5527–5537. doi: 10.1021/ic500196m. [DOI] [PubMed] [Google Scholar]
- 13.a) Sánchez-Carnerero EM, Agarrabeitia AR, Moreno F, Maroto BL, Muller G, Ortiz MJ, de la Moya S. Chem. Eur. J. 2015;21:13488–13500. doi: 10.1002/chem.201501178. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kumar J, Nakashima T, Kawai T. J. Phys. Chem. Lett. 2015;6:3445–3452. doi: 10.1021/acs.jpclett.5b01452. [DOI] [PubMed] [Google Scholar]; c) Gon M, Morisaki Y, Yoshiki C. Eur. J. Org. Chem. 2015:7756–7762. [Google Scholar]; d) Nakabayashi K, Kitamura S, Suzuki N, Guo S, Fujiki M, Imai Y. Eur. J. Org. Chem. 2016:64–69. [Google Scholar]; e) Gon M, Morisaki Y, Sawada R, Chujo Y. Chem. Eur. J. 2016;22:2291–2298. doi: 10.1002/chem.201504270. [DOI] [PubMed] [Google Scholar]
- 14.a) Sakai H, Shinto S, Kumar J, Araki Y, Sakanoue T, Takenobu T, Wada T, Kawai T, Hasobe T. J. Phys. Chem. C. 2015;119:13937–13947. [Google Scholar]; b) Alnoman RB, Rihn S, O´Connor DC, Black A, Costello B, Waddell PG, Clegg W, Peacock RD, Herrebout W, Knight JG, Hall MJ. Chem. Eur. J. 2016;22:93–96. doi: 10.1002/chem.201504484. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Longhi G, Castiglioni E, Villani C, Sabia R, Menichetti S, Viglianisi C, Devlin F, Abbate S. J. Photochem. Photobiol., A. 2016 http://dx.doi.org/10.1016/j.jphotochem.2015.12.011. [Google Scholar]
- 15.Sánchez-Carnerero EM, Moreno F, Maroto BL, Agarrabeitia AR, Bañuelos J, Arbeloa T, López-Arbeloa I, Ortiz MJ, de la Moya S. Chem. Commun. 2013;49:11641–11643. doi: 10.1039/c3cc47570k. [DOI] [PubMed] [Google Scholar]
- 16.a) Beer G, Niederalt C, Grimme S, Daub J. Angew. Chem. Int. Ed. 2000;39:3252–3255. [PubMed] [Google Scholar]; b) Beer G, Rurack K, Daub J. Chem. Commun. 2001:1138–1139. [Google Scholar]; c) Haefele A, Zedde C, Retailleau P, Ulrich G, Ziessel R. Org. Lett. 2010;12:1672–1675. doi: 10.1021/ol100109j. [DOI] [PubMed] [Google Scholar]; d) Lerrick RI, Winstanley TPL, Haggerty K, Wills C, Clegg W, Harrington RW, Bultinck P, Herrebout W, Benniston AC, Hall MJ. Chem. Commun. 2014;50:4714–4716. doi: 10.1039/c4cc00851k. [DOI] [PubMed] [Google Scholar]; e) Bruhn T, Pescitelli G, Jurinovich S, Schaumlöffel A, Witterauf F, Ahrens J, Bröring M, Bringmann G. Angew. Chem. Int. Ed. 2014;53:14592–14595. doi: 10.1002/anie.201408398. [DOI] [PubMed] [Google Scholar]
- 17.a) Gossauer A, Fehr F, Nydegger F, Stöckli-Evans H. J. Am. Chem. Soc. 1997;119:1599–1608. [Google Scholar]; b) Gossauer A, Nydegger F, Kiss T, Sleziak R, Stoeckli-Evans H. J. Am. Chem. Soc. 2004;126:1772–1780. doi: 10.1021/ja030542r. [DOI] [PubMed] [Google Scholar]; c) Kolemen S, Cakmak Y, Kostereli Z, Akkaya EU. Org. Lett. 2014;16:660–663. doi: 10.1021/ol403193f. [DOI] [PubMed] [Google Scholar]; d) Ma X, Azeem EA, Liu X, Cheng Y, Zhu C. J. Mater. Chem. C. 2014;2:1076–1084. [Google Scholar]; e) Zhang S, Wang Y, Meng F, Dai C, Cheng Y, Zhu C. Chem. Commun. 2015;51:9014–9017. doi: 10.1039/c5cc01994j. [DOI] [PubMed] [Google Scholar]; f) Alnoman RB, Rihn S, O'Connor DC, Black FA, Costello B, Waddell PG, Clegg W, Peacock RD, Herrebout W, Knight JG, Hall MJ. Chem. Eur. J. 2016;22:93–96. doi: 10.1002/chem.201504484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rohand T, Baruah M, Qin W, Boens N, Dehaen W. Chem. Commun. 2006:266–268. doi: 10.1039/b512756d. [DOI] [PubMed] [Google Scholar]
- 19.a) Yang L, von Zelewski A, Nguyen HP, Muller G, Labat G, Stoeckli-Evans H. Inorg. Chim. Acta. 2009;362:3853–3856. doi: 10.1016/j.ica.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Field JE, Muller G, Riehl JP, Venkataraman D. J. Am. Chem. Soc. 2003;125:11808–11809. doi: 10.1021/ja035626e. [DOI] [PubMed] [Google Scholar]; c) Sánchez-Carnerero EM, Moreno F, Maroto BL, Agarrabeitia AR, Ortiz MJ, Vo BG, Muller G, de la Moya S. J. Am. Chem. Soc. 2014;136:3346–3349. doi: 10.1021/ja412294s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.a) Kumar J, Nakashima T, Tsumatori H, Mori M, Naito M, Kawai T. Chem. Eur. J. 2013;19:14090–14097. doi: 10.1002/chem.201302146. [DOI] [PubMed] [Google Scholar]; b) Abbate S, Longhi G, Lebon F, Castiglione E, Superchi S, Pisani L, Fontana L, Torricelli F, Coronna T, Villani C, Sabia R, Tommasini M, Lucotti A, Mendola D, Mele A, Lightner DA. J. Phys. Chem. C. 2014;118:1682–1695. [Google Scholar]; c) Amako T, Nakabayashi K, Mori T, Inoue Y, Fujiki M, Imai Y. Chem. Commun. 2014;50:12836–12839. doi: 10.1039/c4cc04228j. [DOI] [PubMed] [Google Scholar]; d) Yuasa J, Ueno H, Kawai T. Chem. Eur. J. 2014;20:8621–8627. doi: 10.1002/chem.201402268. [DOI] [PubMed] [Google Scholar]; e) Kitamura S, Nakabayashi K, Wakabayashi T, Tajima N, Fujiki M, Imai Y. RSC Adv. 2015;5:67449–67453. [Google Scholar]; f) Sheng Y, Shen D, Zhang W, Zhang H, Zhu C, Cheng Y. Chem. Eur. J. 2015;21:13196–13200. doi: 10.1002/chem.201502193. [DOI] [PubMed] [Google Scholar]
- 21.a) Niu S, Ulrich G, Retailleau P, Ziessel R. Tetrahedron Lett. 2011;52:4848–4853. [Google Scholar]; b) Boens N, Leen V, Dehaen W. Chem. Soc. Rev. 2012;41:1130–1172. doi: 10.1039/c1cs15132k. [DOI] [PubMed] [Google Scholar]; c) Bonnier C, Machin DD, Abdi O, Koivisto BD. Org. Biomol. Chem. 2013;11:3756–3760. doi: 10.1039/c3ob40213d. [DOI] [PubMed] [Google Scholar]
- 22.For example see: Duran-Sampedro G, Palao E, Agarrabeitia AR, de la Moya S, Boens N, Ortiz MJ. RSC Adv. 2014;4:19210–19213. Esnal I, Duran-Sampedro G, Agarrabeitia AR, Bañuelos J, García-Moreno I, Macías MA, Peña-Cabrera E, López-Arbeloa I, de la Moya S, Ortiz MJ. Phys. Chem. Chem. Phys. 2015;17:8239–8247. doi: 10.1039/c5cp00193e.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.