ABSTRACT
To explore the effects of hydrogen sulfide (H2S) on renal fibrosis and the expressions of connexins and matrix metalloproteinase (MMP) in diabetic rats. Method: SD rats were divided into 4 groups randomly: control group(n = 12), STZ group (n = 12), STZ+ H2S group (n = 12), and H2S group (n = 12). Diabetic model was induced by intraperitoneal injections of STZ. After 72 h after injection, the rats whose blood glucose were higher than 16.7 mmol/L. STZ+H2S group and H2S group were injected NaHS by intraperitoneal. Control group and STZ group were injected with the same dose of normal saline (NS) in equal doses every day. 8 weeks later, urine were collected. The expression of connexin and matrix metalloproteinase was analyzed by Western blot. We measured the Kidney hydroxyproline content by hydrolysis method. Type II collagen content was detected by immunohistochemical method and Masson staining. Results: Compared with the control group, collagen accumulation was markedly enhanced, and the content of type II collagen, kidney hydroxyproline and timp-2 were boosted in STZ group mice (P < 0.01), while the expressions of CX40,CX43, CX45, MMP-1 and MMP-2 were obviously deceased (P < 0.01). Compared with STZ group, the expressions of CX40, CX43, CX45, MMP-1 and MMP-2 were significantly increased (P < 0.01), while the content of type II collagen, kidney hydroxyproline and timp-2 expression were markedly deceased in STZ+H2S group. Conclusion: H2S may attenuate renal fibrosis by up-regulating the expressions of CX40, CX43, CX45 and regulating the MMPs/TIMPs parameters.
KEYWORDS: connexin, diabetic, fibrosis, hydrogen sulfide, renal
Introduction
Diabetic nephropathy (DN) is the major chronic complications in the end-stage nephropathy.1,2 The pathological change of renal fibrosis exists in the whole course of the occurrence and development of DN; its manifestations include increase of collagenous fiber elements of extracellular matrix in mesangial cells. It has been found that renal interstitial fibrosis is a concomitant pathological change in the occurrence and development of DN.3 The disorder of matrix metalloproteinases and the tissue inhibitors of metalloproteinases (MMPS/TIMPs) have relation with nephropathy fibrosis.4 Studies show that gap junction plays a significant role in ischemic renal injury and the occurrence and development of DN, and Cx43, Cx40 and Cx45 are the main connexins expressed in the kidney. It has been found that constantly lowering Cx43 expression in cells after tissue injury may lead to changes of intercellular junction and intracellular space, thus causing the pathophysiological changes of DN.5,6 As the third important gaseous signal molecule in the body after NO and CO, H2S is involved in regulating renal function, neuron system, cardiovascular system, inflammatory response and gastrointestinal system.7 By building diabetic rat model, this research was to observe renal fibrosis and change of Cxs expression in diabetic rats, as well as the influences of H2S on myocardial fibrosis and Cxs expression.
Materials and methods
Animals and reagents
Sprague-Dawley (SD) rats weighting 288-300g were purchased from the SJA Lab Animal Central of Changsha (Changsha, China). STZ was obtained from MP Biomedicals, LLC. (USA). Sodium hydrosulfide (NaHS) was obtained from Sigma-Aldrich LLC. (USA). Antibodies for Connexin-40 (BA1539-1), Connexin-43 (BA1727), Connexin-45 (BA1728), MMP1 (BA1270), MMP2 (BA0569), TIMP2 (BA0576-2) were obtained from BOSTER (Wuhan, China). Hydroxyproline detection kit was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Anti-rabbit secondary antibody was obtained from Kirkegaard & Perry Laboratories, Inc. (USA).
Model establishment and experimental protocol
The rats were divided into 4 groups: Control group, STZ group, STZ+H2S group and H2S group. Diabetic rats were induced injection of STZ (40 mg/kg). After STZ Dissolved quickly in 0.1 mol/l sodium citrate buffer 72 hours after STZ injection, only those with a glycemia level ≥ 16.7mmol/l.8,9 were considered as successful diabetes model. The control group and STZ group were daily injected with NS for 8 weeks, while the STZ + H2S group and H2S group were intraperitoneally administered with an equivalent volume of NaHS at 100umol/kg10-12 daily for 8 weeks. The rats were killed by chloral hydrate (350 mg/kg).
Histopathological examination
Kidney samples were fixed (4% paraformaldehyde), embedded (Paraffin), sectioned, stained (Van Gieson staining), observed at magnification under a light microscope (*100). The expression of Collagen II was determined by immunohistochemistry. Histological study of the kidneys of STZ group showed basement membrane thickening, and the level of collagen fibers was significantly higher in STZ group than Control group. But in the STZ+H2S group, the level of collagen fibers was significant deceased. The study of immunohistochemistry, the normal rats glomerulus surrounded by the Collagen II. The kidneys of STZ group showed the level of collagen II was significant higher than that in Control group. The level of collagen II was significant deceased in H2S group.
Specimen collection and processing
After 8 weeks, 24h urinary protein (Ualb) and 24h urinary microalbumin excretion were determined by biochemical methods. The urine samples were collected, the urine volume was calculated and the content of urinary protein was detected. The urinary microalbumin excretion were determined in Clinical laboratory of the First Affiliated Hospital of University of South China. BCA Protein Assay Kit measured the urinary protein.
Hydroxyproline content assay
Hydrolysis method was the way to calculate the kidney hydroxyproline. The tissue of rats which were cut into 10 mg pieces, were added into 1ml hydrolysates. Then, the kidney samples were hydrolyzed at 100°C for 20 min. The hydroxyproline content was measured through absorbance was measured by wavelength of 490 nm.
Western blot analysis
Kidney were homogenized in lysis buffer [20 mM Tris–HCl, 150 mM NaCl, H 7.5, 1mM phenylmethylsulphonylfluoride (PMSF), 1% Triton X-100, EDTA and 1 mM Na3VO4, leupeptin]. Then it was centrifuged (12,000 r.p.m. for 10 min at 4°C), collect the supernatant. Protein was assessed (BCA™ Protein Assay Kit). Then sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Then these proteins were transferred to a PVDF membrane and blocked in TBST buffer (50 mM Tris-HCl,150 mM NaCl, pH 7.5, 0.05% Tween-20) for 2 h. The membranes were incubated primary antibody overnight at 4°C after washing. Then it was observed with an image analysis system.
Statistical analysis
Statistical analyses ues by SPSS 18.0. Values were presented as mean ± SD. Statistical differences between groups were assessed by one-way ANOVA. A P value of less than 0.05 was considered to be statistically significant.
Results
Effects of H2S on pathological changes in diabetic rats
Histological study of the normal kidneys of the nondiabetic rats revealed that have not showed any inflammatory changes surrounded by the proximal and distal convoluted tubules in normal group. The kidneys of untreated diabetic rats showed degenerated glomeruli infiltrated by the inflammatory cells and basement membrane thickening. Histological study of the kidneys of STZ group showed that the basement membrane was thickened and the level of collagen fibers was significantly higher in STZ group than Control group. But in the diabetic rats treated with the H2S, the level of collagen fibers was significant deceased (Fig. 1).
Figure 1.
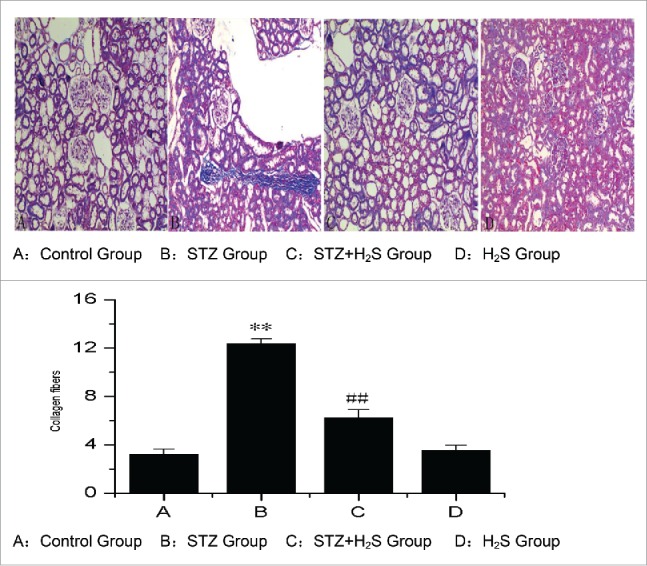
Pathological changes in diabetic rats in each group. (A: Control Group, B: STZ Group, C : STZ+H2S Group, D : H2S Group).
Effects of H2S on expression of collagen II in diabetic rats
Immunohistochemistry study of the normal rats revealed normal glomerulus with Collagen II surrounding. The level of collagen II in the kidneys of STZ group was much higher than that in Control group. The level of collagen II was significant deceased in H2S group (Fig. 2).
Figure 2.
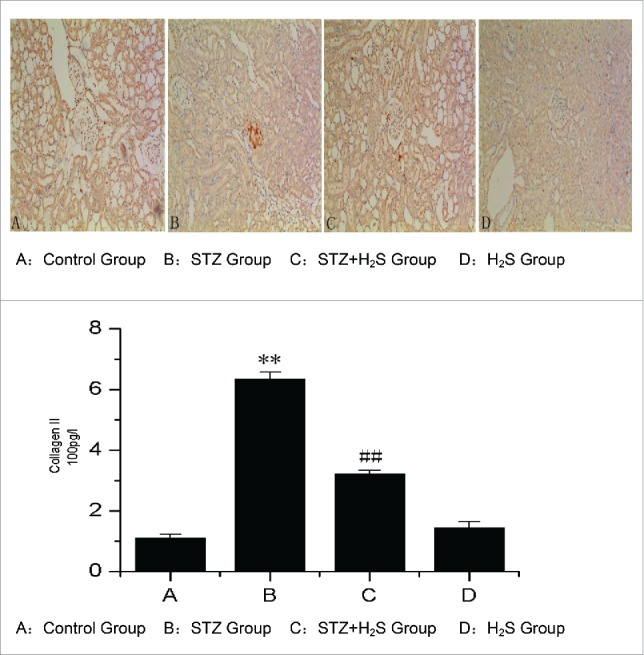
Expression of Collagen II in diabetic rats in each group. (A: Control Group, B: STZ Group, C: STZ+H2S Group, D: H2S Group).
Effects of H2S on 24h urinary protein (Ualb) and 24h urinary microalbumin excretion
We detected the 24h urinary protein (Ualb) and 24h urinary microalbumin excretion. The results showed that, comparing to the Control group, the content of Ualb and 24h urinary microalbumin excretion were both significantly increased in STZ group, but they were deceased in STZ+H2S group than those in STZ group after the intervention of H2S (Table 1).
Table 1.
Content of Ualb and 24 h urinary microalbumin excretion in each group.
| Groups | 24 h urinary protein(Ualb) | 24 h urinary microalbumin excretion |
|---|---|---|
| Control Groups | 11.8 ± 3.3 | 0.5 ± 0.3 |
| STZ Groups | 44.5 ± 6.4a | 13.0 ± 2.6a |
| STZ+H2S Groups | 26.6 ± 6.1b | 5.9 ± 2.4b |
| H2S Groups | 12.6 ± 4.0 | 0.6 ± 0.3 |
(The values are presented as mean ± SEM, aP < 0.01 vs Control group; bP < 0.01 vs STZ group).
Effects of H2S on hydroxyproline content in diabetes rats
Hydroxyproline content in diabetes rats in each group was showed below. Compared with control group, there was a significant higher level of hydroxyproline in STZ group. However, compared with STZ group, it deceased remarkably in STZ+H2S group (Fig. 3).
Figure 3.
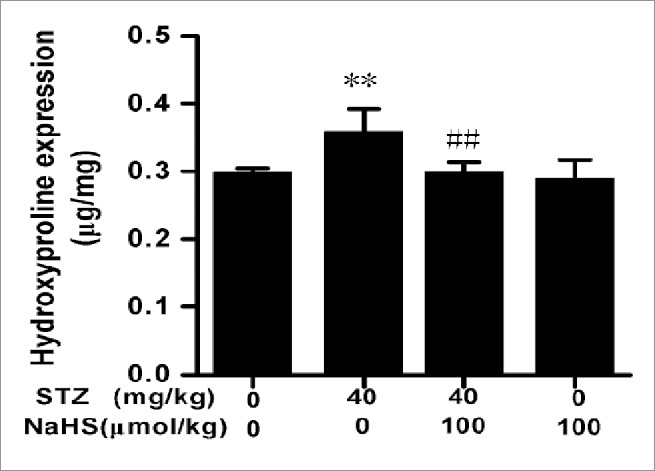
Expression of hydroxyproline in diabetic rats in each group. (**P < 0.01 vs Control group; ##P < 0.01 vs STZ group.
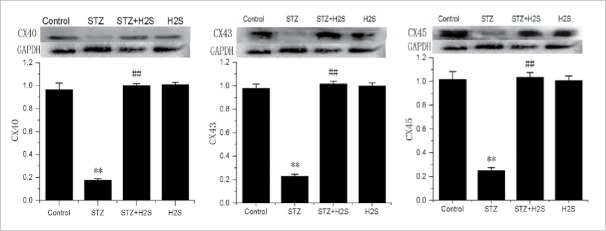
Effects of H2S on CX40, CX43 and CX45 expressions in diabetes rats
As shown in Fig. 4, compared with control group, there were an obvious lower expressions of CX40, CX43 and CX45 in STZ group. However, compared with STZ group, the expressions of CX40, CX43 and CX45 were significantly
Figure 4.
Expressions of CX40, CX43 and CX45 in diabetes rats in each group (normalized by GAPDH; **P < 0.01 vs Control group, ##P < 0.01 vs STZ group).
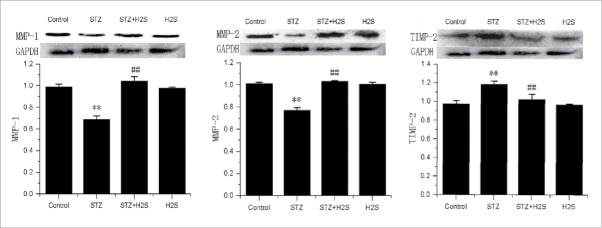
Effects of H2S on MMP-1, MMP-2 and TIMP-2 expressions in diabetes rats
As showed in Fig. 5, compared with control group, there was an obvious higher expression of TIMP-2, but lower expression of MMP-1 and MMP-2 in STZ group. However, compared with STZ group, the expression of TIMP-2 was significantly deceased, while those of MMP-1 and MMP-2 were significantly increased in STZ+H2S group.
Figure 5.
Expression of MMPs and TIMP in the kidney tissues of each group (normalized by GAPDH; **P < 0.01 vs Control group, ##P < 0.01 vs STZ group).
Discussion
DN is the most serious and common diabetic complication. It will soon develop from proteinuria in the early stage to renal hypertension and nephrotic syndrome, and eventually cause renal failure and even death.13 DN has now become the leading protopathy of chronic renal failure in clinic; the three year survival rate of DN patients after the onset of the disease is only about 50%.The typical pathological change of DN is glomerular sclerosis accompanied by such histological characteristics as thickening of renal tubule and glomerulus basement membrane, mesangial extracellular matrix (ECM) expansion, hypernephrotrophy and renal tubule interstitial fibrosis.14,15
Hydrogen sulfide (H2S) is a new signal molecule.Exogenous H2S can protect against diabetes-induced hypertrophyand myocardialfibrosis, dysfunction, oxidative stress,apoptosis.16,17 The pathological change of renal fibrosis exists in the whole course of the occurrence and development of DN; its manifestations include increase of collagenous fiber elements of extracellular matrix in mesangial cells, increase of collagen in renal tubule basement membrane and increase of renal tubule interstitial collagen and fibronection secretion. Collagen II is an important component of ECM. The disequilibrium of its synthesis and degradation is one of the major pathological processes that lead to renal fibrosis of patients with DN. Therefore, the increased synthesis and secretion and lowered degradation of collagen II is one of the major causes or key correlative factors that lead to the development of renal diseases, ECM accumulation and eventually glomerular sclerosis and renal interstitial fibrosis. The accumulations of the aforementioned collagens are often caused by increased mesangial cell synthesis or reduced degradation of metalloproteinases in mesangial matrix.15,16 Collagen II is one of the major members of the collagen family; its abnormally increased expression and irregular arrangement signify the occurrence of fibrosis. By using Masson staining, the present research found the significant increase of darkly stained collagenous fibers and cellular matrix in renal interstitium of diabetic rats; meanwhile, immunohistochemistry test also showed the significant increased expression of collagen II in glomerulus and tubulointerstitial, suggesting apparent fibrosis of renal interstitium in diabetic rats and its association with the reduced degradation of metalloproteinases.
The gap junctions composed of Cxs, the only membrane channel structure for direct information and energy exchanges between adjacent cells, play an important role in regulating cell metabolism, homeostasis, proliferation, differentiation and other physiological processes. Renal Cxs facilitate vascular conduction, tubular purinergic signaling, and juxtaglomerular apparatus calcium signaling. Gap junctions between histocytes participate jointly in multiple pathological processes, and they are also widely found in renal tissue.6,7 It has been found that Cx43 is the most common Cx expressed in the kidney, while Cx45 and Cx40 expressions are also detected to some extent. For normal renal tissue cells, gap junction channel structures closely assembled on adjacent cellular membrane are responsible for information exchange, regulation of messangial cell growth and reproduction, renin secretion, renal blood flow and so on. As the leading connexin in the kidney, Cx43 has a vital influence on the function of gap junction channel.11,12 DN is a dysmetabolism disease and pathological changes of gap junction between renal tissue cells among DN patients have been found. Other studies find that Cxs can inhibit the expressions of cyclin P21 and P27, thus postponing the hypertrophy and aging of mesangial cells in renal tissue and end-stage renal disease.18 In this research, the expressions of connexins in rat renal tissues were determined by Western blot. The result revealed the significant reductions of Cx43, Cx40 and Cx45 expressions in renal tissues of diabetic rats, indicating the potential correlation between the decrease of connexin and the renal interstitium fibrosis of diabetic rats.
H2S is a kind of gaseous signal molecule with extensive physiological functions such as anti-inflammation, anti-oxidation, anti-apoptosis, etc. It has been proved by researches that H2S can ameliorate myocardial fibrosis and DN.19 In this research, we noted that only a small portion of collagenous fibers and basement membranes of glomerular and renal tubule in the renal interstitium of rats were stained blue in the H2S intervention group, but they were lighter than those in the DN group. Meanwhile, positive staining of collagen II in glomerulus and tubulointerstitial also showed significant reduction in the H2S intervention group. Compared with control group, collagen accumulation was markedly enhanced in STZ group, and kidney hydroxyproline and timp-2 were increased in STZ group mice (P < 0.01). Compared with STZ group, the expressions of MMP-1 and MMP-2 were significantly increased, and kidney hydroxyproline and timp-2 were markedly deceased in STZ+H2S group. The expression levels of connexins such as Cx43, Cs40 and Cx45 in rat renal tissues were down-regulated significantly in the DN group, while the expression of Cx43 protein between the renal tissue cells showed an increase in the H2S intervention group, and the expressions of Cx40 and Cx45 were also up-regulated significantly, suggesting that the mechanism by which H2S improves renal tissue fibrosis in diabetic rats may be associated with the upregulation of connexin expression.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Quiroga B, Arroyo D, de Arriba G. Present and Future in the Treatment of Diabetic Kidney Disease. J Diabetes Res 2015; 2015:801348; PMID:25945357; http://dx.doi.org/ 10.1155/2015/801348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ding Y, Choi ME. Autophagy in diabetic nephropathy. J Endocrinol 2015; 224:15-30; http://dx.doi.org/ 10.1530/JOE-14-0437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ding Y, Choi ME. Regulation of autophagy by TGF-β: emerging role in kidney fibrosis. Seminars Nephrol 2014; 34:62-71; http://dx.doi.org/ 10.1016/j.semnephrol.2013.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li YY, McTiernan CF, Feldman AM. Interlpay of matrix metalloproteinases, tissus inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovascular Res 2000; 46:214-24; http://dx.doi.org/ 10.1016/S0008-6363(00)00003-1 [DOI] [PubMed] [Google Scholar]
- [5].Guo YN, Wang JC, Cai GY, Hu X, Cui SY, Lv Y, Yin Z, Fu B, Hong Q, Chen XM. AMPK-mediated downregulation of connexin43 and premature senescence of mesangial cells under high-glucose conditions. Exp Gerontol 2014; 51:71-81; PMID:24423443; http://dx.doi.org/ 10.1016/j.exger.2013.12.016 [DOI] [PubMed] [Google Scholar]
- [6].Liu L, Hu X, Cai GY, Lv Y, Zhuo L, Gao JJ, Cui SY, Feng Z, Fu B, Chen XM. High glucose-induced hypertrophy of mesangial cells is reversed by connexin43 overexpression via PTEN/Akt/mTOR signaling. Nephrol Dialysis Transplantation 2012; 27:90-100; http://dx.doi.org/ 10.1093/ndt/gfr265 [DOI] [PubMed] [Google Scholar]
- [7].Beltowski J. Hydrogen sulfide in pharmacology and medicine - An update. Pharmacological Reports 2015; 67:647-58; PMID:25933982; http://dx.doi.org/ 10.1016/j.pharep.2015.01.005 [DOI] [PubMed] [Google Scholar]
- [8].Zhou X, An G, Lu X. Hydrogen sulfide attenuates the development of diabetic cardiomyopathy. Clin Sci (Lond) March 2015; 128:325-35; PMID:25394291; http://dx.doi.org/ 10.1042/CS20140460 [DOI] [PubMed] [Google Scholar]
- [9].Zhong X, Wang L, Wang Y, Dong S, Leng X, Jia J, Zhao Y, Li H, Zhang X, Xu C, et al.. Exogenous hydrogen sulfide attenuates diabetic myocardial injury through cardiac mitochondrial protection. Mol Cellular Biochem December 2012; 371(1–2):187-98; http://dx.doi.org/ 10.1007/s11010-012-1435-3 [DOI] [PubMed] [Google Scholar]
- [10].Tang ZJ, Zou W, Yuan J, Zhang P, Tian Y, Xiao ZF, Li MH, Wei HJ, Tang XQ. Antidepressant-like and anxiolytic-like effects of hydrogen sulfide in streptozotocin-induced diabetic rats through inhibition of hippocampal oxidative stress. Behavioural Pharmacol August 2015; 26:427-35; http://dx.doi.org/ 10.1097/FBP.0000000000000143 [DOI] [PubMed] [Google Scholar]
- [11].Pan LL, Wang XL, Zhu YZ. Sodium hydrosulfide prevents myocardial dysfunction through modulation of extracellular matrix accumulation and vascular density. Int J Mol Sci 2014; 15:23212-26; PMID:25514418; http://dx.doi.org/ 10.3390/ijms151223212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sun L, Jin H, Sun L, Chen S, Huang Y, Liu J, Li Z, Zhao M, Sun Y, Tang C, et al.. Hydrogen sulfide alleviates myocardial collagen remodeling in association with inhibition of TGF-β/Smad signaling pathway in spontaneously hypertensive rats. Mol Med 2014; 20:503-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dalla Vestra M, Arboit M, Bruseghin M, Fioretto P. The kidney in type 2 diabetes: focus on renal structure. Endocrinologia Y Nutricion 2009; 56:18-20; PMID:20629225; http://dx.doi.org/ 10.1016/S1575-0922(09)73511-9 [DOI] [PubMed] [Google Scholar]
- [14].Bai Y, Wang L, Li Y, Liu S, Li J, Wang H, Huang H. High ambient glucose levels modulates the production of MMP-9 and alpha5(IV) collagen by cultured podocytes. Cellular Physiol Biochem 2006; 17:57-68; http://dx.doi.org/ 10.1159/000091464 [DOI] [PubMed] [Google Scholar]
- [15].Trojanek J. Matrix metalloproteinases and their tissue inhibitors. Postepy Bio Chemi 2012; 58:353-62 [PubMed] [Google Scholar]
- [16].Mazanowska O, Zabińska M, Kościelska-Kasprzak K, Kamińska D, Banasik M, Krajewska M, Madziarska K, Zmonarski SC, Chudoba P, et al.. Advanced age of renal transplant recipients correlates with increased plasma concentrations of interleukin-6, chemokine ligand 2 (CCL2), and matrix metalloproteinase 2, and urine concentrations of CCL2 and tissue inhibitor of metalloproteinase 1. Transplant Proc 2014; 46:2640-3; PMID:25380884; http://dx.doi.org/ 10.1016/j.transproceed.2014.08.030 [DOI] [PubMed] [Google Scholar]
- [17].Hanner F, Sorensen CM, Holstein-Rathlou NH, Peti-Peterdi J. Connexins and the kidney. Am J Physiol Regulatory Integrative Comparative Physiol 2010; 298:1143-55; http://dx.doi.org/ 10.1152/ajpregu.00808.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang X, Chen X, Wu D, Liu W, Wang J, Feng Z, Cai G, Fu B, Hong Q, Du J. Downregulation of connexin 43 expression by high glucose induces senescence in glomerular mesangial cells. J Am Society Nephrol 2006; 17:1532-42; http://dx.doi.org/ 10.1681/ASN.2005070776 [DOI] [PubMed] [Google Scholar]
- [19].Zhou X, Feng Y, Zhan Z, Chen J. Hydrogen sulfide alleviates diabetic nephropathy in a streptozotocin-induced diabetic rat model. J Biol Chem 2014; 289:28827-34; PMID:25164822; http://dx.doi.org/ 10.1074/jbc.M114.596593 [DOI] [PMC free article] [PubMed] [Google Scholar]