ABSTRACT
The western area of the Jilin province, a typical seasonal frost region, is located in the southern Songnen plain of China. Significantly salinized soils are widely distributed on the Songnen plain in western Jilin. Soil salinization can cause degradation of cultivated land and grass, which threatens the human environment. To investigate the treatment of saline-alkali soil, a laboratory test was conducted to evaluate the ability of sulfur-oxidizing bacteria to improve the performance of saline-alkali soil in western Jilin. The results showed that sulfur-oxidizing bacteria treatment was suitable for the soil from pH 7.5 to 8, and 50 ml thiobacillusthiooxidans showed the best improvement to the saline-alkali soil.
KEYWORDS: biological treatment, land salinization, saline-alkali soil, sulfur-oxidizing bacteria, soil improvement
Introduction
Today, saline-alkali soil has a significant negative effect on agricultural production and vegetation growth. However, biological treatment can change the soil's structure and increase microbial activity, which can provide more favorable nutritional conditions for vegetation growth. The improvement of saline-alkali soil by biological treatment can be used as a sort of agricultural resource.1 Bacterial treatment can provide plenty of beneficial microorganisms and organic matter, which produces massive amounts of organic acid.2 Because of this, the physicochemical properties and microbial activity of the soil can be positively changed, and the fertility of the soil can be improved. In this paper, the biological nature of sulfur-oxidizing bacteria was used to improve saline-alkali soil. When the biochemical reaction took place in the saline-alkali soil, the pH and salt content of the soil decreased.
Western Jilin province is a typical seasonal frozen soil distribution area of northeast China, which has massive areas of saline-alkali soil. Soil salinization not only brings serious harm to agricultural production, but also leads to damage to engineering projects such as frost heave and differential settlement of highways.3 In this paper, soil samples were taken from Da'an in Jilin province and treated biologically with sulfur-oxidizing bacteria to provide a basic understanding of a potential technique to prevent soil salinization in western Jilin.
Materials
Soil materials
Soil samples were taken from the Da'an area of Western Jilin province during autumn. 8 samples were collected at depths of 0 cm, 10 cm, 20 cm, 30 cm, 40 cm, 50 cm, 70 cm and 100 cm. As is typical of this kind of field, the surface of the earth was dry and gray-white because of the massive crystals of soluble salt, while the soil underground was wet and either black-brown or gray.
Table 1 shows the total soluble salt content and each kind of salinity composition of the soil samples.
Table 1.
Basic chemical properties of soil samples.
| Soluble salt component content/% |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Depth of soil samples/cm | Total soluble salt content/% | Na+ | K+ | Ca+ | Mg+ | HCO3− | Cl− | SO42− | Organic matter content/ | pH | CEC content/mmol/100 g |
| 0 | 0.740 | 0.02 | 7.50 | 0.22 | 0.15 | 24.94 | 0.38 | 0.02 | 0.26 | 9.0 | 11.58 |
| 10 | 0.250 | 0.14 | 3.39 | 0.21 | 0.12 | 22.48 | 0.30 | 0.02 | 0.39 | 8.5 | 12.11 |
| 20 | 0.180 | 0.08 | 2.12 | 0.31 | 0.21 | 15.63 | 0.20 | 0.08 | 0.32 | 8.2 | 13.48 |
| 30 | 0.150 | 0.08 | 1.75 | 0.16 | 0.21 | 12.99 | 0.10 | 0.06 | 0.31 | 8.2 | 10.87 |
| 40 | 0.060 | 0.08 | 1.38 | 0.21 | 0.16 | 11.24 | 0.10 | 0.00 | 0.28 | 7.9 | 10.60 |
| 50 | 0.080 | 0.08 | 1.11 | 0.08 | 0.21 | 7.78 | 0.05 | 0.02 | 0.29 | 7.8 | 8.86 |
| 70 | 0.060 | 0.01 | 0.88 | 0.08 | 0.29 | 6.20 | 0.05 | 0.02 | 0.24 | 7.7 | 8.56 |
| 100 | 0.090 | 0.01 | 0.78 | 0.12 | 0.31 | 5.20 | 0.05 | 0.04 | 0.09 | 7.6 | 9.86 |
According to Table 1, the pH of 8 of the soil samples was higher than 7, indicating that all of the soil samples were alkaline. The pH increased as the depth increased, meaning that the alkalinity of the soil was reducing with depth.
Bacteria materials
The secreta and wracks of flora and fauna metabolic activity in soil provide luxuriant organic nutrition of microorganisms. The minerals in the soil contain microelements such as sulfur, phosphorus, potassium, iron, magnesium, calcium, boron, molybdenum, zinc, and manganese, among others. Therefore, sulfur-oxidizing bacteria can be cultivated by the culture medium, which is rich in sulfur, phosphorus, potassium, iron, magnesium and calcium.
The Sulfur-oxidizing bacteria was taken from mineral water and mineral debris. After inoculation, it was enriched and purified by thiobacillusthiooxidans and thiobacillusthioparus. The bacteria used in the experiment was purified 7 times.
Purified thiobacillusthioparus liquid was made by the following purge process. Sodium thiosulfate medium was mixed with a inoculater water sample in a pH range of 7.0–7.2. The sample was then cultivated for 7–10 d at a temperature of 28°C to 30°C.
Purified thiobacillusthiooxidans was made by the following purge process: Thiobacillusthiooxidans medium was inoculated with coal mine debris, which has thiobacillusthiooxidans, in a pH range of 4.5–5.0. The sample was then cultivated for 7–10 d at a temperature of 28°C to 30°C.
Theory and methods
Theory
Sulfur-oxidizing bacteria is a kind of chemoautotrophy bacteria, which is widely distributed in soil, fresh water, salt water, hot springs and sulfur mines. It can oxidize hydrogen sulfide and other sulfides into elemental sulfur; sulfide is the electron donor and oxygen is the electron acceptor.4 The reaction equations are as Eq. (1) and (2):5
| (1) |
| (2) |
Sulfur-oxidizing bacteria can also oxidize elemental sulfur into energy, which is needed for cell growth and metabolism. The oxidative product is sulfuric acid, and more soluble sulfate gathers in the water. The biochemical reaction equation is as Eq. (3):6
| (3) |
During the biochemical reaction, sulfur-oxidizing bacteria produce sulfuric acid and take the neutralization reaction to OH in soil. The reaction can lower the pH of the soil.
Methods
In order to compare the improvement effects of different bacteria, schemes were designed to improve the saline-alkali soil samples. Table 2 shows the culture kinds and contents of each scheme. 400 ml water was added to a 600 g soil sample in a small bowl. Then, the bacteria and sulfur were added into the samples. The whole experiment lasted for 20 d. After the experiment ended, the pH of the soil lixivium was tested, and the improvement effect can be discussed by the results.
Table 2.
Bacteria content of different schemes.
| No. of scheme | Thiobacillusthiooxidans/ml | Thiobacillusthioparus/ml | Sulfur powder/g |
|---|---|---|---|
| A | 25 | / | 3 |
| B | 50 | / | 6 |
| C | 100 | / | 12 |
| D | 50 | 10 | 6 |
| E | 15 | 10 | 6 |
| F | / | / | 6 |
Results
According to the experimental methods in this research, pH of soil was tested after the experiment, and the results are shown in Table 3. The variation of the pH as the depth increased is shown in Fig. 1.
Table 3.
Results of saline-alkali soil improvement experiment.
| No. of scheme |
||||||
|---|---|---|---|---|---|---|
| Depth of soil sample | A | B | C | D | E | F |
| 0 | 9 | 9.1 | 9 | 9 | 9 | 8.9 |
| 10 | 8.5 | 8.5 | 8.6 | 8.5 | 8.5 | 8.5 |
| 20 | 8.3 | 8.2 | 8.2 | 8.2 | 8.3 | 8.1 |
| 30 | 8.1 | 8.1 | 8.2 | 8.1 | 8.1 | 8 |
| 40 | 7.6 | 7.5 | 7.6 | 7.6 | 7.5 | 7.4 |
| 50 | 7.4 | 7.3 | 7.5 | 7.4 | 7.4 | 7.2 |
| 70 | 7.1 | 7.1 | 7.3 | 7.1 | 7 | 7.1 |
| 100 | 7.1 | 7 | 7.2 | 7.1 | 7.1 | 7 |
Figure 1.
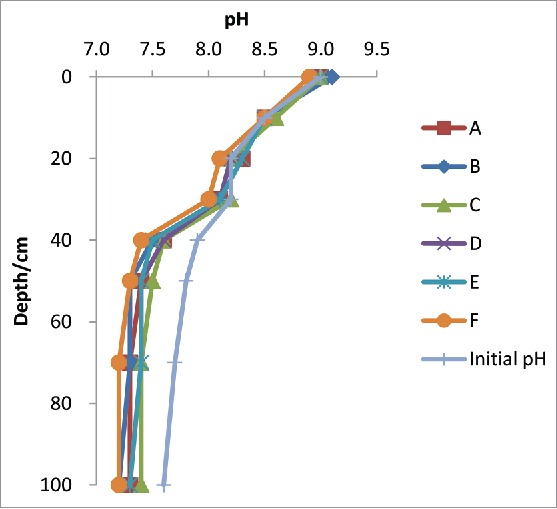
pH of soil samples before and after improvement experiment.
Discussion
According to Table 3 and Fig. 1, the pH of the soil samples all decreased in different degrees after the biological improvement experiment. The pH of the 0 cm soil samples hardly changed. This is likely because the initial pH is too high (pH = 9.0) for the bacteria to survive. The pH of the 20 cm to 30 cm soil samples reduced at a lower rate than the 40 cm to 50 cm soil samples, while the pH of the 70 cm to 100 cm soil samples only slightly reduced. This indicates that for the sulfur-oxidizing bacteria to be the most effective in improving saline-alkali soil, the soil should have an initial pH between 7.5 and 8.0. When the initial pH is too high, the sulfur-oxidizing bacteria cannot survive, and when the initial pH is too low, the soil salinization and alkalinization degree is low, and the improvement effect of biological treatment is low. Also, if the pH of the soil is too low, it is not conducive to crop growth.
When contrasted with different kinds and contents of bacteria, scheme E showed the best improvement effects. Therefore, only sulfur power shows the effects. Between the schemes of thiobacillusthiooxidans bacteria (A to C), 50 ml thiobacillusthiooxidans showed the best effect. This indicates that a moderate amount of thiobavillusthiooxidans is optimal. Between schemes D and E, the improvement effect didn't show much difference between the different contents of mixed bacteria.7 It can therefore be inferred that the content of mixed bacteria did not have a significant effect on the treatment of saline-alkali soil.
Conclusions
Saline-alkali soil is widely distributed in western Jilin. All of the soil samples had pH values of 7 or above, and the pH increased as the depth increased.
Sulfur-oxidizing bacteria can lower the pH of the soil, and this method can be used in western Jilin.
The most effective initial pH of soil samples for improvement by sulfur-oxidizing bacteria is between 7.5 and 8.0.
Of all of the treatments that were tested, 50 ml contantthiobacillusthiooxidans was the most effective in improving saline-alkali soil.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by Jilin Province Science and Technology Development Projects (grant number: No.20150623024TC-13), Scientific Research Project of Jilin Province Department of Education (grant number: [2016] No. 150), and the Natural Science Foundation of China (grant number: NO.41430642, NO.41372267).
References
- [1].Jing Z, Qing W, Xiaoru Li, Tie S, Qianying Q. Research on improving the Saline-sodic soil by sulfur-oxidizing bacteria. J Jilin Univ (Earth Science Edition) 2009; 39:147-51 (in Chinese). [Google Scholar]
- [2].Ningya Z, Ming Z. Research progress of microorganism agents on saline-alkaline soil microbial remediation. Anhui Agri Sci Bull 2013; 19:22-24. [Google Scholar]
- [3].Shuochao B, Qing W, Xinhua B, Zijian W. Characters of saline-alkali soil in Western Jilin and biological treatment. Journal of Pure Applied Microbiology 2013; 809-12. [Google Scholar]
- [4].Bossuyt H, Denef K, Six J, Frey SD, Merckxb R, Paustiana K. Influence of microbial population sand residue quality on aggregate stability. Appl Soil Ecol 2001; 16:195-208; http://dx.doi.org/ 10.1016/S0929-1393(00)00116-5 [DOI] [Google Scholar]
- [5].Robertson Lesley A, Kuenen JG. The colorless sulfur bacteria. Prokaryoters 2006; 6:985-1011; http://dx.doi.org/ 10.1007/0-387-30742-7_31 [DOI] [Google Scholar]
- [6].Craig DT, Carl OW, Francoise G. Rapid microbial production of filamentous sulfur mats at hydrothermal vents. Appl Environ Microbiol 1999; 65:2253-5; PMID:10224031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhang W, Wang ZL, Wang MY. Analyses of the vertical and temporal distributions of sulfate-reducing bacteria in sediments of Lake Erhai, southwest China. Res J Chem Environ 2012; 16:44-511. [Google Scholar]


