Abstract
Background
National registries have not adequately captured concurrent partial hepatic resections or ablations. Therefore, the aim of this analysis was to describe the patterns of concurrent partial resections and ablations in North America.
Methods
Patients undergoing a hepatic resection were identified using the American College of Surgeons-National Surgical Quality Improvement Program Targeted Hepatectomy database. Perioperative outcomes were compared for patients undergoing concurrent “wedge” resections and/or ablations and other subsets.
Results
A total of 2714 patients were identified who met inclusion criteria. Major hepatectomy was performed in 1037 patients (38.2%) while partial lobectomy was performed in 1677 (61.8%) patients. Concurrent “wedge” hepatic resections and ablations were undertaken in 56.0% and 14.2% of patients, respectively, and were more frequently performed among patients undergoing a partial lobectomy and among patients undergoing surgery for colorectal liver metastasis (both p < 0.001). While associated with a decreased incidence of postoperative complications (p = 0.027) and liver failure (p = 0.031) among patients undergoing a major hepatectomy, concurrent therapies were associated with comparable 30-day outcomes for patients undergoing partial lobectomy.
Conclusion
Concurrent “wedge” hepatic resections and ablations are performed in 56.0% and 14.2%, respectively of patients undergoing hepatectomy. Concurrent procedures were not associated with worse clinical outcomes.
Introduction
Liver resection is increasingly performed in the United States and often represents the only potentially curative option for patients presenting with primary and secondary neoplastic diseases of the liver.1, 2, 3, 4, 5 Although careful patient selection, improved operative technique, and more developed perioperative care pathways have resulted in decreased perioperative morbidity and mortality following liver resection, a significant proportion of patients presenting with hepatocellular carcinoma (HCC), colorectal liver metastasis (CRLM) or biliary malignancies are not candidates for curative resection due to inadequate functional hepatic reserve, multifocal disease or a combination of both.3, 6, 7, 8, 9
For patients who are not candidates for surgical resection due to multifocal disease, adjunct locoregional therapies including the concurrent use of thermal or alcohol ablation has been proposed.10, 11, 12 Combining hepatectomy with ablation permits the removal of the largest tumors while simultaneously ablating any smaller residual tumors.11, 13, 14 Using this concurrent treatment, not only are more patients candidates for surgical treatment but surgeons are also able to address all sonographically detectable smaller tumors.11, 13, 14 Additionally, the use of parenchymal-sparing liver resections (PSLRs), particularly for the treatment of CRLM and among those with a low functional hepatic reserve have increased in recent years.12, 15, 16 This approach allows for more radical resections while ensuring the maximum preservation of parenchyma and therefore potentially decreases risk for postoperative liver failure.12, 15, 16
Although recent studies have associated the use of concurrent/combined treatments with improved short and long-term clinical outcomes, information pertaining to outcomes following concurrent treatments is largely limited to single-center studies and therefore may lack external validity.11, 13 Furthermore, as nationally representative datasets are unable to accurately capture and code for concurrent surgical therapies, nationally representative estimates for the incidence and patterns of use for concurrent therapies are lacking.17 Given the need for accurate population-based outcomes data, the objective of the current study was to report the use of concurrent partial hepatic resections and/or ablations using a nationally representative cohort of patients undergoing liver resection. Additionally, the current study sought to evaluate short-term postoperative clinical outcomes among patients undergoing concurrent partial resections or concurrent ablations for neoplastic liver disease.
Methods
Study population and data sources
A set of patients undergoing a liver resection between January 01, 2014 and December 31, 2014 were identified using the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) Hepatectomy Targeted Participant Use Data File (PUF) for 2014.18 For each patient record, the ACS-NSQIP Hepatectomy Targeted PUF includes data pertaining to baseline demographics including patient age, sex and race, as well as detailed clinical variables describing preoperative comorbidity, the primary indication for surgery, intra- and postoperative clinical details, 30-day postoperative morbidity and 30-day postoperative mortality.18 In addition to standard NSQIP variables, the Hepatectomy Targeted PUF also gathers data on neoadjuvant therapy, history of viral hepatitis, Pringle maneuver, biliary reconstruction, drain usage, bile leak, post-hepatectomy liver failure (PHLF) and pathology. All data are prospectively specified and managed by trained surgical reviewers, and the program systematically audits to ensure inter-reviewer reliability and quality assurance.18 As all patient records are de-identified and compliant with the Health Insurance Portability and Accountability Act of 1996, patient consent was waived and the study was approved by the Johns Hopkins University Institutional Review Board.
Patients undergoing an elective major hepatectomy (Current Procedural Terminology [CPT] codes “47122,” “47125,” and “47130”) or a partial lobectomy (CPT code “47120”) were identified.19 Patients undergoing minor “wedge” resections (CPT code “47100”) as the primary procedure were not included. Patients undergoing a surgical resection for benign disease, primary malignancy of the liver, or secondary malignancy of the liver were included in the final study cohort; patients who underwent surgery on an emergent basis or patients who were transferred in from another medical center were excluded. Preoperative patient body mass index (BMI) was classified according the World Health Organization (WHO) classification for BMI, while preoperative performance status was assessed using the American Society of Anesthesiologists (ASA) physical status classification score.20, 21 The receipt of concurrent partial wedge resections and the use of concurrent radiofrequency, microwave or alcohol ablation was determined using the ACS-NSQIP Procedure Targeted module.
Postoperative outcomes
The primary outcomes of interest were bile leak, liver failure, 30-day postoperative morbidity, 30-day postoperative mortality, length-of-stay (LOS) and 30-day readmission. Liver failure was classified as either: grade A-abnormality in laboratory parameters but not requiring change in clinical management; grade B-deviation from clinical management without invasive treatment; or as grade C-deviation from clinical management requiring invasive treatment according to the International Study Group for post-hepatectomy liver failure (PHLF).22 Postoperative morbidity was defined using a composite measure for postoperative complications generated from all postoperative complications specified within the ACS-NSQIP and included surgical site infections, pneumonia, need for intubation, ventilator dependence, venous thromboembolism (pulmonary embolism or deep venous thromboembolism), acute renal failure, urinary tract infections, myocardial infarction, bleeding, sepsis, bile leakage, liver failure, and postoperative coma.19
Statistical analysis
Continuous data were reported as medians with interquartile range (IQR) while categorical data were reported as whole numbers and percentages. The Kruskal–Wallis test was used to compare continuous data while categorical data were compared using Pearson's chi-squared test. Statistical significance was defined by a p-value of <0.05. All statistical analysis were performed using STATA version 14.0 (StataCorp, College Station, TX).
Results
Clinicopathologic and operative characteristics by extent of liver resection
A total of 2714 patients were identified who underwent a liver resection and met inclusion criteria (Table 1). A total of 1037 patients underwent a major hepatectomy (38.2%), while 61.8% (n = 1677) patients underwent a partial lobectomy. When stratified by the extent of surgical resection (major hepatectomy vs. partial lobectomy), several differences in patient, disease and operative characteristics were noted. For example, while patient age, sex and ASA score were comparable between the two patient groups, patients who underwent a major hepatectomy were less likely to present with a higher preoperative BMI (>30.0 kgm−2) compared with patients who underwent a partial lobectomy (major hepatectomy 30.9% vs. partial lobectomy 36.6%; p = 0.011). Furthermore, although secondary liver malignancies were the most common indication for resection within both patient groups, a greater proportion of patients who underwent a major hepatectomy presented with primary liver malignancies (34.3% vs. 25.3%; p < 0.001). While 12.9% of patients underwent a major hepatectomy via a minimally invasive approach, 32.1% of all partial lobectomies were performed either via a laparoscopic and/or robotic approach (p < 0.001, Table 2). Patients undergoing a major hepatectomy also were more likely to have a Pringle maneuver (30.8% vs. 23.9%, p < 0.001), a biliary reconstruction (13.2% vs, 2.9%, p < 0.0001) and a drain placed (57.5% vs, 39.4%, p < 0.001).
Table 1.
Baseline sociodemographic and clinicopathologic characteristics by extent of surgical resection
| Major hepatectomy |
Partial lobectomy |
p-value | Total |
||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Age, year, median (IQR) | 60 (50–68) | 61 (51–69) | 0.050 | 60 (50–68) | |||
| Female | 508 | 49.0% | 892 | 53.2% | 0.033 | 1400 | 51.6% |
| BMI (n = 2685) | 0.011 | ||||||
| <18.5 | 21 | 2.0% | 23 | 1.4% | 44 | 1.6% | |
| 18.5–24.9 | 321 | 31.2% | 453 | 27.3% | 774 | 28.8% | |
| 25.0–29.9 | 368 | 35.8% | 575 | 34.7% | 943 | 35.1% | |
| >30.0 | 318 | 30.9% | 606 | 36.6% | 942 | 34.4% | |
| ASA class (n = 2714) | 0.423 | ||||||
| I/II | 271 | 26.2% | 462 | 27.6% | 733 | 27.0% | |
| III/IV | 765 | 73.8% | 1214 | 72.4% | 1979 | 73.0% | |
| Neoadjuvant therapy (n = 2687) | 408 | 39.8% | 473 | 28.5% | <0.001 | 881 | 32.8% |
| History of viral hepatitis | 106 | 10.2% | 186 | 11.1% | 0.478 | 292 | 10.8% |
| Primary pathology | <0.001 | ||||||
| Benign disease | 164 | 15.8% | 379 | 22.6% | 543 | 20.0% | |
| Primary liver malignancy | 356 | 34.3% | 425 | 25.3% | 781 | 28.8% | |
| Secondary liver malignancy | 517 | 49.9% | 873 | 52.1% | 1390 | 51.2% | |
Table 2.
Operative characteristics by extent of surgical resection
| Major hepatectomy |
Partial lobectomy |
p-value | Total |
||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Operative approach | <0.001 | ||||||
| Open | 902 | 87.2% | 1137 | 67.9% | 2039 | 75.2% | |
| Laparoscopic | 126 | 12.2% | 498 | 29.7% | 624 | 23.0% | |
| Robotic | 7 | 0.7% | 40 | 2.4% | 47 | 1.7% | |
| Pringle maneuver | 319 | 30.8% | 401 | 23.9% | <0.001 | 720 | 26.5% |
| Biliary reconstruction (n = 2678) | 137 | 13.2% | 49 | 2.9% | <0.001 | 186 | 6.9% |
| Intraoperative drain | 596 | 57.5% | 661 | 39.4% | <0.001 | 1257 | 46.3% |
| Concurrent resection | 518 | 50.0% | 1002 | 59.8% | <0.001 | 1520 | 56.0% |
| Median number of concurrent resections | 2 (2–5) | 2 (2–4) | 0.036 | 2 (2–4) | |||
| Concurrent ablation | 97 | 9.4% | 288 | 17.2% | <0.001 | 385 | 14.2% |
| Type of concurrent ablation (n = 385) | 0.391 | ||||||
| Radiofrequency ablation | 73 | 73.3% | 196 | 68.1% | 269 | 69.9% | |
| Microwave ablation | 20 | 20.6% | 74 | 25.7% | 94 | 24.4% | |
| Other ablation | 4 | 4.1% | 18 | 6.3% | 22 | 5.7% | |
Patterns of concurrent partial resections and ablations
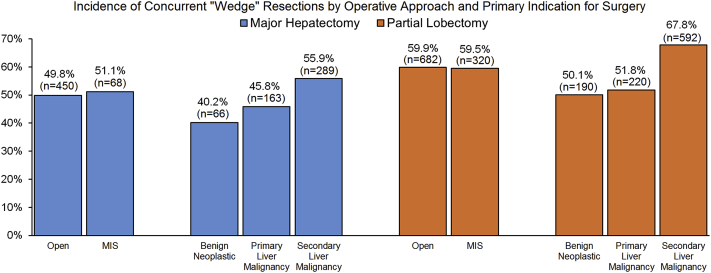
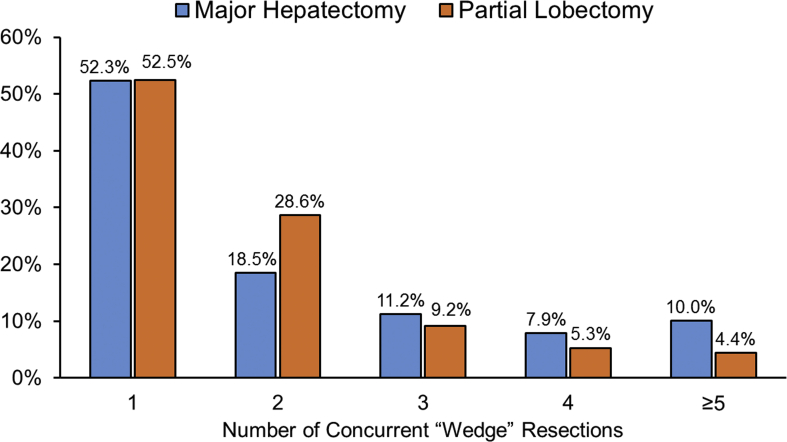
Among the study cohort, a concurrent partial resection was performed in 56.0% (n = 1520) of all patients and was more commonly performed among patients undergoing an initial partial lobectomy (50.0% vs. 59.8%; p < 0.001, Table 2). Of note, while the median number of concurrent partial resections was comparable by the extent of liver resection, the proportion of patients undergoing multiple concurrent partial resections differed when compared between the two patient groups (Supplemental Fig. 1). When stratified by the indication for surgery, concurrent partial resections were more frequently performed among patients undergoing surgery for secondary liver malignancies compared with patients undergoing surgery for primary liver malignancies or benign diseases (secondary liver malignancy vs. primary liver malignancy vs. benign: 63.4% vs. 49.0% vs. 47.2%; both p < 0.001). A similar pattern in concurrent surgical resections was also observed when stratified by the extent of the primary liver resection, with patients who presented with secondary liver malignancies proportionally being more likely to undergo a concurrent wedge resection (major hepatectomy: 55.9% vs. 45.8% vs. 40.2%; both p < 0.001, partial lobectomy: 67.8% vs. 51.8% vs. 50.1%; both p < 0.001, Fig. 1). Of note, no differences in the incidence of concurrent wedge resections were observed by surgical approach (all p > 0.05).
Figure 1.
Incidence of concurrent wedge resections by surgical approach and indication for surgery among patients who underwent major hepatectomy (n = 1037) or partial lobectomy (n = 1677). A major hepatectomy was defined as the resection of four or more liver segments while a partial lobectomy was defined as the resection of three or fewer liver segments
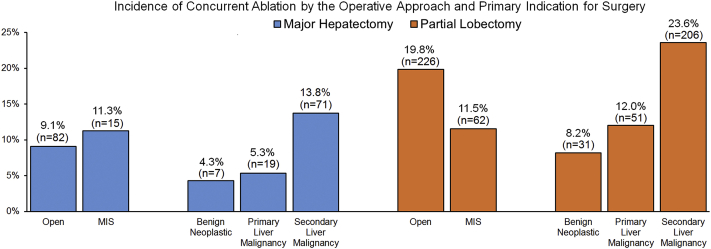
Concurrent ablation was performed in 14.2% (n = 385) of all patients. Among those RFA was performed in 69.9% (n = 269 out of 385) and MWA was performed in 24.4% (n = 94 out of 385), respectively. Compared with patients who underwent a major hepatectomy, patients who underwent a partial hepatectomy were more likely to undergo a concurrent ablation (9.4% vs. 17.2%; p < 0.001). Similarly, the use of concurrent ablation was noted to vary by the primary indication for surgery and the surgical approach. Specifically, concurrent ablations were more commonly used among patients who underwent a liver resection for secondary liver malignancies compared with patients undergoing surgery for primary liver malignancies or benign diseases (secondary liver malignancy vs. primary liver malignancy vs. benign: 19.9% vs. 9.0% vs. 7.0%; both p < 0.001). This pattern in the use of concurrent ablation was also observed when stratified by the type of primary liver resection (major hepatectomy: 13.8% vs. 5.3% vs. 4.3%; both p < 0.001, partial lobectomy: 23.6% vs. 12.0% vs. 8.2%; both p < 0.001, Fig. 2). Of note, although concurrent ablations were more frequently performed with open partial lobectomies (open vs. MIS: 19.8% vs. 11.5%; p < 0.001), the use of concurrent ablation was comparable regardless of the surgical approach among patients who underwent a major hepatectomy (p = 0.414).
Figure 2.
Incidence of concurrent ablations by surgical approach and indication for surgery among patients who underwent major hepatectomy (n = 1037) or partial lobectomy (n = 1677). A major hepatectomy was defined as the resection of four or more liver segments while a partial lobectomy was defined as the resection of three or fewer liver segments
Postoperative clinical outcomes by operative approach
Among all patients who underwent a liver resection, the median LOS was 5 days (IQR: 4–7) while 30-day postoperative mortality was 1.4% (n = 39). Patients who underwent a major hepatectomy demonstrated a longer LOS compared with patients who underwent a partial hepatectomy (6 days [IQR: 5–9] vs. 5 days [IQR: 3–6]; p < 0.001, Table 3). Postoperative mortality was also observed to be higher among patients undergoing a major hepatectomy (2.4% vs. 0.8%, p = 0.001). In contrast, no differences in LOS or postoperative mortality were observed between patients who underwent a concurrent surgical resection or ablation (all p > 0.05, Supplemental Tables 1, 2). Among all patients, 17.4% (n = 472) received a perioperative blood transfusion. Patients undergoing a major hepatectomy were more likely to receive a perioperative blood transfusions compared with patients undergoing a partial lobectomy (25.6% vs. 12.4%, p < 0.001). Of note, when stratified by the extent of surgical resection, the use of perioperative blood transfusions was observed to be lower among patients undergoing a major hepatectomy with a concurrent wedge resection (20.3% vs. 30.8%, p < 0.001) or ablation (15.5% vs. 26.6%, p = 0.017).
Table 3.
Postoperative clinical outcomes by the extent of surgical resection
| Major hepatectomy |
Partial lobectomy |
p-value | Total |
||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Length-of-stay, days, median (IQR) | 6 (5–9) | 5 (3–6) | <0.001 | 5 (4–7) | |||
| Postoperative complicationa | 490 | 47.3% | 423 | 25.2% | <0.001 | 913 | 33.6% |
| Perioperative blood transfusion | 265 | 25.6% | 207 | 12.4% | <0.001 | 472 | 17.4% |
| Postoperative mortality | 25 | 2.4% | 14 | 0.8% | 0.001 | 39 | 1.4% |
| Bile leakage | 150 | 14.5% | 92 | 5.5% | <0.001 | 242 | 8.9% |
| Liver failure | 94 | 9.1% | 31 | 1.9% | <0.001 | 125 | 4.6% |
| Grade of liver failure | 0.250 | ||||||
| Grade A | 40 | 42.6% | 8 | 25.8% | 48 | 38.4% | |
| Grade B | 30 | 31.9% | 13 | 41.9% | 43 | 34.4% | |
| Grade C | 24 | 25.5% | 10 | 32.3% | 34 | 27.2% | |
| 30-day readmission | 134 | 12.7% | 142 | 8.5% | <0.001 | 274 | 10.1% |
Represents composite score denoting any postoperative complication including surgical site infections, pneumonia, need for intubation, ventilator dependence, venous thromboembolism (pulmonary embolism or deep venous thromboembolism), acute renal failure, urinary tract infections, myocardial infarction, bleeding, sepsis, bile leakage, liver failure, and postoperative coma.
A total of 913 (33.6%) patients developed at least one postoperative complication. Postoperative complications were more common among patients who underwent a major hepatectomy compared with patients who underwent a partial lobectomy (47.3% vs. 25.2%; p < 0.001). When stratified by the extent of surgical resection, undergoing a concurrent partial resection (43.8% vs. 50.7%; p = 0.027) or ablation (38.1% vs. 48.2%; p = 0.059) at the time of major hepatectomy was associated with decreased postoperative morbidity. A similar association between concurrent resection/ablation and postoperative morbidity was not observed, however, among patients who underwent a partial lobectomy (both p > 0.05). Postoperatively, 242 patients (8.9%) developed a bile leak. Of note, while the incidence of bile leak was approximately 3 times higher among patients who underwent a major hepatectomy compared with patients who underwent a partial lobectomy (14.5% vs. 5.5%, p < 0.001), the incidence of bile leak was comparable among patients undergoing concurrent wedge resections or ablations and those who did not (all p > 0.05). Similarly, the incidence of post-hepatectomy liver failure was higher among patients who underwent a major hepatectomy compared with patients who underwent a partial lobectomy (9.1% vs. 1.9%, p = 0.031). Concurrent wedge resections (7.1% vs. 11.0%, p = 0.031) and concurrent ablations (3.1% vs. 9.7%, p = 0.031) were associated with a decreased incidence of liver failure among patients who underwent a major hepatectomy. A similar trend, however, was not observed among patients undergoing a partial lobectomy (all p > 0.05). Further, the grade of liver failure was also comparable by the extent of surgical resection, as well as by the receipt of concurrent partial resections or ablations (all p > 0.05). Among all patients, no differences in 30-day readmission were observed by the receipt of concurrent wedge resections or concurrent ablations (all p > 0.05).
Discussion
Liver resection is increasingly performed in the United States and represents the best potentially curative option for many patients with hepatocellular carcinoma, colorectal liver metastasis or biliary malignancies.9 Historically, the most common surgical method of treatment for liver cancers was major hepatectomy, in which an entire liver lobe is excised to remove the tumor.10 Although reports have suggested a 5-year survival rate for selected patients who undergo a complete resection to be as high as 50%, many patients are not amenable to curative resection due to inadequate functional hepatic reserve, multifocal disease or both.4, 15 Among patients who are unable to undergo a curative resection, concurrent therapy in the form of thermal or alcohol ablation, or concurrent wedge resections has been proposed.11, 13, 16, 23 While a limited body of research suggests improved short and long-term clinical outcomes with the use of concurrent/combined treatments, these reports have been limited to single-center studies and therefore may lack external validity.13 Nationally representative estimates for the use of concurrent therapy, as well as population-based outcomes data for patients undergoing concurrent therapies are lacking.17 In the current study, we sought to report on the use of concurrent wedge hepatic resections and/or ablations using a nationally representative database of 2714 patients undergoing liver resection in 2014. The use of concurrent wedge surgical resections was observed in 56.0% of patients and was more frequently performed among patients undergoing a partial lobectomy versus those undergoing a major hepatectomy. Concurrent ablations were performed among 14.2% of patients and also were more frequently performed among patients undergoing a partial lobectomy. Interestingly, when evaluating postoperative clinical outcomes, concurrent therapy was not associated with worse postoperative clinical outcomes. Rather, differences in postoperative outcomes were explained by differences in the extent of surgical resection, suggesting that concurrent therapies can be safely performed and should be considered among a subset of patients undergoing liver resection.
Although the number of liver resections performed has increased by 15–20% over the past two decades, current data regarding the use of concurrent therapies are limited with a majority of existing reports from single-center studies.3, 9 The current study is important as we report for the first time, population-based estimates from the 2014 ACS-NSQIP Hepatectomy Targeted PUF regarding the use of concurrent therapies for liver resection. Specifically, in the current study we defined the use of concurrent wedge resection and ablation to be 56.0% and 14.2%, respectively, among all patients undergoing a liver resection and accrued into the ACS-NSQIP. Estimates for concurrent ablation are consistent with those reported in previous single-center studies.13, 14, 16 For example, in a study of patients undergoing liver resection at the Memorial Sloan Kettering Cancer Center, Kingham and colleagues reported use of concurrent ablation in 19.2% of patients with the number of patients undergoing a concurrent ablation increasing over the study time period.16 In contrast, as the receipt of concurrent wedge resections cannot be accurately captured by traditional service/billing codes using the International Classification of Disease coding lexicon, current estimates regarding the use of concurrent wedge resections do not exist. To the best of our knowledge, herein we report the first estimates for the use of concurrent wedge resection among patients undergoing hepatic resection in North America.
A particular strength of the current study is that we compared postoperative clinical outcomes relative to the use of concurrent therapy. Although postoperative clinical outcomes were comparable by the use of concurrent therapy (concurrent wedge resection and concurrent ablation), when stratified by the extent of surgical resection, undergoing a concurrent wedge resection and major hepatectomy was associated with a decreased incidence of postoperative complications including postoperative liver failure as well as a lower incidence of perioperative blood transfusions. Consistent with results from the current study, a recent report by Choi et al. demonstrated that the use of concurrent radiofrequency ablation and minor hepatectomy was associated with decreased postoperative morbidity, as well as improved long-term outcomes including overall survival and disease recurrence.13 Similarly, Joon Lee et al. reported a decreased incidence of liver failure, use of blood transfusions and postoperative morbidity among patients undergoing a combined procedure compared with patients undergoing hepatectomy alone.14 Taken together, results from the current study and others suggest that hepatectomy can be safely combined with concurrent ablative/surgical resections and should therefore be offered to patients who may not be amenable to surgical resection due to a decreased hepatic functional reserve or multifocal disease. For example, combining hepatectomy with ablation permits the removal of the largest tumors while simultaneously ablating any smaller residual tumors, thereby resulting in a greater proportion of patients being amenable to curative resection while also permitting surgeons to address all sonographically detectable smaller tumors.11, 13, 14 Similarly, combining hepatectomy with additional wedge resections allows for a parenchymal-sparing liver resection (PSLRs), facilitating a more radical resection of all tumors while simultaneously ensuring the maximum preservation of parenchyma and therefore a decreased risk for postoperative liver failure.12, 15, 16
The current study should be interpreted in light of several limitations. First, as the ACS-NSQIP Targeted Hepatectomy PUF contains data for a single calendar year, the current study was unable to report on national trends in the use of concurrent wedge resections and ablations, as well as trends in postoperative clinical outcomes following concurrent procedures. Second, as the ACS-NSQIP only collects data within the immediate 30-day postoperative window, the current analysis was unable to compare differences in long-term clinical outcomes including survival and disease recurrence relative to surgical approach or the use of concurrent wedge resection or ablation. Third, as data pertaining to the use of adjuvant therapies was not available within the database, the effect of/confounding due to the receipt of adjuvant therapy could not be accounted for. Lastly, as the ACS-NSQIP Targeted Hepatectomy PUF collects and reports information only from select, participating hospitals, our data may not reflect the experiences of all hospitals in North America and may be confounded by some selection bias that could not be controlled.
In conclusion, concurrent wedge hepatic resections and ablations are performed in 56.0% and 14.2% of patients undergoing hepatectomy. Concurrent therapies were more frequently undertaken among patients undergoing a partial lobectomy compared with patients undergoing a major hepatectomy and for the treatment of secondary liver malignancies. Although postoperative clinical outcomes were observed to be comparable relative to the use concurrent therapy, major hepatectomy was associated with worse postoperative outcomes. Results of the current study suggest that concurrent wedge resections and ablations can be safely performed and should therefore be considered for patients with extensive disease undergoing surgery.
Footnotes
This study was previously presented as an ePoster at the 12th International Hepato-Pancreatico-Biliary Association (IHPBA) World Congress held in Sao Paulo, Brazil from April 20th to 23rd 2016.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.hpb.2016.06.002.
Author contributions
Study concept and design: Gani, Pitt, Pawlik.
Acquisition and analysis of data: Gani, Thompson, Bentrem, Hall, Pitt, Pawlik.
Drafting of the manuscript: Gani, Pitt, Pawlik.
Critical revision of the manuscript: Gani, Thompson, Bentrem, Hall, Pitt, Pawlik.
Final approval of the manuscript: Gani, Thompson, Bentrem, Hall, Pitt, Pawlik.
Conflicting interests/disclosures
Dr. Vanessa M. Thompson is a current employee of the American College of Surgeons and reports work for the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP). Dr. Bruce L. Hall receives consultant support from the American College of Surgeons for his work/role in the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP). All other authors have no conflicts of interest or financial disclosures to report.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplemental Figure 1.
Number of concurrent wedge resections by the extent of surgical resection.
References
- 1.Forner A., Llovet J.M., Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 2.Foster J.M., Hoshi H., Gibbs J.F., Iyer R., Javle M., Chu Q. Gallbladder cancer: defining the indications for primary radical resection and radical re-resection. Ann Surg Oncol. 2007;14:833–840. doi: 10.1245/s10434-006-9097-6. [DOI] [PubMed] [Google Scholar]
- 3.Mayo S.C., Heckman J.E., Shore A.D., Nathan H., Parikh A.A., Bridges J.F.P. Shifting trends in liver-directed management of patients with colorectal liver metastasis: a population-based analysis. Surgery. 2011;150:204–216. doi: 10.1016/j.surg.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman W.C., Klintmalm G., Hemming A., Vachharajani N., Majella Doyle M.B., DeMatteo R. Surgical treatment of hepatocellular carcinoma in North America: can hepatic resection still be justified? J Am Coll Surg. 2015;220:628–637. doi: 10.1016/j.jamcollsurg.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 5.Shah S.A., Bromberg R., Coates A., Rempel E., Simunovic M., Gallinger S. Survival after liver resection for metastatic colorectal carcinoma in a large population. J Am Coll Surg. 2007;205:676–683. doi: 10.1016/j.jamcollsurg.2007.06.283. [DOI] [PubMed] [Google Scholar]
- 6.Wanebo H.J., Chu Q.D., Vezeridis M.P., Soderberg C. Patient selection for hepatic resection of colorectal metastases. Arch Surg. 1996;131:322–329. doi: 10.1001/archsurg.1996.01430150100019. [DOI] [PubMed] [Google Scholar]
- 7.Page A.J., Gani F., Crowley K.T., Lee K.H.K., Grant M.C., Zavadsky T.L. Patient outcomes and provider perceptions following implementation of a standardized perioperative care pathway for open liver resection. Br J Surg. 2016 doi: 10.1002/bjs.10087. [DOI] [PubMed] [Google Scholar]
- 8.Altekruse S.F., McGlynn K.A., Reichman M.E. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimick J.B., Wainess R.M., Cowan J.A., Upchurch G.R., Knol J.A., Colletti L.M. National trends in the use and outcomes of hepatic resection. J Am Coll Surg. 2004;199:31–38. doi: 10.1016/j.jamcollsurg.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Pawlik T.M., Scoggins C.R., Thomas M.B., Vauthey J.-N. Advances in the surgical management of liver malignancies. Cancer J. 2004;10:74–87. doi: 10.1097/00130404-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Choi D., Lim H.K., Rhim H. Concurrent and subsequent radiofrequency ablation combined with hepatectomy for hepatocellular carcinomas. World J Gastrointest Surg. 2010;2:137–142. doi: 10.4240/wjgs.v2.i4.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cipriani F., Shelat V.G., Rawashdeh M., Francone E., Aldrighetti L., Takhar A. Laparoscopic parenchymal-sparing resections for nonperipheral liver lesions, the diamond technique: technical aspects, clinical outcomes, and oncologic efficiency. J Am Coll Surg. 2015;221:265–272. doi: 10.1016/j.jamcollsurg.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 13.Choi D., Lim H.K., Joh J.-W., Kim S.-J., Kim M.J., Rhim H. Combined hepatectomy and radiofrequency ablation for multifocal hepatocellular carcinomas: long-term follow-up results and prognostic factors. Ann Surg Oncol. 2007;14:3510–3518. doi: 10.1245/s10434-007-9492-7. [DOI] [PubMed] [Google Scholar]
- 14.Lee S.J., Cho E.-H., Kim R., Kim Y.H., Lim C.-S., Kim S.B. Hepatectomy, combined with intraoperative radiofrequency ablation in patients with multiple hepatocellular carcinomas. Korean J Hepato-Biliary-Pancreatic Surg. 2015;19:98–102. doi: 10.14701/kjhbps.2015.19.3.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gold J.S., Are C., Kornprat P., Jarnagin W.R., Gönen M., Fong Y. Increased use of parenchymal-sparing surgery for bilateral liver metastases from colorectal cancer is associated with improved mortality without change in oncologic outcome: trends in treatment over time in 440 patients. Ann Surg. 2008;247:109–117. doi: 10.1097/SLA.0b013e3181557e47. [DOI] [PubMed] [Google Scholar]
- 16.Kingham T.P., Correa-Gallego C., D'Angelica M.I., Gönen M., DeMatteo R.P., Fong Y. Hepatic parenchymal preservation surgery: decreasing morbidity and mortality rates in 4,152 resections for malignancy. J Am Coll Surg. 2015;220:471–479. doi: 10.1016/j.jamcollsurg.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitt H.A., Kilbane M., Strasberg S.M., Pawlik T.M., Dixon E., Zyromski N.J. ACS-NSQIP has the potential to create an HPB-NSQIP option. HPB. 2009;11:405–413. doi: 10.1111/j.1477-2574.2009.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ACS NSQIP Participant Use Data File n.d. https://www.facs.org/quality-programs/acs-nsqip/program-specifics/participant-use (accessed May 9, 2016).
- 19.Spolverato G., Ejaz A., Kim Y., Hall B.L., Bilimoria K., Cohen M. Patterns of care among patients undergoing hepatic resection: a query of the National Surgical Quality Improvement Program-targeted hepatectomy database. J Surg Res. 2015;196:221–228. doi: 10.1016/j.jss.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 20.American Society of Anesthesiologists – American Society of Anesthesiologists n.d. http://www.asahq.org/ (accessed May 1, 2015).
- 21.WHO: Global Database on Body Mass Index n.d. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html (accessed May 1, 2015).
- 22.Rahbari N.N., Garden O.J., Padbury R., Brooke-Smith M., Crawford M., Adam R. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Jarnagin W.R., Gonen M., Fong Y., DeMatteo R.P., Ben-Porat L., Little S. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. discussion 406–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.