Summary
Health-related quality of life (HRQOL) is impaired in chronic viral hepatitis and a direct role of the virus, although suggested, has not been demonstrated. Our aim was to evaluate HRQOL at blood donation before knowledge of the diagnosis of both hepatitis C virus (HCV) and hepatitis B virus (HBV) so as to elucidate this matter.
Methods
Prospectively, 67 sequential patients, 35 with HCV and 32 with HBV, and 67 matched controls were administered the generic Short Form-36 (SF-36) questionnaire. After knowledge of diagnosis, the SF-36 was repeated and a disease-specific questionnaire (Liver Disease Quality of Life, LDQOL-1.0) was also administered. The Wilcoxon test and Mann-Whitney U were used for between-group comparisons.
Results
Before knowledge of diagnosis, patients with HCV had worse HRQOL than controls, with statistically significant changes in 7/8 domains of the SF-36, and also in its physical and mental components. In the HBV group, only 2/8 domains and the physical component were significantly different from controls. After diagnosis, similar changes persisted in the HCV group, whereas two more domains were compromised in the HBV group. Comparisons between the HCV and HBV groups did not show significant differences.
Conclusion
The finding of greater HRQOL impairment in the HCV group before diagnosis confirms the theory that the presence of HCV in the early stage of the disease is associated with worse quality of life.
Introduction
It is widely known that hepatitis C virus (HCV) and hepatitis B virus (HBV) infection may be associated with impairment in health-related quality of life (HRQOL) [1,2]. As the diagnosis of HCV and HBV infection in the early stages of liver disease is frequently made at blood donation, when individuals are unaware of their infectious conditions, this is the optimal moment for assessment of virus-related changes in HRQOL.
HRQOL refers to a patient’s subjective assessment or perception of complete physical, mental and social well-being, including a range of conditions, not limited to medical interventions [3]. A number of different instruments and questionnaires, classified as generic or specific, can be used to measure HRQOL, according to the purpose of the study and the population of interest [4,5].
Late-stage liver disease, with a high degree of fibrosis and liver dysfunction, will surely lead to impairment of HRQOL, regardless of etiology. However, many studies have shown poorer HRQOL even in the early phases of liver disease, especially in HCV patients [6,7].
This worse HRQOL might be explained, at least in part, by the fact that viral diseases may cause stigmatization of patients, leading to feelings of shame and rejection, or have a negative impact on their social relations and self-esteem [8,9]. Other host-related factors, especially psychiatric disorders or various comorbidities, have also been implicated in impairment of HRQOL due to HCV [10]. Nevertheless, the hypothesis of a direct impact of the virus per se has been raised in some studies, and warrants a closer look [11,12].
The main objective of this study was to evaluate HRQOL in voluntary blood donors, unaware of their HCV or HBV status, at first donation and compare it with that of a matched-pair control group of healthy blood donors.
Patients and methods
Study design
This was a prospective, comparative, pre-post study (two successive evaluations in the HCV and HBV groups, before and after knowledge of infection). Outcomes assessment was performed at the end of the study, during outpatient follow-up.
Patients
The study population comprised patients with a positive serologic screening test for HBV or HCV at blood donation. Patients were recruited at the time of callbacks for repeat testing to confirm or rule out infection.
The inclusion criteria were positive HBsAg and reactive anti-HBc enzyme immunoassays at donation and at confirmatory testing for the hepatitis B group and a reagent anti-HCV enzyme immunoassay at donation and reagent enzyme immunoassay at confirmatory testing for the hepatitis C group. The exclusion criteria were failure to confirm infection, prior knowledge of HBV or HCV serology status, and nonadherence to the HRQOL instruments at the two predefined time points for assessment.
Study procedures
The instruments used for assessment of HRQOL — the generic Short-form 36 (SF-36) and the liver disease-specific Liver Disease Quality of Life 1.0 (LDQOL 1.0) — were administered to all HBV-positive and HCV-positive patients. Blood donors with repeatedly nonreactive screening serology (negative controls), matched by age, sex, and race to the HBV and HCV patients, were administered the SF-36 only. After patients and controls had been given the necessary information and provided written informed consent, they were asked to fill out the generic HRQOL questionnaire (SF-36). Patients with poor understanding of the instrument were aided by trained physicians. At outpatient follow-up, after approximately 30 days, the SF-36 was administered again to the HBV and HCV patients, as was the disease-specific LDQOL1.0 questionnaire.
The SF-36 questionnaire [13] is applicable in all disease states and to healthy respondents (such as the negative controls in our sample) as well. It comprises 36 items allocated across eight domains, namely: Physical Functioning, Physical Role Functioning, Emotional Role Functioning, Social Role Functioning, Mental Health, Bodily Pain, Vitality, and General Health Perceptions. The SF-36 also includes two summary scales for scoring: a physical health scale, which encompasses the domains Physical Functioning, Physical Role Functioning, Bodily Pain, and General Health Perceptions; and a mental health scale, which covers the domains Vitality, Social Role Functioning, Emotional Role Functioning, and Mental Health. The disease-specific instrument used in this study, the LDQOL-1 questionnaire [14], includes specific items designed to evaluate symptoms of liver disease, the effects of liver disease on activities of daily living, concentration, memory, sexual functioning, sexual problems, sleep problems, loneliness, hopelessness, the quality of social interaction, health-related distress, and the stigma of liver disease. This questionnaire was previously translated into Brazilian Portuguese and validated in a Brazilian population by our research group [15]. The results of both instruments (SF-36 and LDQOL-1) are based on the scores assigned to items belonging to each domain on a scale of 0 (zero), corresponding to the worst health condition possible, to 100 (one hundred), corresponding to the best health-related quality of life. All blood donors newly diagnosed with HCV or HBV hepatitis were referred for clinical assessment, mostly to our outpatient hepatitis clinic, where further laboratory testing and histopathological examination of liver tissue were performed to determine whether antiviral treatment was indicated and patient outcomes were assessed. The study project had been previously approved by the Research Ethics Commission of the facility where our work was carried out.
Samples were tested at the Serology Laboratories of Fundação Pró-Sangue de São Paulo as described elsewhere [16], using the following assays and reagents:
The Enzygnost® HBsAg 5.0 and Enzygnost® Anti-HBc monoclonal (Dade Behring GmbH, Germany) assays were employed simultaneously for detection of HBV markers and the Murex anti-HCV version 4.0 (Murex Biotech SA (Pty) Ltd., South Africa) assay for detection of HCV markers. The CHIRON® RIBA HCV Strip Immunoblot Assay (Chiron Corporation, Emeryville, CA, USA) was used for confirmatory HCV testing. The presence of at least two bands with an intensity of 1+ or greater on the test strip was required for confirmation.
Statistical analysis
For descriptive analysis, data were expressed as means and standard deviations, medians and ranges, and absolute and relative frequencies as appropriate.
The nonparametric Wilcoxon test was used for comparison of HRQOL before and after knowledge of HBV and HCV infection between donors and their respective controls.
The nonparametric Mann-Whitney U test was used for comparison of SF-36 results among donors before knowledge of HCV and HBV infection and for comparison of LDQOL results among donors after knowledge of HCV and HBV infection.
The significance level was set at 5% (p-value ≤ 0.05).
Results
The study sample comprised 134 voluntary blood donors: 67 with seroreactivity for HBV (n = 32) or HCV (n = 35) and 67 nonreactive controls matched by sex, age, and race. Mean donor and control age was 36.2 ± 9.7 years in the HBV group and 37.1 ± 10.8years in the HCV group. Both groups were predominantly male (68.7% in the HBV and 57.1% in the HCV group).
Comparison between HCV-positive blood donors still unaware of their status and their matched controls using the SF-36 questionnaire showed statistically significant differences in the domains: Physical Functioning (p = 0.038), Bodily Pain (p = 0.003), General Health Perceptions (p < 0.001), Vitality (p = 0.034), Social Role Functioning (p = 0.006), Emotional Role Functioning (p = 0.024) and Mental Health (p = 0.007). On the summary scales, significant differences were found for the physical component (p = 0.002) and mental component (p = 0.028) alike, with lower scores among the HCV patients. The repeat SF-36 administered to patients after knowledge of infection, at clinical and laboratory assessment, revealed significant differences in the domains Physical Role Functioning (p = 0.040), Bodily Pain (p = 0.017), General Health Perceptions (p < 0.001), Vitality (p = 0.019), Social Role Functioning (p = 0.005), Emotional Role Functioning (p = 0.025), and Mental Health (p = 0.034), as well as for the physical component (p = 0.008) of the SF-36 summary scale (Supplementary material, Table S1).
Comparison between HBV-positive blood donors unaware of their serologic status and their matched controls using the SF-36 questionnaire yielded statistically significant differences in the domains Bodily Pain (p = 0.036) and General Health Perceptions (p = 0.007), as well as on the physical component of the summary SF-36 scale (p = 0.004), with consistently lower scores for hepatitis B patients. There were no significant differences in the other SF-36 domains. When repeated after disease awareness, at clinical and laboratory assessment, the SF-36 confirmed significant differences in the Bodily Pain (p = 0.002) and General Health Perceptions (p = 0.006) domains, as well as in the physical component of the summary scale (p = 0.006), as before. Furthermore, scores for the Physical Functioning (p = 0.028) and Vitality (p = 0.046) domains were also significantly different from those of controls, as shown in Supplementary material, Table S2.
Head-to-head comparison between HBV-positive and HCV-positive blood donors prior to knowledge of infection using the SF-36 questionnaire did not show significant differences in any of the eight domains, nor in the physical and mental components of the summary scale. Administration of the LDQOL-1 disease-specific quality of life questionnaire also showed no significant differences between HBV and HCV patients on any of the domains assessed.
Fig. 1 shows a study flowchart including outcomes for all 67patients in the sample. Just over half of patients (56% in the HBV group and 57% in the HCV group) received follow-up at our outpatient hepatitis clinic. Although this is a small series, we found that 60% of blood donors newly diagnosed with hepatitis C had elevated liver enzymes, versus only 22% of those newly diagnosed with hepatitis B. Liver biopsy was indicated in 50% of patients in the HCV group versus 17% in the HBV group, using persistently elevated liver enzymes as a criterion. Histopathological examination of liver biopsy specimens did not show any instances of cirrhosis or advanced fibrosis. Overall, using the criteria set in Latin American consensus guidelines, 35% of patients in the HCV group required antiviral therapy, versus 11% in the HBV group [17,18].
Figure 1.
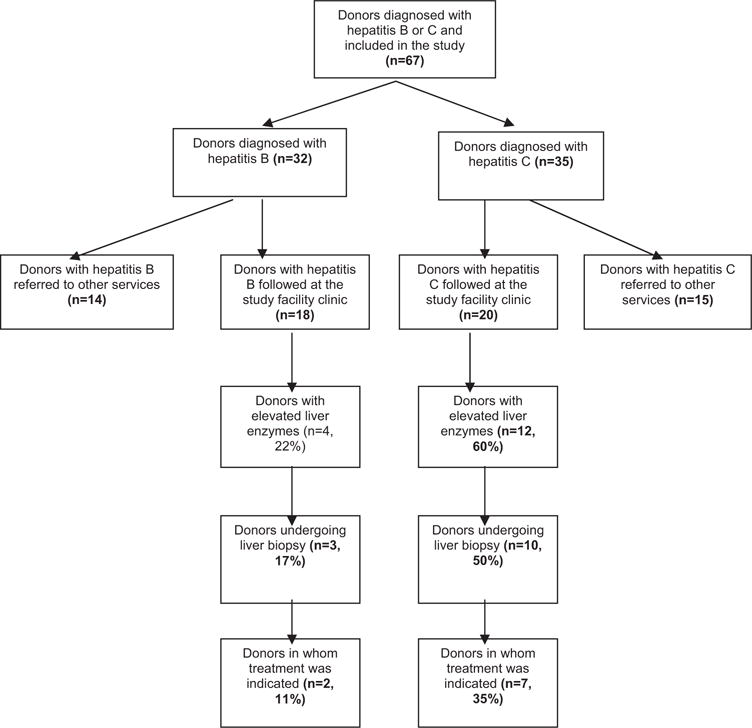
Flowchart of follow-up of blood donors newly diagnosed with HCV and HBV.
Discussion
The poorer quality of life found among blood donors who were unaware of their HCV carrier status in this study strengthen the theory of a direct viral role in HRQOL impairment. Our results run counter to those of a retrospective cohort study of patients with acute hepatitis who were later diagnosed with HCV disease, producing groups of individuals aware and unaware of their serologic status. HRQOL questionnaires administered to both groups prior to disclosure of diagnosis revealed worse HRQOL among patients who were aware of their status [8]. A potential effect of disease stigma or other comorbidities on this outcome cannot be ruled out [19]. Another study carried out in rural Egypt showed no significant differences in HRQOL between individuals unaware of HCV infection and uninfected controls [20]. However, this study may have been biased by poor quality of life in the overall population in rural areas of the country. In the present study, we assessed voluntary blood donors recruited from a major medical center at a cosmopolitan city, within a population that is mostly well-informed and aware of its health conditions.
The foundation for the hypothesis that presence of HCV per se may impair HRQOL even in mild chronic hepatitis stems from a series of 27 HCV-positive patients who were found to have cognitive changes on comparison with 16 controls who had achieved HCV clearance [21], and is supported by specific findings of altered dopaminergic and serotoninergic neurotransmission in HCV-infected patients with chronic fatigue symptoms or cognitive impairments [22]. More recently, a Brazilian research group showed that psychiatric comorbidities might account for HRQOL impairments in patients with HCV [23]. An Irish study of HCV-infected women in whom hepatitis C-associated comorbidities were carefully ruled out also found neurocognitive abnormalities indicative of a neurophysiological mechanism caused by the presence of HCV [24].
Several studies have shown that patients who achieve sustained virologic response (SVR) after therapy exhibit improved HRQOL as compared with nonresponders [25–27]. However, this could be attributed to a reduction in inflammatory activity and even to partial regression of fibrosis and, subsequently, of disease severity, as a recent study showed [28]. Nevertheless, some studies have suggested that HRQOL improves even during antiviral therapy, at early virologic response [7,29], which supports the hypothesis of viral infection itself as the causative factor of HRQOL impairment.
It bears noting that different studies have evinced a very broad range of changes that may affect HRQOL in hepatitis C, from the most basic demographic variables, such as sex and age, to markers of disease severity such as extent of fibrosis and liver dysfunction, to a variety of comorbidities, including psychosocial disorders [19,30–32]. In view of these host factors, some authors continue to deny the role of mere presence of viral infection as a direct determinant of impaired HRQOL in patients with hepatitis C [33].
In this study, we controlled for sex, age, severity of liver disease, and comorbidities; therefore, between-group differences cannot be attributed to these variables. Although severity of liver disease may be related to worse HRQOL, none of the healthy blood donors in both HCV and HBV groups have presented cirrhosis or any sign of hepatic decompensation. Likewise, any psychosocial changes that might influence HRQOL among infected blood donors would have occurred randomly among matched controls as well, although we did not address this issue specifically. Furthermore, assessment of HRQOL before patients were aware of their diagnosis prevented the potential consequences of the stigma of infection, which can influence HRQOL [19]. Therefore, we believe our findings prove that the mere presence of HCV reduces HRQOL among seemingly healthy voluntary blood donors.
The first evaluation of HRQOL in chronic hepatitis B using the SF-36 instrument came from China, and showed lower HRQOL in patients as compared with a population-based control group [34]. Other reports, especially with Asian patients, have demonstrated that asymptomatic carriers have HRQOL similar to that of controls, declining with disease progression to cirrhosis and cancer [2,35,36]. Antiviral therapy leading to reduced levels of HBV-DNA has been associated with significant improvement in HRQOL [37].
In our series, no differences in HRQOL were found on comparison between the HCV and HBV groups at this very early stage of the disease, and both groups were subjected to the same stress of uncertainty regarding a possible infectious disease. Better HRQOL has been reported in untreated HBV patients as compared with HCV patients [38]; conversely, the impact of interferon treatment on HRQOL in HBV patients is less marked than in treated HCV patients, due mainly to the lower incidence of depression and other side effects [39].
Despite the small sample size, the outcomes of our patients are indicative of benign disease in both groups, although liver enzymes were more often elevated in chronic hepatitis C, more patients in this group required liver biopsy and 35% already had treatment indications, versus only 11% in the hepatitis B group.
We chose to administer both a generic instrument (SF-36) and a disease-specific instrument (LDQOL-1) for assessment of HRQOL in our study because these questionnaires assess different aspects of patient quality of life. The generic SF-36 is the most widely used instrument in HRQOL studies, as it provides an assessment of broader aspects of HRQOL and allows comparisons between patients with different diseases, and is applicable in healthy subjects as well [40]. On the other hand, a disease-specific instrument such as the LDQOL-1 is able to detect clinically important changes and specific aspects of a particular disease, particularly in longitudinal studies or among patients with greater disease severity. In our experience, when applied after recurrence of hepatitis C in the post-transplant setting, the LDQOL-1 questionnaire was able to detect HRQOL impairment more accurately than the SF-36 [41]. As our asymptomatic patients were newly diagnosed with either hepatitis C or hepatitis B and were not in the severe stages of the disease, significant differences were found only on comparison with their matched controls, but not between the HBV and HCV groups. In conclusion, a remarkable decrease in HRQOL was detected in HCV-positive blood donors unaware of their condition. This strongly suggests that the virus itself is implicated in HRQOL impairment.
Supplementary Material
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.clinre.2013.08.008.
Footnotes
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
References
- 1.Strauss E, Dias Teixeira MC. Quality of life in hepatitis C. Liver Int. 2006;26:755–65. doi: 10.1111/j.1478-3231.2006.01331.x. [DOI] [PubMed] [Google Scholar]
- 2.Ong SC, Mak B, Aung MO, Li SC, Lim SG. Health-related quality of life in chronic hepatitis B patients. Hepatology. 2008;47:1108–17. doi: 10.1002/hep.22138. [DOI] [PubMed] [Google Scholar]
- 3.Zautra A, Goodhart D. Quality of life indicators: a review of the literature. Community Ment Health Rev. 1979;4(1):3–10. doi: 10.1300/J257v04n01_01. [DOI] [PubMed] [Google Scholar]
- 4.Chen TH, Li L, Kochen MM. A systematic review: how to choose appropriate health-related quality of life (HRQOL) measures in routine general practice? J Zhejiang Univ Sci B. 2005;6:936–40. doi: 10.1631/jzus.2005.B0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patrick DL, Deyo RA. Generic and disease-specific measures in assessing health status and quality of life. Med Care. 1989;27:S217–32. doi: 10.1097/00005650-198903001-00018. [DOI] [PubMed] [Google Scholar]
- 6.Foster GR, Goldin RD, Thomas HC. Chronic hepatitis C virus infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology. 1998;27:209–12. doi: 10.1002/hep.510270132. [DOI] [PubMed] [Google Scholar]
- 7.Teixeira MC, Ribeiro Mde F, Gayotto LC, Chamone Dde A, Strauss E. Worse quality of life in volunteer blood donors with hepatitis C. Transfusion. 2006;46:278–83. doi: 10.1111/j.1537-2995.2006.00712.x. [DOI] [PubMed] [Google Scholar]
- 8.Rodger AJ, Jolley D, Thompson SC, Lanigan A, Crofts N. The impact of diagnosis of hepatitis C virus on quality of life. Hepatology. 1999;30:1299–301. doi: 10.1002/hep.510300504. [DOI] [PubMed] [Google Scholar]
- 9.Minuk GY, Gutkin A, Wong SG, Kaita KD. Patient concerns regarding chronic hepatitis C infections. J Viral Hepat. 2005;12:51–7. doi: 10.1111/j.1365-2893.2005.00553.x. [DOI] [PubMed] [Google Scholar]
- 10.Taliani G, Rucci P, Biliotti E, Cirrincione L, Aghemo A, Alberti A, et al. Therapy expectations and physical comorbidity affect quality of life in chronic hepatitis C virus infection. J Viral Hepat. 2007;14:875–82. doi: 10.1111/j.1365-2893.2007.00883.x. [DOI] [PubMed] [Google Scholar]
- 11.Koff RS. Impaired health-related quality of life in chronic hepatitis C: the how, but not the why. Hepatology. 1999;29:277–9. doi: 10.1002/hep.510290127. [DOI] [PubMed] [Google Scholar]
- 12.Forton DM, Taylor-Robinson SD, Thomas HC. Cerebral dysfunction in chronic hepatitis C infection. J Viral Hepat. 2003;10:81–6. doi: 10.1046/j.1365-2893.2003.00416.x. [DOI] [PubMed] [Google Scholar]
- 13.McHorney CA, Ware JE, Jr, Raczek AE. The MOS. 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Gralnek IM, Hays RD, Kilbourne A, Rosen HR, Keeffe EB, Artinian L, et al. Development and evaluation of the Liver Disease Quality of Life instrument in persons with advanced, chronic liver disease —the LDQOL 1.0. Am J Gastroenterol. 2000;95:3552–65. doi: 10.1111/j.1572-0241.2000.03375.x. [DOI] [PubMed] [Google Scholar]
- 15.Teixeira MC, Strauss E. Tool to evaluate the quality of life in patients with liver disease —LDQOL-1. Translation and adaptation to the Portuguese language. GED. 2004;23:5. [Google Scholar]
- 16.Salles NA, Sabino EC, Barreto CC, Barreto AM, Otani MM, Chamone DF. The discarding of blood units and the prevalence of infectious diseases in donors at the Pro-Blood Foundation/Blood Center of Sao Paulo, Sao Paulo, Brazil. Rev Panam Salud Publica. 2003;13:111–6. doi: 10.1590/s1020-49892003000200011. [DOI] [PubMed] [Google Scholar]
- 17.Mendez-Sanchez N. The Latin American Association for the Study of the Liver in its meeting on diagnosis, management and treatment of hepatitis C. Ann Hepatol. 9(Suppl):7. [PubMed] [Google Scholar]
- 18.Daruich J, Gadano A, Fainboim H, Pessoa M, Cheinquer H. Latin American guideline for the treatment of chronic hepatitis B. Acta Gastroenterol Latinoam. 2007;37:168–77. [PubMed] [Google Scholar]
- 19.Niederau C, Bemba G, Kautz A. Changes in socio-economics, quality of life and knowledge of patients with chronic hepatitis C during the Hepatitis Competence Net Project. Z Gastroenterol. 2008;46:22–33. doi: 10.1055/s-2007-963534. [DOI] [PubMed] [Google Scholar]
- 20.Schwarzinger M, Dewedar S, Rekacewicz C, Abd Elaziz KM, Fontanet A, Carrat F, et al. Chronic hepatitis C virus infection: does it really impact health-related quality of life? A study in rural Egypt. Hepatology. 2004;40:1434–41. doi: 10.1002/hep.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forton DM, Thomas HC, Murphy CA, Allsop JM, Foster GR, Main J, et al. Hepatitis C and cognitive impairment in a cohort of patients with mild liver disease. Hepatology. 2002;35:433–9. doi: 10.1053/jhep.2002.30688. [DOI] [PubMed] [Google Scholar]
- 22.Weissenborn K, Ennen JC, Bokemeyer M, Ahl B, Wurster U, Tillmann H, et al. Monoaminergic neurotransmission is altered in hepatitis C virus infected patients with chronic fatigue and cognitive impairment. Gut. 2006;55:1624–30. doi: 10.1136/gut.2005.080267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batista-Neves S, Quarantini LC, Galvao-de Almeida A, Cardeal M, Lacerda AL, Parana R, et al. Impact of psychiatric disorders on the quality of life of Brazilian HCV-infected patients. Braz J Infect Dis. 2009;13:40–3. doi: 10.1590/s1413-86702009000100009. [DOI] [PubMed] [Google Scholar]
- 24.Lowry D, Coughlan B, McCarthy O, Crowe J. Investigating health-related quality of life, mood and neuropsychological test performance in a homogeneous cohort of Irish female hepatitis C patients. J Viral Hepat. 17:352–9. doi: 10.1111/j.1365-2893.2009.01188.x. [DOI] [PubMed] [Google Scholar]
- 25.Bonkovsky HL, Snow KK, Malet PF, Back-Madruga C, Fontana RJ, Sterling RK, et al. Health-related quality of life in patients with chronic hepatitis C and advanced fibrosis. J Hepatol. 2007;46:420–31. doi: 10.1016/j.jhep.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Younossi Z, Kallman J, Kincaid J. The effects of HCV infection and management on health-related quality of life. Hepatology. 2007;45:806–16. doi: 10.1002/hep.21565. [DOI] [PubMed] [Google Scholar]
- 27.John-Baptiste AA, Tomlinson G, Hsu PC, Krajden M, Heathcote EJ, Laporte A, et al. Sustained responders have better quality of life and productivity compared with treatment failures long after antiviral therapy for hepatitis C. Am J Gastroenterol. 2009;104:2439–48. doi: 10.1038/ajg.2009.346. [DOI] [PubMed] [Google Scholar]
- 28.D’Ambrosio R, Aghemo A, Rumi MG, Ronchi G, Donato MF, Paradis V, Colombo M, et al. A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis. Hepatology. 56:532–543. doi: 10.1002/hep.25606. [DOI] [PubMed] [Google Scholar]
- 29.Quarantini LC, Miranda-Scippa A, Batista-Neves S, Galvao-de-Almeida A, Lacerda AL, Moriyama TS, et al. The effect of early virological response in health-related quality of life in HCV-infected patients. J Med Virol. 2008;80:419–23. doi: 10.1002/jmv.21094. [DOI] [PubMed] [Google Scholar]
- 30.Teuber G, Schafer A, Rimpel J, Paul K, Keicher C, Scheurlen M, et al. Deterioration of health-related quality of life and fatigue in patients with chronic hepatitis C: Association with demographic factors, inflammatory activity, and degree of fibrosis. J Hepatol. 2008;49:923–9. doi: 10.1016/j.jhep.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 31.Bezemer G, Van Gool AR, Verheij-Hart E, Hansen BE, Lurie Y, Esteban JI, Lagging M, et al. Long-term effects of treatment and response in patients with chronic hepatitis C on quality of life. An international, multicenter, randomized, controlled study. BMC Gastroenterol. 12:11. doi: 10.1186/1471-230X-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dias Teixeira MC, de Fatima Gomes de Sa Ribeiro M, Strauss E. A new insight into the differences among non-cirrhotic and cirrhotic patients using the liver disease quality of life instrument (LDQOL) Ann Hepatol. 2005;4:264–71. [PubMed] [Google Scholar]
- 33.Helbling B, Overbeck K, Gonvers JJ, Malinverni R, Dufour JF, Borovicka J, et al. Host-rather than virus-related factors reduce health-related quality of life in hepatitis C virus infection. Gut. 2008;57:1597–603. doi: 10.1136/gut.2007.142844. [DOI] [PubMed] [Google Scholar]
- 34.Wu GC, Zhou WP, Zhao YR, Guo SH, Wang ZY, Zou SB, et al. Long-term health-related quality of life in chronic hepatitis B patients. Zhonghua Gan Zang Bing Za Zhi. 2003;11:275–7. [PubMed] [Google Scholar]
- 35.Lam ET, Lam CL, Lai CL, Yuen MF, Fong DY, So TM. Health-related quality of life of Southern Chinese with chronic hepatitis B infection. Health Qual Life Outcomes. 2009;7:52. doi: 10.1186/1477-7525-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woo G, Tomlinson G, Yim C, Lilly L, Therapondos G, Wong DK, Ungar WJ, et al. Health state utilities and quality of life in patients with hepatitis B. Can J Gastroenterol. 26:445–51. doi: 10.1155/2012/736452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JH, Kwon SY, Lee YS, Lee JH, Lee CH. Virologic response to therapy increases health-related quality of life for patients with chronic hepatitis B. Clin Gastroenterol Hepatol. 10:291–6. doi: 10.1016/j.cgh.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 38.Bondini S, Kallman J, Dan A, Younoszai Z, Ramsey L, Nader F, et al. Health-related quality of life in patients with chronic hepatitis B. Liver Int. 2007;27:1119–25. doi: 10.1111/j.1478-3231.2007.01558.x. [DOI] [PubMed] [Google Scholar]
- 39.Marcellin P, Lau GK, Zeuzem S, Heathcote EJ, Pockros PJ, Reddy KR, et al. Comparing the safety, tolerability and quality of life in patients with chronic hepatitis B vs chronic hepatitis C treated with peginterferon alpha-2a. Liver Int. 2008;28:477–85. doi: 10.1111/j.1478-3231.2008.01696.x. [DOI] [PubMed] [Google Scholar]
- 40.Slavenburg S, van Oijen MG, Spiegel BM. Comparison of health-related quality of life between populations. Liver Int. 2008;28:285–6. doi: 10.1111/j.1478-3231.2007.01622.x. [author reply: 286–7] [DOI] [PubMed] [Google Scholar]
- 41.Gotardo DR, Strauss E, Teixeira MC, Machado MC. Liver transplantation and quality of life: relevance of a specific liver disease questionnaire. Liver Int. 2008;28:99–106. doi: 10.1111/j.1478-3231.2007.01606.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


