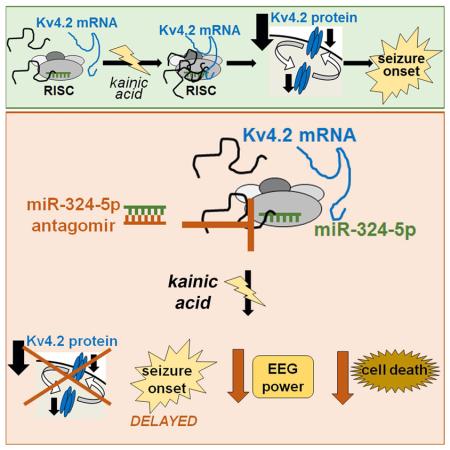
Abstract
Seizures are bursts of excessive synchronized neuronal activity, suggesting that mechanisms controlling brain excitability are compromised. The voltage-gated potassium channel Kv4.2, a major mediator of hyperpolarizing A-type currents in the brain, is a crucial regulator of neuronal excitability. Kv4.2 expression levels are reduced following seizures and in epilepsy, but the underlying mechanisms remain unclear. Here, we report that Kv4.2 mRNA is recruited to the RNA-induced silencing complex shortly after status epilepticus in mice and after kainic acid treatment of hippocampal neurons, coincident with reduction of Kv4.2 protein. We show that the microRNA miR-324-5p inhibits Kv4.2 protein expression and that antagonizing miR-324-5p is neuroprotective and seizure-suppressive. MiR-324-5p inhibition also blocks kainic acid-induced reduction of Kv4.2 protein in vitro and in vivo and delays kainic acid-induced seizure onset in wild type but not in Kcnd2 knockout mice. These results reveal an important role for miR-324-5p-mediated silencing of Kv4.2 in seizure onset.
Keywords: microRNA, Kv4.2, seizure, excitotoxicity, miR-324-5p
Graphical abstract
Introduction
Seizures are episodes of synchronized brain activity, but the underlying molecular mechanisms are not fully understood. The voltage-gated potassium channel Kv4.2 (KCND2; potassium channel, voltage-gated Shal-related subfamily D, member 2) is a major mediator of transient A-type currents in the brain and is important for controlling neuronal excitability (Jerng et al., 2004). Knockout of Kv4.2 or its auxiliary subunits DPP6 (Dipeptidyl-peptidase-like protein 6) and KChIP2 (K channel-interacting protein 2) in mice reduces A-type currents, increases dendritic excitability and enhances susceptibility to provoked seizures (Chen et al., 2006, Barnwell et al., 2009, Sun et al., 2011, Wang et al., 2013). Mutations in KCND2 have been found in humans with epilepsy (Singh et al., 2006, Lee et al., 2014) further suggesting that compromised Kv4.2 function increases the brain’s vulnerability to develop seizures.
Reduced expression and impaired function of Kv4.2 were observed in at least three different rodent models of acquired epilepsy (pilocarpine-induced temporal lobe epilepsy, traumatic brain injury and ischemic insult) (Monaghan et al., 2008, Lei et al., 2012, Lei et al., 2014, Bernard et al., 2004) and in rats acutely following evoked seizure (Francis et al., 1997, Tsaur et al., 1992). Reduction of Kv4.2 expression may thus be a pathological mechanism contributing to seizures and epilepsy; however, the molecular mechanisms regulating Kv4.2 expression during neuronal hyperactivity are unknown, and it is not clear if downregulation of Kv4.2 expression contributes to seizure onset.
MicroRNAs recognize and bind specific sequences on their target mRNAs via the RNA-induced silencing complex (RISC) followed by RNA degradation or translational suppression (Pasquinelli, 2012). Several recent studies have demonstrated that seizures in rodents cause differential expression of microRNAs, and select microRNAs were shown to be involved in modulating seizure susceptibility and epilepsy-induced neuroinflammation (e.g. Brennan et al., 2016; reviewed in Reschke and Henshall, 2015). The underlying molecular mechanisms and the targeted mRNAs that are regulated by microRNAs during seizures are not well established. In particular, it is not known if microRNA-mediated silencing of the potassium channel Kv4.2, a crucial regulator of neuronal excitability, plays a role in the reduction of Kv4.2 expression in epileptic mice or after status epilepticus.
This study reveals microRNA-mediated silencing as a mechanism promoting downregulation of Kv4.2 protein expression following status epilepticus. We demonstrate that the microRNA miR-324-5p reduces Kv4.2 protein expression and further show that antagonizing miR-324-5p is seizure-suppressive, which is mediated partially through Kv4.2. Our work provides insight into how Kv4.2 is downregulated after seizures and highlights the potency of a microRNA targeting an ion channel to regulate the onset of provoked seizures.
Results
Kv4.2 mRNA is recruited to the RISC during neuronal hyperactivity
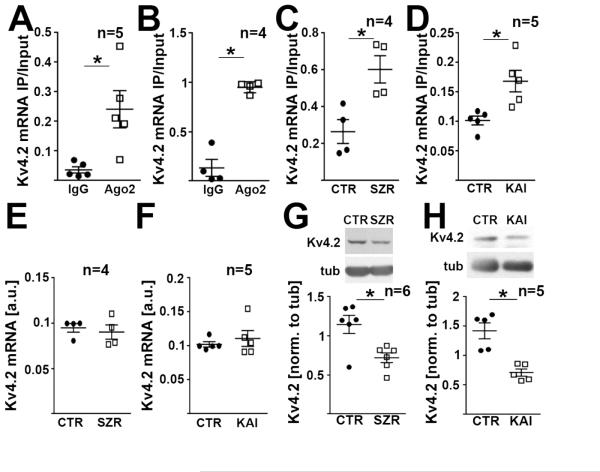
To investigate whether microRNA-induced silencing regulates Kv4.2 expression in the brain, we quantified Kv4.2 mRNA in Ago2-specific immunoprecipitates (IPs) from brain tissue and neurons. Ago2 is an essential component of the RNA-induced silencing complex (RISC) that associates with mRNAs prone to microRNA-mediated silencing (Bartel, 2009). Using quantitative real-time PCR, we detected enrichment of Kv4.2 mRNA in Ago2-IPs from mouse hippocampal lysates (Fig. 1A) and cultured mouse hippocampal neurons (Fig. 1B) compared to IPs with normal mouse IgG (western blots confirming Ago2 IP shown in Fig. S1A). To analyze if the RISC is involved in the regulation of Kv4.2 expression during seizures and excitotoxicity-inducing neuronal hyperactivity, we quantified the association of Kv4.2 mRNA with Ago2 30 minutes following onset of kainic acid-induced status epilepticus in mice as well as 4 hours following kainic acid exposure of cultured hippocampal neurons in vitro. Onset of status epilepticus was defined behaviorally according to the Racine scale. Kainic acid-induced neuronal hyperactivity increased the association of Kv4.2 mRNA with Ago2 in hippocampus and cultured hippocampal neurons (Fig. 1C,D), whereas total Kv4.2 mRNA levels were unchanged (Fig. 1E,F). Both treatments also led to reduced Kv4.2 protein levels (Fig. 1G,H). A similar reduction of Kv4.2 protein was observed 30 minutes and 3 hours after onset of pilocarpine-induced status epilepticus (Fig. S1B).
Figure 1. Kv4.2 mRNA is regulated by the RNA-induced silencing complex during seizure.
(A,B) Kv4.2-specific qRT-PCRs of mRNA isolated from immunoprecipitations (IPs) with an Ago2-specific antibody using hippocampal tissue (A) and cultured hippocampal neurons (B) from mice show enrichment of Kv4.2 mRNA in Ago2 IPs compared to IgG (paired t-tests; A: *p=0.037, B: *p=0.002). See Fig. S1A for western blots confirming Ago2 IP. (C,D) Kv4.2 mRNA association with Ago2 in hippocampus is increased 30 min after onset of seizure (SZR) induced by i.p. injection of kainic acid (C, paired t-test, *p=0.0005), and 4 hrs following neurotoxicity-inducing kainic acid treatment in cultured hippocampal neurons (KAI) (D, 10 μM kainic acid, paired t-test, *p=0.034). (E-H) Seizures or excitotoxicity do not affect total Kv4.2 mRNA levels (E,F), but lead to significantly reduced Kv4.2 protein levels (G,H) (conditions as above, paired t-tests, E: p=0.545; F: p=0.375; G: *p=0.006; H:*p=0.021). Kv4.2 protein was still reduced in the hippocampus 3 hrs after onset of status epilepticus (Fig. S1B). N indicated in the figure, error bars represent SEM.
MiR-324-5p is a specific suppressor of Kv4.2 protein expression
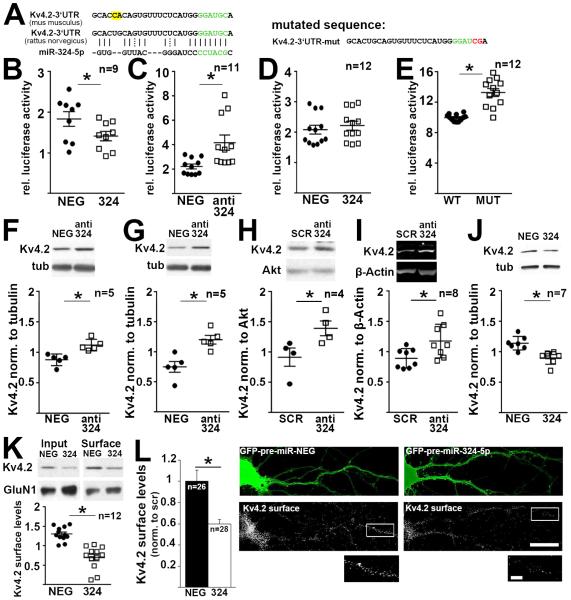
Using online software tools (microRNA.org, TargetScan) we identified miR-324-5p as a putative candidate microRNA targeting Kv4.2 mRNA with a highly complementary seed region found in mouse, rat and human (Fig. 2A). To assess if miR-324-5p regulates Kv4.2 expression, we generated a luciferase reporter construct containing the rat Kv4.2 3’UTR. Luciferase assays in Neuro2A (N2A) cells showed that miR-324-5p negatively regulates Kv4.2 reporter expression (Fig. 2B-E). Co-expression of the reporter construct with pre-miR-324-5p reduced luciferase activity (Fig. 2B), and treatment of the cells with a locked-nucleic acid (LNA)-modified “antagomir” containing the miR-324-5p antisense sequence increased luciferase activity of the reporter (Fig. 2C). A reporter construct bearing a double point mutation in the seed region-targeted sequence of the Kv4.2 3’UTR was not affected by overexpression of pre-miR-324-5p (Fig. 2D), and had increased basal luciferase activity compared to the wild type sequence, presumably due to of lack of inhibition by endogenous miR-324-5p in N2A cells (Fig. 2E).
Figure 2. MiR-324-5p represses recombinant and endogenous Kv4.2 protein expression.
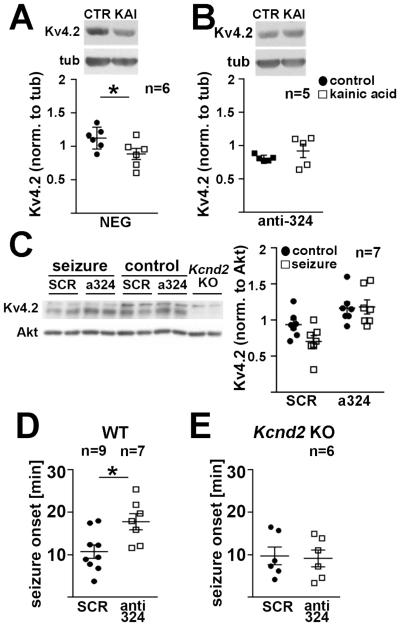
(A) MiR-324-5p target region on mouse and rat Kv4.2 3’UTRs, seed region is shown in green. Two nucleotides differ in the mouse and rat 3’UTRs (highlighted in yellow), but do not affect miR-324-5p targeting. Luciferase constructs were based on the rat sequence. (B,C) Neuro2a cells were transfected with Kv4.2 firefly luciferase 3’UTR reporters and pre-microRNA expressing plasmids (B) or antagomirs (C) (paired t-tests, B: *p=0.0015; C: *p=0.0012). (D,E) Mutating two nucleotides in the seed region of the Kv4.2 3’UTR (shown on top, mutated nucleotides in red) abolishes the inhibitory effect of miR-324-5p overexpression (D, paired t-test, p=0.005), and increases luciferase activity compared to the wild type reporter (E, paired t-test, *p<0.0001). Luciferase activity was normalized to co-transfected Renilla firefly, pre-microRNA or antagomir sequences with no known homology served as control (NEG). (F,G) MiR-324-5p inhibition with a sequence-specific antagomir increases endogenous Kv4.2 protein expression as analyzed by western blots in cultured cortical (F) and hippocampal (G) neurons, and in cortical (H) and hippocampal (I) tissue of mice i.c.v. injected with anti-miR-324-5p or scrambled (SCR) antagomirs 24 hrs prior tissue harvest (paired t-tests, F: *p=0.048; G: *p=0.025; unpaired t-tests, H: *p=0.05; I: *p=0.025). MiR-324-5p overexpression using pre-microRNA expressing lentiviral particles reduces Kv4.2 total (J, paired t-test, *p=0.019) and cell surface expression in cultured cortical neurons (K, paired t-test, *p=0.0007, n indicates separate IPs from 6 independent experiments from 3 cultures), as well as cell surface expression in cultured hippocampal neurons (L, Mann-Whitney U test, *p=0.0027, n represents individual neurons from 3 cultures). Cell surface expression was measured using surface biotinylation and western blot analysis in K, and using quantitative immunofluorescence under non-permeabilizing conditions in transfected cultured hippocampal neurons in L. Neurons in L were identified by GFP co-expressed by the pre-miRNA plasmid and by morphology. Primary and secondary dendritic segments (15-50 μm), 60-100 μm apart from the cell body, were analyzed. Examples of analyzed dendritic segments are shown in the magnifications on the bottom (white box). Scale bar is 25 μm in the overview images, and 10 μm in the inset. Images were deconvolved and maximum intensity projections of 10 stacks (15μm total) are shown. Contrast was adjusted equally for both images. Quantification of GluN1 surface expression in biotinylation experiments shown in K revealed no significant differences (Fig. S2H). Mir-324-5p expression patterns in the mouse brain are shown in Fig. S2A,B. Kv4.2 mRNA levels were not affected by the miR-324-5p antagomir or by overexpressing pre-miR-324-5p (Fig. S2C-E,G). Cultured neurons were between 11 and 15 days in vitro. N indicated in the figure; error bars are SEM.
MiR-324-5p regulates endogenous Kv4.2 protein levels
Next, we examined if miR-324-5p controls endogenous Kv4.2 protein levels in neurons. Previous reports have shown that miR-324-5p is expressed in the rodent brain (Kim et al., 2004, Capitano et al., 2015). Using fluorescence in situ hybridization analyses we confirmed expression of miR-324-5p in the mouse cortex (data not shown), and showed strong expression in both somatic and dendritic areas of the hippocampus, whereas the signal for miR-330, a previously identified somatic microRNA (Kye et al., 2007), was confined to the cell bodies (Fig. S2A,B). Inhibition of miR-324-5p in cultured cortical and hippocampal neurons using antagomirs significantly increased endogenous Kv4.2 protein levels as quantified by western blotting (Fig. 2F,G). mRNA levels were not affected (Fig. S2C). In vivo, intracerebroventricular (i.c.v.) injection of a miR-324-5p-specific antagomir in mice resulted in significant upregulation of Kv4.2 protein in the cortex and hippocampus after 24 hours (Fig. 2H,I), but mRNA levels were unchanged (Fig. S2D,E). Reduction of miR-324-5p by the antagomir was confirmed by qRT-PCR (Fig. S2F). Virus-mediated overexpression of pre-miR-324-5p significantly reduced Kv4.2 protein in cortical neurons (Fig. 2J) but did not change Kv4.2 mRNA levels (Fig. S2G).
Kv4.2 is a transmembrane protein that mediates potassium currents across the plasma membrane. Using surface biotinylation and western blot analyses in cultured cortical neurons (Fig. 2K), as well as non-permeabilizing fluorescence immunocytochemistry in hippocampal neurons (Fig. 2L), we detected significantly reduced Kv4.2 cell surface expression following miR-324-5p overexpression. By contrast, GluN1 cell surface expression was not affected (western blot in Fig. 2K, quantification in Fig. S2H). Non-permeabilizing fluorescence immunocytochemistry was validated by analyzing Kv4.2 cell surface levels after kainic acid treatment, which showed a significant reduction (Fig. S2I). Previous studies reported that Kv4.2 mRNA translation is regulated by the Fragile X Mental Retardation Protein (FMRP) (Gross et al., 2011, Lee et al., 2011). Using hippocampal Fmr1 knockout (KO) neurons we showed that FMRP is not necessary for miR-324-5p-mediated regulation of Kv4.2 (Fig. S2J).
Antagonizing miR-324-5p delays seizure onset, reduces EEG total power and prevents cell death after intra-amygdala kainic acid injection
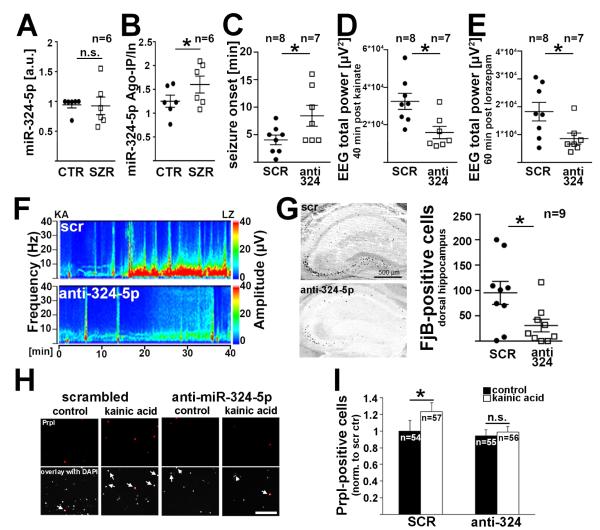
We next tested if miR-324-5p is affected by seizures. Total hippocampal miR-324-5p levels were unchanged 30 minutes after onset of status epilepticus induced by intraperitoneal (i.p.) injection of kainic acid (Fig. 3A), but association of miR-324-5p with the RISC component Ago2 was increased (Fig. 3B). To test if inhibition of miR-324-5p affects seizures, we induced status epilepticus by intra-amygdala injection of kainic acid (Jimenez-Mateos et al., 2012) in mice that had received scrambled or miR-324-5p-specific antagomirs by i.c.v. injection 24 hours prior to seizure induction (timeline shown in Fig. S3A). Antagonizing miR-324-5p significantly delayed seizure onset (Fig. 3C) and also reduced the EEG total power compared to scrambled control (Fig. 3D-F, Fig. S3B-F). Fluoro-Jade B staining 72 hours after status epilepticus showed a neuroprotective effect of anti-miR-324-5p in the hippocampus, resulting in less neuronal death (Fig. 3G, Fig. S3G-I). The neuroprotective effect of miR-324-5p inhibition was confirmed in vitro in cultured hippocampal neurons: kainic acid treatment (10 μM, 4 hours) induced cell death in neurons transfected with control antagomir but not in neurons transfected with miR-324-5p-specific antagomirs (Fig. 3H,I).
Figure 3. Inhibition of miR-324-5p reduces kainic acid-induced seizure severity and excitotoxicity.
(A,B) Total miR-324-5p levels are unchanged 30 min following seizure onset induced by i.p. injection of kainic acid (A, paired t-test, p=0.908), but association of miR-324-5p with Ago2 is increased (B, paired t-test, *p=0.030). (C-F) I.c.v. injections of miR-324-5p antagomirs delayed seizure onset following intra-amygdala kainic acid injection (C, independent t-test, *p=0.048) and reduced total EEG power 40 minutes post kainic acid (D, independent t-test, *p=0.010) and 60 min post lorazepam (E, independent t-test, *p=0.032). Lorazepam was used to end the seizures. Example EEGs are shown in F, additional analyses and timeline of the experiment are shown in Fig. S3A-F. (G) Antagonizing miR-324-5p reduces seizure-induced cell death in the dorsal hippocampus as shown by Fluoro-Jade B staining (independent t-test, *p=0.025). Similar results were obtained for ventral hippocampus, CA1 and CA3 subfields (Fig. S3G-I). (H,I) Propidium iodide staining in living hippocampal neurons (14 days in vitro) shows reduced kainic acid-induced cell death in neurons transfected with miR-324-5p antagomirs compared to control antagomirs. Neurons were analyzed 4 hrs following treatment with 10 μM kainic acid, and DAPI staining was used as a counter-stain. Scale bar is 200 μm. Contrasts were adjusted equally for all example images in H. Shown in I is the relative increase in propidium iodide-positive cells normalized to control (Kruskal-Wallis test p=0.036; Dunn’s posthoc tests *p=0.036; n.s.p>0.9999). N indicated in the figure, error bars represent SEM.
Antagonizing miR-324-5p prevents kainic acid-induced downregulation of Kv4.2 and delays seizure onset in wild type but not in Kcnd2 KO mice
We next analyzed if miR-324-5p-specific antagomirs blocked Kv4.2 reduction following kainic acid-induced excitotoxicity in vitro or status epilepticus in vivo. Kainic acid treatment significantly reduced Kv4.2 protein expression in cultured hippocampal neurons transfected with control antagomirs (Fig. 4A) but not in neurons that were transfected with miR-324-5p-specific antagomirs (Fig. 4B). Similarly, in vivo, seizures evoked by i.p. injection of kainic acid reduced hippocampal Kv4.2 expression in mice that had received i.c.v. injections of scrambled antagomirs but not in mice that had received miR-324-5p-specific antagomirs 24 hours prior seizure induction (Fig. 4C).
Figure 4. Kv4.2 contributes to the seizure-suppressing effect of miR-324-5p inhibition.
(A,B) Antagonizing miR-324-5p prevents kainic acid-induced reduction of Kv4.2 protein levels in cultured neurons (paired t-tests; A, negative control: *p=0.011; B, anti-miR324-5p: p=0.324). Antagomirs were expressed for less than 24 hrs. (C) I.c.v. injection of miR-324-5p antagomirs prevents reduction in Kv4.2 expression in the hippocampus one hour after kainic acid-induced seizure (2-way ANOVA; effect of antagomir *p=0.0002, effect of seizure p=0.198; interaction p=0.145; Sidak’s posthoc tests (ctr-seizure): scrambled, p=0.110; miR-324-5p: p=0.99). (D,E) I.c.v. injection of miR-324-5p antagomirs delays seizure onset after i.p. injection of kainic acid in wild type (D, independent t-test, *p=0.013), but not in Kcnd2 KO mice (E, independent t-test, p=0.84). Seizure onset was determined by cortical EEG recordings. Mortality rates and EEG power analyses are shown in Fig. S4. Seizures were terminated after 90 minutes by i.p. injection of diazepam. N indicated in the figure, error bars represent SEM.
MiR-324-5p was experimentally confirmed to silence a few genes and is predicted to target many others (HumanTargetScan 7.1, Agarwal et al., 2015; Table S1). To assess the specific impact of Kv4.2 on the miR-324-5p-mediated seizure-suppressing effects we tested if blocking miR-324-5p affects seizure onset in the absence of Kv4.2. We induced status epilepticus by i.p. kainic acid injection in wild type and Kcnd2 KO mice (Guo et al., 2005) and assessed seizure onset using cortical EEG recordings. As with the amygdala model of status epilepticus (Fig. 3C), antagonizing miR-324-5p prolonged the latency to seizure onset in wild type mice (Fig. 4D). This effect was abolished in Kcnd2 KO mice (Fig. 4E). In contrast to a previous study (Barnwell et al., 2009), we did not detect differences in the latency to seizure onset between wild type and Kcnd2 KO mice (Fig. 4D,E). This may be caused by different doses of kainic acid and genetic backgrounds used, which were shown to affect the phenotype (Barnwell et al., 2009). However, like Barnwell and colleagues, we observed an increased seizure-induced mortality rate (50% Kcnd2 KO mice versus 22% wild type mice, scrambled antagomir-injected, Fig. S4A). Moreover, EEG total power was higher in Kcnd2 KO mice (Fig. S4B). Notably, the miR-324-5p-specific antagomir reduced EEG total power regardless of genotype, although statistical significance was not reached (Fig. S4B). This suggests that, in contrast to regulation of latency to seizure onset, other miR-324-5p targets, apart from Kv4.2, might contribute to the effect of miR-324-5p inhibition on EEG total power.
Discussion
Reduced expression and function of the voltage-gated potassium channel Kv4.2 have been associated with epilepsy and seizures, but the molecular mechanisms leading to downregulation of Kv4.2 following seizures are unknown. Here, we present data supporting a model in which microRNA-induced silencing decreases Kv4.2 protein expression during excitotoxic events and seizures. We identify miR-324-5p as a specific regulator of Kv4.2 protein expression and show that miR-324-5p plays a role in regulating seizures and neuronal excitotoxicity: blocking miR-324-5p prevents kainic acid-mediated reduction of Kv4.2 protein, delays kainic acid-induced seizure onset, decreases EEG total power after kainic acid treatment and is neuroprotective. MiR-324-5p inhibition does not delay seizure onset in mice that lack Kv4.2, supporting an important role of Kv4.2 in mediating the seizure-delaying effect of miR-324-5p.
Previous studies have detected reduced expression of Kv4.2 mRNA and protein in the hippocampus several hours following seizures (Francis et al., 1997, Tsaur et al., 1992). In this study, we analyzed early events regulating Kv4.2 expression shortly after seizure and demonstrate that Kv4.2 mRNA is recruited to the RISC 30 minutes after kainic acid-induced status epilepticus (Fig. 1C), suggesting that increased microRNA-induced silencing of Kv4.2 contributes to Kv4.2 protein downregulation in the hippocampus (Fig. 1G). In line with this hypothesis, inhibition of miR-324-5p, a microRNA which we show suppresses Kv4.2 protein expression in the brain (Fig. 2F-L), counteracts kainic acid-induced downregulation of Kv4.2 protein (Fig. 4A-C). Two other microRNAs, miR-223-3p and miR-301a-3p, were shown to regulate Kv4.2 in the heart (Panguluri et al., 2013, Liu et al., 2016), but their function in neurons and during seizures is unknown.
We did not detect changes in total miR-324-5p levels shortly after seizure (Fig. 3A). A previous study identified miR-324-5p as one of several microRNAs that were downregulated in the hippocampus 24 hours following pilocarpine-induced status epilepticus (Kretschmann et al., 2015), but this result was not reproduced in a similar study using kainic acid to induce seizures (Jimenez-Mateos et al., 2011). Another study showed hippocampal upregulation of miR-324-5p in a rat epilepsy model 60 days following lithium-pilocarpine-induced status epilepticus (Song et al., 2011). In the future, it will be interesting to analyze if miR-324-5p and Kv4.2 expression are co-regulated at later time points after status epilepticus. We show that miR-324-5p is recruited to the RISC 30 min after seizure (Fig. 3B), suggesting early dynamic changes in the composition of miRISC during neuronal hyperexcitation. These findings add to recent discoveries that microRNA association with the RISC is not static, but dynamic changes remodeling RISC can occur in response to synaptic activity (Muddashetty et al., 2011, Jimenez-Mateos et al., 2012).
A prerequisite for our observation that changes in microRNA-induced translational silencing have an immediate impact on Kv4.2 protein expression (within 30 minutes to 4 hours, Fig. 1G,H; 4A-C) is that the turnover rate of Kv4.2 protein must be fast. A study in COS-1 cells using recombinant Kv4.2 protein has shown that the half-life of Kv4.2 is indeed relatively short, namely two hours (Shibata et al., 2003). In cultured neurons, Kv4.2 protein is rapidly degraded within minutes in response to glutamatergic activation (Lee et al., 2011). Thus, under physiological conditions in the brain the turnover rate of Kv4.2 might be even faster than two hours. We speculate that the reduction in Kv4.2 evident 30 minutes after seizure is a combination of increased Kv4.2 degradation and reduced Kv4.2 synthesis. Nonetheless, blocking miR-324-5p-mediated translational suppression of Kv4.2 abolishes kainic acid-induced Kv4.2 reduction (Fig. 4A-C), implying that increased Kv4.2 protein synthesis can overcome excitotoxicity-induced Kv4.2 degradation.
It was previously shown that Kv4.2 mRNA is translationally regulated by the mRNA-binding protein FMRP (Lee et al., 2011, Gross et al., 2011). The exact mechanisms and consequences of loss of FMRP on Kv4.2 expression and function remain controversial, as both decreased and increased levels and function of Kv4.2 have been shown in Fmr1 KO mice (Kalmbach et al., 2015, Gross et al., 2011, Lee et al., 2011, Routh et al., 2013). FMRP interacts with the microRNA pathway (Jin et al., 2004) and cooperates with microRNAs to regulate translation of certain mRNA targets (Muddashetty et al., 2011, Edbauer et al., 2010). Our results suggest that FMRP is not necessary for the gene silencing effects of miR-324-5p on Kv4.2 (Fig. S2J), but future studies are needed to investigate the relationship between FMRP- and microRNA-mediated translational control of Kv4.2 and its significance for seizure-induced downregulation of Kv4.2.
Manipulation of miR-324-5p is expected to affect multiple mRNA targets in addition to Kv4.2, since microRNAs usually regulate numerous mRNAs. Our results in Kcnd2 KO mice show that a miR-324-5p-specific antagomir does not delay seizure onset in the absence of Kv4.2 and thus support a specific role of Kv4.2 in the delay in seizure onset mediated by miR-324-5p inhibition. However, our analyses also suggest that Kv4.2 is dispensable for the effects of miR-324-5p inhibition on EEG total power (Fig. S4B), and it is unclear if the observed neuroprotective effect (Fig. 3G-I) is mediated through Kv4.2. There are a few experimentally confirmed targets of miR-324-5p that might be involved in neuroprotection after miR-324-5p inhibition, and other potassium channels are predicted to be targets that may contribute to seizure suppression (Table S1). In the future, it will be interesting to assess the contribution of individual targets of miR-324-5p to the neuroprotective and seizure-suppressing effects of miR-324-5p inhibition.
Experimental Procedures Mice and Materials
For a list of mice, antibodies, drugs and source and sequence of antagomirs, FISH probes, and qRT-PCR primers see Supplemental Experimental Procedures.
Ago2 co-Immunoprecipitation
Ago2 co-IPs were done using a mouse monoclonal Ago 2 antibody and Protein G-coupled agarose. For details, see Supplemental Experimental Procedures.
RNA isolation and qRT-PCR
RNA was extracted using Trizol® (Life Technologies), followed by cDNA preparation and qRT-PCR analysis using SYBR green or Taqman assays. For details, see Supplemental Experimental Procedures.
Luciferase assays
Luciferase assays were carried out using the Dual-Glo® Luciferase Assay System (Promega) and a Luminometer (VeritasTM, Promega). For details, see Supplemental Experimental Procedures.
Fluorescence in situ hybridization
Fluorescence in situ hybridization on mouse brain slices was performed as described in Muddashetty et al., 2011. For details, see Supplemental Experimental Procedures.
Intracerebroventricular injection of microRNAs
For i.c.v. injections of miR-324-5p-specific or scrambled antagomirs, mice received a 2 μl infusion of 0.5 nmol of either scrambled or miR-324-5p specific antagomirs in aCSF. For experiments shown in Fig. 3C-G a cannula was implanted for kainic acid injection at the time of antagomir injection. Mice were euthanized to quantify miR-324-5p or Kv4.2 protein and mRNA after 24 hrs. Alternatively, status epilepticus was induced after 24 hrs by i.p. or intra-amygdala kainic acid injection.
Seizure models
For experiments shown in Fig. 1C,E,G, 3A,B, 4C-E, and S4 seizures were induced in male C57BL/6 mice or male Kcnd2 KO mice in C57BL/6 background (4E,S4) by i.p. injection of kainic acid (15 mg/kg). Control mice received PBS injections. For experiments in Fig. 1C,E,G and 3A,B, mice were between postnatal days 24 and 28 and were euthanized 30 min after behavioral seizure onset. In Fig. 4C-E, mice were between 6 and 9 weeks old (20-23g). In Fig. 4C mice were euthanized 90 min after injection of kainic acid. Behavioral seizure onset was defined as a full tonic-clonic seizure. In Fig. 4D,E, seizure onset was identified by cortical EEG recordings using a wireless EEG system (TA11ETAF10) from Data Sciences International. A seizure was defined as sudden onset of high amplitude activity (>2x background) and a duration greater than ten seconds. Seizures were terminated by subcutaneous injection of diazepam 90 min after seizure onset (10 mg/kg). Experiments shown in Fig. 3C-F and S3 were done as described (Jimenez-Mateos et al., 2012). Also see Supplemental Experimental Procedures.
Surface biotinylation
Cultured Cortical neurons at 12 days in vitro were transduced with lentiviral particles expressing pre-miR-324-5p or control sequence. After 5-6 days, surface biotinylation was performed as in Gross et al., 2011. For details, see Supplemental Experimental Procedures.
Fluorescence immunocytochemistry
For fluorescence immunocytochemistry under non-permeabilizing conditions, neurons between days 10 and 12 in vitro were transfected with plasmids expressing GFP and pre-miR-324-5p or a control sequence using Lipofectamine 2000 (Invitrogen). After 24 hrs neurons were stained for Kv4.2 without detergents. Cells were imaged using a Nikon Eclipse Ti inverted microscope. Hippocampal pyramidal neurons were identified according to their morphology. Images were deconvolved using AutoQuant X2 (Media Cybernetics) or NIS Elements (Nikon), and fluorescence intensities in primary and secondary dendritic segments (15-50 μm in length, 15μm stack), 60-100 μm apart from the cell body, were quantified using ImageJ (NIH). For details (including image processing), see Supplemental Experimental Procedures.
Propidium iodide staining
Propidium iodide staining was performed as described in Gauthier et al., 2012. For details, see Supplemental Experimental Procedures.
Fluoro-Jade B staining
Fluoro-Jade B staining (EMD Millipore) was performed and analyzed as described previously (Moran et al., 2013). Mice were sacrificed 72 hrs after seizure induction. Postfixed sections were incubated in 0.006% potassium permanganate solution, followed by 0.001% FJB solution, and imaged using a Nikon 2000s epifluorescence microscope and a Hamamatsu Orca 285 camera.
Statistical analyses
Data was analyzed using GraphPad Prism6. Data was tested for normality using Shapiro-Wilk tests and appropriate parametric or non-parametric tests were used as indicated in each figure legend. Significance level was set to α<0.05. For 2-group/2-factor analyses, 2-way ANOVA with Tukey’s posthoc assays were performed, except of Fig. 3I, where a Kruskall-Wallis test followed by Dunn’s posthoc analyses was performed, as the data was not normally distributed. Data are expressed as means with standard errors of the mean. Repetitions of experiments are indicated in the legends.
Supplementary Material
Acknowledgements
This research was supported by a Research Grant by the Epilepsy Foundation (C.G.), an Emory University Research Council grant (C.G.), a Trustee Award by the Cincinnati Children’s Research Foundation (C.G.), NIH Grants 1R01NS092705 (C.G.), 1R21NS089080 (G.J.B.), 1R21DA033478 (G.J.B.), and 2R01NS062806 (S.C.D.), Science Foundation Ireland grants 13/SIRG/2014 (E.M.J), 13/SIRG/2098, 12/COEN/18 (T.E.), and 13/IA/1891 (D.C.H.), Health Research Board (HRA-POR-2013-325) (D.C.H.) and the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 602130 (D.C.H.). The authors thank Dr. Jeanne Nerbonne for providing the Kcnd2 KO mice, and Ashwini Poopal, Nina Patel, Qiaoyun Gong, and Nada El-Sayed for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
C.G., X.Y. and G.J.B. perceived of the study. C.G., X.Y., L.X., R.Y.K.P, S.C.D, D.C.H. and G.J.B. designed and analyzed experiments, C.G., X.Y., T.E., D.T., L.X., S.W.D., K.T.T., L.M.S. and E.M.J. performed and analyzed experiments, S.R. analyzed experiments. C.G. coordinated and supervised the study and wrote the manuscript. All authors read and revised the manuscript.
References
- AGARWAL V, BELL GW, NAM J-W, BARTEL DP. Predicting effective microRNA target sites in mammalian mRNAs. eLife. 2015;4:e05005. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNWELL LF, LUGO JN, LEE WL, WILLIS SE, GERTZ SJ, HRACHOVY RA, ANDERSON AE. Kv4.2 knockout mice demonstrate increased susceptibility to convulsant stimulation. Epilepsia. 2009;50:1741–51. doi: 10.1111/j.1528-1167.2009.02086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTEL DP. MicroRNAs: Target Recognition and Regulatory Functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNARD C, ANDERSON A, BECKER A, POOLOS NP, BECK H, JOHNSTON D. Acquired dendritic channelopathy in temporal lobe epilepsy. Science. 2004;305:532–5. doi: 10.1126/science.1097065. [DOI] [PubMed] [Google Scholar]
- BRENNAN GP, DEY D, CHEN Y, PATTERSON KP, MAGNETTA EJ, HALL AM, DUBE CM, MEI Y-T, BARAM TZ. Dual and opposing roles of microRNA-124 in epilepsy are mediated through inflammatory and NRSF-dependent gene networks. Cell reports. 2016;14:2402–2412. doi: 10.1016/j.celrep.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAPITANO F, CAMON J, FERRETTI V, LICURSI V, VITO F, RINALDI A, VINCENTI S, MANNIRONI C, FRAGAPANE P, BOZZONI I, OLIVERIO A, NEGRI R, PRESUTTI C, MELE A. microRNAs Modulate Spatial Memory in the Hippocampus and in the Ventral Striatum in a Region-Specific Manner. Molecular Neurobiology. 2015:1–13. doi: 10.1007/s12035-015-9398-5. [DOI] [PubMed] [Google Scholar]
- CHEN X, YUAN L-L, ZHAO C, BIRNBAUM SG, FRICK A, JUNG WE, SCHWARZ TL, SWEATT JD, JOHNSTON D. Deletion of Kv4.2 Gene Eliminates Dendritic A-Type K+ Current and Enhances Induction of Long-Term Potentiation in Hippocampal CA1 Pyramidal Neurons. J. Neurosci. 2006;26:12143–12151. doi: 10.1523/JNEUROSCI.2667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDBAUER D, NEILSON JR, FOSTER KA, WANG CF, SEEBURG DP, BATTERTON MN, TADA T, DOLAN BM, SHARP PA, SHENG M. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron. 2010;65:373–84. doi: 10.1016/j.neuron.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANCIS J, JUGLOFF DG, MINGO NS, WALLACE MC, JONES OT, BURNHAM WM, EUBANKS JH. Kainic acid-induced generalized seizures alter the regional hippocampal expression of the rat Kv4.2 potassium channel gene. Neurosci Lett. 1997;232:91–4. doi: 10.1016/s0304-3940(97)00593-4. [DOI] [PubMed] [Google Scholar]
- GAUTHIER SA, TIZON B, SAHOO S, LEVY E. In vitro assays measuring protection by proteins such as cystatin C of primary cortical neuronal and smooth muscle cells. Methods Mol Biol. 2012;849:275–87. doi: 10.1007/978-1-61779-551-0_19. [DOI] [PubMed] [Google Scholar]
- GROSS C, YAO X, PONG DL, JEROMIN A, BASSELL GJ. Fragile X mental retardation protein regulates protein expression and mRNA translation of the potassium channel Kv4.2. J Neurosci. 2011;31:5693–8. doi: 10.1523/JNEUROSCI.6661-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUO W, JUNG WE, MARIONNEAU C, AIMOND F, XU H, YAMADA KA, SCHWARZ TL, DEMOLOMBE S, NERBONNE JM. Targeted deletion of Kv4.2 eliminates I(to,f) and results in electrical and molecular remodeling, with no evidence of ventricular hypertrophy or myocardial dysfunction. Circ Res. 2005;97:1342–50. doi: 10.1161/01.RES.0000196559.63223.aa. [DOI] [PubMed] [Google Scholar]
- JERNG HH, PFAFFINGER PJ, COVARRUBIAS M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Molecular and Cellular Neuroscience. 2004;27:343–369. doi: 10.1016/j.mcn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- JIMENEZ-MATEOS EM, BRAY I, SANZ-RODRIGUEZ A, ENGEL T, MCKIERNAN RC, MOURI G, TANAKA K, SANO T, SAUGSTAD JA, SIMON RP, STALLINGS RL, HENSHALL DC. miRNA Expression profile after status epilepticus and hippocampal neuroprotection by targeting miR-132. Am J Pathol. 2011;179:2519–32. doi: 10.1016/j.ajpath.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIMENEZ-MATEOS EM, ENGEL T, MERINO-SERRAIS P, MCKIERNAN RC, TANAKA K, MOURI G, SANO T, O'TUATHAIGH C, WADDINGTON JL, PRENTER S, DELANTY N, FARRELL MA, O'BRIEN DF, CONROY RM, STALLINGS RL, DEFELIPE J, HENSHALL DC. Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat Med. 2012;18:1087–94. doi: 10.1038/nm.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIN P, ZARNESCU DC, CEMAN S, NAKAMOTO M, MOWREY J, JONGENS TA, NELSON DL, MOSES K, WARREN ST. Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nat Neurosci. 2004;7:113–7. doi: 10.1038/nn1174. [DOI] [PubMed] [Google Scholar]
- KALMBACH BE, JOHNSTON D, BRAGER DH. Cell-Type Specific Channelopathies in the Prefrontal Cortex of the fmr1-/y Mouse Model of Fragile X Syndrome. eNeuro. 2015;2 doi: 10.1523/ENEURO.0114-15.2015. ENEURO.0114-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM J, KRICHEVSKY A, GRAD Y, HAYES GD, KOSIK KS, CHURCH GM, RUVKUN G. Identification of many microRNAs that copurify with polyribosomes in mammalian neurons. Proceedings of the National Academy of Sciences. 2004;101:360–365. doi: 10.1073/pnas.2333854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRETSCHMANN A, DANIS B, ANDONOVIC L, ABNAOF K, VAN RIKXOORT M, SIEGEL F, MAZZUFERI M, GODARD P, HANON E, FRÖHLICH H, KAMINSKI RM, FOERCH P, PFEIFER A. Different MicroRNA Profiles in Chronic Epilepsy Versus Acute Seizure Mouse Models. Journal of Molecular Neuroscience. 2015;55:466–479. doi: 10.1007/s12031-014-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KYE MJ, LIU T, LEVY SF, XU NL, GROVES BB, BONNEAU R, LAO K, KOSIK KS. Somatodendritic microRNAs identified by laser capture and multiplex RT-PCR. Rna. 2007;13:1224–34. doi: 10.1261/rna.480407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE H, LIN MC, KORNBLUM HI, PAPAZIAN DM, NELSON SF. Exome sequencing identifies de novo gain of function missense mutation in KCND2 in identical twins with autism and seizures that slows potassium channel inactivation. Hum Mol Genet. 2014;23:3481–9. doi: 10.1093/hmg/ddu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE HY, GE WP, HUANG W, HE Y, WANG GX, ROWSON-BALDWIN A, SMITH SJ, JAN YN, JAN LY. Bidirectional regulation of dendritic voltage-gated potassium channels by the fragile X mental retardation protein. Neuron. 2011;72:630–42. doi: 10.1016/j.neuron.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEI Z, DENG P, LI J, XU ZC. Alterations of A-type potassium channels in hippocampal neurons after traumatic brain injury. J Neurotrauma. 2012;29:235–45. doi: 10.1089/neu.2010.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEI Z, ZHANG H, LIANG Y, CUI Q, XU Z, XU ZC. Reduced expression of I channels is associated with postischemic seizures in hyperglycemic rats. J Neurosci Res. 2014;92:1775–84. doi: 10.1002/jnr.23445. [DOI] [PubMed] [Google Scholar]
- LIU X, ZHANG Y, DU W, LIANG H, HE H, ZHANG L, PAN Z, LI X, XU C, ZHOU Y, WANG L, QIAN M, LIU T, YIN H, LU Y, YANG B, SHAN H. MiR-223-3p as a Novel MicroRNA Regulator of Expression of Voltage-Gated K+ Channel Kv4.2 in Acute Myocardial Infarction. Cellular Physiology and Biochemistry. 2016;39:102–114. doi: 10.1159/000445609. [DOI] [PubMed] [Google Scholar]
- MONAGHAN MM, MENEGOLA M, VACHER H, RHODES KJ, TRIMMER JS. Altered expression and localization of hippocampal A-type potassium channel subunits in the pilocarpine-induced model of temporal lobe epilepsy. Neuroscience. 2008;156:550–62. doi: 10.1016/j.neuroscience.2008.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORAN C, SANZ-RODRIGUEZ A, JIMENEZ-PACHECO A, MARTINEZ-VILLAREAL J, MCKIERNAN RC, JIMENEZ-MATEOS EM, MOONEY C, WOODS I, PREHN JH, HENSHALL DC, ENGEL T. Bmf upregulation through the AMP-activated protein kinase pathway may protect the brain from seizure-induced cell death. Cell Death Dis. 2013;4:e606. doi: 10.1038/cddis.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUDDASHETTY RS, NALAVADI VC, GROSS C, YAO X, XING L, LAUR O, WARREN ST, BASSELL GJ. Reversible inhibition of PSD-95 mRNA translation by miR-125a, FMRP phosphorylation, and mGluR signaling. Mol Cell. 2011;42:673–88. doi: 10.1016/j.molcel.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANGULURI SK, TUR J, CHAPALAMADUGU KC, KATNIK C, CUEVAS J, TIPPARAJU SM. MicroRNA-301a Mediated Regulation of Kv4.2 in Diabetes: Identification of Key Modulators. PLoS One. 2013;8:e60545. doi: 10.1371/journal.pone.0060545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASQUINELLI AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 2012;13:271–82. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]
- RESCHKE CR, HENSHALL DC. microRNA and Epilepsy. Adv Exp Med Biol. 2015;888:41–70. doi: 10.1007/978-3-319-22671-2_4. [DOI] [PubMed] [Google Scholar]
- ROUTH BN, JOHNSTON D, BRAGER DH. Loss of functional A-type potassium channels in the dendrites of CA1 pyramidal neurons from a mouse model of fragile X syndrome. J Neurosci. 2013;33:19442–50. doi: 10.1523/JNEUROSCI.3256-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIBATA R, MISONOU H, CAMPOMANES CR, ANDERSON AE, SCHRADER LA, DOLIVEIRA LC, CARROLL KI, SWEATT JD, RHODES KJ, TRIMMER JS. A fundamental role for KChIPs in determining the molecular properties and trafficking of Kv4.2 potassium channels. J Biol Chem. 2003;278:36445–54. doi: 10.1074/jbc.M306142200. [DOI] [PubMed] [Google Scholar]
- SINGH B, OGIWARA I, KANEDA M, TOKONAMI N, MAZAKI E, BABA K, MATSUDA K, INOUE Y, YAMAKAWA K. A Kv4.2 truncation mutation in a patient with temporal lobe epilepsy. Neurobiol Dis. 2006;24:245–53. doi: 10.1016/j.nbd.2006.07.001. [DOI] [PubMed] [Google Scholar]
- SONG Y-J, TIAN X-B, ZHANG S, ZHANG Y-X, LI X, LI D, CHENG Y, ZHANG J-N, KANG C-S, ZHAO W. Temporal lobe epilepsy induces differential expression of hippocampal miRNAs including let-7e and miR-23a/b. Brain Research. 2011;1387:134–140. doi: 10.1016/j.brainres.2011.02.073. [DOI] [PubMed] [Google Scholar]
- SUN W, MAFFIE JK, LIN L, PETRALIA RS, RUDY B, HOFFMAN DA. DPP6 establishes the A-type K(+) current gradient critical for the regulation of dendritic excitability in CA1 hippocampal neurons. Neuron. 2011;71:1102–15. doi: 10.1016/j.neuron.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSAUR ML, SHENG M, LOWENSTEIN DH, JAN YN, JAN LY. Differential expression of K+ channel mRNAs in the rat brain and down-regulation in the hippocampus following seizures. Neuron. 1992;8:1055–67. doi: 10.1016/0896-6273(92)90127-y. [DOI] [PubMed] [Google Scholar]
- WANG HG, HE XP, LI Q, MADISON RD, MOORE SD, MCNAMARA JO, PITT GS. The auxiliary subunit KChIP2 is an essential regulator of homeostatic excitability. J Biol Chem. 2013;288:13258–68. doi: 10.1074/jbc.M112.434548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.