Abstract
Cardiovascular disease is the number one cause of death worldwide. A powerful strategy for cardioprotection would be to identify specific molecules or targets that mimic ischemic preconditioning (IP), where short non-lethal episodes of ischemia and reperfusion prior to myocardial infarction result in dramatic reduction of infarct sizes. Since 1960 researchers believed that adenosine has a strong cardio-protective potential. In fact, with the discovery of cardiac IP in 1986 by Murry et al., adenosine was the first identified molecule that was used in studying the underlying mechanism of IP. Today we know, based on genetic studies, that adenosine is crucial for IP mediated cardio-protection and that the adenosine receptors ADORA1, ADORA2a and ADORA2b play an important role. However, the ADORA2b receptor is the only receptor so far which has been found to play a role in human and murine myocardial ischemia. With recent advances using tissue specific mice for the ADORA2b, we were able to uncover cardiomyocytes and endothelia as the responsible cell type for cardiac IP. Using a wide search for ADORA2b downstream targets, our group identified the circadian rhythm protein, Period 2 (PER2), as a novel target for IP mediated cardioprotection. Mechanistic studies on PER2 mediated cardioprotection revealed an important role for PER2 in optimizing cardiac metabolism through activation of oxygen saving pathways. Thus, cardiomyocyte or endothelial expressed ADORA2b or the downstream circadian rhythm protein PER2 are key targets for cardiac IP and could represent novel strategies to treat or prevent MI.
Keywords: Adora2b, Ischemic Preconditioning, Ischemia-reperfusion Injury, Cardiomyocytes, Myocardial Infarction, Cardiac Period 2, CD73
Introduction
Myocardial ischemia (MI) is the leading cause of death worldwide. In the United States, every 42 seconds someone has a heart attack and each minute someone dies from a heart disease-related event (1). Despite better outcomes with early coronary artery reperfusion strategies for the treatment of acute MI, morbidity and mortality remains significant. Thus, there is an urgent need to explore novel and innovative cardioprotective therapies (2).
For almost five decades, studies have suggested that adenosine is critical for protection from MI (3, 4). Described mechanisms of adenosine dependent cardioprotection include alterations of cell metabolism, vasodilatation of coronary arteries or inhibition of inflammatory responses (3, 5, 6). Adenosine mediates its effects through four adenosine receptors [ARs; ADORA1, ADORA2a, ADORA2b and ADORA3 (7–9)]. All ARs have been associated with cardiac tissue protection in different settings. In particular, the ADORA1 and ADORA2b have been implicated in ischemic preconditioning (IP) (10–12) and postconditioning (13, 14) of the heart. Both represent powerful cardioprotective mechanisms where the heart tissue at risk is exposed to short repeated non-lethal ischemic periods either prior to the onset of ischemia or at the onset of reperfusion (15–17). However, the ADORA2b is the only 1 of the 4 adenosine receptors whose cardiac expression is induced by ischemia in both mice and humans and whose function is implicated in ischemic pre- and postconditioning (18). More recent studies found that ADORA2b mediated cardioprotection works through modulation of metabolic and oxygen sensing pathways that also seem to be activated by intense daylight (19). Due to its special role, the current review focuses on recent advances on understanding ADORA2b mechanisms in cardioprotection.
Adenosine Receptor Mediated Cardioprotection
Adenosine is a purine nucleoside whose concentration increases during exercise, stress (4) or inflammation (20) and more general, during imbalances of oxygen supply, as it occurs during myocardial ischemia (21). Adenosine has several effects on the heart with coronary artery vasodilation being the most famous (4). Adenosine is able to mediate its effects through binding to a family of G protein-coupled receptors known as ADORA1, ADORA2a, ADORA2b or ADORA3. All of these receptors are expressed in the myocardium and have been implicated in the role of cardioprotection during ischemia (22). E.g. the ADORA1 has been found to delay the onset of ischemic contracture through a specific agonist. As such application of an ADORA1 agonist prior to ischemia reduced infarct sizes and improved post-ischemic functions in many different animal models (23). Other studies using Adora1−/− mice found ADORA1 to be critical in preconditioning (12) or postconditioning (14) of the heart.
The ADORA2a plays a major role in adenosine’s coronary vasodilatory properties (4). Thus, this receptor is expressed predominantly in coronary endothelial cells as well as in coronary smooth muscle cells (24, 25). Interestingly, studies have shown that activation of the ADORA2a causes inhibition of CD4+ T cell accumulation and activation in the reperfused heart which attenuates myocardial infarct sizes during reperfusion (26). However, there is also evidence for activation of the ADORA2a during ischemic postconditioning of the heart. As such it has been found that a genetic deletion of the Adora2a in mice results in larger infarct sizes following an ischemic postconditioning protocol (27).
Much less is known about the ADORA3 than the other adenosine receptors in the same family. Nevertheless, studies on Adora3 deficient mice found that infarct sizes following 30 minutes of coronary occlusion and 24 hours of reperfusion are smaller in Adora3−/− mice than WT mice. This study suggests that mice lacking this receptor are more resistant to the development of irreversible ischemic injury. In contrast to the other adenosine receptors, these results indicate that the activation of the ADORA3 can be injurious to the myocardium during times of ischemia.
Cardioprotection by CD73 and ADORA2b
A variety of factors regulate the healing process after tissue damage from MI. One such factor is adenosine, which is generated by the dephosphorylation of adenine nucleotides such as ATP (7). Initial studies had demonstrated that the observed cardiac tissue protection was dominantly mediated by extracellular adenosine generation via hypoxia-inducible CD73, the key enzyme of extracellular adenosine generation (28, 29). However, based on the lack of genetic mouse models and the use of pharmacologic inhibitors, contrary results were also found (30). Almost a decade thereafter, Cd73−/− mice were found to have significantly larger infarct sizes and no cardioprotection by IP when compared to their littermate controls (11). This was the first genetic evidence for CD73-dependent cardioprotection. Furthermore, in proof of principle studies, it was found that soluble CD73, when administered to Cd73−/− mice, could restore infarct sizes to a wildtype phenotype. These studies indicated that treatment with CD73 could represent a potential novel therapy during acute MI. Interestingly, it was also found that Cd73−/− mice had significantly attenuated adenosine levels at baseline or during IP, supporting the original idea that CD73 represents the key enzyme of extracellular adenosine generation (21). After genetic confirmation for adenosine generation being a crucial part of cardioprotection mediated by IP, the next set of studies looked at the contribution of individual ARs. This study was unique as it was the first study performing a head-to head comparison of all 4 adenosine receptor deficient mice (11). Surprisingly, while some cardioprotection by IP was observed in the ADORA1, ADORA2a, or the ADORA3 deficient mouse, the Adora2b−/− mouse had even bigger infarcts sizes following IP treatment prior to ischemia. This was the first genetic evidence showing a pivotal role for the ADORA2b in IP mediated cardioprotection. Finally, in pharmacologic studies, the authors found that ADORA2b antagonism increased infarct sizes, whereas a novel ADORA2b agonist exhibited a significant attenuation of the infarct size (11).
The Role of ADORA2b Signaling on Bone Marrow Derived Cells in Myocardial Ischemia
To further understand the mechanism by which the ADORA2b confers cardioprotection during myocardial ischemia, a follow up study looked at adenosine signaling in bone marrow derived cells (31). In fact, it had been shown that Adora2b−/− mice had higher levels of tumor necrosis factor α [TNFα; (32)]. This cytokine is released by inflammatory cells and plays a role in cardiac ischemia-reperfusion (IR) injury through apoptosis of cardiomyocytes (33). Polymorphonuclear leukocytes (PMNs) represent a significant source of TNFα and thus the authors hypothesized that the inhibition of pro-inflammatory cytokine release through the ADORA2b could be responsible for its cardioprotective effects. To study the role of ADORA2b signaling on inflammatory cells, the authors transplanted wildtype (WT) mouse bone marrow into Adora2b−/− mice or Adora2b−/− mouse bone marrow into WT mice. In the first treatment group, bone marrow transplantation from WT to knockout mice, the authors found a decrease in infarct size following IR injury. However, in the inverse treatment group, an increase in infarct size was observed. Next, neutrophil-depleted WT mice or controls were treated with a specific ADORA2b agonist and then underwent IR injury. Here it was found that neutrophil depletion had the same effect on reducing the infarct size as treatment of normal WT mice with an ADORA2b agonist. However, neutrophil depleted animals did not show a further reduction in infarct sizes upon ADORA2b agonist treatment. Subsequent studies on human PMNs revealed that ADORA2b agonist treatment significantly dampened TNFα release from activated PMNs (Figure 1A). These results show that ADORA2b signaling on bone marrow derived cells, such as PMNs, represents one important mechanism for cardioprotection during cardiac IR injury (31).
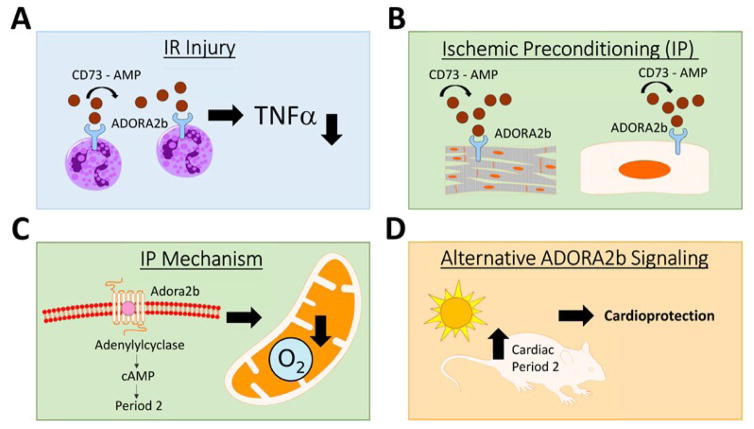
Figure 1. Role of ADORA2b activation in IR-injury and IP mediated cardioprotection.
CD73 generates extracellular adenosine via phosphohydrolysis of AMP. Adenosine can then bind to the ADORA2b and activate it. During ischemia and reperfusion (IR) injury, activation of the ADORA2b on polymorph nuclear leukocytes (PMNs) attenuates myocardial infarct sizes by reducing TNF levels (A). During ischemic preconditioning (IP) of the heart, activation of the ADORA2b on cardiomyocytes or endothelial cells confers cardioprotection (B). IP-cardioprotection is mediated through a downstream protein known as Period 2 (PER2) via cAMP. PER2 stabilization allows the oxygen deprived myocardium to switch its metabolism to a more oxygen-efficient metabolism thereby decreasing infarct sizes during ischemia (C). Alternative cardiac PER2 stabilization can be achieved by exposure of mice to intense light which also leads to reduced infarct sizes in a murine model for myocardial ischemia (D).
Differential Tissue-Specific Function of ADORA2b in Cardioprotection
Although these previously mentioned studies had shown the cardioprotective effects of ADORA2b signaling for IR injury of the heart, the specific cell type responsible for preconditioning effects or protection from reperfusion injury in mice was still not fully elucidated yet. Therefore, to study whether endothelial cells, cardiomyocytes or only bone marrow derived cells were responsible for the beneficial effects of ADORA2b activation during cardiac IP or IR injury of the heart, tissue specific deletion of the Adora2b was achieved using the Cre-Lox system (34). These studies confirmed that mice with a bone marrow specific knockout of the Adora2b showed significantly increased infarct sizes following IR injury. However, IP mediated infarct size reduction of the heart was unchanged in mice with a bone marrow cell specific deletion of the Adora2b. These data suggest that the ADORA2b on bone-marrow derived inflammatory cells does not play an important role in mediating the cardioprotection that is observed by cardiac IP. In fact, detailed immunostaining experiments revealed that the ADORA2b was strongly upregulated in endothelia or cardiomyocytes following IP of the heart. In fact, in mice with an endothelial Adora2b knockout, the infarct size limiting effect following IP of the heart was significantly blunted. Interestingly, mice with a cardiomyocyte specific Adora2b deletion had even increased infarct sizes following IP treatment prior to ischemia. These data indicate, that while ADORA2b on bone marrow derived cells play an important cardioprotective role during IR injury, cardiac IP might be exclusively mediated by ADORA2b signaling on cardiomyocytes and to some extent on endothelia (Figure 1B). In general, these studies demonstrate that activation of the ADORA2b on different tissues can represent different therapeutic targets and strategies (34).
ADORA2b signals through the Circadian Rhythm Protein PER2
To further understand the mechanism by which ADORA2b elicits cardioprotection mediated by IP, studies were performed to look at downstream responses. To identify the transcriptional responses elicited by IP, microarrays were performed in either WT or Adora2b−/−. The gene identified with the highest differential expression between these two groups was the circadian clock gene Period 2 (Per2). Per2 is a member of the Period family of genes which is expressed in a circadian (24-hour) pattern in the suprachiasmatic nucleus of the hypothalamus (SCN). From here the circadian expression of PER2 is regulated in all other tissues and organs (35). In Adora2b−/− mice, it was found that cardiac PER2 did not fluctuate according to a circadian pattern of expression as compared to WT littermates. Following studies in Per2−/− mice revealed abolished cardioprotection by IP, diminished amounts of cellular energy stores during ischemia and lost capability to generate lactate through anaerobic glycolysis. These studies indicate that PER2 promotes a metabolic switch during ischemia as cardio-adaptive mechanism. Typically, during ischemic periods, the myocardium will adapt to limited oxygen availability toward a more oxygen-efficient utilization of carbohydrates (36). However, Per2−/− mice were unable to perform this switch due to the deficiency of specific hypoxia signaling pathway (Figure 1C).
Based on the observation that light is the main regulator of circadian proteins, the authors achieved stabilization of cardiac PER2 by exposing wildtype animals to intense light. In fact, after intense light exposure, WT mice were observed to have a transcriptional induction of metabolic pathways that were associated with smaller infarct sizes following ischemia and reperfusion of the heart (Figure 1D). However, Per2−/− mice were not protected by intense light suggesting that the observed cardioprotection could be PER2 specific.
Further analysis of the circadian expressed cardiac PER2 protein, found that WT mice showed diurnal variations in infarct sizes with the smallest infarct sizes at zeitgeiber time 12 (ZT12). ZT12 is the time of lights off or the onset of locomotor activity for mice, however also the time when cardiac PER2 levels were the highest. Indeed, at ZT0, when cardiac PER levels were the lowest, infarct sizes were significantly bigger when compared to ZT12. Despite the fact that mice are nocturnal, these findings were similar to findings seen in humans. In fact, a recent study has shown that patients have larger infarct sizes in the early morning compared to any other time of day (37). Interestingly, melatonin, as output of circadian rhythms peaks at 3AM in men and at 5AM in mice. In fact, in both species melatonin is suppressed with light ‘on’. Moreover, melatonin is synthesized and secreted during the dark period, independent of whether the animal is diurnal or nocturnal (38). This indicates that being ‘nocturnal’ might be a different mechanism than light controlled circadian rhythm proteins and that the observed interrelation ship between cardiac PER2 levels and infarct sizes could be the same in mice and men.
It seems surprising that a circadian rhythm protein was found downstream of ADORA2b signaling. However, it has been shown that cAMP, the second messenger of ADORA2b signaling (Figure 1C) represents a core element of PER2 regulation (39). In fact, very early studies found that CD73, the key enzyme of extracellular adenosine generation has a circadian expression pattern (40). In addition, other studies found that mice kept at constant darkness had altered fatty acid metabolism. A search for soluble signaling molecules in the blood serendipitously identified 5′AMP and adenosine as possible candidates (41). Moreover, recent genetic studies confirmed, that mice that lack any adenosine generation have altered locomotor and metabolic activities (42).
Conclusion
Adenosine as cardio-protective agent has become the point of interest since Berne’s theory of adenosine as mediator of coronary blood flow (4). Since then adenosine had been found to be critical for ischemic preconditioning of the heart – one of the most powerful infarct size reducing mechanism at the bench (29, 30, 43–45). While all adenosine receptors have been found to play a role in cardioprotection, detailed follow up studies are still missing. What we know for the ADORA2b is, its regulation in human and mice during cardiac ischemia (6), its critical role for cardiac ischemic preconditioning through cardiomyocytes or endothelia cells (34) and its protective effect during IR injury through signaling on inflammatory cells (31). A potential downstream target of ADORA2b signaling represents PER2, a nuclear receptor that controls other transcription factors as cofactor (46) and is key regulator of many metabolic processes (47, 48). However, specific drugs such as ADORA2b agonists have not been introduced into clinical practice yet (9). Hopefully with a better understanding of molecular mechanisms, more specific drugs targeting downstream pathways of ADORA2b signaling will be able to find their way from bench to bedside.
Acknowledgments
Source of financial support for the work: National Heart, Lung, and Blood Institute (NIH-NHLBI), Bethesda, MD, USA, Grant 1R01HL122472-01A1 to T.E.
Footnotes
Conflict of Interest: no conflicts declared.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 3.Peart JN, Headrick JP. Adenosinergic cardioprotection: multiple receptors, multiple pathways. Pharmacol Ther. 2007;114:208–221. doi: 10.1016/j.pharmthera.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Katori M, Berne RM. Release of adenosine from anoxic hearts. Relationship to coronary flow. Circ Res. 1966;19:420–425. doi: 10.1161/01.res.19.2.420. [DOI] [PubMed] [Google Scholar]
- 5.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 6.Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM, et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med. 2012;18:774–782. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koeppen M, Eckle T, Eltzschig HK. Interplay of hypoxia and A2B adenosine receptors in tissue protection. Adv Pharmacol. 2011;61:145–186. doi: 10.1016/B978-0-12-385526-8.00006-0. [DOI] [PubMed] [Google Scholar]
- 8.Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature. 2014;509:310–317. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eltzschig HK, Bonney SK, Eckle T. Attenuating myocardial ischemia by targeting A2B adenosine receptors. Trends Mol Med. 2013;19:345–354. doi: 10.1016/j.molmed.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuno A, Critz SD, Cui L, Solodushko V, Yang XM, Krahn T, Albrecht B, Philipp S, Cohen MV, Downey JM. Protein kinase C protects preconditioned rabbit hearts by increasing sensitivity of adenosine A2b-dependent signaling during early reperfusion. J Mol Cell Cardiol. 2007;43:262–271. doi: 10.1016/j.yjmcc.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, et al. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 12.Lankford AR, Yang JN, Rose’Meyer R, French BA, Matherne GP, Fredholm BB, Yang Z. Effect of modulating cardiac A1 adenosine receptor expression on protection with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2006;290:H1469–1473. doi: 10.1152/ajpheart.00181.2005. [DOI] [PubMed] [Google Scholar]
- 13.Philipp S, Yang XM, Cui L, Davis AM, Downey JM, Cohen MV. Postconditioning protects rabbit hearts through a protein kinase C-adenosine A2b receptor cascade. Cardiovasc Res. 2006;70:308–314. doi: 10.1016/j.cardiores.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Xi L, Das A, Zhao ZQ, Merino VF, Bader M, Kukreja RC. Loss of myocardial ischemic postconditioning in adenosine A1 and bradykinin B2 receptors gene knockout mice. Circulation. 2008;118:S32–37. doi: 10.1161/CIRCULATIONAHA.107.752865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granfeldt A, Lefer DJ, Vinten-Johansen J. Protective ischaemia in patients: preconditioning and postconditioning. Cardiovasc Res. 2009;83:234–246. doi: 10.1093/cvr/cvp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank A, Bonney M, Bonney S, Weitzel L, Koeppen M, Eckle T. Myocardial ischemia reperfusion injury: from basic science to clinical bedside. Semin Cardiothorac Vasc Anesth. 2012;16:123–132. doi: 10.1177/1089253211436350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckle T. About Dogs, Mice, and Men: From Ischemic Preconditioning to Anesthetic Postconditioning of the Heart. Semin Cardiothorac Vasc Anesth. 2014;18:247–248. doi: 10.1177/1089253214542253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang G, FitzGerald GA. (Almost) everything is illuminated: adenosine shines a light on cardioprotection. Circ Res. 2012;111:965–966. doi: 10.1161/CIRCRESAHA.112.279752. [DOI] [PubMed] [Google Scholar]
- 19.Bonney S, Hughes K, Harter PN, Mittelbronn M, Walker L, Eckle T. Cardiac period 2 in myocardial ischemia: clinical implications of a light dependent protein. Int J Biochem Cell Biol. 2013;45:667–671. doi: 10.1016/j.biocel.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koeppen M, Eckle T, Eltzschig HK. The hypoxia-inflammation link and potential drug targets. Curr Opin Anaesthesiol. 2011;24:363–369. doi: 10.1097/ACO.0b013e32834873fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5′-nucleotidase (CD73) Purinergic Signal. 2006;2:351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McIntosh VJ, Lasley RD. Adenosine receptor-mediated cardioprotection: are all 4 subtypes required or redundant? J Cardiovasc Pharmacol Ther. 2012;17:21–33. doi: 10.1177/1074248410396877. [DOI] [PubMed] [Google Scholar]
- 23.Lasley RD, Rhee JW, Van Wylen DG, Mentzer RM., Jr Adenosine A1 receptor mediated protection of the globally ischemic isolated rat heart. J Mol Cell Cardiol. 1990;22:39–47. doi: 10.1016/0022-2828(90)90970-d. [DOI] [PubMed] [Google Scholar]
- 24.Olanrewaju HA, Mustafa SJ. Adenosine A(2A) and A(2B) receptors mediated nitric oxide production in coronary artery endothelial cells. Gen Pharmacol. 2000;35:171–177. doi: 10.1016/s0306-3623(01)00107-0. [DOI] [PubMed] [Google Scholar]
- 25.Hussain T, Linden J, Mustafa SJ. 125I-APE binding to adenosine receptors in coronary artery: photoaffinity labeling with 125I-azidoAPE. J Pharmacol Exp Ther. 1996;276:284–288. [PubMed] [Google Scholar]
- 26.Yang Z, Day YJ, Toufektsian MC, Xu Y, Ramos SI, Marshall MA, French BA, Linden J. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation. 2006;114:2056–2064. doi: 10.1161/CIRCULATIONAHA.106.649244. [DOI] [PubMed] [Google Scholar]
- 27.Norton ED, Jackson EK, Turner MB, Virmani R, Forman MB. The effects of intravenous infusions of selective adenosine A1-receptor and A2-receptor agonists on myocardial reperfusion injury. Am Heart J. 1992;123:332–338. doi: 10.1016/0002-8703(92)90643-a. [DOI] [PubMed] [Google Scholar]
- 28.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitakaze M, Node K, Minamino T, Komamura K, Funaya H, Shinozaki Y, Chujo M, Mori H, Inoue M, Hori M, et al. Role of activation of protein kinase C in the infarct size-limiting effect of ischemic preconditioning through activation of ecto-5′-nucleotidase. Circulation. 1996;93:781–791. doi: 10.1161/01.cir.93.4.781. [DOI] [PubMed] [Google Scholar]
- 30.Miki T, Miura T, Bunger R, Suzuki K, Sakamoto J, Shimamoto K. Ecto-5′-nucleotidase is not required for ischemic preconditioning in rabbit myocardium in situ. Am J Physiol. 1998;275:H1329–1337. doi: 10.1152/ajpheart.1998.275.4.H1329. [DOI] [PubMed] [Google Scholar]
- 31.Koeppen M, Harter PN, Bonney S, Bonney M, Reithel S, Zachskorn C, Mittelbronn M, Eckle T. Adora2b signaling on bone marrow derived cells dampens myocardial ischemia-reperfusion injury. Anesthesiology. 2012;116:1245–1257. doi: 10.1097/ALN.0b013e318255793c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli P, et al. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006;116:1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994;94:1621–1628. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seo SW, Koeppen M, Bonney S, Gobel M, Thayer M, Harter PN, Ravid K, Eltzschig HK, Mittelbronn M, Walker L, et al. Differential Tissue-Specific Function of Adora2b in Cardioprotection. J Immunol. 2015;195:1732–1743. doi: 10.4049/jimmunol.1402288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G, Lee CC, Bradley A. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- 36.Wolff AA, Rotmensch HH, Stanley WC, Ferrari R. Metabolic approaches to the treatment of ischemic heart disease: the clinicians’ perspective. Heart Fail Rev. 2002;7:187–203. doi: 10.1023/a:1015384710373. [DOI] [PubMed] [Google Scholar]
- 37.Suarez-Barrientos A, Lopez-Romero P, Vivas D, Castro-Ferreira F, Nunez-Gil I, Franco E, Ruiz-Mateos B, Garcia-Rubira JC, Fernandez-Ortiz A, Macaya C, et al. Circadian variations of infarct size in acute myocardial infarction. Heart. 2011;97:970–976. doi: 10.1136/hrt.2010.212621. [DOI] [PubMed] [Google Scholar]
- 38.Pevet P. Melatonin in animal models. Dialogues Clin Neurosci. 2003;5:343–352. doi: 10.31887/DCNS.2003.5.4/ppevet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MH. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science. 2008;320:949–953. doi: 10.1126/science.1152506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frederiks WM, Marx F, Bosch KS, Van Noorden CJ. Diurnal variation in 5′-nucleotidase activity in rat liver. A quantitative histochemical study. Histochemistry. 1987;87:439–443. doi: 10.1007/BF00496815. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Kaasik K, Blackburn MR, Lee CC. Constant darkness is a circadian metabolic signal in mammals. Nature. 2006;439:340–343. doi: 10.1038/nature04368. [DOI] [PubMed] [Google Scholar]
- 42.O’Brien WG, 3rd, Berka V, Tsai AL, Zhao Z, Lee CC. CD73 and AMPD3 deficiency enhance metabolic performance via erythrocyte ATP that decreases hemoglobin oxygen affinity. Sci Rep. 2015;5:13147. doi: 10.1038/srep13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 44.Kitakaze M, Hori M, Morioka T, Minamino T, Takashima S, Sato H, Shinozaki Y, Chujo M, Mori H, Inoue M, et al. Infarct size-limiting effect of ischemic preconditioning is blunted by inhibition of 5′-nucleotidase activity and attenuation of adenosine release. Circulation. 1994;89:1237–1246. doi: 10.1161/01.cir.89.3.1237. [DOI] [PubMed] [Google Scholar]
- 45.Kloner RA, Bolli R, Marban E, Reinlib L, Braunwald E. Medical and cellular implications of stunning, hibernation, and preconditioning: an NHLBI workshop. Circulation. 1998;97:1848–1867. doi: 10.1161/01.cir.97.18.1848. [DOI] [PubMed] [Google Scholar]
- 46.Yang X. A wheel of time: the circadian clock, nuclear receptors, and physiology. Genes Dev. 2010;24:741–747. doi: 10.1101/gad.1920710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grimaldi B, Bellet MM, Katada S, Astarita G, Hirayama J, Amin RH, Granneman JG, Piomelli D, Leff T, Sassone-Corsi P. PER2 controls lipid metabolism by direct regulation of PPARgamma. Cell Metab. 2010;12:509–520. doi: 10.1016/j.cmet.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonney S, Kominsky D, Brodsky K, Eltzschig H, Walker L, Eckle T. Cardiac Per2 functions as novel link between fatty acid metabolism and myocardial inflammation during ischemia and reperfusion injury of the heart. PLoS One. 2013;8:e71493. doi: 10.1371/journal.pone.0071493. [DOI] [PMC free article] [PubMed] [Google Scholar]