Abstract
The adult bovine (Bos taurus) intervertebral disc is primarily comprised of two major tissue types: The outer annulus fibrosus (AF) and the central nucleus pulposus (NP). We isolated several primary cell lineages of passage (P) 0 cells from the AF tissue omitting typically used enzymatic tissue digestion protocols. The cells grow past p10 without signs of senescence in DMEM + 10% FCS on 0.1% gelatin coated/uncoated surfaces of standard cell culture plates and survive freeze-thawing. Preliminary analysis of the AF derived cells for expression of the two structural genes Col1a1 and Col2a1 was performed by PISH recapitulating the expression observed in vivo.
Keywords: Bovine, Intervertebral disc, Annulus fibrosus, Cell line, In situ hybridization
Resource table.
| Name of resource | Adult bovine (Bos taurus) intervertebral disc annulus fibrosus (AF) cell lines. |
| Institution | Department of Biology, Clarkson University, 8 Clarkson Avenue, Potsdam, NY 13699, USA. |
| Person who created resource | Petra Kraus and Thomas Lufkin |
| Contact person and email | Petra Kraus, pkraus@clarkson.edu; Thomas Lufkin, tlufkin@clarkson.edu |
| Date archived/stock date | Established April 23rd 2016, frozen as passage 3 on May 9th 2016 |
| Type of resource | Cell line derived from the annulus fibrosus tissue of the intervertebral disc of an adult Bos taurus |
| Link to directly related literature that employed/validated this resource | In press |
| Information in public databases | N/A, in press |
1. Resource details
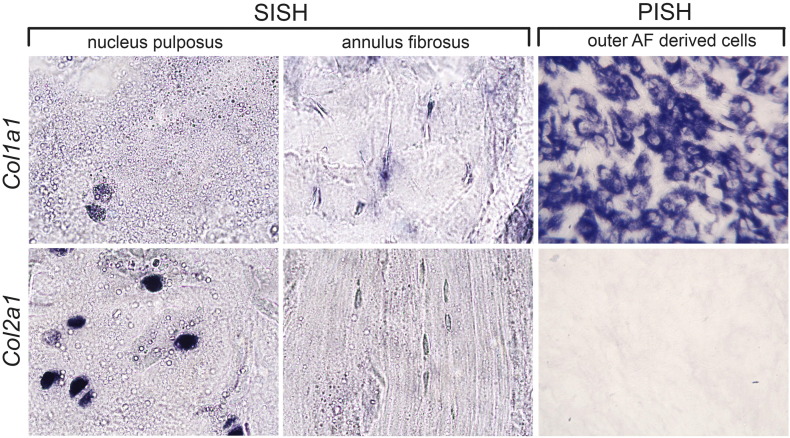
Several cell lines were isolated during independent experiments from dissected annulus fibrosus (AF) tissue of mature bovine intervertebral discs (IVD) via a reproducible non-enzyme driven protocol. The cell lines were frozen at low passage number and they recovered well after freeze-thawing (see Fig. 1). Preliminary characterization of the AF cells was carried out with bovine specific RNA probes derived from bovine genomic DNA using plate RNA in situ hybridisation (PISH) [1] for Col1a1 and Col2a1 expression, two structural proteins found in the mature IVD [2]. Less type-II collagen fibers were described for the outer AF in rabbit [3] correlating with a common notion that type II collagen is higher in the NP than the AF [2], [4]. The dissected outer AF of mature bovine IVDs was the source for our AF cell lines and we did not detect Col2a1 expression by either RNA in situ hybridization (SISH) [5], [6], [7], [8] on sections of the outer AF tissue or by PISH on the cells derived from the outer AF, while Col2a1 expression was very prominent in cells of the NP as shown by SISH on the same section (Fig. 2). The discrepancy between our findings and that of increased Col2a1 expression in the bovine AF over the NP reported by Minoque et al. [9] might reflect differences in defining the AF. Minoque's Microarray expression analysis indicated increased expression of Col1a1 in AF cells [9]. We see Col1a1 expression in AF and NP cells by SISH in vivo and by PISH in vitro (Fig. 1).
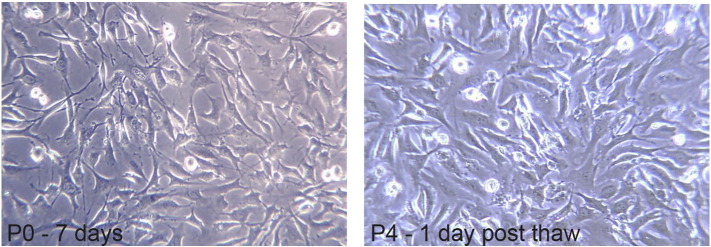
Fig. 1.
Cells derived from the outer annulus fibrosus (AF) at P0 with 7 days in culture are shown on the left, while cells from the same cell line at P4 are shown on the right one day after a freeze thaw cycle. Images were taken using phase contrast at 200 × on a Zeiss Primo Vert scope with a Moticam 2 2.0MP digital camera.
Fig. 2.
7um paraffin cross sections of the mature bovine IVD show cells expressing Col1a1 mRNA by cells of the nucleus pulposus (NP) and outer annulus fibrosus (AF) by section in situ hybridization (SISH) and cells derived from this region of the outer AF by plate in situ hybridization (PISH). Col2a1 expression was detected in the NP by SISH, but absent from AF tissue and cells derived therefrom. SISH images were taken at 400 × on a MoticBA310 compound scope with a Moticam 1SP 1.3MP digital camera and PISH images at 200 × on a Zeiss Primo Vert scope with a Moticam 2 2.0MP digital camera.
2. Materials and methods
Skinned bovine tails were collected fresh from local abattoirs, remained chilled and were processed within 2 h. Tail pieces were immersed in 10% Povidone-Iodine solution, rinsed with tap water, followed by immersion in 70% EtOH prior to removing all fat and muscle tissue. IVDs were dissected away from adjacent vertebrae endplates, briefly dipped in 70% EtOH and rinsed with 1 × PBS/10% Gentamicin prior to separating the outer AF from the remaining IVD tissue. Outer AF tissue was cut into smaller pieces using sterile procedures and placed in uncoated as well as 0.1% gelatin coated 35 mm culture dishes (Falcon). Sterile filtered FBS-HI with 10% Gentamicin and 5 μg/ml Amphotericin B (all GIBCO) was added prior to the incubation at 37C, 5% CO2 and atmospheric O2. After 24 h the FBS mix was diluted 1:1 with standard DMEM based growth medium containing 1 × DMEM with 4.5 g/l glucose, 1 × Pyruvate, 1 × Glutamax, 1 × nonessential amino acids, 10% v/v HI-FBS, 0.48% v/v Gentamicin (all GIBCO), 0.12 mM beta-mercapthoethanol (Sigma) and additional 5 μg/ml Amphotericin B and the tissue. Following 48 h of incubation cells had attached to the bottom of the wells and were expanded in fresh standard DMEM based growth medium (see above). Cell lines derived in such manner from AF tissue could be passaged with 0.05% Trypsin/EDTA (GIBCO) at 1:10 dilutions for more than 10 passages without slowing down in population growth or dramatic changes in morphology (Fig. 1). Early and late passages were subjected to plate RNA in situ hybridization (PISH) [1] for preliminary gene expression analysis (Fig. 2).
3. Verification and authentication
During embryogenesis, the AF part of the IVD is believed to be of sclerotomal origin [10], [11]. Cultured cells derived from the outer AF of mature bovine caudal IVDs with our procedure were assayed for the expression of two major collagen genes Col1a1 and Col2a1. The observed in vitro expression of these two genes mirrored the in vivo expression in cells of the mature the AF: Presence of Col1a1 expression and absence of Col2a1 expression in cells of the outer AF (Fig. 2).
References
- 1.Kraus P., Kocsis V., Williams C., Youngs B., Lufkin T. Plate in situ hybridization (PISH) as a time and cost effective RNA expression assay to study phenotypic heterogeneity in a population of cultured murine cells at single cell resolution. Biotechnol. Lett. 2015;37:1573–1577. doi: 10.1007/s10529-015-1833-1. [DOI] [PubMed] [Google Scholar]
- 2.McCann M.R., Seguin C.A. Notochord cells in intervertebral disc development and degeneration. J. Dev. Biol. 2016;4:1–18. doi: 10.3390/jdb4010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J., Liu C., Guo Q., Yang H., Li B. Regional variations in the cellular, biochemical, and biomechanical characteristics of rabbit annulus fibrosus. PLoS One. 2014;9 doi: 10.1371/journal.pone.0091799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sive J.I., Baird P., Jeziorsk M., Watkins A., Hoyland J.A., Freemont A.J. Expression of chondrocyte markers by cells of normal and degenerate intervertebral discs. Mol. Pathol. 2002;55:91–97. doi: 10.1136/mp.55.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X., Li X., Wang W., Lufkin T. Dlx5 and Dlx6: an evolutionary conserved pair of murine homeobox genes expressed in the embryonic skeleton. Ann. N. Y. Acad. Sci. 1996;785:38–47. doi: 10.1111/j.1749-6632.1996.tb56242.x. [DOI] [PubMed] [Google Scholar]
- 6.Kraus P., Lufkin T. Mammalian Dlx homeobox gene control of craniofacial and inner ear morphogenesis. J. Cell. Biochem. Suppl. 1999;32-33:133–140. doi: 10.1002/(sici)1097-4644(1999)75:32+<133::aid-jcb16>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 7.Wang W., Lo P., Frasch M., Lufkin T. Hmx: an evolutionary conserved homeobox gene family expressed in the developing nervous system in mice and Drosophila. Mech. Dev. 2000;99:123–137. doi: 10.1016/s0925-4773(00)00488-3. [DOI] [PubMed] [Google Scholar]
- 8.Zhao F., Lufkin T., Gelb B.D. Expression of Tfap2d, the gene encoding the transcription factor Ap-2 delta, during mouse embryogenesis. Gene Expr. Patterns. 2003;3:213–217. doi: 10.1016/s1567-133x(02)00067-4. [DOI] [PubMed] [Google Scholar]
- 9.Minogue B.M., Richardson S.M., Zeef L.A., Freemont A.J., Hoyland J.A. Transcriptional profiling of bovine intervertebral disc cells: implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthritis Res. Ther. 2010;12:R22. doi: 10.1186/ar2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christ B., Wilting J. From somites to vertebral column. Ann. Anat. 1992;174:23–32. doi: 10.1016/s0940-9602(11)80337-7. [DOI] [PubMed] [Google Scholar]
- 11.Sivakamasundari V., Lufkin T. Bridging the gap: understanding embryonic intervertebral disc development. Cell Dev. Biol. 2012;1 [PMC free article] [PubMed] [Google Scholar]