Abstract
IMPORTANCE
Immunomodulatory anticancer drugs, such as the anti–programmed death-1 drug pembrolizumab, have shown promising results in trials, and more patients will receive such treatments. Little is known about cutaneous adverse events (AEs) caused by these drugs and their possible correlation with treatment response.
OBJECTIVE
To describe the frequency and spectrum of cutaneous AEs linked with pembrolizumab and their possible correlation with treatment response.
DESIGN, SETTING, AND PARTICIPANTS
A single-institution, retrospective medical record review was conducted of patients with cancer who were treated with pembrolizumab from March 1, 2011, to May 28, 2014. The review comprised 83 consecutive patients who were enrolled in 2 clinical trials, received at least 1 dose of pembrolizumab, and had at least 1 follow-up visit. Patients were grouped according to the following therapeutic regimen for pembrolizumab: 43 received 10 mg/kg every 3 weeks, 24 received 10 mg/kg every 2 weeks, and 16 received 2 mg/kg every 3 weeks. Sixty-six patients were treated for melanoma, 15 patients for lung cancer, 1 patient for prostate cancer, and 1 patient for Merkel cell carcinoma. Median follow-up was 15 weeks (range, 2-105 weeks). The analysis was conducted from March 1 to September 30, 2014.
MAIN OUTCOMES AND MEASURES
Occurrence, severity, and type of cutaneous AEs, as well as disease progression and response to pembrolizumab treatment.
RESULTS
Thirty-five patients (42%) developed cutaneous AEs attributed to pembrolizumab. The most common cutaneous AEs were macular papular eruption (24 [29%]), pruritus (10 [12%]), and hypopigmentation (7 [8%]). All 7 patients who developed hypopigmentation were treated for melanoma. Survival analyses showed that patients who developed cutaneous AEs had significantly longer progression-free intervals in all 3 groups (pembrolizumab, 10 mg/kg, every 3 weeks, P = .001; pembrolizumab, 10 mg/kg, every 2 weeks, P = .003; pembrolizumab, 2 mg/kg, every 3 weeks, P = .009) compared with patients who did not develop cutaneous AEs.
CONCLUSIONS AND RELEVANCE
Pembrolizumab therapy was associated with cutaneous AEs in 42% of patients. The development of cutaneous AEs, especially of hypopigmentation in patients with melanoma, could point toward better treatment response.
The immune system recognizes and eliminates transformed cells and protects against cancer. Cell clones that evade this immunosurveillance can multiply and lead to malignant neoplasms. Cancer cells develop different mechanisms to avoid this immunosurveillance.1
In one of these evasion mechanisms, tumor cells express programmed death ligand 1 (PDL-1) and PDL-2. Both PDL-1 and PDL-2 bind to the programmed death-1 (PD-1) receptor, which can be expressed on CD4+ T cells, CD8+ T cells, natural killer T cells, and B cells. The interaction of PDL-1 and PD-1 leads to an inactivation of immune cells and prevents an effective immune response. Tumor cells that express PDL-1 are believed to use this interaction to suppress T-lymphocyte action and induce adaptive immune resistance.2,3
Newly developed monoclonal antibodies such as pembrolizumab and nivolumab are designed to block the interaction between the tumor cell and the immune system, facilitating the immune response to cancer. Both antibodies target PD-1 and have shown promising results in clinical trials in patients with melanoma, non-small cell lung cancer, and renal cell cancer.4-6 Therapy with pembrolizumab at any dose level led to a tumor response in 38% of patients with metastatic melanoma; 52% of patients responded to a dosage of 10 mg/kg every 2 weeks.5 The US Food and Drug Administration recently approved pembrolizumab and nivolumab for the treatment of patients with unresectable or metastatic melanoma and disease progression following ipilimumab therapy, and, if the neoplasm is positive for a BRAF V600 mutation, combination therapy with a BRAF inhibitor.7
Similar to other therapies, anti–PD-1 treatment is associated with a number of adverse events (AEs) such as hypothyroidism, gastrointestinal tract disorders, generalized symptoms like fatigue or myalgia, increased aminotransferase levels, respiratory disorders, and skin disorders. Macular papular eruption, pruritus, and vitiligo were reported in 21%, 21%, and 9%, respectively, of patients receiving anti–PD-1 treatment.5 These and other cutaneous AEs can affect patients’ quality of life and can lead to dose reduction or therapy discontinuation.
Owing to the promising results seen with anti–PD-1 treatment, we expect more patients to receive anti-PD-1 treatment in the near future. We describe cutaneous AEs and their correlation with disease progression and eosinophil serum count in 83 patients treated with pembrolizumab.
Methods
Patients
The University of California, San Francisco, Institutional Review Board approved this retrospective cohort study on patients enrolled in 2 clinical trials (NCT01295827 and NCT01866319). Patients provided written consent for the study. All patients received pembrolizumab treatment and were observed at the University of California, San Francisco. Patients who received at least 1 cycle of pembrolizumab treatment and completed at least 1 follow-up visit were included. Seventy-one patients were included in trial NCT012958278 and 12 in trial NCT01866319.9 Sixty-six patients were treated for melanoma, 15 patients for lung cancer, 1 patient for prostate cancer, and 1 patient for Merkel cell carcinoma. No patient was unavailable for follow-up.
Data Collection
We reviewed patients’ medical records from the beginning of each trial (March 1, 2011) to May 28, 2014. Data collection and analysis was performed from March 1, 2014, to September 30, 2014. We collected the following data: patient demographics, trial number, therapeutic regimen, time in trial, number of cycles of pembrolizumab received, type of disease, stage of disease, previous cancer therapies, known allergies, time point of disease progression, history of skin disease, cutaneous AEs attributed to pembrolizumab, time point of cutaneous AEs, treatment of cutaneous AEs and response to such treatment, eosinophil count before the first pembrolizumab cycle, and eosinophil count at the time point of cutaneous AEs. Adverse events were graded based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.03.10 Disease progression was defined according to immune-related response criteria.11
Statistical Analysis
Statistical analyses were performed using Stata, version 12.0 (StataCorp). To compare groups, we used the Pearson χ2 or Fisher exact test (categorical variables) and Wilcoxon rank-sum test (continuous variables). For comparisons of absolute eosinophil count numbers across time, we used the Wilcoxon signed-rank test. Time to disease progression was calculated from the first pembrolizumab cycle to progression. For patients with disease progression, time to progression corresponded to the time receivingtreatment. Kaplan-Meier curves were plotted for time to event analyses, and for comparisons we used the log-ranktest. Coxpro-portional hazards regression analyses were carried out on time to progression to calculate crude and adjusted hazard ratios with 95% CIs for different study groups. The proportional hazard assumption was assessed with Schoenfeld residuals. P < .05 was considered significant.
Results
Frequency and Spectrum of Cutaneous AEs and Eosinophil Count
We included 83 patients in our study (31 women and 52 men); median age at the beginning of the study was 66 years (range, 18-90 years). Median follow-up was 15 weeks (range, 2-105 weeks) and median number of treatment cycles was 6 (range, 1-51 cycles). Thirty-four patients were still receiving pembrolizumab treatment at the end of the study. Table 1 reports the characteristics of the included patients.
Table 1.
Characteristics of 83 Patients Treated With Pembrolizumab
| Characteristic | Adverse Eventsa | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 mg/kg Every 3 Weeks (n = 43) |
P Value | 10 mg/kg Every 2 Weeks (n = 24) |
P Value | 2 mg/kg Every 3 Weeks (n = 16) |
P Value | ||||
| No Cutaneous (n = 24) |
Cutaneous (n = 19) |
No Cutaneous (n = 17) |
Cutaneous (n = 7) |
No Cutaneous (n = 7) |
Cutaneous (n = 9) |
||||
| Age, median (range), y | 65.9 (36.6-89.6) |
67.4 (55.2-87.7) |
.64b | 67.6 (38.5-88.8) |
64.1 (40.4-84.4) |
.82b | 57.3 (43.2-73.0) |
57.0 (18.4-72.0) |
.79b |
| Sex | .56c | .61d | .63d | ||||||
| Male | 16 (67) | 11 (58) | 14 (82) | 5 (71) | 2 (29) | 4 (44) | |||
| Female | 8 (33) | 8 (42) | 3 (18) | 2 (29) | 5 (71) | 5 (56) | |||
| Previous allergy | .25c | .48d | >.99d | ||||||
| No | 14 (58) | 14 (74) | 10 (59) | 3 (43) | 4 (57) | 4 (44) | |||
| Yes | 10 (42) | 5 (26) | 7 (41) | 4 (57) | 3 (43) | 5 (56) | |||
| Tumor type | >.99d | .66d | >.99d | ||||||
| Melanoma | 17 (71) | 15 (79) | 13 (76) | 6 (86) | 7 (100) | 8 (89) | |||
| Lung cancer | 6 (25) | 4 (21) | 4 (24) | 1 (14) | 0 | 0 | |||
| Prostate cancer | 1 (4) | 0 | 0 | 0 | 0 | 0 | |||
| Merkel cell carcinoma | 0 | 0 | 0 | 0 | 0 | 1 (11) | |||
| Tumor stage | .24d | >.99d | >.99d | ||||||
| 3 | 3 (13) | 0 | 2 (12) | 0 | 7 (100) | 9 (100) | |||
| 4 | 21 (88) | 19 (100) | 15 (88) | 7 (100) | 0 | 0 | |||
| Previous chemotherapy | .61c | >.99d | >.99d | ||||||
| No | 12 (50) | 11 (58) | 12 (71) | 5 (71) | 5 (71) | 6 (67) | |||
| Yes | 12 (50) | 8 (42) | 5 (29) | 2 (29) | 2 (29) | 3 (33) | |||
| Previous immunotherapy | .11c | >.99d | .21d | ||||||
| No | 16 (67) | 8 (42) | 9 (53) | 5 (71) | 0 | 3 (33) | |||
| Yes | 8 (33) | 11 (58) | 8 (47) | 2 (29) | 7 (100) | 6 (67) | |||
| Previous target therapy | .55d | .13d | .55d | ||||||
| No | 17 (71) | 15 (79) | 11 (65) | 7 (100) | 5 (71) | 8 (89) | |||
| Yes | 7 (29) | 4 (21) | 6 (35) | 0 | 2 (29) | 1 (11) | |||
| Baseline eosinophil count, absolute No., median (range), 109/L |
0.12 (0.01-0.44) |
0.13 (0.04-0.44) |
.57b | 0.13 (0.04-0.66) |
0.16 (0.03-0.46) |
.37b | 0.08 (0.04-0.27) |
0.10 (0.07-0.31) |
.12b |
| Cycles of pembrolizumab, median (range), No. |
4.5 (1-24) | 17 (2-35) | >.001b | 6 (1-23) | 10 (6-51) | .02b | 4 (1-21) | 18 (4-33) | .05b |
SI conversion factor: To convert eosinophil counts to billion cells per liter, multiply by 0.001.
Data are presented as number (percentage) of patients unless otherwise indicated.
Two-sample Wilcoxon rank-sum test.
χ2 Test.
Fisher exact test.
Patients were grouped according to the therapeutic regimen for pembrolizumab: 43 received 10 mg/kg every 3 weeks, 24 received 10 mg/kg every 2 weeks, and 16 received 2 mg/kg every 3 weeks. Thirty-five patients (42%) developed cutaneous AEs attributed to pembrolizumab therapy (Table 2). Twenty-six patients developed 1 cutaneous AE, 7 developed 2 cutaneous AEs, and 2 developed 3 cutaneous AEs (macular papular eruption, pruritus, and hypopigmentation). The most prevalent cutaneous AEs were macular papular eruption (24 [29%]), pruritus (10 [12%]), and hypopigmentation (7 [8%]). Figure 1 and Figure 2 show representative clinical pictures. Table 2 shows the distribution of cutaneous AEs in the 3 treatment-regimen groups.
Table 2.
Cutaneous Adverse Events in Patients Treated With Pembrolizumab
| Adverse Event | No. (%) | |||
|---|---|---|---|---|
| Total (N = 83) |
10 mg/kg Every 3 Weeks (n = 43) |
10 mg/kg Every 2 Weeks (n = 24) |
2 mg/kg Every 3 Weeks (n = 16) |
|
| Any cutaneous adverse event | 35 (42) | 19 (44) | 7 (29) | 9 (56) |
| Grade | ||||
| 1 | 31 of 35 (89) | 16 of 19 (84) | 6 of 7 (86) | 9 of 9 (100) |
| 2 | 2 of 35 (6) | 2 of 19 (11) | 0 | 0 |
| 3 | 2 of 35 (6) | 1 of 19 (5) | 1 of 7 (14) | 0 |
| 4 | 0 | 0 | 0 | 0 |
| Macular papular eruption | 24 (29) | 15 (35) | 3 (13) | 6 (38) |
| Pruritus | 10 (12) | 6 (14) | 2 (8) | 2 (13) |
| Hypopigmentation | 7 (8) | 4 (9) | 1 (4) | 2 (13) |
| Xerosis | 2 (2) | 0 | 2 (8) | 0 |
| Keratosis | 2 (2) | 2 (5) | 0 | 0 |
| Facial erythema | 1 (1) | 1 (2) | 0 | 0 |
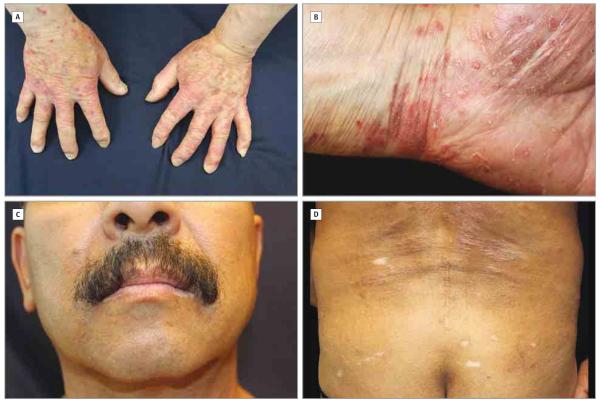
Figure 1. Cutaneous Adverse Events During Treatment With Pembrolizumab.
A, Macular papular eruption involving the dorsal surfaces of both hands. B, Macular papular eruption involving the palmar surface of both hands. C, Spontaneous hypopigmentation (vitiligo) on and around lips. D, Scaly macular papular eruption on the lower back with some hypopigmented postinflammatory regions.
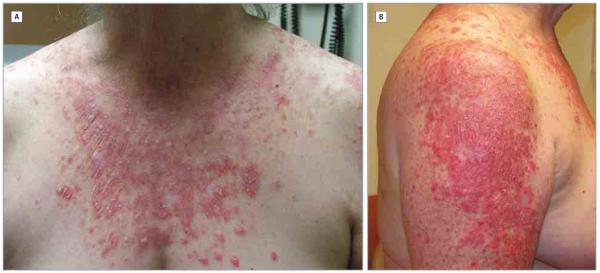
Figure 2. Grade 3 Cutaneous Adverse Events in a Patient Treated With Pembrolizumab.
A, Macular papular eruption with predilection sites on photoexposed areas of the chest. B, Macular papular eruption with predilection sites on photoexposed areas of the arm.
A macular papular eruption was diagnosed in 24 patients, of which 8 (33%) developed the eruption after the first treatment cycle. The median number of pembrolizumab cycles before eruption occurrence was 3 (range, 1-29 cycles). Mild and moderate eruptions were treated with low-dose topical corticosteroids (betamethasone valerate, 0.1%, or hydrocortisone acetate, 1%) and moisturizing ointments. The treatment of more severe cutaneous AEs is described below. Nine (38%) eruptions resolved completely. The ongoing eruptions were well tolerated and did not cause modifications in the pembrolizumab treatment regimen.
Pruritus was diagnosed in 10 patients, of which 6 had no other skin manifestations. The median number of treatment cycles received before the development of pruritus was 3 (range, 1-17 cycles). All 10 patients were treated topically with moisturizing ointments and creams and, for persistent cases of pruritus, with oral antihistamines. Five patients experienced complete resolution of pruritic symptoms.
Seven patients developed hypopigmentation, all of whom were treated for melanoma. The median number of pembrolizumab cycles before development of hypopigmentation was 8 (range, 5-14 cycles). In 3 patients who also developed a macular papular eruption, the hypopigmentation always appeared after the onset of the eruption.
None of the patients developed grade 4 cutaneous AEs. Two patients developed a grade 3 cutaneous AE. In particular, 1 patient had a psoriasis exacerbation with development of new plaques on the back, extremities, and face. The new plaques occurred after 3 cycles of pembrolizumab treatment, and treatment was withheld for 1 week. This patient was treated with systemic (prednisone, 10 mg/d) and topical (triamcinolone acetonide, 0.1%, twice a day) corticosteroids and had a partial response. The second patient with a grade 3 cutaneous AE developed erythematous papules and pustules coalescing to large plaques across the body after 4 treatment cycles. The lesions were more prominent on photoexposed skin. Pembrolizumab was withheld for 7 weeks and this patient was treated with systemic (prednisone, 60 mg/d, tapered to discontinuation) and topical (clobetasol propionate ointment, 0.05%, twice a day) corticosteroids, with a partial response (Figure 2).
Baseline eosinophil count for all patients with cutaneous AEs (P = .43), and only patients who developed macular papular eruptions or pruritus (P = .92) did not differ significantly from the eosinophil count at the time of development of cutaneous AEs.
Cutaneous AEs and Disease Progression
To analyze associations between cutaneous AEs and time to disease progression, we divided the patients into 3 groups according to the treatment schedule. Baseline characteristics did not differ between patients who developed cutaneous AEs and those who did not, in all 3 groups. Patients who developed cutaneous AEs received a higher number of pembrolizumab cycles (Table 1).
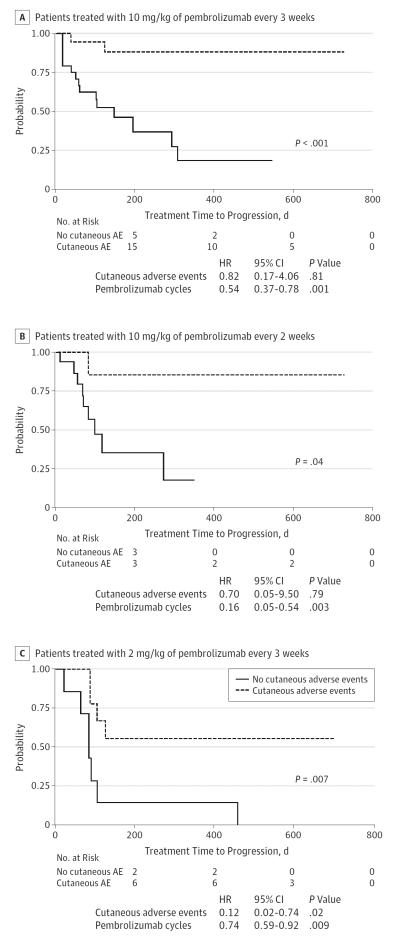
Survival analyses showed that patients who developed cutaneous AEs had a significantly longer progression-free survival in all 3 groups (pembrolizumab, 10 mg/kg every 3 weeks, P < .001; pembrolizumab, 10 mg/kg every 2 weeks, P = .04; pembrolizumab, 2 mg/kg every 3 weeks, P = .007) (Figure 3). In patients treated with the lowest dose of pembrolizumab, the hazard ratio maintained its statistical significance also in multivariate analysis.
Figure 3. Kaplan-Meier Survival Curves for Progression-Free Survival and Multivariate Cox Proportional Hazard Regression Models Corrected for the Number of Pembrolizumab Cycles.
A, Patients treated with 10 mg/kg every 3 weeks. B, Patients treated with 10 mg/kg every 2 weeks. C, Patients treated with 2 mg/kg every 2 weeks. Patients who developed cutaneous adverse events have a longer progression-free interval in all treatment groups. HR indicates hazard ratio.
Discussion
Our study describes the incidence and the spectrum of cutaneous AEs in a cohort of 83 patients treated with pembrolizumab. Thirty-five patients (42%) developed cutaneous AEs that were attributed to pembrolizumab. The cutaneous safety profile was overall favorable: no grade 4 AEs were observed and the 2 cases of grade 3 cutaneous AEs were manageable. Previous studies testing the safety and activity profiles of different pembrolizumab dose regimens reported similar numbers of AEs.4,5 The frequencies of cutaneous AEs during pembrolizumab treatment are similar to those previously described during ipilimumab treatment, which is another immunomodulatory drug used for the treatment of cancer.12,13
Macular papular eruptions were the most common cutaneous AE in our cohort (24 [29%]). Photoexposed areas were more frequently involved, and in 7 patients, the eruptions involved the hands or feet (Figure 1). No patient reported involvement of mucous membranes. These clinical features are similar to those commonly termed as maculopapular drug exanthema, which is frequently observed with other drugs such as antibiotics, chemotherapeutics, and targeted cancer therapies.12,14 Most of our patients developed eruptions after the first dose of pembrolizumab. The high incidence, fast onset, and distribution of the rashes point toward a type A adverse drug reaction, according to the commonly used classification proposed by Rawlins and Thompson.15 However, we could not observe any correlations in our patients between cutaneous AEs and dose, which are usually seen in type A adverse drug reactions. Macular papular eruptions and pruritus also occur with high incidence in patients treated with ipilimumab. In these patients, a correlation between serum eosinophil count and development of eruptions and pruritus was described.16 In our cohort, none of the cutaneous AEs were associated with changes in serum eosinophil count, suggesting a different pathogenic mechanism.
Survival analyses showed that patients who developed cutaneous AEs had significantly longer progression-free intervals, regardless of the treatment regimen. We must interpret these findings with caution because patients who progress interrupt the pembrolizumab treatment and do not have the same cumulative dose of the drug as those who do not progress and continue taking it. Therefore, they have less chance and time to develop a cutaneous AE. Indeed, patients who received more pembrolizumab cycles had a longer progression-free survival and developed more cutaneous AEs. However, other authors have already identified cutaneous AEs as positive prognostic factors for other immune-modulatory cancer treatments,17,18 and in one of our treatment groups, the development of cutaneous AEs maintained its significant role in the risk of progression when corrected for the number of pembrolizumab cycles.
Such cutaneous manifestations could be the product of more potent immune system activation owing to immune-modulatory drugs. Supporting this hypothesis, we observed that only patients affected by melanoma developed hypopigmentation. In fact, melanocytes and melanoma have common antigens (eg, MART-1, gp100, and tyrosinase) and lymphocyte populations directed against the tumor could cross-react with normal melanocytes and cause skin hypo-pigmentation.19,20 This is a well-known positive prognostic factor in patients with melanoma.18,21-23 In our study, of the 7 patients who developed hypopigmentation, only 1 developed disease progression.
Conclusions
Altogether, our experience with 83 patients receiving pembrolizumab showed a favorable cutaneous safety profile. The majority of our patients responded well to topical corticosteroids, such as betamethasone, 0.1%, and hydrocortisone, 1% to 2%, and to oral antipruritics, paired with moisturizing ointments. The development of cutaneous AEs, especially of hypopigmentation in patients with melanoma, might be a positive prognostic sign.
Acknowledgments
Funding/Support: This study was supported by the National Cancer Institute of the National Institutes of Health under award number K08CA155035 and the Melanoma Research Alliance.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Sanlorenzo and Vujic had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sanlorenzo, Vujic, Daud, Rappersberger, Ortiz-Urda.
Acquisition, analysis, or interpretation of data: Sanlorenzo, Vujic, Daud, Algazi, Gubens, Luna, Lin, Quaglino, Ortiz-Urda.
Drafting of the manuscript: Sanlorenzo, Vujic.
Critical revision of the manuscript for important intellectual content: Vujic, Daud, Algazi, Gubens, Luna, Lin, Quaglino, Rappersberger, Ortiz-Urda.
Statistical analysis: Sanlorenzo, Vujic.
Obtained funding: Rappersberger, Ortiz-Urda.
Administrative, technical, or material support: Algazi, Lin, Ortiz-Urda.
Study supervision: Algazi, Quaglino, Rappersberger, Ortiz-Urda.
Conflict of Interest Disclosures: Drs Gubens, Daud, and Algazi report research support to their institution by Merck involving work under consideration. Dr Gubens also reports research support to his institution by Abbvie, Celgene, Genentech/Roche, and Novartis, and advisory board involvement with Celgene (travel only), Genentech/Roche (travel only), and Pfizer.DrDaud reports research support to his institution by BMS, Genentech, GlaxoSmithKline, Novartis, OncoSec, Pfizer, and Roche, and stock ownership in OncoSec. Dr Daud also acts as consultant for Amgen, BMS, Celgene, Genentech, Median Technologies, and OncoSec. Dr Algazi reports research support to his institution by AstraZeneca, BMS, GlaxoSmithKline, Novartis, OncoSec, and Pfizer. No other disclosures were reported.
Additional Contributions: The authors are grateful to Timothy Dattels, MBA, for his generous support.
REFERENCES
- 1.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121(1):1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 3.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19(7):813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 4.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration [Accessed October 28, 2014];Pembrolizumab. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm412861.htm.
- 8.US National Institutes of Health [Accessed June 1, 2015];Study of pembrolizumab (MK-3475) in participants with progressive locally advanced or metastatic carcinoma, melanoma, or non-small cell lung carcinoma (P07990/MK-3475-001/KEYNOTE-001) https://clinicaltrials.gov/ct2/show/NCT01295827.
- 9.US National Institutes of Health [Accessed June 1, 2015];Study to evaluate the safety and efficacy of two different dosing schedules of pembrolizumab (MK-3475) compared to ipilimumab in participants with advanced melanoma (MK-3475-006/KEYNOTE-006) https://clinicaltrials.gov/ct2/show/NCT01866319.
- 10.US Department of Health and Human Services [Accessed September 30, 2014];Common terminology criteria for adverse events (CTCAE) updated June 14, 2010. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 11.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 12.Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapy for cancer: part II: targeted therapy. J Am Acad Dermatol. 2014;71(2):217.e1–e217.e11. doi: 10.1016/j.jaad.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Kähler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges. 2011;9(4):277–286. doi: 10.1111/j.1610-0387.2010.07568.x. [DOI] [PubMed] [Google Scholar]
- 14.Bigby M. Rates of cutaneous reactions to drugs. Arch Dermatol. 2001;137(6):765–770. [PubMed] [Google Scholar]
- 15.Rawlins M, Thompson J. Pathogenesis of adverse drug reactions. In: Davies DM, editor. Textbook of Adverse Drug Reactions. Oxford University Press; Oxford, England: 1977. [Google Scholar]
- 16.Jaber SH, Cowen EW, Haworth LR, et al. Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Arch Dermatol. 2006;142(2):166–172. doi: 10.1001/archderm.142.2.166. [DOI] [PubMed] [Google Scholar]
- 17.Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti–CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8(1):e53745. doi: 10.1371/journal.pone.0053745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phan GQ, Attia P, Steinberg SM, White DE, Rosenberg SA. Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol. 2001;19(15):3477–3482. doi: 10.1200/JCO.2001.19.15.3477. [DOI] [PubMed] [Google Scholar]
- 19.Houghton AN, Eisinger M, Albino AP, Cairncross JG, Old LJ. Surface antigens of melanocytes and melanomas: markers of melanocyte differentiation and melanoma subsets. J Exp Med. 1982;156(6):1755–1766. doi: 10.1084/jem.156.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch SA, Bouchard BN, Vijayasaradhi S, Yuasa H, Houghton AN. Antigens of melanocytes and melanoma. Cancer Metastasis Rev. 1991;10(2):141–150. doi: 10.1007/BF00049411. [DOI] [PubMed] [Google Scholar]
- 21.Nordlund JJ, Kirkwood JM, Forget BM, Milton G, Albert DM, Lerner AB. Vitiligo in patients with metastatic melanoma: a good prognostic sign. J Am Acad Dermatol. 1983;9(5):689–696. doi: 10.1016/s0190-9622(83)70182-9. [DOI] [PubMed] [Google Scholar]
- 22.Quaglino P, Marenco F, Osella-Abate S, et al. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a single-institution hospital-based observational cohort study. Ann Oncol. 2010;21(2):409–414. doi: 10.1093/annonc/mdp325. [DOI] [PubMed] [Google Scholar]
- 23.Bystryn JC, Rigel D, Friedman RJ, Kopf A. Prognostic significance of hypopigmentation in malignant melanoma. Arch Dermatol. 1987;123(8):1053–1055. [PubMed] [Google Scholar]