Abstract
Sepsis is a life-threatening condition caused by the inflammatory response to invading organisms. Polymyxin B-immobilized fiber column direct hemoperfusion (PMX-DHP) is used to reduce blood endotoxin levels and modulate circulating inflammatory cytokine levels in sepsis patients. Here we report that severe sepsis caused by an infection of the gram-negative bacterium Pantoea agglomerans in a patient with small cell lung carcinoma was treated successfully with antibiotics and PMX-DHP. The patient, a 49-year-old Japanese male smoker whose condition was complicated with hyponatremia due to SIADH (syndrome of inappropriate secretion of antidiuretic hormone), rapidly developed sepsis and disseminated intravascular coagulation (DIC) after the administration of cisplatin and irinotecan. Despite initial antibiotics therapy, severe host responses including hypotension, high body temperature and tachycardia were noted. We initiated PMX-DHP, and the patient's Sequential Organ Failure Assessment score was greatly reduced and his DIC improved immediately. To our knowledge, this is the first reported case of PMX-DHP therapy for severe sepsis caused by P. agglomerans infection. Although the efficacy of PMX-DHP in sepsis is not well defined, PMX-DHP therapy should be considered in cases of sepsis from gram-negative infections.
Keywords: Pantoea agglomerans, SCLC, SIADH, PMX-DHP, Sepsis
1. Introduction
Small cell lung carcinoma (SCLC) is a common cancer type in bronchogenic carcinomas. The features of SCLC include rapid growth, early disseminated metastasis and highly responsivity to chemotherapy. Although chemotherapy for SCLC can effectively reduce the tumor size, some SCLC patients undergoing chemotherapy are exposed to severe infection, which is attributed to their immunocompromised condition. Pantoea agglomerans (P. agglomerans) is a gram-negative bacterium found in soil, water, plant and the intestinal tracts of human and animals [1], [2], [3].
Little has been reported on the infectious conditions of P. agglomerans [4], but P. agglomerans has described as a pathogen for arthritis [5], pneumonia [6], peritonitis [7], and sepsis [3], [4], [8] in immunocompromised hosts, including patients undergoing chemotherapy. Polymyxin B-immobilized fiber column direct hemoperfusion (PMX-DHP) therapy has been used to remove lipopolysaccharides (LPS) from gram-negative organisms in sepsis patients. Although it has been documented that PMX-DHP improved patients' Sequential Organ Failure Assessment (SOFA) score and reduced 28-day mortality in a prospective randomized controlled trial [9], the results obtained to date regarding the efficacy of PMX-DHP in sepsis are controversial [10].
Here, we report that severe sepsis caused by a P. agglomerans infection in an SCLC patient undergoing cisplatin-based chemotherapy was treated successfully with antibiotics and PMX-DHP.
2. Clinical report
A 49-year-old Japanese male smoker with SCLC was admitted for chemotherapy. His performance status (PS) was good (PS = 0). He had been diagnosed with SCLC 3 months prior to this admission (Fig. 1). At that time, he had been treated with cisplatin (CDDP: 60 mg/m2) and irinotecan (CPT-11: 60 mg/m2) as first-line chemotherapy. Since the chest CT scan on his admission to our institution revealed the reduction of tumor size (reduction rate: 75%, Fig. 1) by chemotherapy, he was admitted for the administration of a third cycle of chemotherapy. On day 6 of the third cycle, he had become febrile with severe diarrhea. Despite the intravenous administration of sulbactam/ampicillin sodium (SBT/ABPC), life-threatening severe sepsis developed and he was transferred to the intensive care unit (ICU) the same day.
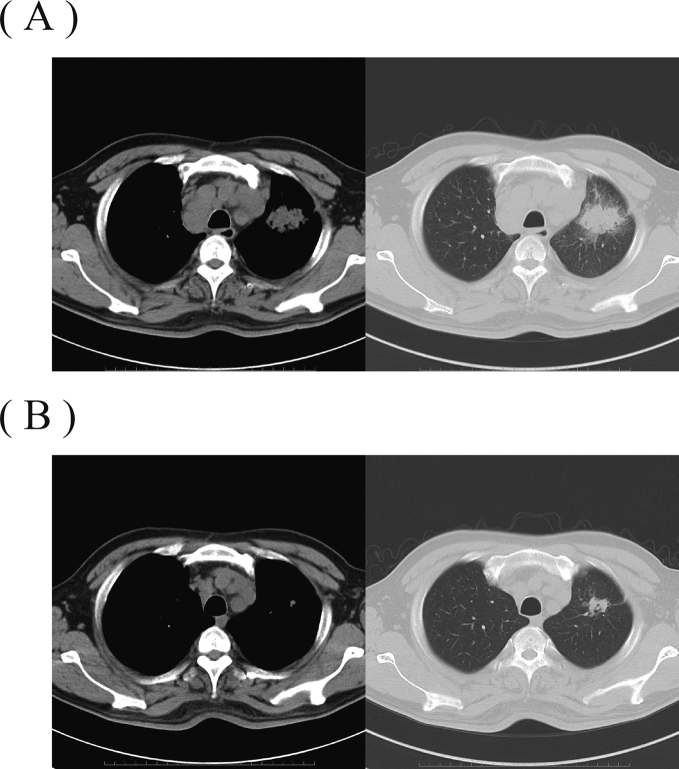
Fig. 1.
Chest CT at the diagnosis of SCLC and 3 months later at admission. (A) Chest CT at the diagnosis of SCLC (3 months prior to this admission) in the patient, a 49-year-old Japanese male smoker. The chest CT shows the primary tumor shadow in the left superior lobe and lymph node metastasis in the mediastinum. (B) Chest CT on admission. Reductions of size in both the primary tumor shadow and the lymph node metastasis are observed.
The physical examination on transfer to the ICU revealed the body temperature of 38.9 °C, blood pressure 67/42 mmHg, regular pulse 152 beats/min and oxygen saturation 86% under 3 L/min of oxygen. The patient's skin was not flushed, and lung auscultation was the normal respiratory sound.
The results of the laboratory data and an arterial blood gas analysis under 3 L/min of oxygen (Table 1) showed a white blood cell count 19,100/mm3, hemoglobin 10.2 g/dL, platelet count 18,000/mm3, C-reactive protein (CRP) 24.4 mg/dl, endotoxin 8.4 pg/mL, pH 7.23, PaCO2 23.2 Torr, PaO2 45.9 Torr, and lactic acid 7.7 mmol/L. The SOFA score was 11 (PaO2/FiO2 ratio 139, platelet count 18,000/mm3, serum bilirubin 1.4 mg/dL, dopamine <5 μg/kg/min, Glasgow Coma Scale 13, and serum creatinine 1.33 mg/dL), and the disseminated intravascular coagulation (DIC) score was 5 (systemic inflammatory response syndrome [SIRS] score 4, platelet count 18,000/mm3, prothrombin time ≥ 1.2, and fibrinogen degradation products 8.38 mg/dL).
Table 1.
The laboratory data on admission.
| <Hematology> | <Biochemistry> | <Coagulation> | |||
|---|---|---|---|---|---|
| WBC | 19100/m3 | TP | 5.73 g/dl | PT-INR | 1.74 |
| Neut. | 93.9% | BUN | 20.0 mg/dl | APTT | 36.1 sec |
| Lymph. | 2.8% | Cre | 1.33 mg/dl | D-dimer | 5.12 μg/mL |
| Mono. | 2.9% | AST | 17 IU/L | FDP | 9.2 μg/mL |
| RBC | 317 × 104/mm3 | ALT | 20 IU/L | Fibrinogen | 476 mg/mL |
| Hb | 10.2 g/dl | LDH | 248 IU/L | <Others> | |
| Plt | 1.8 × 104/mm3 | Na | 127 mEq/L | ADH | 1.1 pg/dl |
| K | 3.0 mEq/L | Cortisol | 39.7 μg/dl | ||
| Cl | 90 mEq/L | urine Na | 127 mEq/L | ||
| CRP | 24.4 mg/dl | plasma osmolality | 264 mOsm/Kg | ||
| Endotoxin | 8.4 pg/dl | urine osmolality | 524 mOsm/Kg | ||
The laboratory data revealed that the patient's SCLC was complicated by syndrome of inappropriate secretion of antidiuretic hormone (SIADH; plasma osmolality 264 mOsm/kg, urine osmolality 524 mOsm/kg, urine sodium concentration 65 mEq/L, serum sodium 127 mEq/L, serum creatinine 0.61 mg/dL, plasma cortisol 39.7 μg/dl, and antidiuretic hormone 1.1 pg/mL). A chest X-ray showed no abnormal shadows, such as consolidations, except for shadows of the main tumor and lymph node metastasis (Fig. 2). Although a sputum culture was negative, blood cultures from the intermediate cubital vein and right radial artery revealed P. agglomerans.
Fig. 2.
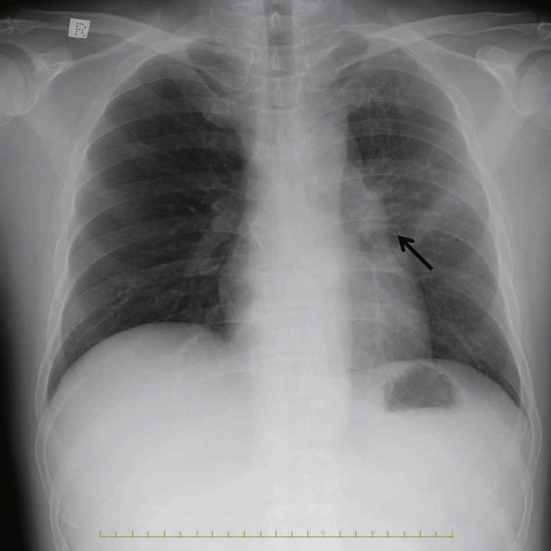
Chest X-ray on transfer to the intensive care unit. Chest X-ray on transfer to the ICU. The chest X-ray shows enlargement of left hilar shadow (arrow) without consolidations.
After the patient's transfer to the ICU, his antibiotic therapy was changes to tazobactam/piperacillin (TAZ/PIPC) and gentamicin (GM) for a total of 14 days, and he underwent PMX-DHP for 2 consecutive days (Fig. 3). Additionally, red cell concentrates-mannitol adenine phosphate (RCC-MAP), platelet concentrates-leukocytes reduced (PC-LR) and fresh frozen plasma-leukocytes reduced (FFP-LR) were transfused and recombinant thrombomodulin was administered for the patient's DIC.
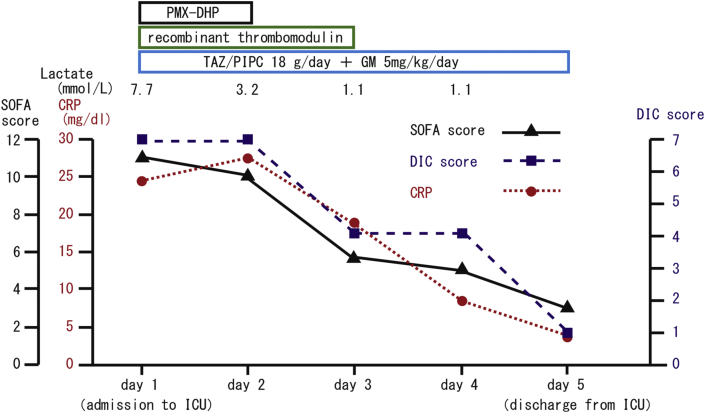
Fig. 3.
Clinical course in the intensive care unit. Changes in the SOFA score, DIC score, CRP level and lactate level. After PMX-DHP treatment and the administration of antibiotics, the SOFA score, DIC score, and CRP level dropped from 11 to 3, from 7 to 1, and from 24.4 mg/dl to 3.3 mg/dl, respectively during the patient's 5-day stay in the ICU.
On day 9, the patient recovered from the septic shock, and the administration of the vasopressor was discontinued. He showed a good response to these therapies, including the antibiotics and PMX-DHP, and he was released from the ICU on day 11. On day 12 of the third cycle of chemotherapy, the TAZ/PIPC and GM were discontinued and his treatment was switched to daily intravenous flomoxef for 7 days. By the end of the antibiotics therapy, the blood cultures were negative. Although the patient underwent second- and third-line chemotherapy, carboplatin + etoposide and amrubicin, respectively, he died due to lymphangitis carcinomatosa at 15 months after the SCLC diagnosis.
3. Discussion
This is the first report of the clinical presentation and course of treatment by PMX-DHP for septic shock due to a P. agglomerans infection in a patient with SCLC with SIADH.
P. agglomerans is a gram-negative rod bacterium commonly found in soil, plants and the feces of humans and animals. Nosocomial infection caused by P. agglomerans is rare [3]. Few reports are available on sepsis due to P. agglomerans in patients with cancer, while reports of 16 cases are available in the English literature [3], [4], [8], [11]. The mortality rate of patients with sepsis due to P. agglomerans among adults and neonates were 11.1% [4] and 55.5% [12], respectively. With regard to the effective antimicrobial treatment of P. agglomerans infection, the antimicrobial susceptibility in patients with bacteremia was measured as follows: fosfomycin (FOM), 33%; ampicillin (ABPC), 56%; cefazolin (CEZ), 61%; cefotaxime (CTX), 100%; ceftazidime (CAZ), 100%; piperacillin-tazobactam (PIPC/TAZ), 100%; imipenem (IPM), 100%; ciprofloxacin (CPFX), 100%; gentamicin (GM), 100% and amikacin (AMK), 100% [4].
In the present patients' case, the susceptibility (such as the minimal inhibitory concentration [MIC]) of cultured P. agglomerans showed characteristics similar to those of a previous report as follows: ABPC, intermediate (MIC, 16.0 μg/mL); CEZ, susceptible (MIC <4.0 μg/mL); CTX, susceptible (MIC <8.0 μg/mL); CAZ, susceptible (MIC <1.0 μg/mL); PIPC/TAZ, susceptible (MIC <16.0 μg/mL); meropenem (MEPM), susceptible (MIC <1.0 μg/mL); CPFX, susceptible (MIC <0.5 μg/mL); GM, susceptible (MIC <1.0 μg/mL) and AMK, susceptible (MIC <4.0 μg/mL). In a cohort of adults, the patients with bacteremia who received effective empirical antibiotics had a high cure rate (88.9%) [4]. Thus, the antibiotics recommended as empiric antimicrobial therapy were PIPC/TAZ and carbapenem, according to the antimicrobial susceptibility.
Several routes of P. agglomerans infection such as catheter [13] and thorn injury [5] have been proposed. In our patient's case, although central venous and pulmonary artery catheters were not used and no bacterial body grew from sputum culture and the chest X-ray showed no consolidations on transfer to the ICU, grade 3 diarrhea appeared (Common Terminology Criteria for Adverse Events, ver. 4.0) after irinotecan-based chemotherapy. Intestinal mucositis is a common side effect of irinotecan [14], and it can cause bacteremia [15]. The intestinal epithelial damage caused by irinotecan treatment leads to diarrhea and bacterial/endotoxin translocation due to the disruption of the epithelial barrier, as severe adverse effects [14], [16].
It is unclear how our patient acquired a septic infection of P. agglomerans, but it is possible that irinotecan-induced intestinal mucositis cause the infection. The fact that P. agglomerans is one of the gut bacteria, the non-use of central venous and pulmonary artery catheters, our finding that the sputum culture grew no bacterial body, and the presence of severe diarrhea all support this possibility.
In our patient, we noted some parameters associated with poor prognosis in sepsis; i.e., serum lactic acid [17], serum procalcitonin [18] and hyponatremia [19]. In addition, hyponatremia induced by SIADH (a paraneoplastic syndrome) is a strong prognostic factor in SCLC patients [20]. In our patient, the levels of plasma osmolality, urine osmolality, urine sodium, serum sodium, serum creatinine and antidiuretic hormone, the non-use of diuretic agents, and the normal thyroid and adrenal function satisfied the diagnostic criteria of SIADH [21]. These data suggest that the mortality rate of this type of case is very high.
With regard to the treatment of sepsis, this patient was treated by PMX-DHP with the administration of antibiotics. Sepsis is defined as a systemic response to infection with the presence of organ dysfunction [22]. It has been proposed that the mechanism of the progression of sepsis is an overwhelming reaction by innate immunity products, infectious products and host products, such as Toll-like receptor (TLR), LPS and high mobility group box 1 (HMGB1), respectively [22]. PMX-DHP removes integral components of sepsis, including HMGB1 and LPS [23] and modulates the circulating cytokine levels, such as those of interleukin (IL)-1 and IL-6 [24]. Although the effectiveness of PMX-DHP in sepsis is controversial, our patient's SOFA score was greatly reduced and his DIC improved immediately after PMX-DHP treatment with antibiotics. PMX-DHP may thus have contributed to the improvement of sepsis in this case.
In conclusion, we have described the case of an SCLC patient with severe sepsis caused by P. agglomerans infection who was treated successfully with antibiotics and PMX-DHP. Although the efficacy of PMX-DHP in sepsis is not well established, the combined use of PMX-DHP and antibiotics should be considered in cases of sepsis from gram-negative infections.
Conflicts of interest
The authors declare that there were no conflicts of interest.
References
- 1.De Champs C., Le Seaux S., Dubost J.J., Boisgard S., Sauvezie B., Sirot J. Isolation of Pantoea agglomerans in two cases of septic monoarthritis after plant thorn and wood sliver injuries. J. Clin. Microbiol. 2000;38:460–461. doi: 10.1128/jcm.38.1.460-461.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kageyama B., Nakae M., Yagi S., Sonoyama T. Pantoea punctata sp. nov., Pantoea citrea sp. nov., and Pantoea terrea sp. nov. isolated from fruit and soil samples. Int. J. Syst. Bacteriol. 1992;42:203–210. doi: 10.1099/00207713-42-2-203. [DOI] [PubMed] [Google Scholar]
- 3.Christakis G.B., Perlorentzou S.P., Aslanidou M., Savva L., Zarkadis I.K. Bacteremia caused by Pantoea agglomerans and Enterococcus faecalis in a patient with colon cancer. J. BUON. 2007;12:287–290. [PubMed] [Google Scholar]
- 4.Cheng A., Liu C.Y., Tsai H.Y., Hsu M.S., Yang C.J., Huang Y.T., Liao C.H., Hsueh P.R. Bacteremia caused by Pantoea agglomerans at a medical center in Taiwan, 2000-2010. J. Microbiol. Immunol. Infect. 2013;46:187–194. doi: 10.1016/j.jmii.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Kratz A., Greenberg D., Barki Y., Cohen E., Lifshitz M. Pantoea agglomerans as a cause of septic arthritis after palm tree thorn injury; case report and literature review. Arch. Dis. Child. 2003;88:542–544. doi: 10.1136/adc.88.6.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shubov A., Jagannathan P., Chin-Hong P.V. Pantoea agglomerans pneumonia in a heart-lung transplant recipient: case report and a review of an emerging pathogen in immunocompromised hosts. Transpl. Infect. Dis. 2011;13:536–539. doi: 10.1111/j.1399-3062.2011.00630.x. [DOI] [PubMed] [Google Scholar]
- 7.Lim P.S., Chen S.L., Tsai C.Y., Pai M.A. Pantoea peritonitis in a patient receiving chronic ambulatory peritoneal dialysis. Nephrol. Carlt. 2006;11:97–99. doi: 10.1111/j.1440-1797.2006.00552.x. [DOI] [PubMed] [Google Scholar]
- 8.Liberto M.C., Matera G., Puccio R., Lo Russo T., Colosimo E., Foca E. Six cases of sepsis caused by Pantoea agglomerans in a teaching hospital. New Microbiol. 2009;32:119–123. [PubMed] [Google Scholar]
- 9.Cruz D.N., Antonelli M., Fumagalli R., Foltran F., Brienza N., Donati A., Malcangi V., Petrini F., Volta G., Bobbio Pallavicini F.M. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 2009;301:2445–2452. doi: 10.1001/jama.2009.856. [DOI] [PubMed] [Google Scholar]
- 10.Payen D.M., Guilhot J., Launey Y., Lukaszewicz A.C., Kaaki M., Veber B., Pottecher J., Joannes-Boyau O., Martin-Lefevre L., Jabaudon M. Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: a multicenter randomized control trial. Intensive Care Med. 2015;41:975–984. doi: 10.1007/s00134-015-3751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uche A. Pantoea agglomerans bacteremia in a 65-year-old man with acute myeloid leukemia: case report and review. South Med. J. 2008;101:102–103. doi: 10.1097/SMJ.0b013e31815d3ca6. [DOI] [PubMed] [Google Scholar]
- 12.Lalas K.M., Erichsen D. Sporadic Pantoea agglomerans bacteremia in a near-term female: case report and review of literature. Jpn. J. Infect. Dis. 2010;63:290–291. [PubMed] [Google Scholar]
- 13.Cruz A.T., Cazacu A.C., Allen C.H. Pantoea agglomerans, a plant pathogen causing human disease. J. Clin. Microbiol. 2007;45:1989–1992. doi: 10.1128/JCM.00632-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong D.V., Lima-Junior R.C., Carvalho C.B., Borges V.F., Wanderley C.W., Bem A.X., Leite C.A., Teixeira M.A., Batista G.L., Silva R.L. The adaptor protein Myd88 is a key signaling molecule in the pathogenesis of irinotecan-induced intestinal mucositis. PLoS One. 2015;10:e0139985. doi: 10.1371/journal.pone.0139985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbers A.H., de Haan A.F., van der Velden W.J., Donnelly J.P., Blijlevens N.M. Mucositis not neutropenia determines bacteremia among hematopoietic stem cell transplant recipients. Transpl. Infect. Dis. 2014;16:279–285. doi: 10.1111/tid.12195. [DOI] [PubMed] [Google Scholar]
- 16.Nakao T., Kurita N., Komatsu M., Yoshikawa K., Iwata T., Utusnomiya T., Shimada M. Irinotecan injures tight junction and causes bacterial translocation in rat. J. Surg. Res. 2012;173:341–347. doi: 10.1016/j.jss.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Park J.H., Lee J., Park Y.S., Lee C.H., Lee S.M., Yim J.J., Kim Y.W., Han S.K., Yoo C.G. Prognostic value of central venous oxygen saturation and blood lactate levels measured simultaneously in the same patients with severe systemic inflammatory response syndrome and severe sepsis. Lung. 2014;192:435–440. doi: 10.1007/s00408-014-9564-y. [DOI] [PubMed] [Google Scholar]
- 18.Huang M.Y., Chen C.Y., Chien J.H., Wu K.H., Chang Y.J., Wu K.H., Wu H.P. Serum procalcitonin and procalcitonin clearance as a prognostic biomarker in patients with severe sepsis and septic shock. Biomed. Res. Int. 2016;2016:1758501. doi: 10.1155/2016/1758501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tseng M.H., Cheng C.J., Sung C.C., Chou Y.C., Chu P., Chen G.S., Lin S.H. Hyponatremia is a surrogate marker of poor outcome in peritoneal dialysis-related peritonitis. BMC Nephrol. 2014;15:113. doi: 10.1186/1471-2369-15-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermes A., Waschki B., Reck M. Hyponatremia as prognostic factor in small cell lung cancer–a retrospective single institution analysis. Respir. Med. 2012;106:900–904. doi: 10.1016/j.rmed.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Ellison D.H., Berl T. Clinical practice. the syndrome of inappropriate antidiuresis. N. Engl. J. Med. 2007;356:2064–2072. doi: 10.1056/NEJMcp066837. [DOI] [PubMed] [Google Scholar]
- 22.Vincent J.L., Opal S.M., Marshall J.C., Tracey K.J. Sepsis definitions: time for change. Lancet. 2013;381:774–775. doi: 10.1016/S0140-6736(12)61815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueno T., Ikeda T., Ikeda K., Taniuchi H., Suda S., Yeung M.Y., Matsuno N. HMGB-1 as a useful prognostic biomarker in sepsis-induced organ failure in patients undergoing PMX-DHP. J. Surg. Res. 2011;171:183–190. doi: 10.1016/j.jss.2009.11.708. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu T., Hanasawa K., Sato K., Umeki M., Koga N., Naganuma T., Sato S., Shimonishi T., Ikeda T., Matsuno N. Direct hemoperfusion with polymyxin-B-immobilized fiber columns improves septic hypotension and reduces inflammatory mediators in septic patients with colorectal perforation. Langenbecks Arch. Surg. 2009;394:303–311. doi: 10.1007/s00423-008-0395-2. [DOI] [PubMed] [Google Scholar]