Abstract
The diagnosis of sarcoidosis in a patient living with HIV infection is an uncommon event and a challenge for clinicians. Clinical manifestations are variable and fluctuating depending to adherence to ARV therapy and to the level of CD4 count. We analyze here one chronic case in which sarcoidosis appeared clinically two years after pulmonary tuberculosis. The course of the disease was influenced and prolonged by frequent interruptions of antiretroviral therapy. Moreover the diagnosis and the decision to treat have been delayed by the need of exclusion of other pathologies, principally tuberculosis reactivation/reinfection, other mycobacterial diseases, hematologic malignancies. We propose a simplified flowchart for diagnosis and follow up of sarcoidosis, which may also be applied to patients with HIV infection. Diagnosis of latent tuberculosis infection (LTBI) may be difficult in these patients, because the immunological paradox of sarcoidosis. For this reason, following exclusion of active tuberculosis, we advise to submit all sarcoidosis patients to IPT (isoniazid preventive therapy), when immunosuppressive therapy is started.
Keywords: Sarcoidosis, HIV infection, Diagnostic flowchart
1. Introduction
Sarcoidosis is a granulomatous multisystem chronic disease of inflammatory origin. It is distributed worldwide, with a prevalence of about 4.7–64 in 100,000 and incidence of 1.0–35.5/100,000 p.y. Seventy % of patients are aged 25–45 years and in Europe and Japan a second peak in incidence occurs in women aged more than 50 years. It is the second most common form of interstitial lung disease after idiopathic pulmonary fibrosis [10], [18].
The diagnosis of sarcoidosis is made principally by exclusion of other pathologies, together with histological evidence of typical granulomas in tissues biopsy, mostly lung, skin and lymph nodes. Similar to sarcoidosis is the combination of erythema nodosum, bilateral hilar adenopathy and arthritis, called Löfgren syndrome, which diagnosis is clinical, it doesn't need biopsy and has a relatively good prognosis [2], [10], [18].
Skin, liver and eyes are the most common localizations outside the lungs. This fact suggests that exposure to airborne antigens (microbial antigens end/or pollutants) plays a role in triggering the disease. Mycobacterial and propionibacterial antigens have been identified in biopsies of patients affected by sarcoidosis [4], [8]. Mycobacterial catalase-peroxidase was detected by immunoblotting in 50% of tissue samples from patients with sarcoidosis. Genomic fragments of Propionibacterium acnes and/or P. granulosus were detected in almost all tissue samples of patients with sarcoidosis coming from Europe (mainly England, Germany and Italy) and Japan. Bacterial and mycobacterial antigens may persist in macrophage phagosomes because they have membranes with high lipid content and many of their glycoproteins are insoluble and resistant to degradation.
A primary feature of sarcoidosis is the presence of CD4+ T-cells that interact with antigen presenting cells to initiate the formation and maintenance of granulomas. The triggering antigens favor progressive accumulation and activation of selective T-cell clones. These activated CD4+ cells differentiate into type 1 helper T (Th1)–like cells and secrete predominantly interleukin-2 and interferon-γ, increase macrophage TNF-α production, and amplify the local cellular immune response with granulomas formation. Granulomas are compact, centrally organized collections of macrophages and epithelioid cells encircled by lymphocytes. Macrophages, chronically stimulated by cytokines, differentiate into epithelioid cells, gain secretory and bactericidal capability, lose some phagocytic capacity, and fuse to form multinucleated giant cells. In more mature granulomas, fibroblasts and collagen encase the central cluster of cells, and in some cases, sclerosis occurs, altering the organ architecture and function and leading, for example, to fibrosis of lung tissue. In Sarcoidosis is also present an “immunological paradox” [14]: despite extensive local inflammation, anergy may develop, as indicated by suppression of the immune response to cell antigens such as tuberculin. Amplification of CD25bright FoxP3 regulatory T-cells, a subset of CD4+ T-lymphocytes, in active sarcoidosis may account for this anergy by abolishing interleukin-2 production and strongly inhibiting T-cell proliferation, without inhibiting the secretion of TNF-α and to a lesser extent IFN-γ [10].
When patients infected by HIV have reduced circulating CD4+ T-lymphocytes or dysfunctional T-cell mediated immunity, they present a low incidence of sarcoidosis. As HIV has become a chronic disease with immunity restoration made possible by antiretroviral therapy, it is likely that sarcoidosis may increase in incidence. In fact sarcoidosis diagnosis in HIV patients is associated with CD4+ T-lymphocytes counts greater than 200 cells/μl [6], [14]. Case reports of coexistent sarcoidosis and HIV infection describes sarcoidosis as a manifestation just occurring when the patient is restoring T-cell mediated immunity, during the first 9 months (median) of antiretroviral therapy [16]. Sarcoidosis in HIV patients seems in this way to be a clinical manifestation of IRIS – immune reconstitution syndrome, or a reactivation of previous disease.
We describe here one case of sarcoidosis in an HIV positive patient followed at the National Institute of Infectious Diseases “Lazzaro Spallanzani” – Rome during the period from 2003 to 2011.
2. Case presentation
CF, a 26-years-old man of North African origin, was hospitalized for persistent cough on July 2003. Chest x-ray showed at this moment bilateral hilar adenopathy and tenuous infiltration of the upper right lobe. HRTC showed multiple irregular parenchymal opacities of alveolar interstitial type at the upper lobes and bilateral hilar enlargement.
Diagnosis of pulmonary TB was made after bronchoscopy on July 2003, for positivity of M. tuberculosis DNA and RNA in bronchoalveolar lavage fluid. Culture of sputum and BAL fluid were negative. Tuberculin skin test was negative. Anti TB therapy was started on July and continued for six months until January 2004.
HIV infection had been diagnosed just one month before this hospitalization, on June 2003, with a CD4+ count of 371/mmc (19.6%), HIVRNA 139,000 cp/ml (wild type) and HCV coinfection (HCV type 1a). Antiretroviral therapy with (azt + 3tc) + nvp was also started on June 2003. During the first year he attained a good viro-immunological response, but later he became less compliant, and on November 2004 he was lost to follow up.
On April 2006 he was readmitted at day service during a period of jail detention: CD4+ were 374/mmc (26%), HIV viremia was 3055 cp/ml and HCV RNA titre was 3,097,802 cp/ml. ART therapy was switched to tdf + 3tc + lpv/r. Chest x-ray showed pulmonary apical bilateral infiltrates without pleural effusion, but the patient was lost to follow up for a second time before doing further investigations.
After two years, on March 2008, he was conducted to the service for consultation: he was in good conditions, but since 2007 he was developing chronic cutaneous maculopapular lesions at the right arm. Adherence to ART therapy was suboptimal and he was highly viremic, with 166,469 cp/ml of HIVRNA on July 2008. Therapy was modified to (tdf + ftc) + lpv/r. During the following year he developed multiple symmetric lymphadenopathies (neck, axillae and groin). Chest x-ray showed: bilateral fibronodular apical-subclavian lesions with hilar enlargement and prominence of the left second cardiac arch. CT-scan revealed mediastinal and intra-abdominal periaortic severe adenopathy (4 cm diameter).
Biopsy of the forearm cutaneous lesions showed granulomatous infiltration of sarcoid type without caseous necrosis. BAAR or fungi were not detected by specific stains of the tissue.
He was hospitalized in another institution for dyspeptic symptoms on October 2009. Total body CT-scan showed enlarged intra-abdominal lymph nodes (max 2.5 cm in diameter), hilar bilateral adenopathy (3 cm in diam.) and apical pulmonary fibrosis with reticulonodular infiltrates of lymphatic origin (galaxy sign). Biopsy of an axillary lymph node showed foci of chronic granulomatous epithelioid inflammation with giant multinucleated cells. No mycobacteria spp, fungi or other parasites were demonstrated by molecular or histological techniques. Bronchoscopy was also performed with BAL and the research of malignant cells and mycobacteria spp was negative (MTB and atypical mycobacteria) by PCR and culture. He was dismissed on November 2009 with the diagnosis of: “Chronic granulomatous lymphadenitis, Chronic Active Hepatitis HCV-related, Duodenitis in patient with HIV infection C3, Opioid addiction undergoing pharmacological therapy”.
Afterwards he continued ART therapy with better adherence, but after few months he presented general malaise, weight loss, persistent nausea and anorexia with emesis, increasing groin adenopathy and was newly hospitalized for the clinical suspect of lymphoma. Node biopsy showed: “Chronic granulomatous non necrotising epithelioid gigantocellular lymphadenitis with confluent foci as observed in sarcoidosis”. PCR for M. tuberculosis DNA and for atypical mycobacteria from node sections was negative. Culture for M tuberculosis and other mycobacteria was also negative. IGRA test (Quantiferon-TB test) was negative. Functional respiratory tests were also performed: Walking test was normal, Spirometry showed moderate-severe obstructive disease, with reduction of CO diffusion. Salivary glands echography showed parenchymal disomogeneity with ectasic ducta. Eye examination was normal.
Steroid therapy was started on July 2010 with prednisone at the dose of 1 mg/kg/die: after one month lymph nodes were considerably reduced; the patient was gaining weight; he had neither fever nor constitutional symptoms. He continued ART therapy with (azt + 3tc) + lpv/r.
Corticosteroid therapy was continued for 6 months (including one month of voluntary auto-suspension), but on March 2011 AST/ALT values were increased (446/315 mU/ml). Liver biopsy was decided, but the patient was definitely lost to follow up for personal reasons.
3. Discussion
This clinical history has several critical points that we wish to stress out. The first point is the diagnosis of pulmonary tuberculosis, which came quite at the same time of HIV infection diagnosis, when the patient had about 300 CD4+ T-lymphocytes/mmc. TB infection was demonstrated only by M. tuberculosis DNA and RNA detection in BAL fluid by PCR. Microscopic examination and culture of sputum and BAL fluid were negative. Radiological findings were specific, with hilar bilateral enlargement and multiple irregular opacities of alveolar interstitial type at the upper lobes. At this moment a reactivation of a previous tubercular infection was probably occurring, that is a common event in the evolution of uncontrolled HIV infection.
Treatment of tubercular infection, together with anti-HIV therapy, re-established T-cells compartment. But the patient, for several reasons, was not regular in assuming therapies. After anti-TB therapy was completed (but not directly observed by our service), ART therapy was interrupted and modified several times during the following 3–5 years. This fact was coincident with the development of generalized and important adenopathy, together with the appearance of radiographic signs of interstitial lung disease.
He underwent to repeated hospitalizations in order to exclude hematologic malignancies, reactivation of tuberculosis, atypical mycobacterial or fungal infections. Bronchoscopy with BAL fluid examination for the research of infectious agents and malignant cells was also performed, but without a differential cells count and lymphocyte subset analysis. SACE test was never requested at admission, maybe because it was prominent the suspect of malignancy, evoked by conspicuous lymphadenopathy, and it was never requested during the twelve months period of follow up. When symptoms became too severe and diagnosis of sarcoidosis was made by node biopsy, excluding infections and cancer, steroid therapy was finally started. The patient achieved for the first time a sustained virological response to HIV therapy, but, after 6 months of steroids, he had a flare of transaminases due to reactivation of chronic HCV infection. Steroid therapy was stopped. We don't know if sarcoidosis exacerbated after steroids withdrawal or if it relapsed during the following years.
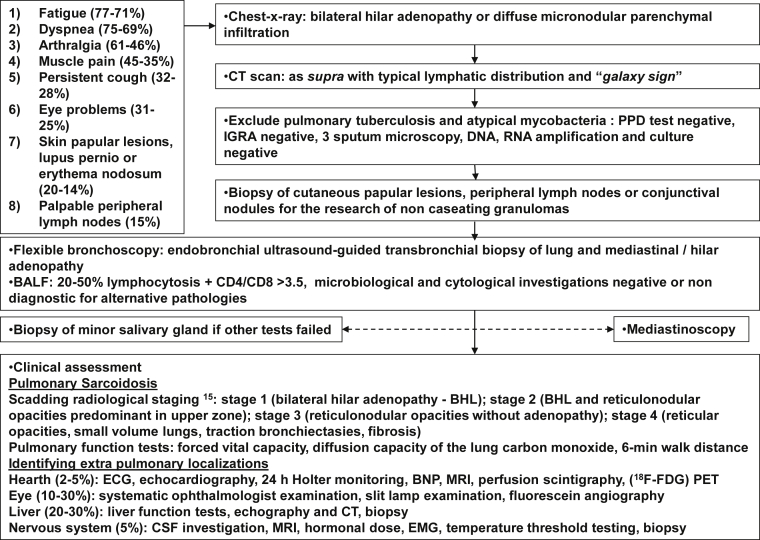
Below we propose a decisional “step-by-step” flowchart for Sarcoidosis diagnosis (Fig. 1F) in order to facilitate this elusive diagnosis. We want to point out that, in case of radiological signs of lung granulomas, in HIV patients undergoing immune reconstitution (particularly during the first 9 months of HAART therapy) it is essential to explore the possibility that the patient is affected by a chronic granulomatous disease such as sarcoidosis.
Fig. 1.
Flowchart for Sarcoidosis diagnosis [15].
Our patient was submitted to corticosteroid therapy with a favorable evolution of adenopathy. Unfortunately, pulmonary function tests were not repeated at follow up.
The decision to start steroid therapy in a patient infected by HIV is not simple, because clinicians may fear to arm the patient with iatrogenic immunosuppression in case of LTBI. In our case, the fact that anti TB therapy was completed before diagnosis of sarcoidosis and that all microbiological examinations for M. tuberculosis and atypical mycobacteria were negative, contributed to increase the level of security for the patient when corticosteroid therapy was requested.
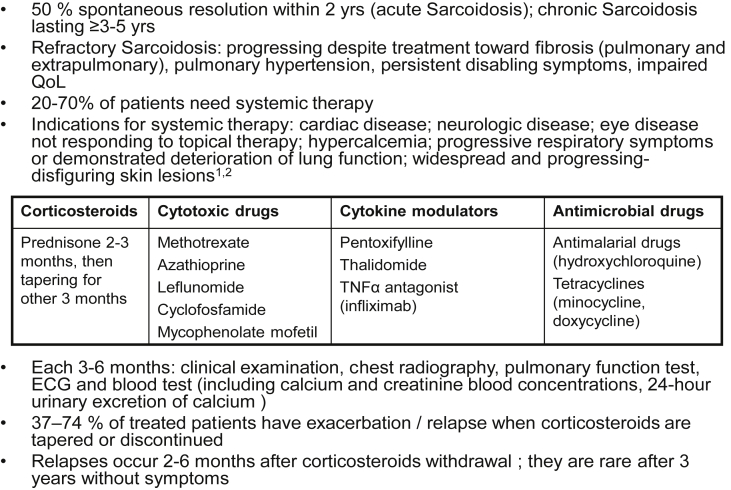
In Fig. 2 we stress some relevant points extracted from literature [2], [3], useful for clinicians in the decision of starting immunosuppressive therapy and for the evaluation at follow up.
Fig. 2.
Therapy and follow up of Sarcoidosis.
SACE determination has a limited diagnostic utility in sarcoidosis. Two recent population-based studies [9], [17] compared the results of this test between patients with and without diagnosis of sarcoidosis: Ungprasert et al. [17] confirmed a poor sensitivity (41.4%) and specificity (89.9%), with a Positive Predictive Value (PPV) of 25.4% and Negative Predictive Value (NPV) of 95%. Flood-Page et al. [9], conversely, affirmed that high values of sACE (>100 IU/L) have a Positive Predictive Value of 88%, arriving to 96% if excluding patients with primary biliary cirrhosis and hypersensitive pneumonitis. Since 1980 until now different Authors agree on the fact that ACE test is of value as a serum marker for response to therapy in those cases that were positive at diagnosis [3].
A lymphocyte differential count in BAL fluid ≥25% suggests granulomatous lung disease, including sarcoidosis [13]. In a selected population of sarcoidosis symptomatic patients, Danila et al. [5] found 45 ± 19% lymphocytes in BAL fluid cells, but in patients empirically pretreated with systemic corticosteroids this feature is less pronounced, with 39 ± 15% lymphocytes in the differential count. In severe cases the number of neutrophils may also be augmented [7].
The CD4/CD8 ratio in BAL fluid of patients with proven diagnosis of sarcoidosis is highly variable. In 1997 Kantrow and colleagues [12] published the results of CD4/CD8 ratio in BAL fluid of 86 patients: 42% of subjects showed a ratio greater than 4, and 12% had a ratio less than 1, with a median value of 3.35.
Even if it is recognized that lymphocytosis and an elevated CD4/CD8 ratio in BAL fluid may strongly suggest sarcoidosis, it is so necessary that the diagnosis is finally proven by biopsy of affected tissues, specific clinical and radiological signs and by exclusion of other pathologies [13].
In HIV patients with sarcoidosis CD4/CD8 ratio in BAL fluid is even more variable, following the immunological condition at diagnosis, the activity of the disease and the assumption of HAART. In general, the ratio may be is less elevated than in non HIV patients, or normal [1], [6], [16].
4. Conclusion
As often described in the literature the diagnosis of sarcoidosis was delayed by the worry of clinicians to be in front of a case of lymphoma or mycobacterial infection and, by the other side, by the patient social instability. The decision to start steroid therapy was also delayed because clinicians repeated several times the research of active mycobacterial infection to be reassured to not put in arm the patient. By the side of patient, the symptoms of sarcoidosis (fatigue, cough and dyspnea) were probably negatively affecting adherence to HAART therapy and, finally, they were directly and proportionally linked to it by a causal relationship.
Clinical presentation and diagnosis of sarcoidosis in HIV-positive patients is similar to that of other non co-infected patients, but it is particularly influenced by the fact that cellular immunity in HIV patients is highly variable, following ART therapy start and/or discontinuation, with the possibility of crossing the threshold of 200 CD4+/mmc several times. The prominence of adenopathy in our patient may be related to the fact that the inflammatory process was probably relapsing each time that HAART was restarted. We don't know if this irregularity may facilitate relapses or the evolution to pulmonary fibrosis of sarcoidosis.
The hypothesis of mycobacterial and/or bacterial antigens triggering the pathogenic mechanism of sarcoidosis is also intriguing [19]. When we are evaluating a sarcoidosis patient co-infected with HIV, we may also consider to be in front of a person at increased risk to develop active tuberculosis during or after immunosuppressive therapy, even if there is anergy to PPD test and/or IGRA tests are negative at diagnosis, as this fact is explained by the immunological paradox. For this reason it should be interesting to propose TB prophylaxis to all HIV positive patients affected by sarcoidosis when immunosuppressive therapy is considered. Further studies are needed in order to evaluate the risk of developing active tuberculosis in HIV patients with sarcoidosis after immunosuppressive therapy, if they were or not previously submitted to anti-TB prophylaxis or therapy. We do not know if with iatrogenic immunosuppression we can also induce active infection by atypical mycobacteria. These questions may also be formulated regarding others HIV patients in HAART, when they are affected by autoimmune disorders demanding immunosuppressive therapy [11].
Conflict of interest
None.
Acknowledgements
We thanks Prof. Cesare Saltini for revision of the text.
Prof. Cesare Saltini
Cattedra di Malattie dell'Apparato Respiratorio
Dipartimento di Biomedicina e Prevenzione
Università di Roma “Tor Vergata”
References
- 1.Almeida F.A., Sager J.S., Eiger G. Coexistent sarcoidosis and HIV infection: an immunological paradox? J. Infect. 2006 Mar;52(3):195–201. doi: 10.1016/j.jinf.2005.05.009. Epub 2005 Aug 3. [DOI] [PubMed] [Google Scholar]
- 2.Badgwell C., Rosen T. Cutaneous Sarcoidosis therapy updated. J. Am. Acad. Dermatol. 2007;56(1):69–83. doi: 10.1016/j.jaad.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 3.Baughman R.P., Nunes H., Sweiss N.J., Lower E.E. Established and experimental medical therapy of pulmonary sarcoidosis. Eur. Respir. J. 2013;41(6):1424–1438. doi: 10.1183/09031936.00060612. [DOI] [PubMed] [Google Scholar]
- 4.Brownell I., Ramirez-Valle F., Sanchez M., Pristowsky S. Evidence for mycobacteria in sarcoidosis. Am. J. Respir. Cell Mol. Biol. 2011;45:899–905. doi: 10.1165/rcmb.2010-0433TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danila E., Jurgauskienė L., Norkūnienė J., Malickaitė R. BAL fluid cells in newly diagnosed pulmonary sarcoidosis with different clinical activity. Ups. J. Med. Sci. Mar. 2009;114(1):26–31. doi: 10.1080/03009730802579729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doffman S.R., Miller R.F. Interstitial lung disease in HIV. Clin. Chest Med. 2013;34:293–306. doi: 10.1016/j.ccm.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Drent M., Mansour K., Linssen C. Bronchoalveolar lavage in sarcoidosis. Semin. Respir. Crit. Care Med. 2007;28:486–495. doi: 10.1055/s-2007-991521. [DOI] [PubMed] [Google Scholar]
- 8.Eishi Y., Suga M., Ishige I. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J. Clin. Microbiol. 2002;40(1):198–204. doi: 10.1128/JCM.40.1.198-204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flood-Page P.T., Brothers H. A re-examination of the value of serum angiotensin converting enzyme levels in the diagnosis of sarcoidosis within a South Wales population. Am. J. Respir. Crit. Care Med. 2015;191:A3745. [Google Scholar]
- 10.Iannuzzi M.C., Rybicki B.A., Teirstein A.S. Medical progress. Sarcoidosis N. Engl. J. Med. 2007;357:2153–2165. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 11.Iordache L., Launay O., Bouchaud O., Jeantils V. Autoimmune diseases in HIV-infected patients: 52 cases and literature review. Autoimmun. Rev. 2014;13:850–857. doi: 10.1016/j.autrev.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Kantrow S.P., Meyer K.C., Kidd P., Raghu G. The CD4/CD8 ratio in BAL fluid is highly variable in sarcoidosis. Eur. Respir. J. 1997;10:2716–2721. doi: 10.1183/09031936.97.10122716. [DOI] [PubMed] [Google Scholar]
- 13.Meyer K.C., Ganesh R., Baughman R.P., Brown K.K., Costabel U., du Bois R.M., Drent M., Haslam P.L., Dong Soon Kim, Nagai S., Rottoli P., Saltini C., Selman M., Strange C., Wood B., on behalf of the American Thoracic Society Committee on BAL in Interstitial Lung Disease An official american thoracic society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am. J. Respir. Crit. Care Med. May 1, 2012;185(9):1004–1014. doi: 10.1164/rccm.201202-0320ST. [DOI] [PubMed] [Google Scholar]
- 14.Miyara M., Amoura Z., Parizot C., Badoual C. The immune paradox of Sarcoidosis and regulatory T cells. JEM. 2006;203:359–370. doi: 10.1084/jem.20050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scadding J.G. Prognosis of intrathoracic Sarcoidosis in England: a review of 136 cases after five years' observation. BMJ. 1961;2:1165–1172. doi: 10.1136/bmj.2.5261.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trevenzoli M., Cattelan A.M., Marino F., Marchioro U., Cadrobbi P. Sarcoidosis and HIV infection: a case report and a review of the literature. Postgrad. Med. J. 2003;79:535–538. doi: 10.1136/pmj.79.935.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ungprasert P., Carmona E.M., Crowson C.S., Matteson E.L. Diagnostic utility of angiotensin converting enzyme in sarcoidosis: a population-based study. Am. J. Respir. Crit. Care Med. 2016;193:A5033. doi: 10.1007/s00408-015-9826-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valeyre D., Prasse A., Nunes H., Uzunhan Y., Brillet P.-Y., Müller-Quernheim J. Sarcoidosis. Lancet. 2014;383:1155–1167. doi: 10.1016/S0140-6736(13)60680-7. [DOI] [PubMed] [Google Scholar]
- 19.Van Enschot J.W.T., van Balkom R.H.H. Sarcoidosis following Mycobacterium tuberculosis infection: coincidence or consequence. Respir. Med. Case Rep. 2013;9:11–14. doi: 10.1016/j.rmcr.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]