Abstract
Aims
Due to the demographic development there is an increasing number of senior citizens with left ventricular systolic dysfunction (LVSD), defined as ejection fraction (EF) < 40%. Unfortunately there are under‐diagnosis and under‐treatment in the elderly of this serious condition. Echocardiography is the gold standard to diagnose LVSD, but access is limited. Simple screening methods may ensure reduction of undetected cases, and this study investigates if electrocardiogram (ECG) can be used to screen for LVSD in the geriatric population.
Methods and results
A total of 260 persons aged 75 to 92 years had an echocardiography, a 12 leads ECG, and NT‐proBNP; 61 had EF < 40%, and of these 60 had an abnormal ECG. EF < 40% was significantly related to atrial fibrillation (A), pacing (P), LBBB (L), Q‐waves (Q), and QRS duration ≥ 120 ms (D). EF < 40%, atrial fibrillation, pacing, and LBBB were related to NT‐proBNP > 35 pmol/L. When APL was absent, NT‐proBNP had discriminatory value regarding LVSD in the presence of Q‐waves or QRS duration > 120 ms. Algorithms to screen for LVSD had sensitivity >90% and specificity >80% and claimed at least one of five (A/P/L/Q/D), one of 4 (A/P/L/Q), or one of three (A/Q/D) ECG changes. The optimal algorithm to reduce the need for diagnostic echocardiographies included four (A/P/L/Q) ECG changes and measurement of NT‐proBNP when Q‐waves were the only ECG change present.
Conclusions
Ninety percent of LVSD may be detected, and when there is atrial fibrillation, pacing or LBBB, or QRS ≥ 120 ms/Q‐waves and NT‐proBNP>35 pmol/L, a diagnostic echocardiography should be considered.
Keywords: Heart failure, Screening, ECG, Geriatric, NT‐proBNP, Echocardiography
Introduction
Chronic left ventricular systolic dysfunction (LVSD) represents the final stage in most heart diseases. It has a prevalence of 2% of the entire population and 10% of the geriatric population (75+),1, 2, 3 many asymptomatic, undetected, and untreated.4, 5, 6, 7, 8, 9, 10, 11, 12 The number of senior citizens increases, and consequently LVSD also increases.13, 14, 15, 16, 17, 18
LVSD may severely affect functional capacity, life‐quality, and prognosis, but treatments are capable of improving morbidity and mortality, even in the asymptomatic.1, 19 However, LVSD is under‐diagnosed and under‐treated in the elderly,2, 4, 8, 14, 15 especially in elderly women13 and screening for LVSD is mandatory to improve treatment of senior citizens and to alleviate symptoms, delay progression, and improve prognosis.1, 3, 5, 7, 12, 13, 18, 19
Clinical criteria alone are an insufficient basis for the diagnosis of LVSD, and the detection of LVSD also cannot rely on clinical signs and symptoms alone, because these may be non‐specific and obscured by co‐morbidity.1, 5, 13, 15 Echocardiography is the gold standard to diagnose LVSD2, 6, 8, 18, 20, 21, 22and may help to determine the underlying cause of LVSD; but as access is limited, alternative screening‐methods are needed.1, 3, 9, 10, 15, 17
Electrocardiogram (ECG) and NT‐proBNP are inexpensive and easily obtained. They reflect anatomy, physiology, and electro‐physiology, and LVSD is associated with an abnormal ECG and elevated NT‐proBNP. ECG and NT‐proBNP have independent diagnostic utility, and the combination of ECG and NT‐proBNP seems to improve screening for LVSD.1, 2, 3, 4, 5, 6, 9, 10, 20, 22 However, there has been little attempt to categorize the ECG abnormalities that may occur in LVSD, and to correlate ECG parameters and NT‐proBNP,15, 23 and there has not been as yet a systematic exploration as to what combinations of ECG incides and NT‐proBNP could accomplish in diagnosing LVSD.23
The present study hypotheses are, that (A) LVSD may be characterized by specific ECG changes, (B) these ECG changes may be useful to detect LVSD in the elderly high‐risk population, and (C) NT‐proBNP in the presence of these ECG changes may be useful to select subjects to a diagnostic echocardiography and subsequent medical intervention.
Materials and methods
This study was part of an investigation about screening for LVSD in an elderly study population. It is in compliance with the Declaration of Helsinki and approved by the local ethics committee and the Danish Data Protection Agency. For recruitment of participants the study was announced locally in the Danish Association of Senior Citizens (Aeldresagen), in a newspaper article, and in the department of cardiology and the heart failure clinic. In total, 260 persons, 75 years and older, with risk factors for heart disease, or with known heart disease, as well as healthy persons, gave written informed consent. Table 1 shows the baseline characteristics of the study participants.
Table 1.
Baseline characteristics of the study population
| Characteristics | N = 260 | % |
|---|---|---|
| Age | 80 (75–92) | |
| Females | 134 | 51.5 |
| Males | ||
| BMI | 25.5 (15.5–39.8) | |
| Smoking | ||
| ‐ Never | 104 | 40 |
| ‐ Present | 28 | 11 |
| ‐ Ex‐smoker | 128 | 49 |
| Alcohol | ||
| ‐ Never | 35 | 13 |
| ‐ Fewer than 14 drinks a week | 194 | 75 |
| ‐ More than 14 drinks a week | 31 | 12 |
| History of | ||
| ‐AMI | 55 | 21 |
| ‐ PCI | 39 | 15 |
| ‐ CABG | 30 | 12 |
| ‐ Valvular substitution | 10 | 4 |
| ‐ Dilated cardiomyopathy | 15 | 6 |
| ‐ Systolic heart failure | 72 | 28 |
| ‐ Arrhythmias | 92 | 35 |
| ‐ Atrial fibrillations | 75 | 29 |
| ‐ Pacemaker | 29 | 11 |
| ‐ Hypertension | 173 | 67 |
| ‐ Hypercholesterolemia | 144 | 55 |
| ‐ Diabetes mellitus | 37 | 14 |
| ‐ Thyroid disease | 31 | 12 |
| ‐ Stroke and TCI | 43 | 17 |
| ‐ Peripheral arterial disease | 23 | 9 |
| ‐ Lung disease | 67 | 26 |
| ‐ Autoimmune disease | 20 | 8 |
| ‐ Renal disease, moderate‐severe | 21 | 8 |
| ‐ Cancer, all types, former and present | 68 | 26 |
| ‐ Musculoskeletal disease | 160 | 62 |
| Medical treatment | N | % |
| ‐ ASA | 111 | 43 |
| ‐ Marevan | 52 | 21 |
| ‐ Statins | 109 | 42 |
| ‐ Diuretics | 120 | 46 |
| ‐ Aldosteron‐antagonist | 26 | 10 |
| ‐ ACE‐inhibitors | 76 | 29 |
| ‐ ATII | 46 | 18 |
| ‐ Beta‐blockers | 110 | 42 |
| ‐ Digoxin | 23 | 9 |
| ‐ Prolonged nitro | 27 | 10 |
It is a case–control study, which compares ECG in subjects with LVSD (cases) with ECG in subjects without LVSD (controls). In case–control studies it is recommended to include a number of controls up to 4 times the number of cases, and this is the reason for the set up and the composition of the study population. The purpose is to examine if there are significant differences in ECG changes in the two groups, in order to evaluate if ECG can be used as a screening tool and as a first step in the detection of LVSD.
While resting in supine position all of the 260 subjects had a 12 lead ECG and then a blood sample taken, and within the same hour a transthoracal echocardiography by an experienced level 3 echocardiographer using General Electric Vingmed Vivid 7 or 9 and MJS probe 1.5–4.0 MHz and following guidelines from the Danish Society of Cardiology. Echocardiography divided the study participants into two groups, one with LVSD (defined as ejection fraction (EF) < 40%)20 and one without LVSD (EF ≥ 40%) and also in classes with EF < 30%, 30–40%, and ≥40%.20
ECG 12 was evaluated by an experienced cardiologist according to the Minnesota Code24 and a structured data sheet (Table 2). The observer was blinded to any subject data as well as to the automatic ECG description. The QRS duration was measured automatically on the ECG 12 (MUSE Cardiology Information System). QRS >120 ms is consistent with probable LVSD.1, 23, 25, 26
Table 2.
Left ventricular systolic dysfunction is characterized by specific electrocardiogram (ECG) changes
| N | PPV (%) | NPV (%) | LVSD | P value | ||
|---|---|---|---|---|---|---|
| Yes = 60 | No = 200 | |||||
| Sensitivity (%) | Specificity (%) | |||||
| NT‐proBNP ≥ 35 | 131 | 44 | 98 | 95 | 63 | <0.001 |
| Abnormal ECG | 166 | 36 | 100 | 100 | 47 | <0.001 |
| QRS ≥ 120 ms | 60 | 67 | 90 | 67 | 90 | <0.001 |
| LBBB | 15 | 73 | 80 | 18 | 98 | <0.001 |
| Pace rhythm | 26 | 88 | 84 | 38 | 98 | <0.001 |
| Atrial fibrillation | 51 | 55 | 85 | 47 | 89 | <0.001 |
| Q‐waves | 23 | 57 | 80 | 22 | 95 | <0.001 |
| r‐waves | 35 | 40 | 80 | 23 | 90 | 0.011 |
| Q‐ and r‐waves | 45 | 44 | 81 | 33 | 88 | <0.001 |
| Incomplete RBBB | 19 | 37 | 78 | 12 | 94 | 0.14 |
| RBBB | 15 | 33 | 78 | 8 | 95 | 0.33 |
| 1. AV | 25 | 20 | 77 | 8 | 90 | 0.70 |
| LAH | 40 | 28 | 78 | 18 | 86 | 0.47 |
| LPH | 6 | 33 | 77 | 3 | 98 | 0.55 |
| T‐change | 18 | 17 | 76 | 5 | 93 | 0.50 |
| Hypertrophy | 7 | 29 | 77 | 3 | 98 | 0.73 |
| Frequency < 50 | 11 | 27 | 77 | 5 | 96 | 0.74 |
| Frequency > 100 | 4 | 50 | 77 | 3 | 99 | 0.20 |
| SVES (>3/10 sec.) | 4 | 0 | 77 | 0 | 98 | 0.27 |
| VES (>3/10 sec.) | 3 | 33 | 77 | 2 | 99 | 0.67 |
| SSS | 3 | 0 | 77 | 0 | 99 | 0.34 |
LVSD = left ventricular systolic dysfunction, N = number, NPV = negative predictive value of ECG change, PPV = positive predictive value of ECG change; Sensitivity, specificity, and P value of ECG change in relation to LVSD. LVSD defined as EF < 40 and abnormal ECG.
NT‐proBNP was measured with the Elecsys assay and equipment of Roche Diagnostics. NT‐proBNP < 35 pmol/L (300 pg/mL) has a high negative predictive value and practically precludes LVSD,19 even though elevated values are reduced by treatment for LVSD and BMI > 30.2, 19 NT‐proBNP > 35 pmol/L corresponds to possible LVSD or increased risk for developing heart disease and LVSD, but NT‐proBNP may be elevated for other reasons like cor pulmonale, atrial fibrillation,27 diastolic dysfunction, valvular heart diseases,2 and with reduced kidney function.4, 19 Thus NT‐proBNP is known to have a high sensitivity but a low specificity as a diagnostic marker of LVSD. As these co‐morbidities are frequent in the geriatric population, the value of NT‐proBNP is less important in the elderly and has less prognostic power, and a higher rule‐in value is required in the elderly to render LVSD probable, compared with younger persons with less co‐morbidity.19
Statistical analysis
The statistical analysis was performed with STATA 12 (StataCorp, College Station, Texas, USA).
With EF < 40% as reference, each ECG change was analysed for positive and negative predictive values, as well as sensitivity and specificity (Table 2). The positive predictive value is the likelihood of LVSD and EF < 40%, when a certain ECG change is present, opposite to the negative predictive value which is the likelihood of a normal EF ≥ 40%, when the ECG change is absent. Sensitivity is the likelihood that an ECG change is present, when EF < 40%, opposite to specificity which is the likelihood that the ECG change is absent, when EF > 40%.28
ECG changes predictive for LVSD were utilized in simple algorithms intended to select persons to diagnostic echocardiography (Table 3). The algorithms were compared regarding sensitivity, specificity, number of NT‐proBNP‐measurements, and number of diagnostic echocardiographies, to distinguish the algorithm with superior ability to detect LVSD.
Table 3.
Electrocardiogram (ECG) changes associated with left ventricular systolic dysfunction (LVSD) can be used to screen for LVSD in the elderly population. Sensitivity and specificity of algorithms (A1–5/B1–5) to recognize LVSD in the study population and to select those to be submitted to echocardiography clarifying whether LVSD or not (LVSD = 60, no LVSD = 200)
| Q‐waves (alone) | |||||
|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | A5 | |
| Sensitivity (%) | 96.7 (58) | 93.3 (56) | 98.3 (59) | 91.7 (55) | 96.7 (58) |
| Specificity (%) | 77.0 (154) | 82.5 (165) | 77.0 (154) | 85.0 (170) | 82.5 (165) |
| % Echocardiography | 40.0 (104) | 35.0 (91) | 40.4 (105) | 32.7 (85) | 35.8 (93) |
| % NT‐proBNP | 0 (0) | 0 (0) | 0 (0) | 5.8 (15) | 11.2 (29) |
| Q‐waves and r‐waves joined* | |||||
| B1 | B2 | B3 | B4 | B5 | |
| Sensitivity (%) | 96.7 (58) | 95.0 (57) | 98.3 (59) | 93.3 (56) | 96.7 (58) |
| Specificity (%) | 70.5 (141) | 76.0 (152) | 70.5 (141) | 82.5 (165) | 80.0 (160) |
| % Echocardiography | 45.0 (117) | 40.4 (105) | 45.4 (118) | 35.0 (91) | 37.7 (98) |
| % NT‐proBNP | 0 (0) | 0 (0) | 0 (0) | 11.2 (29) | 16.2 (42) |
Atrial fibrillation, Afli (A), Q‐waves (Q), pace rhythm (P), LBBB (L), and QRS duration > 120 ms (D).
NT‐proBNP = NT‐B. LVSD = left ventricular systolic dysfunction.
A1: Afli/QRS ≥ 120/Q‐waves = ADQ (at least one of these three ECG changes).
A2: Afli/Pace/LBBB/Q‐waves = APLQ (at least one of these four ECG changes).
A3: Afli/Pace/LBBB/QRS ≥ 120/Q‐waves = APLDQ (at least one of these five ECG changes).
A4: Afli/Pace/LBBB/(Q‐waves(−APL) + NT‐proBNP ≥ 35) = APL(Q(−APL) + NT‐B ≥ 35) (at least one of the four).
A5: Afli/Pace/LBBB/(Q‐waves/QRS ≥ 120(−APL) + NT‐B>35) = APL(QD(−APL) + NT‐B> = 35) (at least one of the five).
B1–B5: Q‐waves alone replaced by Q‐waves and r‐waves joined.
Table 4 presents prevalence ratios (PR) of LVSD among patients with a given ECG change relative to LVSD among patients without the ECG change. Multivariate models illustrate the simultaneous predictive power of the ECG changes. The univariate PRs were obtained by binomial regression while the multivariate PRs were obtained as margins from a multivariate logistic regression.
Table 4.
Univariate and logistic models associating electrocardiogram changes to left ventricular systolic dysfunction
| Univariate | Multivariate without QRS and NT‐proBNP | Multivariate without NT‐proBNP | Multivariate | |
|---|---|---|---|---|
| PR | PR | PR | PR | |
| Pace rhythm | 5.59 (4.04–7.76) | 29.2 (9.88–86.2) | 11.3 (3.53–36.3) | 9.67 (2.81–33.3) |
| Atrial fibrillation | 3.59 (2.39–5.37) | 5.65 (2.75–11.6) | 5.79 (2.73–12.3) | 2.82 (1.29–6.18) |
| LBBB | 3.67 (2.47–5.44) | 12.2 (4.14–36.0) | 3.76 (1.09–13.0) | 2.88 (0.82–10.1) |
| Q‐waves | 2.85 (1.83–4.43) | 6.94 (2.86–16.8) | 6.82 (2.70–17.2) | 6.60 (2.44–17.9) |
| QRS ≥ 120 ms | 6.67 (4.24–10.5) | 4.25 (1.84–9.85) | 3.91 (1.63–9.40) | |
| NT‐proBNP ≥ 35 | 18.7 (6.01–58.2) | 8.21 (2.59–26.0) |
LVSD = left ventricular systolic dysfunction, PR = prevalence ratio.
For each of the simple algorithms in Table 3 a corresponding algorithm was constructed based on logistic regression including the same ECG changes and NT‐proBNP interaction. The simple and logistic‐based algorithms resulted in almost identical sensitivity and specificity.
Using pre‐specified cut‐offs decreases the risk of over‐fitting compared with using data‐driven optimal cut‐offs. However, the pre‐specified cut‐offs seem close to optimal in the present data (Figure 1).
Figure 1.
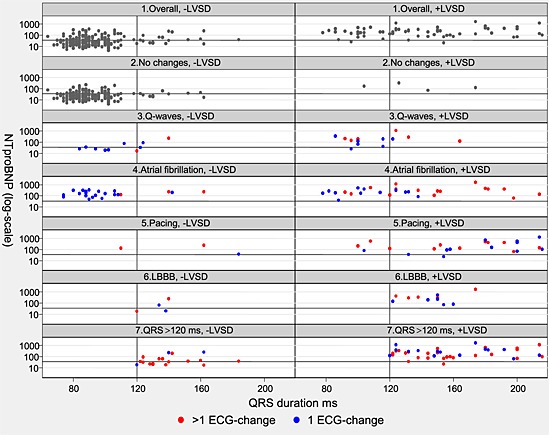
Scatter plots of N terminal pro brain natriuretic peptide (NT‐proBNP) (Y), with 35 pmol/L marked by a line; and QRS duration (X), duration 120 ms marked by a line, for study participants without left ventricular systolic dysfunction (LVSD) (−LVSD) and with LVSD (+LVSD): 1. Overall (all participants); 2. No changes (no Q‐waves, no atrial fibrillation, no pacing, no left bundle branch block (LBBB)); 3. Q‐waves; 4. Atrial fibrillation; 5. Pacing; 6. LBBB; 7. QRS ≥ 120 ms (QRS duration ≥120 ms). Plot 3–7: blue dot represents an isolated electrocardiogram (ECG) change; red dot represents two or more ECG changes. NT‐proBNP < 35 pmol/L almost excludes LVSD, atrial fibrillation, LBBB, and pacing. QRS duration > 120 ms is significantly related to LVSD.
Bootstrap validation of the algorithms in Table 3 was conducted. The average sensitivity/specificity across 1000 bootstrap samples was identical to the apparent sensitivity/specificity in the original data. This was expected, since the simple algorithms were not based on data‐driven model selection.
Results
According to expert‐echocardiography at entry 61 subjects had EF < 40% (31 had EF < 30%, 30 had EF 30–40%) and 199 had EF ≥ 40%. Of the 61 subjects with EF < 40%, 60 had an abnormal ECG, and one patient with EF 30–40% had a normal ECG. In accordance with previous studies, LVSD in this study was defined not only as EF < 40% but also as an abnormal ECG. This definition of LVSD was chosen for practical reasons and to simplify the screening procedure, even at the expense of one patient with EF < 40%. Consequently this study consisted of 60 participants with LVSD and 200 without LVSD.
Table 2 shows that an abnormal ECG was found in 166 subjects, 60 (100%) with LVSD and 106 (53%) without. Atrial fibrillation (A), every kind of pacing (P), complete left bundle branch block LBBB (L), r‐waves and Q‐waves (Q), and QRS duration >120 ms (D) possess power to predict LVSD, both in univariate and multivariate analyses. Other ECG changes were not statistically significantly associated with LVSD.
Table 3 shows, that these ECG changes may be used in algorithms to screen for LVSD in the high‐risk elderly population. An algorithm was simplified from five ECG changes (Table 3 A3: APLQD) to four ECG changes (Table 3 A2: APLQ) and to three ECG changes (Table 3 A1: AQD), because QRS > 120 ms and risk of LVSD is almost interchangeable with LBBB and pacing. Simplification is also justified regarding markers of myocardial infarction, because Q‐waves dominate by far relative to r‐waves (r‐waves excluded from A1–5, included in B1–5, Table 3).
NT‐proBNP > 35 pmol/L was found in 131 subjects, 57 (95%) patients with LVSD and 68 (34%) without LVSD, including 55 with an abnormal ECG but no LVSD. The three patients with LVSD and NT‐proBNP < 35 had EF 30–40% and were well‐treated for LVSD.
Figure 1 illustrates that NT‐proBNP 35 pmol/L was a suitable cut point. NT‐proBNP was >35pmol/L in 51/52 with atrial fibrillation, 24/26 with pacing, 13/15 with LBBB, 49/60 with QRS ≥ 120 ms. and in 16/23 with Q‐waves. Thus subjects with atrial fibrillation, pacing, and LBBB could be referred directly to echocardiography, and NT‐proBNP only had discriminatory value regarding LVSD in the presence of Q‐waves, r‐waves, or QRS > 120 ms, provided absence of atrial fibrillation, pacing, and LBBB.
Discussion
Preventing LVSD by targeting its preclinical stages in the high risk geriatric population may be among the best strategies to retard the progression of heart failure and to reduce the overall societal burden of this disorder,7 and this is the main purpose with the present study. Detection of LVSD rests on improved identification of patients and selection of the right patients for echocardiography, and this study concludes that ECG and NT‐proBNP are effective tools to screen for LVSD in a high risk elderly population. The value of ECG lies in easily recognizable abnormalities, which carry important and unambiguously diagnostic information,9, 23, 25 and the present study reveals distinct ECG correlates to LVSD, and that some major ECG abnormalities correlate to LVSD and to NT‐proBNP>35 pmol/L. Thus the study‐hypotheses seem right, and it seems that the senior citizens may now be screened for LVSD in a simple way by use of our unique and original algorithms which are predominantly based on ECG correlates to LVSD, and in a minority supplied by NT‐proBNP assessment.
This study asserts, together with previous studies,1, 2, 3, 4, 6, 9, 10, 12, 18, 23, 25 that a normal ECG deems EF < 40% unlikely, and severe LVSD with EF < 30% seems to be excluded. This simplified use of ECG has been the basis for prior studies about screening for LVSD, where ECG has been dichotomized into normal (high negative predictive value corresponding to rule‐out of LVSD) and abnormal (high sensitivity to detect LVSD, but low specificity and high false positive rate). This reduces the number of subjects referred to echocardiography; still the false positive rate is substantial.6, 15
The present study categorizes the ECG abnormalities that may occur in LVSD, extends the knowledge about the discriminatory value of ECG concerning LVSD, and notably further reduces the false positive rate and the number of diagnostic echocardiographies. The case–control design allows calculation of sensitivity, specificity, positive predictive value, and negative predictive value of separate ECG abnormalities and thus reveals the ECG changes that correlate with LVSD. Few systematic and detailed case–control studies about ECG changes in association with LVSD have been performed and no study, like the present, in an elderly population.
This study proves that LVSD may be characterized by five specific ECG changes, which are Q‐waves (Q),10, 11 atrial fibrillation (A),22, 27 pace rhythm (P),29 LBBB (L)11, 22 and QRS duration> 120 ms (D).2, 3, 9, 12, 23, 25 These ECG changes are reliable LVSD correlates and mark an increased risk for LVSD. Q‐waves mirror previous myocardial infarction, many of which are clinically unrecognized in the elderly.11 Atrial fibrillation may cause or aggravate LVSD, which on the other hand may promote development of atrial fibrillation.27 Depolarization delay with prolongation of QRS duration and LBBB on its own or caused by ventricular pacing causes dyskinesia and in due time can lead to adverse ventricular remodelling and LVSD26 which may be reversed by cardiac resynchronization therapy. The broader the QRS duration in LBBB, the greater the risk of LVSD.23, 30 The more ventricular pacing, the greater the risk of LVSD.29 LBBB and atrial fibrillation can also be part of different cardiovascular disorders leading to LVSD.26, 28, 29 Other studies have associated the same ECG changes with LVSD and prediction of increased mortality. Besides, left ventricular hypertrophy has been appointed to be a predictor of both diastolic and systolic heart failure.1, 2, 3, 9, 12, 23
Previous studies on screening for LVSD with ECG and/or NT‐proBNP4, 15, 17, 18, 21 have been characterized by heterogeneity in design, study size, subjects and populations and comorbidity, clinical scores and objective evidence of LVSD, number of participating physicians and training in ECG, ECG details and definitions, NT‐proBNP‐cut‐points, and statistical significance; and correlation of ECG changes and NT‐proBNP has only been sparsely investigated.15, 21 Atrial fibrillation is associated with higher levels of NT‐proBNP, independent of EF.27
It seems that this study is the first to include a relatively large number of high risk subjects from the geriatric population (Table 1). Expert cardiologists performed and assessed the echocardiographies, and one ECG expert evaluated all 260 ECGs in details. This enabled not only a thorough analysis of ECG changes in relation to LVSD (Table 2), but also construction of screening algorithms (Table 3) based on the ECG changes and combined with some use of NT‐proBNP (Figure 1).
Besides, this study is the first to relate ECG changes to NT‐proBNP. Figure 1 is unique and illustrates the correlation between ECG abnormalities and NT‐proBNP‐values. As might be expected, NT‐proBNP > 35 pmol/L correlates significantly with the ECG abnormalities most strongly associated with LVSD, and confirms the significant relation between these ECG abnormalities and LVSD. Thus there is an agreement between these two independent methods about LVSD. This internal study‐validation supports the study conclusion and strengthens the study algorithms.
The ECG algorithms (Table 3 A1–3) had a diagnostic specificity about 80% and consequently many false positive LVSD, which therefore may lead to unnecessary diagnostic echocardiographies. The algorithms with the ECG changes and additional measurement of NT‐proBNP (Table 3 A4–5) increase the diagnostic specificity beyond 80% and thereby reduce the needed number of diagnostic echocardiographies. ECG is less expensive than NT‐proBNP, and according to this study NT‐proBNP has restricted value as supplement to ECG when screening for LVSD. It is new knowledge that measurement of NT‐proBNP is unnecessary in subjects with atrial fibrillations, pacing, or LBBB, who should be referred directly to echocardiography.
Table 3 allows comparison between algorithms. The 4‐ECG algorithm (Table 3 A2: APLQ) reduced the needed number of diagnostic echocardiographies (N = 91), and adding in A4 a limited number of NT‐proBNP‐measurements (N = 15), the number of echocardiographies was further reduced (N = 85), with preservation of an acceptable sensitivity and improvement of specificity. Referral to echocardiography with a suspicion of LVSD was based on: (A2) at least one of 4 (APLQ) or (A4) at least one of 3 (APL) or (Q‐waves (no APL) and NT‐proBNP > 35 pmol/L) (Table 3).
Table 3 shows recipes to screen for LVSD. In other words, in the absence of atrial fibrillation, pacing, LBBB, and Q‐waves they were refrained from echocardiography; but in the presence of atrial fibrillation, pacing, and LBBB they were referred directly to echocardiography; and when atrial fibrillation, pacing, or LBBB was absent, but Q‐waves were present, NT‐proBNP was measured and if >35 pmol/L they were referred to echocardiography (Figure 2).
Figure 2.
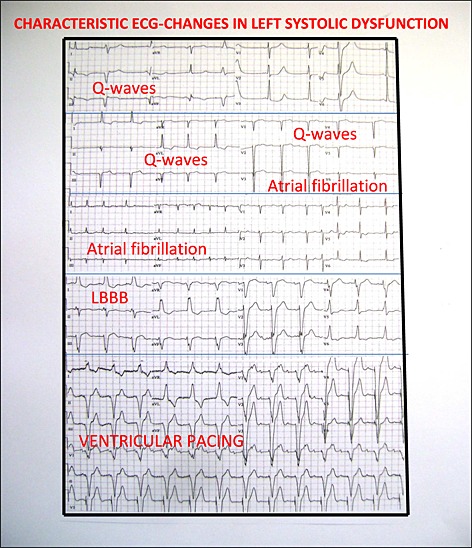
Characteristic electrocardiogram changes in left ventricular systolic dysfunction.
The novelty of the present study perhaps lies not in the description of ECG correlates to LVSD, even though there has been a lack of systematic investigations and explorations of this matter, but a novelty for sure is what combinations of ECG abnormalities and NT‐proBNP can accomplish in diagnosing LVSD and the recommendation to use the study algorithms to screen for LVSD in the elderly high risk population.
A prior study found ECG and NT‐proBNP to be of almost equal value as pre‐screening tools, but because ECG is the less expensive than NT‐proBNP, a strategy to perform handheld echocardiography after ECG provided the greatest cost savings and was the most cost effective. In that study ECG was categorized as normal or abnormal on the basis of a wide array of ECG changes, and the mean specificity of ECG was 58%,6 as compared with about 80% in the present study, which is based on ECG correlates to LVSD. This favours the present study algorithms as pre‐screening tests with the ability to selects subjects to hand‐held echocardiography, which is another independent tool to screen for LVSD, in order to further reduce the needed number of standard echocardiographies and increase cost effectiveness.6, 20
When planning to screen a population, several factors have to be taken into account. The condition or disease to screen for should be important concerning prevalence, morbidity, or mortality, and cure or effective treatment should be present. The screening methods should be easily available and usable, reliable, secure, and inexpensive. These requirements are fulfilled in LVSD in the elderly. LVSD is a serious condition, treatable, and because LVSD accumulates in the elderly, screening for LVSD in the high risk geriatric population is likely to be both beneficial and cost effective. Screening of younger and low risk populations for LVSD is not cost effective and is not recommended.2, 5, 6
When a big population is screened for LVSD, it is mandatory to have a clear‐cut definition of the problem looked for in order to avoid diagnostic uncertainty with increased risk of false positive/negative diagnoses.2 The priority should be to find the patients who have LVSD beyond doubt, defined as EF < 40%. An algorithm capable of detecting everyone with heart failure does not exist, but discovering about 90%, as in this study, seems acceptable, and it is considerably better than what is presently achieved in daily clinical practice. All 31 study patients with EF < 30% had an abnormal ECG, all had at least one of the five ECG changes, and all had NT‐proBNP > 35 pmol/L; thus no one with EF < 30% was missed.
Even the best of algorithms is limited by the type of population in which it is developed, and validation of the study results should be performed in other populations.3 The algorithms should also be validated against automated ECG interpretation.28
In the presence of ECG correlates to LVSD, it is suggested, that a recommendation to consider LVSD should appear automatically on the ECG 12, and this might become an option, in order to improve and facilitate screening for LVSD in the elderly in primary care. In this way no special expertise is required to select the persons who should go on to be tested for LVSD.17, 28 Figure 2 illustrates the ECG correlates. Alternatively to the automated interpretation of the ECG, a physician with knowledge about and interest in ECG, preferably a trained cardiologist, should evaluate the ECG and decide whether or not to measure NT‐proBNP, whether or not to recommend handheld echocardiography, and whether or not to recommend diagnostic standard echocardiography.
A screening procedure can never be all inclusive. In return for simplicity and operability a certain amount of inaccuracy has to be accepted. It is important that a person who passes a screening test is informed about this, the screening personnel too, and if there is a conflict between the test results and the patient's condition, then other diagnostic means should be employed.6 Regular and repeated screening by ECG could make op for the incompleteness of this screening‐method,4, 5, 21 and in the elderly 75+ with no prior diagnosis of LVSD and with no other indication for echocardiography it is suggested to perform annual screening for LVSD.4 In many countries, like the Scandinavians, a yearly health visit is offered in primary care, and it seems opportune to include ECG in order to screen for LVSD at that occasion.
In conclusion, the number of senior citizens increases and so does the number of patients with LVSD. Screening and early treatment can become safeguards to assure optimal quality of life and besides diminish the burden on the health system. A few distinct and easily recognizable ECG abnormalities are useful in screening for LVSD in an elderly population as they may detect 90% of patients with EF < 40%.The main unique new finding of this study is the recommendation to perform echocardiography when there are atrial fibrillation, pacing, and LBBB, and when Q‐waves are associated with NT‐proBNP > 35 pmol. Danish senior citizens 75+ are offered an annual health care visit, and the present study seems to justify future inclusion of ECG in order to detect and treat elderly with LVSD.
Conflict of Interest
None declared.
Funding
This work was supported by
Region Zealand (DK) research foundation of cross‐sectional health projects
Danish Foundation TrygFonden
Region Zealand (DK) health scientific research foundation
Roche Diagnostics A/S, Industriholmen 59, DK‐2650 Hvidovre (kits to measure NT‐proBNP)
Acknowledgement
We want to thank Anders Galløe, Ph. D. and consultant cardiologist, for editing the manuscript before submission.
Olesen, L. L. , and Andersen, A. (2016) ECG as a first step in the detection of left ventricular systolic dysfunction in the elderly. ESC Heart Failure, 3: 44–52. doi: 10.1002/ehf2.12067.
References
- 1. Cincin A, Ozben B, Erdogan O. Diagnostic utility of specific electrodiagraphical parameters in predicting left ventricular function. Exp Clin Cardiol 2012;17:210–214. [PMC free article] [PubMed] [Google Scholar]
- 2. Nielsen OW. Epidemiology of heart failure and clinical application of natriuretic peptide testing in primary care. Faculty of Health Sciences. University of Copenhagen. Academic dissertation. 2008.
- 3. Gillespie N. The diagnosis and management of chronic heart failure in the older patient. Br Med Bull 2005;75:49–62. [DOI] [PubMed] [Google Scholar]
- 4. Murtagh G, Dawkins IR, O´Connell R, Badabhagni M, Patel A, Tallon E, O´Hanlon R, Ledwidge MT, McDonald KM. Screening to prevent heart failure (STOP‐HF): expanding the focus beyond asymptomatic left ventricular systolic dysfunction. Eur J Heart Fail 2012;14:480–486. [DOI] [PubMed] [Google Scholar]
- 5. Goode KM, Clark AL, Bristow JA, Sykes KB, Cleland JGF. Screening for left ventricular systolic dysfunction in high‐risk patients in primary‐care: a cost–benefit analysis. Eur J Heart Fail 2007;9:1186–1195. [DOI] [PubMed] [Google Scholar]
- 6. Galasko GI, Barnes SC, Collinson P, Lahiri A, Senior R. What is the most cost‐effective strategy to screen for left ventricular systolic dysfunction: natriuretic peptides, the electrocardiogram, hand‐held echocardiography, traditional echocardiography, or of their combination? Eur Heart J 2006;27:193–200. [DOI] [PubMed] [Google Scholar]
- 7. Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation 2003;108:977–982. [DOI] [PubMed] [Google Scholar]
- 8. Fuat A, Hungin APS, Murphy JJ. Barriers to accurate diagnosis and effective management of heart failure in primary care: qualitative study. BMJ 2003;326:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ng LL, Loke I, Davies JE, Khunti K, Stone M, Abrahms KR, Chin DT, Squire IB. Identification of previously undiagnosed left ventricular systolic dysfunction: community screening using natriuretic peptides and electrocardiography. Eur J Heart Fail 2003;5:775–782. [DOI] [PubMed] [Google Scholar]
- 10. Nielsen OW, Hansen JF, Hilden J, Larsen CT, Svanegaard J. Risk assessment of left ventricular systolic dysfunction in primary care: cross sectional study evaluating a range of diagnostic tests. BMJ 2000;320:220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sheifer SE, Gersh BJ, Yanez D, Anes PA, Burke GL, Manolio TA. Prevalence, predisposing factors, and prognosis of clinically unrecognized myocardial infarction in the elderly. J Am Coll Cardiol 2000;35:119–126. [DOI] [PubMed] [Google Scholar]
- 12. Davie AP, Francis CM, Love MP, Caruana L, Starkey IR, Shaw TRD, Sutherland GR, McMurray JJV. Value of the electrocardiogram in identifying heart failure due to left ventricular systolic dysfunction. BMJ 1996;312:222–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Olesen LL. The Danish National Quality Project about heart failure indicates lack of reporting and treatment of the elderly. Ugeskr Laeger 2015;177:338–339. [PubMed] [Google Scholar]
- 14. Forman DE, Cannon CP, Hernandez AF, Liang L, Yancy C, Fonarow GC. Influence of age on the management of heart failure: findings from Get With the Guidelines‐Heart Failure (GWTG‐HF). Am Heart J 2009;157:1010–1017. [DOI] [PubMed] [Google Scholar]
- 15. Davenport C, Cheng EYL, Kwok YTT, Lai AHO, Wakabayashi T, Hyde C, Connock M. Assessing the diagnostic test accuracy of natriuretic peptides and ECG in the diagnosis of left ventricular systolic dysfunction: a systematic review and meta‐analysis. Br J Gen Practice 2006;56:48–56. [PMC free article] [PubMed] [Google Scholar]
- 16.E W, Sengupta M, Velkoff VA, DeBarros KA. 65+ in the United States: 2005. Current population reports. Washington, DC: Government Printing Office; 2005: 23–209. http://www.Census.Gov/prod/2006pubs/p23‐209.pdf
- 17. Khunti K, Squire I, Abrams KR, Sutton AJ. Accuracy of a 12‐lead electrocardiogram in screening patients with suspected heart failure for open access echocardiography: a systematic review and meta‐analysis. Eur J Heart Fail 2004;6:571–576. [DOI] [PubMed] [Google Scholar]
- 18. Fox KF, Cowie MR, Wood DA, Coats AJS, Poole‐Wilson PA, Sutton GC. A Rapid Access Heart Failure Clinic provides a prompt diagnosis and appropriate management of new heart failure presenting in the community. Eur J Heart Fail 2000;2:423–429. [DOI] [PubMed] [Google Scholar]
- 19. Maisel A, Mueller C, Adams K, Anker SD, Aspromonte N, Cleland JGF, Cohen‐Solal A, Dahlstrom U, DeMaria A, Di Somma S, Filippatos GS, Fonarow GC, Jourdain P, Komajda M, Liu PP, McDonagh T, McDonald K, Mebazaa A, Nieminen MS, Peacock WF, Tubaro M, Valle R, Vanderhyden M, Yancy CW, Zannad F, Braunwald E. State of the art: using natriuretic levels in clinical practice. Eur J Heart Fail 2008;10:824–839. [DOI] [PubMed] [Google Scholar]
- 20. Olesen LL, Andersen A, Thaulow S. Hand‐held echocardiography is useful for diagnosis of left systolic dysfunction in an elderly population. Dan Med 2015;62:A5100. [PubMed] [Google Scholar]
- 21. Madhok V, Falk G, Rogers A, Struhers AD, Sullivan FM, Fahey T. The accuracy of symptoms, signs and diagnostic tests in diagnosis of left ventricular dysfunction in primary care: a diagnostic accuracy systematic review. Fam Pract 2008;9:56, doi: 10.1186/1471-2296-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boonman‐de Winter LJM, Rutten FH, Cramer MJ, Landman MJ, Zuithoff NPA, Liem AH, Hoes AW. Efficiently screening heart failure in patients with type 2 diabetes. Eur J Heart Fail 2015;17:187–195. [DOI] [PubMed] [Google Scholar]
- 23. Madias JE. The resting electrocardiogram in the management of patients with congestive heart failure: established applications and new insights. Pacing Clin Electrophysiol 2007;30:123–128. [DOI] [PubMed] [Google Scholar]
- 24. http://www.learningace.com/doc/191199/51f25519884ce11938bfbe8f338a2018/mncode
- 25. Khan NK, Goode KM, Cleland JGF, Rigby AS, Freemantle N, Eastaugh J, Clark AL, de Silva R, Calvert MJ, Swedberg K, Komajda M, Mareev V, Follath F. Prevalence of ECG abnormalities in an international survey of patients with suspected or confirmed heart failure at death or discharge. Eur J Heart Fail 2007;9:491–501. [DOI] [PubMed] [Google Scholar]
- 26. Zannad F, Huvelle E, Dickstein K, van Veldhuisen DJ, Stellbrink C, Køber L, Cazeau S, Ritter P, Maggioni AP, Ferrari R, Lechat P. Left bundle branch block as a risk factor for progression to heart failure. Eur J Heart Fail 2007;9:7–14. [DOI] [PubMed] [Google Scholar]
- 27. Correll P. Atrial fibrillation in systolic heart failure: prevalence, incidence and prognosis. University of Copenhagen. Ph. D. thesis. 2007.
- 28. Jensen MSA, Thomsen JL, Jensen SE, Lauritzen T, Engberg M. Electrocardiogram interpretation in general practice. Fam Pract 2005;22:109–113. [DOI] [PubMed] [Google Scholar]
- 29. Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, Lamas GA. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003;107:2932–2937. [DOI] [PubMed] [Google Scholar]
- 30. Dhingra R, Pencina MJ, Wang TJ, Nam BH, Benjamin EJ, Levy D, Larson MG, Kannel WB, D´Agostino RB, Vasan RS. Electrocardiographic QRS duration and the risk of congestive heart failure. The Framingham Heart Study. Hypertension 2006;47:861–867. [DOI] [PubMed] [Google Scholar]


