Abstract
Aims
Data on the prognostic role of left and right bundle branch blocks (LBBB and RBBB), and nonspecific intraventricular conduction delay (IVCD; QRS ≥ 110 ms, no BBB) in acute heart failure (AHF) are controversial. Our aim was to investigate electrocardiographic predictors of long‐term survival in patients with de novo AHF and acutely decompensated chronic heart failure (ADCHF).
Methods and Results
We analysed the admission electrocardiogram of 982 patients from a multicenter European cohort of AHF with 3.9 years' mean follow‐up. Half (51.5%, n = 506) of the patients had de novo AHF. LBBB, and IVCD were more common in ADCHF than in de novo AHF: 17.2% vs. 8.7% (P < 0.001) and 20.6% vs. 13.2% (P = 0.001), respectively, and RBBB was almost equally common (6.9% and 8.1%; P = 0.5), respectively. Mortality during the follow‐up was higher in patients with RBBB (85.4%) and IVCD (73.7%) compared with patients with normal ventricular conduction (57.0%); P < 0.001 for both. The impact of RBBB on prognosis was prominent in de novo AHF (adjusted HR 1.93, 1.03–3.60; P = 0.04), and IVCD independently predicted death in ADCHF (adjusted HR 1.79, 1.28–2.52; P = 0.001). Both findings were pronounced in patients with reduced ejection fraction. LBBB showed no association with increased mortality in either of the subgroups. The main results were confirmed in a validation cohort of 1511 AHF patients with 5.9 years' mean follow‐up.
Conclusions
Conduction abnormalities predict long‐term survival differently in de novo AHF and ADCHF. RBBB predicts mortality in de novo AHF, and IVCD in ADCHF. LBBB has no additive predictive value in AHF requiring hospitalization.
Keywords: Acute heart failure, Ventricular conduction, Bundle branch block, Prognosis, de novo
Introduction
Acute heart failure (AHF) is the leading cause of hospitalization for patients aged over 65 years in the Western world, and long‐term survival with AHF is dismal. Prolonged QRS duration with or without bundle branch block (BBB) is both frequent and has been associated with increased mortality and morbidity in several studies in chronic heart failure.1, 2 However, the role of ventricular conduction abnormalities in the pathophysiology and prognosis of AHF is not well established. Only few studies have investigated the prognostic impact of specific types of ventricular conduction abnormalities, that is, right bundle branch block (RBBB) or left bundle branch block (LBBB), in the long‐term survival of AHF, and the findings have been controversial. This may in part be due to differences in the baseline characteristics of the patient cohorts and in the length of follow‐up period.3, 4, 5, 6
Patients with new‐onset (de novo) AHF differ significantly in their medical history, clinical presentation, and long‐term survival from those with acutely decompensated chronic heart failure (ADCHF).7 Whether differences exist in the prevalence of ventricular conduction abnormalities and their effect on long‐term mortality in a comparison between patients with de novo AHF and ADCHF remains unknown. In this study, we aimed to examine the characteristics in the admission electrocardiogram (ECG) in a large multicentre European cohort of patients hospitalized for AHF and to assess the differences in their impact on long‐term prognosis in patients with de novo AHF and ADCHF.
Methods
Patients
Data from two independent prospectively collected cohorts were combined for this analysis. The FINN‐AKVA (Finnish Acute Heart Failure Study) study is a prospective, national multicentre study, which enrolled 620 consecutive patients with AHF in 2004 in Finland.8 Vital status at 5 years after the index hospitalization and time of death were obtained from the National Population Registry. The admission ECG was available for 595 (96%) patients. The BASEL V study (B‐type Natriuretic Peptide for Acute Shortness of Breath EvaLuation; 2006–2007) recruited patients presenting to the emergency department with a chief complaint of shortness of breath.9 For the present analysis, only patients with an adjudicated diagnosis of AHF (n = 387) were included, in all of which the admission ECG was available. These together resulted in a cohort of 982 AHF patients with a mean follow‐up period of 3.9 years (95% CI 3.7–4.0 years); the median follow‐up time was 5 years. The end point of interest was all‐cause mortality. Final AHF diagnosis was confirmed by the local investigators based on all clinical, laboratory, and imaging information.
The ECGs in each cohort were analysed by two to three researchers (medical doctors) specially trained for and assigned to the task. Rhythm and conduction abnormalities were characterized in the admission ECG. RBBB and LBBB were identified by standard criteria.10 Intraventricular conduction delay (IVCD) was defined as QRS duration ≥ 110 ms without fulfilling the criteria of either BBB.11 Patients with a previous history of heart failure were regarded as ADCHF, whereas the others had de novo AHF. All patients provided their written informed consent. Both studies were approved by local Ethics Committees and conducted in concordance with the Declaration of Helsinki.
Statistical analyses
The statistical analyses were performed with SPSS 21 statistical software (IBM Corp, Armonk, NY, USA). Results are shown as numbers and percentages (%), means with standard deviation (SD) or medians with interquartile range (IQR) for variables not normally distributed. Dichotomous variables were compared using the chi‐square test and continuous variables using Student's t‐test or the Mann–Whitney U‐test as appropriate. Analysis of variance served for multiple group comparisons and was corrected with the Bonferroni method. Mortality analyses were performed using Kaplan–Meier (KM) survival curves and Cox proportional hazard ratios. Hazard ratios (HR) are shown with 95% confidence intervals (CI).
Age, sex, and comorbidities previously shown to associate with prognosis or regarded as clinically significant, such as coronary artery disease, previous myocardial infarction, hypertension, and chronic obstructive pulmonary disease, as well as estimated glomerular filtration rate and smoking, were included in the multivariate models. When analysing all patients as one group, the history of chronic heart failure was also included in the model. Because one‐third of the patients had missing natriuretic peptide values, a separate model including available N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) data was built. Rhythm on the admission ECG was tested in a multivariable model but was neither independently associated with outcome nor did it improve the model performance, so it was not retained in further analyses. KM survival curves were plotted with cases alive censored at their latest contact date. Mortality rates at the end of the follow‐up period were estimated with KM survival tables. Groups were compared by the log‐rank test. In all analyses, P‐values <0.05 were regarded as statistically significant.
Validation procedure
Additional ECG data on 1511 patients with AHF from the Faculty Hospital in Brno, part of the Czech AHEAD registry,12 served for validation of the main findings. All patients with data available on QRS duration in their admission ECG (76.9%) were included. Criteria for determining the presence of RBBB, LBBB, or IVCD were the same as in the derivation cohort. The mean follow‐up period was 5.9 years (95% CI 5.8–6.1 years, range 0.0–8.0 years). In the multivariate mortality analyses with Cox proportional hazard ratios, the same variables were included in the models as in the derivation cohort, with the exception of smoking and NT‐proBNP, which were available in only 675 (44.7%) and 72 patients (4.8%), respectively. Instead, a model including left ventricular (LV) ejection fraction (LVEF), which was available in 1421 patients (94.0%), was used.
Results
Study population
The patients' mean age was 75.8 years (95% CI 75.2–76.5), and 474 (48.3%) of them were women; 506 (51.5%) of the patients had de novo AHF, and 476 (48.5%) had ADCHF. Patient characteristics and medical history are shown in Table 1. Patients with de novo AHF were younger and had fewer cardiac comorbidities than did those with ADCHF. AHF was caused more often by acute coronary syndrome (28.9% vs. 18.8%; P < 0.001) and by atrial fibrillation or flutter (27.5% vs. 20.0%; P = 0.006) in patients with de novo AHF than in those with ADCHF. LVEF was higher in patients with de novo AHF than with ADCHF (47% vs. 43%; P < 0.001). Overall, 497 deaths occurred during follow‐up, of which 300 were in the ADCHF group and 197 in the de novo AHF group. The mortality rate at 5 years was 61.6%, being significantly higher in patients with ADCHF than with de novo AHF (76.3% vs. 47.4%; P < 0.001).
Table 1.
Characteristics of study population in the subgroups of de novo AHF and acutely decompensated chronic heart failure (ADCHF), mean (SD) or n (%)
| De novo AHF | ADCHF | P‐value | |
|---|---|---|---|
| n = 506 | n = 476 | ||
| Age, years | 74.7 (10.9) | 77.1 (9.9) | <0.001 |
| Range | 39–101 | 38–96 | |
| Women | 246 (48.6) | 228 (47.9) | 0.8 |
| Medical history | |||
| Hypertension | 303 (59.9) | 310 (65.1) | 0.09 |
| Chronic atrial fib./flutter | 127 (25.7) | 157 (33.3) | 0.009 |
| Coronary artery disease | 183 (36.2) | 311 (65.3) | <0.001 |
| Previous myocardial infarction | 71 (14.0) | 183 (38.4) | <0.001 |
| Dyslipidemia | 144 (28.5) | 140 (29.6) | 0.7 |
| Diabetes mellitus | 151 (29.8) | 153 (32.1) | 0.4 |
| COPD | 85 (16.8) | 92 (19.3) | 0.3 |
| Smoking | 85 (16.8) | 55 (11.6) | 0.02 |
| BMI (n = 718) | 27.3 (6.4) | 27.7 (5.9) | 0.4 |
| eGFR, mL/min/1.73 m2 | 65 (30) | 55 (26) | <0.001 |
| LVEDD; mm (n = 530) | 54 (9) | 58 (12) | <0.001 |
| LVEF% (n = 638) | 47 (15) | 43 (17) | <0.001 |
| RBBB | 41 (8.1) | 33 (6.9) | 0.5 |
| LBBB | 44 (8.7) | 82 (17.2) | <0.001 |
| IVCD | 67 (13.8) | 98 (22.2) | 0.001 |
Atrial fib, atrial fibrillation; BMI, body mass index; eGFR, estimated glomerular filtration rate; COPD, chronic obstructive pulmonary disease; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction.
ECG characteristics
Sinus rhythm was more common in de novo AHF than in ADCHF patients (60.7% vs. 48.7%; P < 0.001) on admission ECG, and atrial fibrillation was more common in those with ADCHF (42.2% vs. 34.5%; P = 0.01). Mortality rates at 5 years in patients with sinus rhythm, atrial fibrillation or flutter, and other rhythms did not significantly differ in de novo AHF (51.1%, 44.1%, and 34.8%, respectively; P = 0.3) or ADCHF (73.7%, 77.6, and 81.6%, respectively; P = 0.6). Data on duration of the PQ interval were available only from the FINN‐AKVA cohort and were for 288 (29.3%) patients. The median PQ interval was 160 ms (IQR 140–180 ms) in de novo AHF and 180 ms (IQR 160–200 ms) in ADCHF (P < 0.001). In univariate analysis, the increasing duration of PQ‐interval seemed to be associated with increased mortality (unadjusted HR 1.04; 95% CI 1.01–1.08; P = 0.02 for each 10 ms increase in the duration), but not when adjusted for age and sex (HR 1.01; 95% CI 0.98–1.05; P = 0.5).
Ventricular conduction abnormalities
Median duration on the QRS was 102 ms (IQR 90–126 ms); it was longer in patients with ADCHF than with de novo AHF (107 ms, IQR 92–136 ms vs. 100 ms, IQR 88–118 ms; P < 0.001). RBBB was similarly common in ADCHF and de novo AHF (6.9% and 8.1%; P = 0.5), whereas LBBB and IVCD were more common in ADCHF (17.2% vs. 8.7%; P < 0.001, and 22.2% vs. 13.8%; P = 0.001). Characteristics of patients in each group of ventricular conduction abnormality are reported in Supporting Information Table S1. Patients with RBBB and LBBB were older than those either with IVCD or without conduction abnormality. Each of the three conduction abnormality was more common in men than in women (RBBB 10.2% vs. 4.6%; P = 0.001), (LBBB 15.4% vs. 10.1%; P = 0.01), and (IVCD 21.5% vs. 13.9%; P = 0.003). Patients with LBBB and IVCD had more often history of coronary artery disease and lower LVEF values than those with RBBB or normal ventricular conduction.
Ventricular conduction abnormalities associated with mortality
Mortality during follow‐up was higher in patients with a ventricular conduction abnormality (71.5% vs. 55.1%) than in those with normal QRS width: adjusted HR 1.4 (95% CI 1.1–1.8; P = 0.004). However, increasing duration of the QRS (continuous variable) as such was not associated with increased mortality: unadjusted HR 1.02 (95% CI 0.99–1.06; P = 0.3) in de novo AHF and HR 1.00 (95% CI 0.97–1.03; P = 0.8) in ADCHF for each 10 ms increase in QRS duration. We observed higher mortality rates with each 10 ms increase in QRS duration between 100 and 140 ms, but QRS width over 140 ms did not show further increase in mortality. The KM curves in Figure 1 illustrate the differences in survival with RBBB and IVCD between de novo AHF and ADCHF, and Table 2 and Figure 2 summarizes the Cox proportional hazard ratios for mortality with each type of ventricular conduction abnormality in AHF.
Figure 1.
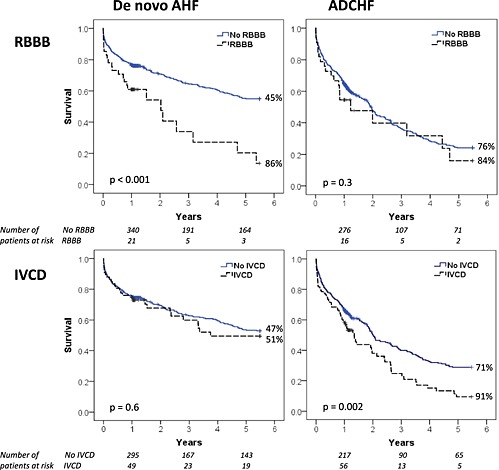
Kaplan–Meier survival curves in patients in de novo AHF (left) and ADCHF (right) with and without RBBB (top) and IVCD (bottom). Mortality rates at the end of the follow‐up period for each subgroup are indicated at the end of the curves. Cases censored during follow‐up are depicted with crosses within the lines. P‐value for difference between groups by log‐rank test.
Table 2.
Cox proportional hazard ratios (HR) with 95% confidential intervals for mortality for each type of ventricular conduction abnormality in all patients and in the subgroups de novo AHF and ADCHF
| Unadjusted HR | P | Adjusted HR | P | Adjusted HR, NT‐proBNP included (n=607) | P | |
|---|---|---|---|---|---|---|
| RBBB, all | 1.64 (1.20–2.24) | 0.002 | 1.38 (1.00–1.90) | 0.05 | 1.72 (1.13–2.61) | 0.01 |
| De novo AHF | 2.21 (1.42–3.42) | <0.001 | 1.54 (0.98–2.42) | 0.06 | 1.93 (1.03–3.60) | 0.04 |
| ADCHF | 1.25 (0.79–1.96) | 0.3 | 1.20 (0.76–1.91) | 0.4 | 1.47 (0.80–2.68) | 0.2 |
| LBBB, all | 1.02 (0.78–1.34) | 0.9 | 0.84 (0.64–1.11) | 0.2 | 0.87 (0.63–1.21) | 0.4 |
| De novo AHF | 1.09 (0.66–1.79) | 0.7 | 0.94 (0.62–1.68) | 0.9 | 1.03 (0.53–2.00) | 0.9 |
| ADCHF | 0.83 (0.60–1.15) | 0.3 | 0.77 (0.55–1.08) | 0.13 | 0.80 (0.55–1.18) | 0.3 |
| IVCD, all | 1.43 (1.14–1.80) | 0.002 | 1.27 (1.01–1.59) | 0.04 | 1.55 (1.18–2.04) | 0.002 |
| De novo AHF | 1.10 (0.73–1.65) | 0.6 | 1.08 (0.71–1.64) | 0.7 | 1.16 (0.70–1.91) | 0.6 |
| ADCHF | 1.53 (1.16–2.01) | 0.003 | 1.38 (1.04–1.82) | 0.03 | 1.79 (1.28–2.52) | 0.001 |
Both multivariate models are adjusted for age, sex, coronary artery disease, previous myocardial infarction, hypertension, chronic obstructive pulmonary disease, smoking, and glomerular filtration rate, as well as previous heart failure when all patients were analysed.
Figure 2.

Adjusted Cox proportional hazard ratios (♦) with 95% confidence intervals  for each type of conduction abnormality in all patients (solid lines) and in the subgroups of de novo AHF and ADCHF (dashed lines) in the derivation cohort.
for each type of conduction abnormality in all patients (solid lines) and in the subgroups of de novo AHF and ADCHF (dashed lines) in the derivation cohort.
The RBBB was related to increased mortality in all patients (adjusted HR 1.7; 95% CI 1.1–2.6; P = 0.01) and in particular in those with de novo AHF (adjusted HR 1.9; 95% CI 1.03–3.6; P = 0.04). In an exploratory analysis categorizing patients by LVEF, the association of RBBB with mortality in de novo AHF was stronger in patients with impaired systolic function (LVEF < 40%) (adjusted HR 3.4; 95% CI 1.1–10.4; P = 0.03) than in patients with more preserved LVEF (adjusted HR 1.5; 95% CI 0.7–3.1; P = 0.3). In contrast, IVCD was independently predictive of poor prognosis overall (adjusted HR 1.6; 95% CI 1.2–2.0; P = 0.002) and pronouncedly in those with ADCHF (adjusted HR 1.8; 95% CI 1.3–2.5; P = 0.001). Again, the effect on outcome was related to impairment of LV systolic function, with adjusted HR 2.7 (95% CI 1.6–4.5; P < 0.001) for IVCD in patients with LVEF < 40% compared with HR 1.2 (95% CI 0.7–2.0; P = 0.6) in patients with preserved LVEF.
Validation data
In the validation cohort, 978 patients (64.7%) had de novo AHF, and 533 (35.3%) had ADCHF. The mean age of the patients was 70.4 years (SD 12.5), and 636 (42.1%) of them were women. Baseline characteristics of the derivation and validation cohorts are shown in Supporting Information Table S2. Compared with the derivation cohort, patients in the validation cohort were younger, were more often men, and had more cardiovascular comorbidities. RBBB was present in 130 patients (8.6%), LBBB in 167 patients (11.1%), and IVCD in 161 patients (10.7%). AHF was caused by acute coronary syndrome more often in the validation than in the derivation cohort (49% vs. 24%; P < 0.001), and the patients in the validation cohort were more often critically ill; cardiogenic shock was present in 14.2% compared with 2.1% in the derivation cohort (P < 0.001).
The total mortality rate during follow‐up in the validation cohort was 65.8% (875 deaths). In de novo AHF patients, the mortality rate was 56.6%, and for those with ADCHF, 81.9% (P < 0.001). As in the derivation cohort, the presence of RBBB in the admission ECG was independently associated with increased mortality rate in the de novo AHF patients (adjusted HR 1.5, 95% CI 1.1–2.1; P = 0.006), but not in the ADCHF patients. In contrast, IVCD was independently associated with increased mortality rate in patients with ADCHF (adjusted HR 1.5, 95% CI 1.1–2.0; P = 0.007), but not in those with de novo (Supporting Information Figure S1). Overall, these results were very similar to the derivation cohort, as illustrated also by the KM survival curves in Supporting Information Figure S2. Again, the associations to mortality were stronger in patients with impaired LV function (LVEF < 40%). More specifically, for the de novo patients with RBBB, the adjusted HR was 2.0 (95% CI 1.3–3.3; P = 0.003) if LVEF < 40%, while in patients with preserved LVEF, the adjusted HR was 1.4 (95% CI 0.90‐2.03; P= 0.1). For the ADCHF patients with impaired systolic function (LVEF < 40%) the adjusted HR for IVCD was 1.7 (95% CI 1.2‐2.4; P = 0.002) compared with adjusted HR 1.2 (95% CI 0.7–2.1; P = 0.5) if LVEF was preserved.
Discussion
This study shows the association of different types of ventricular conduction abnormalities with mortality in AHF. In addition, differences in ventricular conduction abnormalities between de novo AHF and ADCHF patients are here described, to our knowledge, for the first time. The data come from a large multicentre European cohort of patients hospitalized for AHF with long‐term mortality follow‐up, and the main results are validated in a large, independent cohort of AHF from another European centre. We show that RBBB is associated with increased mortality, in particular in patients with de novo AHF. In contrast, IVCD is an independent predictor of poor prognosis in patients with ADCHF. The effect on mortality of both conduction abnormalities are related to impairment of LV systolic function. LBBB was not associated with poorer long‐term survival overall, or in either of the patient subgroups. These results remained very similar in the validation cohort, even though the patient characteristics differed slightly, and the clinical picture of AHF was more severe and more often induced by acute coronary syndrome compared with the derivation cohort.
The prevalence of RBBB and LBBB in this study was similar to other studies of AHF.5, 6 Few studies of AHF have analysed IVCD as such, especially with the use of QRS duration ≥110 ms as the definition for IVCD, as we did. Recent reports have, however, suggested that even QRS duration between 110 and 120 ms is associated with adverse outcome in other populations,11, 13, 14 and furthermore, QRS duration around 110 ms also corresponded to optimal cut‐off for worse survival in our cohort (data not shown). Nevertheless, IVCD prevalence in this study is consistent with that in earlier observations regarding increased QRS duration.1 Comparing de novo and ADCHF patients, we found that the QRS duration was longer and that IVCD as well as LBBB were more frequent in the latter group. RBBB in both groups, in contrast, was almost equally common. Of note, even in de novo AHF, prevalence of ventricular conduction abnormalities is markedly higher than in the general population.13
The RBBB, as we show, was a predictor of long‐term mortality in patients with de novo AHF. De novo AHF and ADCHF patients with RBBB had similar mortality rates, but ADCHF patients showed no increased risk of death associated with RBBB, as their overall mortality was high. Abdel‐Qadir et al. found RBBB to be a predictor of mortality in AHF patients, but in their study, it merely reflected the older age and comorbidities of their patients with RBBB, rather than being an independent risk factor for mortality.6 Here, RBBB was related to increased mortality especially in de novo AHF patients, who were younger and had fewer comorbidities, and even when adjusted for age, sex, medical history, and NT‐proBNP, the strength of this association persisted. RBBB has been associated with previous myocardial infarctions,5 increased systolic pulmonary artery pressure,15 and right ventricle (RV) dysfunction in chronic heart failure patients.16, 17 RV dysfunction is an independent predictor of worse survival in chronic heart failure,18, 19 and recently also found in AHF.20 In that study of consecutively recruited AHF patients, RV dysfunction was found to be present in as much as a fourth of patients, and 70% of them also had pulmonary hypertension assessed with echocardiography.
In our study, while the patients with RBBB in general had higher LVEF values than all other patient groups, the negative impact of RBBB on survival seemed to be stronger in those with reduced LVEF, as observed in earlier studies as well.5, 15, 21 Indeed, the presence of RBBB in manifest LV heart failure may reflect a more severe underlying cardiac disorder with more markedly impaired LV function. Constantly elevated LV filling pressures and secondary pulmonary hypertension leading to a biventricular failure through LV–RV coupling mechanisms negatively impact long‐term prognosis.3, 22 Furthermore, in the setting of AHF, RBBB might be an indicator of acute RV pressure overload induced also by hypoxia‐triggered increase in pulmonary vascular resistance.3, 23 These considerations might partly explain the lack of association of RBBB with increased risk of adverse outcomes in other populations.14, 24, 25
Contrary to some earlier reports,4, 6 in our study, LBBB was not associated with poorer long‐term survival overall, or in either of the patient subgroups. In general, LBBB is associated with advanced LV dysfunction and systolic heart failure.21, 24, 25, 26 Even so, in our study, patients with LBBB had the lowest LVEF but were not at increased risk of death even in univariate analysis. In current guidelines,27, 28 LBBB and mechanical dyssynchrony are targets for cardiac resynchronization therapy to improve prognosis in chronic heart failure. However, in the setting of acute cardiac decompensation, LBBB may simply reflect the severity of cardiac disease and comorbidities, as Tabrizi et al. 29 showed in highly symptomatic chronic HF patients and Stenestrand et al. 30 in acute myocardial infarction patients with LBBB having no additive prognostic value.
Each episode of decompensation is known to substantially worsen the long‐term survival in heart failure.31 Furthermore, there has been increasing focus on the importance of time‐to‐therapy for prognosis in AHF.32, 33 Symptoms of LV decompensation (i.e. dyspnea) may trigger earlier and more aggressive medical interventions in the emergency setting compared with those with peripheral edema (venous congestion and RV failure) as principal sign and symptom of decompensation. In addition, because LBBB is currently a well‐recognized marker of cardiac disease by healthcare providers, this may influence both immediate and long‐term management. Our data suggest that the presence of any conduction abnormality should be regarded as associated with worse prognosis in AHF.
Finally, in the present study, IVCD was more common than either LBBB or RBBB, and almost as frequent as any BBB in AHF. IVCD, like LBBB, has been related to older age, comorbidities, and advanced LV dysfunction.34 Prolonged QRS has, however, been independently associated with increased mortality both in chronic heart failure35 and in AHF.2 Our study contributes to knowledge of the role of IVCD as a prognostic marker in AHF with three important findings. First, we have extended the effect of QRS prolongation on mortality in AHF to include patients with QRS ≥ 110 ms. Secondly, we found that its detrimental effect on prognosis mainly affected patients with ADCHF. Finally, our results suggest that prolonged QRS duration without BBB associates with mortality in patients with evident LV systolic dysfunction. We hypothesize that IVCD is a subtle marker of general myocardial incapability, or electrical failure, which reflects ventricular contractility and in the long term increases the risk of death. Moreover, as a marker of disturbed conduction in the ventricles, QRS prolongation may predispose to arrhythmic or sudden death.11, 13 These effects become evident only with longer exposure, such as in patients with previous history of heart failure or with long‐term follow‐up, or both.
Limitations
We acknowledge that our study has some limitations. Five‐year follow‐up data were not available for the entire study population, but the validation cohort had a mean follow‐up of more than 5 years. In addition, high mortality rates in the study population further limited the number of patients with complete follow‐up times. Especially for RBBB, the numbers of patients and events in the subgroup analyses were small and should be interpreted cautiously. There were no data on pulmonary pressures in the studied cohorts. Data on specific causes of death, especially cardiac or sudden death, would have been of value in addition to all‐cause mortality. Finally, echocardiography was not mandatory during the index hospitalization, and data on LVEF were available in only two‐thirds of the patients in the derivation cohort. However, while NT‐proBNP was measured in very few patients in the validation cohort, the majority had LVEF measured, which strengthens our observations on the association between ventricular conduction abnormalities, LVEF, and outcome.
Conclusions
In patients hospitalized for AHF, ventricular conduction abnormalities are common. The prevalence of RBBB, LBBB, and IVCD (QRS ≥ 110 ms) differs between de novo AHF and ADCHF. RBBB and IVCD are associated with poor long‐term prognosis and should be considered in the risk stratification of patients hospitalized for AHF. RBBB indicates poor survival particularly in patients with de novo AHF, whereas IVCD is an independent predictor of death in patients with ADCHF. The effect of these ventricular conduction abnormalities on prognosis seems to be related to LV systolic dysfunction.
Acknowledgements
The FINN‐AKVA study group: V‐P. Harjola, K. Siirilä‐Waris, MS. Nieminen, Helsinki, University Central Hospital; J. Melin, Central Finland Central Hospital; K. Peuhkurinen, Kuopio University Hospital; M. Halkosaari, Keski‐Pohjanmaa Central Hospital; K. Hänninen, Kymenlaakso Central Hospital; T. Ilva, T. Talvensaari, Kanta‐Häme Central, Hospital; H. Kervinen, Hyvinkää Hospital; K. Kiilavuori, Jorvi Hospital; K. Majamaa‐Voltti, Oulu University Hospital; H. Mäkynen, V. Virtanen, Tampere University Hospital; T. Salmela‐Mattila, Rauma Hospital; K. Soininen, Kuusankoski Hospital; M. Strandberg, H. Ukkonen, Turku University Hospital; I. Vehmanen, Turku Hospital; E.‐P. Sandell, Orion, Pharma, Espoo, Finland. Study nurses: K. Hautakoski, Keski‐Pohjanmaa Central Hospital; J. Lamminen, Hyvinkää Hospital; M.‐L. Niskanen, Kuopio University Hospital; M. Pietilä, Helsinki University Central Hospital; and O. Surakka, Central Finland Central Hospital.
Conflict of Interest
None declared.
Funding
This work was supported by grants from Paulo Foundation, the Finnish Foundation for Cardiovascular Research, and an unrestricted grant from Orion Pharma for the part of the FINN‐AKVA cohort. Heli Tolppanen also received grants from Aarne Koskelo Foundation.
The BASEL V study was supported by the Swiss National Science Foundation, the Swiss Heart Foundation, the University Hospital Basel, Abbott, BRAHMS, and Critical Diagnostics.
The data from Brno (AHEAD‐core) were supported by the Project of Conceptual Development of Research Organization (Department of Health) 65269705 (University Hospital Brno) and by the European Regional Development Fund—Project FNUSA‐ICRC (CZ.1.05/1.1.00/02.0123).
Supporting information
Figure S1. Adjusted Cox proportional hazard ratios (♦) with 95% confidence intervals (‐‐‐) for each type of conduction abnormality in all patients (solid lines) and in the subgroups of de novo AHF and ADCHF (dashed lines) in the validation cohort.
Figure S2. Kaplan–Meier survival curves of patients with de novo AHF (left) and ADCHF (right) in the validation cohort with and without RBBB (above) and IVCD (below). Cases censored during follow‐up are depicted with crosses.
Supporting info item
Supporting info item
Supporting info item
Tolppanen, H. , Siirila‐Waris, K. , Harjola, V.‐P. , Marono, D. , Parenica, J. , Kreutzinger, P. , Nieminen, T. , Pavlusova, M. , Tarvasmaki, T. , Twerenbold, R. , Tolonen, J. , Miklik, R. , Nieminen, M. S. , Spinar, J. , Mueller, C. , and Lassus, J. (2016) Ventricular conduction abnormalities as predictors of long‐term survival in acute de novo and decompensated chronic heart failure. ESC Heart Failure, 3: 35–43. doi: 10.1002/ehf2.12068.
References
- 1. Kashani A, Barold SS. Significance of QRS complex duration in patients with heart failure. J Am Coll Cardiol 2005; 46: 2183–92. [DOI] [PubMed] [Google Scholar]
- 2. Wang NC, Maggioni AP, Konstam MA, Zannad F, Krasa HB, Burnett JC Jr, Grinfeld L, Swedberg K, Udelson JE, Cook T, Traver B, Zimmer C, Orlandi C, Gheorghiade M. Efficacy of vasopressin antagonism in heart failure outcome study with tolvaptan I. Clinical implications of QRS duration in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction. JAMA 2008; 299: 2656–66. [DOI] [PubMed] [Google Scholar]
- 3. Mueller C, Laule‐Kilian K, Klima T, Breidthardt T, Hochholzer W, Perruchoud AP, Christ M. Right bundle branch block and long‐term mortality in patients with acute congestive heart failure. J Intern Med 2006; 260: 421–8. [DOI] [PubMed] [Google Scholar]
- 4. Huvelle E, Fay R, Alla F, Cohen Solal A, Mebazaa A, Zannad F. Left bundle branch block and mortality in patients with acute heart failure syndrome: a substudy of the EFICA cohort. Eur J Heart Fail 2010; 12(2): 156–63. [DOI] [PubMed] [Google Scholar]
- 5. Barsheshet A, Goldenberg I, Garty M, Gottlieb S, Sandach A, Laish‐Farkash A, Eldar M, Glikson M. Relation of bundle branch block to long‐term (four‐year) mortality in hospitalized patients with systolic heart failure. Am J Cardiol 2011; 107: 540–4. [DOI] [PubMed] [Google Scholar]
- 6. Abdel‐Qadir HM, Tu JV, Austin PC, Wang JT, Lee DS. Bundle branch block patterns and long‐term outcomes in heart failure. Int J Cardiol 2011; 146: 213–8. [DOI] [PubMed] [Google Scholar]
- 7. Lassus JP, Siirila‐Waris K, Nieminen MS, Tolonen J, Tarvasmaki T, Peuhkurinen K, Melin J, Pulkki K, Harjola VP, group F‐As . Long‐term survival after hospitalization for acute heart failure—differences in prognosis of acutely decompensated chronic and new‐onset acute heart failure. Int J Cardiol 2013; 168: 458–62. [DOI] [PubMed] [Google Scholar]
- 8. Siirila‐Waris K, Lassus J, Melin J, Peuhkurinen K, Nieminen MS, Harjola VP, Group F‐AS. Characteristics, outcomes, and predictors of 1‐year mortality in patients hospitalized for acute heart failure. Eur Heart J 2006; 27 3011–7. [DOI] [PubMed] [Google Scholar]
- 9. Breidthardt T, Irfan A, Klima T, Drexler B, Balmelli C, Arenja N, Socrates T, Ringger R, Heinisch C, Ziller R, Schifferli J, Meune C, Mueller C. Pathophysiology of lower extremity edema in acute heart failure revisited. Am J Med 2012; 125: 1124 e1–e8. [DOI] [PubMed] [Google Scholar]
- 10. Surawicz B, Childers R, Deal BJ, Gettes LS, Bailey JJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, Rautaharju PM, van Herpen G, Wagner GS, Wellens H, American Heart Association E , Arrhythmias Committee CoCC , American College of Cardiology F , Heart Rhythm S . AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2009; 53: 976–81. [DOI] [PubMed] [Google Scholar]
- 11. Zimetbaum PJ, Buxton AE, Batsford W, Fisher JD, Hafley GE, Lee KL, O'Toole MF, Page RL, Reynolds M, Josephson ME. Electrocardiographic predictors of arrhythmic death and total mortality in the multicenter unsustained tachycardia trial. Circulation 2004; 110: 766–9. [DOI] [PubMed] [Google Scholar]
- 12. Parenica J, Spinar J, Vitovec J, Widimsky P, Linhart A, Fedorco M, Vaclavik J, Miklik R, Felsoci M, Horakova K, Cihalik C, Malek F, Spinarova L, Belohlavek J, Kettner J, Zeman K, Dusek L, Jarkovsky J, investigators AM . Long‐term survival following acute heart failure: the Acute Heart Failure Database Main registry (AHEAD Main). Eur J Intern Med 2013; 24: 151–60. [DOI] [PubMed] [Google Scholar]
- 13. Aro AL, Anttonen O, Tikkanen JT, Junttila MJ, Kerola T, Rissanen HA, Reunanen A, Huikuri HV. Intraventricular conduction delay in a standard 12‐lead electrocardiogram as a predictor of mortality in the general population. Circulation, Arrhythmia and Electrophysiology 2011; 4: 704–10. [DOI] [PubMed] [Google Scholar]
- 14. Zhang ZM, Rautaharju PM, Prineas RJ, Loehr L, Rosamond W, Soliman EZ. Ventricular conduction defects and the risk of incident heart failure in the Atherosclerosis Risk in Communities (ARIC) Study. J Card Fail 2015; 21: 307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hong SJ, Oh J, Kang SM, Youn JC, Han S, Jeon ES, Cho MC, Kim JJ, Yoo BS, Chae SC, Oh BH, Choi DJ, Lee MM, Ryu KH, Kor HFR. Clinical implication of right bundle branch block in hospitalized patients with acute heart failure: data from the Korean Heart Failure (KorHF) Registry. Int J Cardiol 2012; 157: 416–8. [DOI] [PubMed] [Google Scholar]
- 16. Cinca J, Mendez A, Puig T, Ferrero A, Roig E, Vazquez R, Gonzalez‐Juanatey JR, Alonso‐Pulpon L, Delgado J, Brugada J, Pascual‐Figal D, investigators of the Spanish Heart Failure N . Differential clinical characteristics and prognosis of intraventricular conduction defects in patients with chronic heart failure. Eur J Heart Fail 2013; 15: 877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pellicori P, Joseph AC, Zhang J, Lukaschuk E, Sherwi N, Bourantas CV, Loh H, Clark AL, Cleland JG. The relationship of QRS morphology with cardiac structure and function in patients with heart failure. Clinical research in cardiology: official journal of the German Cardiac Society 2015. [DOI] [PubMed] [Google Scholar]
- 18. de Groote P, Millaire A, Foucher‐Hossein C, Nugue O, Marchandise X, Ducloux G, Lablanche JM. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol 1998; 32: 948–54. [DOI] [PubMed] [Google Scholar]
- 19. Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol 2001; 37: 183–8. [DOI] [PubMed] [Google Scholar]
- 20. Aronson D, Darawsha W, Atamna A, Kaplan M, Makhoul BF, Mutlak D, Lessick J, Carasso S, Reisner S, Agmon Y, Dragu R, Azzam ZS. Pulmonary hypertension, right ventricular function, and clinical outcome in acute decompensated heart failure. J Card Fail 2013; 19: 665–71. [DOI] [PubMed] [Google Scholar]
- 21. Lewinter C, Torp‐Pedersen C, Cleland JG, Kober L. Right and left bundle branch block as predictors of long‐term mortality following myocardial infarction. Eur J Heart Fail 2011; 13: 1349–54. [DOI] [PubMed] [Google Scholar]
- 22. Steimle AE, Stevenson LW, Chelimsky‐Fallick C, Fonarow GC, Hamilton MA, Moriguchi JD, Kartashov A, Tillisch JH. Sustained hemodynamic efficacy of therapy tailored to reduce filling pressures in survivors with advanced heart failure. Circulation 1997; 96: 1165–72. [DOI] [PubMed] [Google Scholar]
- 23. Nieminen MS, Bohm M, Cowie MR, Drexler H, Filippatos GS, Jondeau G, Hasin Y, Lopez‐Sendon J, Mebazaa A, Metra M, Rhodes A, Swedberg K, Priori SG, Garcia MA, Blanc JJ, Budaj A, Cowie MR, Dean V, Deckers J, Burgos EF, Lekakis J, Lindahl B, Mazzotta G, Morais J, Oto A, Smiseth OA, Garcia MA, Dickstein K, Albuquerque A, Conthe P, Crespo‐Leiro M, Ferrari R, Follath F, Gavazzi A, Janssens U, Komajda M, Morais J, Moreno R, Singer M, Singh S, Tendera M, Thygesen K, Guideline ESCCfP . Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J 2005; 26: 384–416. [DOI] [PubMed] [Google Scholar]
- 24. Haataja P, Nikus K, Kahonen M, Huhtala H, Nieminen T, Jula A, Reunanen A, Salomaa V, Sclarovsky S, Nieminen MS, Eskola M. Prevalence of ventricular conduction blocks in the resting electrocardiogram in a general population: the Health 2000 Survey. Int J Cardiol 2013; 167: 1953–60. [DOI] [PubMed] [Google Scholar]
- 25. Sumner G, Salehian O, Yi Q, Healey J, Mathew J, Al‐Merri K, Al‐Nemer K, Mann JF, Dagenais G, Lonn E, Investigators H. The prognostic significance of bundle branch block in high‐risk chronic stable vascular disease patients: a report from the HOPE trial. J Cardiovasc Electrophysiol 2009; 20: 781–7. [DOI] [PubMed] [Google Scholar]
- 26. Eriksson P, Hansson PO, Eriksson H, Dellborg M. Bundle‐branch block in a general male population: the study of men born 1913. Circulation 1998; 98: 2494–500. [DOI] [PubMed] [Google Scholar]
- 27. Brignole M, Auricchio A, Baron‐Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE, Guidelines ESCCfP , Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document R, Kirchhof P, Blomstrom‐Lundqvist C, Badano LP, Aliyev F, Bansch D, Baumgartner H, Bsata W, Buser P, Charron P, Daubert JC, Dobreanu D, Faerestrand S, Hasdai D, Hoes AW, Le Heuzey JY, Mavrakis H, McDonagh T, Merino JL, Nawar MM, Nielsen JC, Pieske B, Poposka L, Ruschitzka F, Tendera M, Van Gelder IC, Wilson CM. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 2013; 34: 2281–329. [DOI] [PubMed] [Google Scholar]
- 28. Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, American College of Cardiology F , American Heart Association Task Force on Practice G , Heart Rhythm S . 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device‐based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2013; 127: e283–352. [DOI] [PubMed] [Google Scholar]
- 29. Tabrizi F, Englund A, Rosenqvist M, Wallentin L, Stenestrand U. Influence of left bundle branch block on long‐term mortality in a population with heart failure. Eur Heart J 2007; 28: 2449–55. [DOI] [PubMed] [Google Scholar]
- 30. Stenestrand U, Tabrizi F, Lindback J, Englund A, Rosenqvist M, Wallentin L. Comorbidity and myocardial dysfunction are the main explanations for the higher 1‐year mortality in acute myocardial infarction with left bundle‐branch block. Circulation 2004; 110: 1896–902. [DOI] [PubMed] [Google Scholar]
- 31. Lassus J, Harjola VP, Sund R, Siirila‐Waris K, Melin J, Peuhkurinen K, Pulkki K, Nieminen MS, group F‐AS . Prognostic value of cystatin C in acute heart failure in relation to other markers of renal function and NT‐proBNP. Eur Heart J 2007; 28: 1841–7. [DOI] [PubMed] [Google Scholar]
- 32. Maisel AS, Peacock WF, McMullin N, Jessie R, Fonarow GC, Wynne J, Mills RM. Timing of immunoreactive B‐type natriuretic peptide levels and treatment delay in acute decompensated heart failure: an ADHERE (Acute Decompensated Heart Failure National Registry) analysis. J Am Coll Cardiol 2008; 52: 534–40. [DOI] [PubMed] [Google Scholar]
- 33. Mebazaa A, Yilmaz MB, Levy P, Ponikowski P, Peacock WF, Laribi S, Ristic AD, Lambrinou E, Masip J, Riley JP, McDonagh T, Mueller C, deFilippi C, Harjola VP, Thiele H, Piepoli MF, Metra M, Maggioni A, McMurray J, Dickstein K, Damman K, Seferovic PM, Ruschitzka F, Leite‐Moreira AF, Bellou A, Anker SD, Filippatos G. Recommendations on pre‐hospital & early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine. Eur J Heart Fail 2015; 17: 544–58. [DOI] [PubMed] [Google Scholar]
- 34. Iuliano S, Fisher SG, Karasik PE, Fletcher RD, Singh SN. Department of Veterans Affairs Survival Trial of Antiarrhythmic Therapy in Congestive Heart F. QRS duration and mortality in patients with congestive heart failure. Am Heart J 2002; 143: 1085–91. [DOI] [PubMed] [Google Scholar]
- 35. Shamim W, Francis DP, Yousufuddin M, Varney S, Pieopli MF, Anker SD, Coats AJ. Intraventricular conduction delay: a prognostic marker in chronic heart failure. Int J Cardiol 1999; 70: 171–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Adjusted Cox proportional hazard ratios (♦) with 95% confidence intervals (‐‐‐) for each type of conduction abnormality in all patients (solid lines) and in the subgroups of de novo AHF and ADCHF (dashed lines) in the validation cohort.
Figure S2. Kaplan–Meier survival curves of patients with de novo AHF (left) and ADCHF (right) in the validation cohort with and without RBBB (above) and IVCD (below). Cases censored during follow‐up are depicted with crosses.
Supporting info item
Supporting info item
Supporting info item


