Abstract
Clinical hepatocyte transplantation is hampered by low engraftment rates and gradual loss of function resulting in incomplete correction of the underlying disease. Preconditioning with partial hepatectomy improves engraftment in animal studies. Our aim was to study safety and efficacy of partial hepatectomy preconditioning in clinical hepatocyte transplantation. Two patients with Crigler‐Najjar syndrome type I underwent liver resection followed by hepatocyte transplantation. A transient increase of hepatocyte growth factor was seen, suggesting that this procedure provides a regenerative stimulus. Serum bilirubin was decreased by 50%, and presence of bilirubin glucuronides in bile confirmed graft function in both cases; however, graft function was lost due to discontinuation of immunosuppressive therapy in one patient. In the other patient, serum bilirubin gradually increased to pretransplant concentrations after ≈600 days. In both cases, loss of graft function was temporally associated with emergence of human leukocyte antigen donor‐specific antibodies (DSAs). In conclusion, partial hepatectomy in combination with hepatocyte transplantation was safe and induced a robust release of hepatocyte growth factor, but its efficacy on hepatocyte engraftment needs to be evaluated with additional studies. To our knowledge, this study provides the first description of de novo DSAs after hepatocyte transplantation associated with graft loss.
Keywords: translational research/science, cellular transplantation (non‐islet), liver transplantation/hepatology, regenerative medicine, liver (native) function/dysfunction, liver disease: congenital, immune regulation
Short abstract
The authors report two cases of hepatocyte transplantation with partial hepatectomy preconditioning in patients with Crigler‐Najjar syndrome type I, in which after initial successful allograft function, donor hepatocytes are lost in association with emergence of donor‐specific human leukocyte antigen antibodies.
Abbreviations
- +
positive
- μkat
microkatals
- ALT
alanine aminotransferase
- ANA
antinuclear antibodies
- AST
aspartate aminotransferase
- ATP
adenosine triphosphate
- BDG
bilirubin diglucuronide
- BMG
bilirubin monoglucuronide
- bp
base pair
- CDC
complement‐dependent cytotoxicity
- CN‐I
Crigler‐Najjar syndrome type I
- CRP
C‐reactive protein
- D/C
immunosuppression discontinued
- del
deletion
- DSA
donor‐specific antibody
- EGF
epidermal growth factor
- ELISA
enzyme‐linked immunosorbent assay
- FACS
fluorescence‐activated cell sorting
- Hep‐Tx
hepatocyte transplantation
- HGF
hepatocyte growth factor
- HLA
human leukocyte antigen
- LCU
luminescent counting units
- LTX
liver transplantation
- MFI
mean fluorescence intensity
- NA
not available
- ND
below threshold limit of 1000 MFI
- PRA
panel reactive antibodies
- TNF‐α
tumor necrosis factor α
- Tx
transplant
- UCB
unconjugated bilirubin
- UGT1A1
uridine diphosphoglucuronate glucuronosyltransferase 1A1
Introduction
Crigler‐Najjar syndrome type I (CN‐I) is a rare autosomal recessive metabolic liver disease caused by complete deficiency of uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1). Patients are at risk of developing severe and irreversible brain injury due to neurotoxicity of unconjugated hyperbilirubinemia. Therapy consists of daily phototherapy for 8–14 h, converting hydrophobic unconjugated bilirubin into water‐soluble isomers 1; however, phototherapy becomes less effective with increasing age and constitutes a substantial impairment of quality of life. Finally, most patients undergo liver transplantation, reducing the risk of brain injury.
Hepatocyte transplantation is a novel treatment for CN‐I because replacement of a fraction of UGT1A1 decreases serum bilirubin. To date, 10 patients with CN‐I have received hepatocyte transplantations 2. In most patients, serum bilirubin decreased. Nevertheless, all patients eventually underwent liver transplantation because of graft loss or insufficient improvement of quality of life.
Insufficient engraftment and long‐term function of donor hepatocytes are the major limitations in clinical hepatocyte transplantation. Although hepatocytes have an enormous capacity to proliferate, they do not express this capacity unless a mitotic stimulus is present. Liver resection is the strongest proliferation stimulus, and it improves hepatocyte engraftment in animal studies 3.
The aim of this study was to evaluate safety and outcome of partial hepatectomy preconditioning in clinical hepatocyte transplantation. In addition, growth factors and cytokines controlling liver regeneration were studied. Finally, recipients were evaluated for human leukocyte antigen (HLA) antibodies, which are associated with graft failure in islet, kidney and heart transplantation 4, 5. In hepatocyte transplantation, testing for HLA antibodies is rarely reported, and their role has not been established.
Methods
Patients
CN‐I was confirmed by lack of bilirubin conjugates in bile and UGT1A1 mutation analysis. Patient 1, a 13‐year‐old boy, was found to be homozygous for 1124C > T mutation resulting in an amino acid change at codon 375 (S375F). Patient 2, an 11‐year‐old girl, was found to be a compound heterozygote for 1–2_14 deletions (del) of 16 base pairs (bp) resulting in a premature stop codon and 608_631 del 24 bp resulting in the loss of eight amino acids. Both received 7–9 h of phototherapy and were unresponsive to phenobarbital. No signs of encephalopathy were noted, and electroencephalography was normal. Patient 1 was 158 cm tall (45th percentile) and weighed 69 kg (>95th percentile) with a BMI of 27.6 (>95th percentile), and patient 2 was 151 cm tall (70th percentile) and weighed 40 kg (50th percentile) with a BMI of 17.3 (40th percentile). Serologic tests for hepatitis B and C and human immunodeficiency virus were negative for both patients. Liver chemistry was normal except that both patients showed slightly elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in serum (ALT: 0.6–2 microkatals (μkat) per liter [reference <1.2 μkat/l]; AST: 0.7–1.1 μkat/l [reference <0.7 μkat/l]). Hepatic ultrasound in patient 1 showed irregular echogenicity. Pretransplant liver biopsy showed fibrosis stage 2 (Batts and Ludwig classification) and no signs of inflammation or steatosis. In patient 2, abdominal ultrasound and pretransplant liver histology were normal.
Approval by the regional ethics committee (2010/840‐31) and informed consent from the patients and parents were obtained.
Hepatocyte isolation
Hepatocytes were isolated from deceased donor livers by collagenase perfusion using CIzyme (VitaCyte LLC, Indianapolis, IN) (Table 1). Cell number, hepatocyte yield, cytochrome P450, caspase activity and adenosine triphosphate (ATP) content were analyzed, as described previously 6. Immunocytochemistry of cell smears was performed on a Leica Bond‐III immunostainer using the following antibodies: CD45, CD31 and CK18 (Novocastra) and CD68 (Dako).
Table 1.
Hepatocyte donors
| Characteristics | Donor 1 | Donor 2 | Donor 3 | Historical controls |
|---|---|---|---|---|
| Age | 29 years | 40 years | 4 mo | 6 mo to 85 years |
| Sex | F | M | F | M (99)/F (110) |
| Blood group | 0 | A | 0 | |
| Cold ischemia time (h) | 11 | 15–35 | 5 | |
| Hepatocyte suspension time (h) | 5–7 | 3–5 | 1.5–17 | |
| Description | Segments 1–3 | Whole liver | Whole liver | |
| Yield (106/g) | 9 | 20 | 65 | 7.4 ± 0.5 |
| Viability (%) | 94 | 87–100 | 97 | 76.8 ± 1.0 |
| ATP (LCU/min/ng) | 110.37 | 47.84 | 202.49 | 44.68 ± 4.71 |
| CYP1A2 (LCU/min/ng) | 19.55 | 2.92 | 3.62 | 39.79 ± 34.00 |
| CYP2C9 (LCU/min/ng) | 0.41 | 0.03 | 0.07 | 0.23 ± 0.03 |
| CYP3A7 (LCU/min/ng) | 0.16 | 0.02 | 0.54 | 0.75 ± 0.13 |
| CYP3A4 (LCU/min/ng) | 5.01 | 0.76 | 0.30 | 2.86 ± 0.50 |
| Caspase 3/7 (LCU/min/ng) | 9.89 | 1.46 | 5.65 | 13.73 ± 5.40 |
Donors 1 and 3 were used for patient 1, and donor 2 was used for patient 2. Organs were flushed in situ with University of Wisconsin solution. Liver function tests of the donors were normal, and serologic tests were negative for hepatitis B and C and human immunodeficiency virus. All donors tested negative for a mutation usually found in Gilbert́s syndrome in the promoter region of the UGT1A1 gene. Cold ischemia time indicates time from cold perfusion in the donor until start of hepatocyte isolation. Hepatocyte suspension time indicates time from hepatocyte isolation to hepatocyte infusion. To minimize hepatocyte suspension time, donor liver 2 was separated into three pieces to allow for repeated isolation. Yield is the number of viable hepatocytes per gram of processed tissue. All values are expressed as LCU/min per content in double‐strand DNA. ATP, adenosine triphosphate; LCU, luminescent counting units.
Partial hepatectomy and hepatocyte transplantation
A catheter was advanced to the main portal vein under fluoroscopy guidance accessing the umbilical vein or the mesenteric vein. Liver resection of segment 2/3 was performed before the first transplantation by cavitron ultrasonic surgical aspirator.
Hepatocytes were infused by a pump with continuous monitoring of portal pressure. Doppler ultrasound of the liver was performed regularly. Immunosuppression consisted of induction with basiliximab and 500 mg methylprednisolone followed by taper to 5 mg prednisolone daily. Tacrolimus was given with trough concentrations of 10–13 ng/ml for the first month and 6–8 ng/ml thereafter. Patient 1 received mycophenolate mofetil 1 g twice daily during the first 6 days.
Immunological investigation
Complement‐dependent cytotoxicity (CDC) and flow cytometry crossmatch (fluorescence‐activated cell sorting [FACS]) were performed, as described previously 7. Luminex‐based LABScreen‐PRA and Single Antigen assay (One Lambda) were used to test for anti‐HLA antibodies before and every 3–4 mo after transplant. Complement binding was evaluated by C1q assay with single‐antigen beads. Reactivity was normalized for background and expressed as mean fluorescence intensity (MFI). MFI values >1000 were considered positive.
Autoantibodies and UGT1A1 antibodies
Antinuclear antibodies (ANA) were analyzed by indirect immunofluorescence (Immuno Concepts) and multiplex ANA assay (BioPlex 2200; Bio‐Rad). Antimitochondrial and liver‐specific autoantibodies were analyzed by line immunoassay (Euroimmun). Anti–smooth muscle antibodies were evaluated by indirect immunofluorescence assay (Kallestad). Antibodies against UGT1A1 were evaluated by enzyme‐linked immunosorbent assay (ELISA) 8.
Bilirubin conjugates
Bilirubin conjugates in bile were analyzed, as described previously 8.
Growth factors and cytokines
Human hepatocyte growth factor (HGF) was quantified by ELISA (R&D Systems). Serum epidermal growth factor (EGF), tumor necrosis factor α (TNF‐α) and IL‐6 were analyzed by the Luminex human cytokine kit (Merck).
Liver tissue engraftment
Male donor cells were detected by polymerase chain reaction for the sex‐determining region Y (SRY) gene (Quantifiler DNA Kit; Applied Biosystems).
Results
Hepatocyte viability and function
Cold ischemia time ranged from 5 to 35 h. Viability was 87–100%, and hepatocyte yield was 9–65 × 106 per gram. ATP content, cytochrome P450 and caspase 3/7 activities were adequate compared with historical controls (Table 1). The majority of cells were CK18 positive with low‐level contamination by nonparenchymal cells (Figure S1).
Intraportal infusion
Portal pressure was unchanged after liver resection but increased after each hepatocyte infusion (Figure 1) and normalized within 24 h after infusion. Repeated Doppler ultrasound showed normal portal vein flow without thrombosis. Heart rate, oxygen saturation, central venous pressure and pulmonary artery pressure did not change during the procedure.
Figure 1.
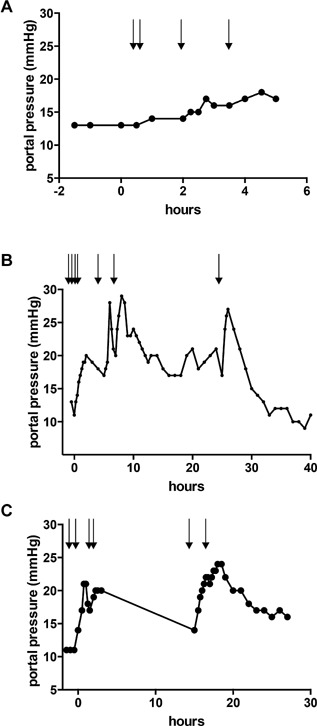
Portal pressure during hepatocyte infusion. Portal pressure was monitored continuously before and after hepatocyte infusion. (A) Portal pressure during first hepatocyte transplantation in patient 1. (B) Portal pressure during second hepatocyte transplantation in patient 1. (C) Portal pressure during hepatocyte transplantation in patient 2. Arrows indicate infusion of one hepatocyte batch of 0.7–1.2 × 109 cells each.
Clinical course
Patient 1 received two transplantations from different donors 3 mo apart, with 2.2 and 9 × 109 hepatocytes, respectively (Table 1). Total bilirubin ranged before transplant from 416 to 450 µmol/l and increased after surgery to peak on day 7 at 490 µmol/l, followed by a decline to 320–350 µmol/l (Figure 2). A decrease to 220–240 µmol/l was observed after the second transplant. After each transplantation, serum conjugated bilirubin and ALT transiently increased. On day 212, a sudden increase of total bilirubin associated with diagnosis of crusted scabies was noted. Despite lowering of the tacrolimus dose and anthelmintic treatment, scabies recurred. The interpretation was that the hepatocyte graft was lost. Because of recurring scabies and risk of encephalopathy, immunosuppression was discontinued, and the patient listed for liver transplantation. Twelve days after discontinuation of immunosuppression, the patient was readmitted for intensified phototherapy due to increased serum bilirubin. During this stay, the patient developed fever, abdominal pain and elevated C‐reactive protein, ALT, pancreatic amylase (5.8 μkat/l) and leukocytes (18.1 × 109/l) (Figure 2). Total bilirubin decreased to 300 μmol/l, and conjugated bilirubin temporarily increased to 51 μmol/l. Hepatic ultrasound was normal, without signs of thrombosis, cholestasis or gallstones. The patient was treated with intravenous antibiotics and recovered without sequelae within 7 days. On day 580, the patient underwent liver transplantation with an ABO‐identical organ from a deceased donor. Serum bilirubin normalized within 2 days, and postoperative course was uncomplicated. A mild rejection at 4 months was reversed by increasing the tacrolimus dose. At 1 year after transplantation, the patient was in good condition with excellent graft function.
Figure 2.
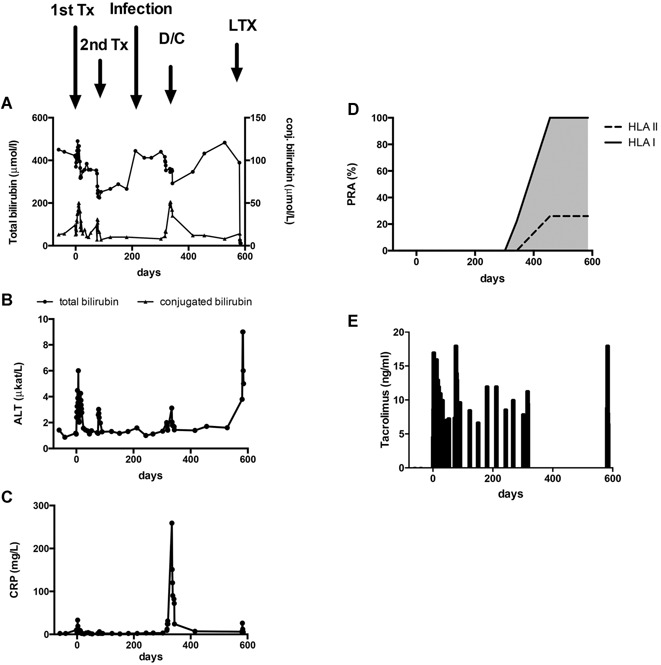
Follow‐up of patient 1. Biochemical follow‐up before and after hepatocyte transplantation. (A) Total serum bilirubin (●) and conjugated serum bilirubin (▴). (B) ALT. (C) CRP. Arrows indicate time points of first and second hepatocyte transplantation, scabies manifestation, presumed rejection, and liver transplantation. (D) Development of de novo PRA to class I and II HLA antigens. HLA class I antibodies to A1, A2, A3, A11, A25, A26, A29, A30, A31, A 32, A33, A34, A36, A43, A66, A68, A69, A74, A80, B8, B44, B45, B59, B76, and B82 and class II antibodies to DR4, DR12, DR53, DQ2, DQ4, DQ8, DQ9, and HLACw7 could be detected. HLA A1, A2, A11, B8, B44, DR4, DR12, and DQ2 were donor‐specific antibodies. (E) Tacrolimus whole‐blood concentrations. ALT, alanine aminotransferase; CRP, C‐reactive protein; D/C, immunosuppression discontinued; LTX, liver transplantation; PRA, panel reactive antibodies; Tx, transplant.
Patient 2 received 5.3 × 109 viable hepatocytes in six infusions in a single transplantation event over 17 h. Serum bilirubin before transplantation ranged from 400 to 450 µmol/l and increased after transplantation to 540 µmol/l on postoperative day 17, followed by a continuous decline to 190 µmol/l, and remained at 50% of pretransplant concentrations for >6 mo (Figure 3). ALT increased temporally after transplantation to 7.4 μkat/l and remained elevated between 1.3 and 2.8 μkat/l thereafter. After 6 mo, serum bilirubin increased progressively over a period of 500 days to pretransplant values. Due to increasing risk of brain injury, patient 2 was listed for liver transplantation. On day 951, the patient received an ABO‐identical organ from a deceased donor. Serum bilirubin normalized within 2 days, and the postoperative course was uncomplicated. At 6 weeks after transplantation, the patient's condition and graft function were excellent.
Figure 3.
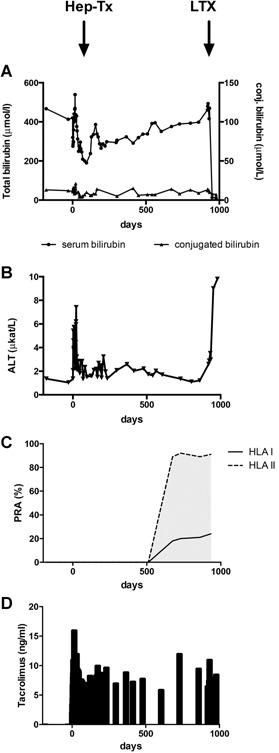
Follow‐up of patient 2. Biochemical follow‐up before and after hepatocyte transplantation. (A) Total serum bilirubin (●) and conjugated serum bilirubin (▴). (B) ALT. (C) Development of de novo PRA to class I and II HLA antigens. Antibodies to HLA B8, B37, B49, B50, DR4, DR7, DR9, DR12, DR52, DR53, and DQ2 were present. HLA antibodies to B8, B50, DR4, DQ2, and DR7 were donor specific. (D) Tacrolimus whole‐blood concentrations. ALT, alanine aminotransferase; Hep‐Tx, hepatocyte transplantation; LTX, liver transplantation; PRA, panel reactive antibodies.
Safety
No procedure‐related complications were noted. Liver resection was performed without transfusion of blood products. Two major complications were noted in patient 1. One was mycophenolate intoxication on day 7, with blood concentration of 8.7 times the upper limit associated with diarrhea and abdominal pain. The other complication was the scabies infection. No major adverse events were noted in patient 2.
Bilirubin conjugates
Pretransplant bile did not contain bilirubin diglucuronides and contained only small amounts of bilirubin monoglucuronide, whereas posttransplant samples showed 4% (patient 2) and 57% (patient 1) bilirubin diglucuronides (Figure 4).
Figure 4.
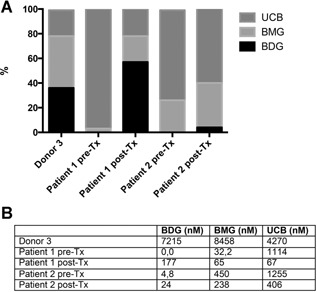
Analysis of bile glucuronides. Bile collected from liver donor 3 and from hepatocyte recipients before and 2 mo (patient 2) and 4 mo (patient 1) after transplantation of hepatocytes was analyzed by high‐performance liquid chromatography for bilirubin conjugates. Bile was collected before transplantation and from donor liver by direct puncture of the gallbladder during surgery. Posttransplant bile was collected through a nasoduodenal tube. (A) Percentage of UCB, BMG, and BDG of total bilirubin. (B) Absolute concentrations of BDG, BMG, and UCB. BDG, bilirubin diglucuronide; BMG, bilirubin monoglucuronide; Tx, transplant; UCB, unconjugated bilirubin.
Growth factors and cytokines
HGF and EGF were released in patient 1, whereas in patient 2, only HGF was released after partial hepatectomy and hepatocyte transplantation. Interestingly, HGF and EGF concentrations increased even after the second infusion in patient 1, without partial hepatectomy. In contrast, IL‐6 and TNF‐α decreased after transplantation, and concentrations remained low (Figure 5).
Figure 5.
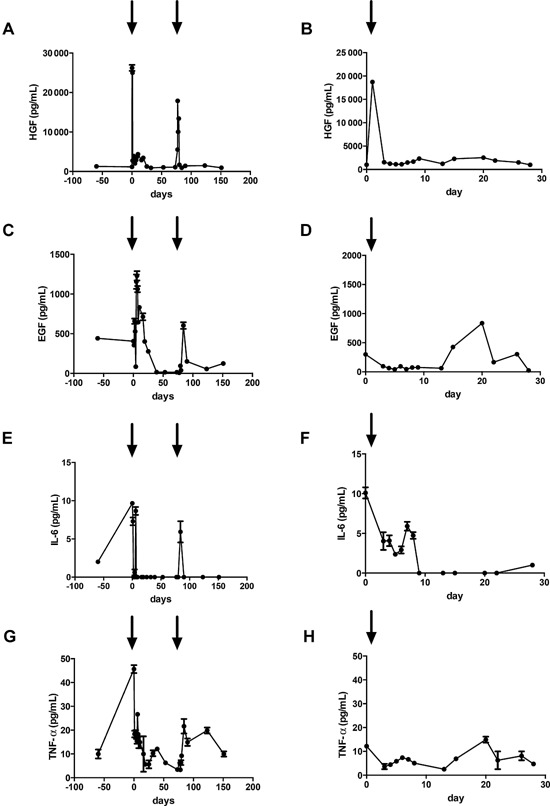
Growth factors and cytokines. Analysis of serum growth factors and cytokines before and after hepatocyte transplantation in patient 1 (A, C, E, G) and in patient 2 (B, D, F, H). Arrows indicate time points of hepatocyte transplantation. EGF, epidermal growth factor; HGF, hepatocyte growth factor; TNF‐α, tumor necrosis factor α.
Assessment of antibody formation
Donor and recipient HLA types are summarized in Table 2. Pretransplant CDC and FACS crossmatch were negative for all three cell infusions. Both patients tested negative for HLA antibodies before transplantation. Neither patient received blood transfusions during the study period.
Table 2.
HLA type of donors and recipients
| HLA type | ||||||
|---|---|---|---|---|---|---|
| A | B | C | BW | DRB1 | DQB1 | |
| Patient 1 | 24 | 18, 35:08 | 04, 12 | 04, 06 | 11 | 03:01 |
| Donor 1 | 02 | 15, 27 | 02, 03 | 04, 06 | 04, 08 | 03:02 |
| Donor 3 | 01, 11 | 08, 44 | 05, 07 | 04, 06 | 04, 12 | 03(7) |
| Patient 2 | 02, 66:01 | 14, 53 | 04, 08 | 04, 06 | 10, 11 | 03:01, 05:01 |
| Donor 2 | 02, 66 | 08, 50 | 06, 07 | 06 | 04, 07 | 02, 08 |
Patient 1 developed HLA antibodies 27 days after discontinuation of immunosuppression, corresponding to 1 week after admission due to fever. Panel reactive antibodies (PRA) increased to 100% for class I and 26% for class II, and a subset of these were donor‐specific antibodies directed against both donors (Table 3; Figure 2). Analysis of complement binding showed C1q binding to HLA Cw7. Repeated FACS crossmatch with serum from days 456 and 580 and stored lymphocytes from donors 1 and 3, respectively, were positive for both T and B cells.
Table 3.
HLA antibody analysis of patient 1
| C1q | Day342 | Day 456 | Day 580 | Day 780 | |
|---|---|---|---|---|---|
| B76 | 8891 | 5676 | 8284 | 2846 | |
| B45 | 8726 | 6285 | 9109 | 2793 | |
| B44* | 5761 | 5841 | 8768 | 2610 | |
| B8* | 5322 | 5482 | 8202 | 2636 | |
| A1* | 3371 | 5919 | 9084 | 5609 | |
| B82 | 2597 | 3344 | 5424 | 2314 | |
| A11* | 2474 | 5380 | 7831 | 6095 | |
| A36 | 2306 | 3495 | 5253 | 2301 | |
| A80 | 1901 | 2595 | 4095 | ND | |
| A2 | 1385 | 1955 | 2791 | 2377 | |
| A43 | 1383 | 2632 | 4919 | ND | |
| A66 | ND | 3149 | 5693 | 5296 | |
| A34 | ND | 3125 | 4977 | 4718 | |
| A3 | ND | 3086 | 4997 | ND | |
| A74 | ND | 3058 | 4958 | 3765 | |
| A31 | ND | 2977 | 4899 | 2849 | |
| A29 | ND | 2917 | 4501 | ND | |
| A68 | ND | 2869 | 4435 | 5646 | |
| A26 | ND | 2821 | 5730 | ND | |
| A33 | ND | 2634 | 4470 | 3360 | |
| A30 | ND | 2561 | 4057 | 4665 | |
| A69 | ND | 2352 | 4564 | 6202 | |
| A25 | ND | 2224 | 4237 | ND | |
| Cw7 | + | ND | 1640 | 2418 | ND |
| B59 | ND | ND | 1359 | ND | |
| A32 | ND | ND | 1319 | ND | |
| DQ4 | NA | 2637 | 4028 | ND | |
| DQ9 | NA | 2011 | 3006 | ND | |
| DQ8 | NA | 1929 | 2949 | ND | |
| DQ2* | NA | 1260 | 1916 | ND | |
| DQ7 | NA | 1136 | 1753 | ND | |
| DR4* | NA | 1050 | 1949 | ND | |
| DR12* | NA | ND | 1626 | ND | |
| DR53 | NA | ND | 1337 | ND |
*Donor‐specific antibody.
MFI values and C1q positivity for patient 1 on days 342, 456, 580, and 780. Day 780 is 220 days after liver transplantation. C1q reactivity was normalized to background and MFI values >1000 were considered positive. +, positive; MFI, mean fluorescence intensity; NA, not available; ND, below threshold limit of 1000 MFI.
In patient 2, de novo HLA antibodies were detected 670 days after hepatocyte transplantation on maintenance immunosuppression. PRA increased to 18% for class I and 89% for class II, and a subset of these were donor specific and C1q positive (Figure 3; Table 4). Repeated FACS crossmatch with serum taken at days 674 and 740 and stored donor lymphocytes were positive for both T and B cells.
Table 4.
HLA antibody analysis of patient 2
| C1q | Day 674 | Day 740 | Day 814 | Day 1062 | |
|---|---|---|---|---|---|
| B37 | + | 4475 | 4075 | 2809 | ND |
| B8* | 4129 | 3448 | 2523 | 3402 | |
| B50* | 1491 | 1307 | ND | 8352 | |
| B49 | 1194 | 1005 | ND | 5774 | |
| DR7* | 8712 | 6607 | 5819 | 11119 | |
| DR53 | + | 7108 | 5497 | 5165 | 10471 |
| DP3 | + | 6434 | 4661 | 4661 | 9725 |
| DR4 * | + | 5988 | 9055 | 8942 | 11835 |
| DP9 | + | 5764 | 4528 | 4463 | 7306 |
| DP20 | + | 5729 | 3930 | 4248 | 6896 |
| DP6 | + | 5587 | 4455 | 4386 | 9566 |
| DQ2 * | + | 5261 | 3793 | 2917 | 3524 |
| DP14 | 4975 | 3597 | 4001 | 8258 | |
| DR9 | 4401 | 2960 | 2949 | 4829 | |
| DR12 | + | 4061 | 2046 | 2631 | 1070 |
| DR52 | + | 3783 | 2560 | 2505 | ND |
| DP17 | + | 3674 | 2756 | 2986 | 10029 |
| DP5 | 2121 | 1565 | 1523 | 9277 | |
| DP11 | + | 1993 | 1437 | 1348 | 9099 |
| DP1 | 1956 | 1443 | 1390 | 9372 | |
| DP19 | + | 1589 | 1205 | 1139 | 10847 |
Donor‐specific antibody.
MFI values and C1q positivity for patient 2 on days 674, 740, 814, and 1062 after hepatocyte transplantation. Day 1062 is 111 days after liver transplantation. C1q reactivity was normalized to background, and MFI values >1000 were considered positive. +, positive; MFI, mean fluorescence intensity; NA, not available; ND, below threshold limit of 1000 MFI.
Analysis of serum from both patients at time points with high levels of HLA antibodies did not show the presence of anti‐UGT1A1 antibodies or ANA, antimitochondrial, liver‐specific, or anti‐smooth muscle autoantibodies.
Analysis of liver tissue engraftment
Histology of explanted liver revealed no inflammation, steatosis or signs of portal microthrombi; however, fibrosis grade was increased in both patients, in patient 1 from grade 2 to 2–3 and in patient 2 from grade 1 to 2, compared with pretransplant biopsies (Figure S2). In patient 2, donor cell engraftment was analyzed by SRY analysis. Male cells could be detected only at low levels in the single‐protocol biopsy taken 2 mo after hepatocyte transplantation, whereas samples before hepatocyte transplantation and 52 liver biopsies covering the entire liver explant did not show amplification (data not shown).
Discussion
A major limitation of clinical hepatocyte transplantation is the limited repopulation of the liver with donor hepatocytes. In animal models, 50–90% of the liver can be replaced using surgical, genetic or chemical preconditioning 2. A consensus meeting in 2011 suggested liver resection preconditioning to increase repopulation with donor hepatocytes 9. At experienced hepatobiliary centers, perioperative safety and experience have increased, and liver resection has been used previously in an ex vivo gene therapy trial for familial hypercholesterolemia 10.
This study evaluated safety and feasibility of partial hepatectomy preconditioning and hepatocyte transplantation in CN‐I. Both patients tolerated liver surgery well, without procedure‐related complications. A concern was related to the risk of increased portal pressure after resection potentiating the risk of portal thrombosis 11. In this study, portal pressure did not increase after liver resection but strongly increased after hepatocyte infusion; however, the increase in portal pressure observed was comparable to previous reports without liver resection 12. Another concern was related to the risk of surgical complications in consecutive liver transplantation due to distorted anatomy 13. In both patients, adhesions close to the resection site were found but did not result in any complications, prolonged operation time or bleeding.
The mitogenic stimulus of liver resection was evaluated. At 2 h after liver resection, serum HGF increased 20‐fold and remained elevated for up to 25 days. A similar pattern could be seen for EGF; however, TNF‐α and IL‐6 decreased after transplantation. A possible explanation of this decline might be the immunosuppressive medication known to inhibit TNF‐α and IL‐6 expression 14. Interestingly, serum HGF also increased after the second hepatocyte transplantation in patient 1. In contrast to the first transplantation, serum HGF normalized within 2 days after hepatocyte infusion. We hypothesize that the increase in HGF by hepatocyte transplantation was caused by portal microembolization with donor hepatocytes, similar to preoperative portal vein embolization in liver surgery 15. This also coincided with the increase in portal pressure, which led us to speculate that increased portal pressure during the infusion might not be simply a side effect but actually could be beneficial for engraftment.
To date, 10 cases of human hepatocyte transplantation for patients with CN‐I have been reported 2. Patient ages ranged from 1 to 10 years, and weight ranged from 7.4 to 38 kg. The cell dose administered in these studies was 160–680 × 106 per kilogram of body weight, corresponding to a theoretical liver mass of 4–17% 2. In comparison to these studies, the patients reported in our study were older and heavier. The cell dose administered was significantly lower, with 133 and 166 × 106 hepatocytes per kilogram of body weight corresponding to liver masses of 3.3% and 4.2%, respectively. Notwithstanding this low dose, both patients showed a strong decrease of serum bilirubin. The most sensitive measurement of hepatic bilirubin glucuronidation activity is the presence of bilirubin glucuronide in bile. Only one study investigated bile for bilirubin conjugates 16. Similar to Fox et al, we detected bilirubin mono‐ and diglucuronides after transplantation.
Two major complications unrelated to the surgical procedure occurred in patient 1. First, he presented on day 7 with mycophenolate intoxication. Mycophenolate is primarily metabolized by glucuronidation by UGT1A9 and UGT2B7 but not UGT1A1 17; however, accumulation of mycophenolate is explained by the mutation involving exon 4 of the UGT1A1 gene and thus affecting all UGT1A isoforms 18. Second, 6 mo after transplantation, patient 1 presented with crusted scabies (Scabies norvegica) infection, a particularly severe form found in immune‐compromised patients.
The best immunosuppressive protocol for clinical hepatocyte transplantation is unknown, and most centers have adopted the immunosuppressive protocol used in liver transplantation. Hepatocytes have tolerogenic properties, and initially it was believed that immunosuppressive therapy might not be necessary 19; however, Allen et al reported a cellular alloresponse directed against donor class I HLA associated with hepatocyte graft loss 19. No DSAs were detected in this patient. Bumgardner et al showed that hepatocyte rejection was not associated with DSAs in mice 20. We report the emergence of DSAs directed against both class I and II and associated with graft loss. Interestingly, in patient 1, DSAs directed against both hepatocyte donors could be detected. In this patient, antibody formation was induced by discontinuation of immunosuppression, whereas in patient 2, DSAs developed during adequate immunosuppressive therapy. Our results suggest involvement of humoral immunity in rejection of allogenic hepatocytes. A limitation of our study is that we had no histopathologic evidence of humoral rejection, and cellular immunity was not investigated. Nevertheless, our results show a high level of DSAs long term after hepatocyte transplantation associated with graft loss, emphasizing the need for studies of HLA antibodies in hepatocyte transplantation. Preformed and de novo DSAs are well‐known risk factors for decreased graft survival after kidney, heart and islet transplantation 4, 5. Until recently, DSAs were considered irrelevant in liver transplantation; however, current data suggest that DSAs may cause allograft injury and affect overall outcome 4. Emergence of DSAs after hepatocyte transplantation is concerning. Just as in islet transplantation, multiple donor infusions are often required, potentiating the risk of broad sensitization. This may have a negative impact if subsequent hepatocyte, liver or kidney transplantation should be necessary.
To our knowledge, this study is the first report of de novo DSAs after clinical hepatocyte transplantation. Graft function loss was temporally associated with antibody formation. Partial hepatectomy alone or with hepatocyte infusion induced a robust release of HGF into the circulation, and a relatively low cell dose provided significant hepatocyte function. The influence of cell number, infusion flow rates and portal pressure on HGF release and the efficacy of partial hepatectomy preconditioning are worthy of additional investigation.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information
Figure S1: Immunocytochemistry. Cell smears from three representative hepatocyte isolations were stained for (A) hepatocyte marker CK18, (B) macrophage/Kupffer cell marker CD68, (C) hematopoetic marker CD45 and (D) endothelial marker CD31. Negative control (E) was treated without primary antibody. (F) Percentage of positively stained cells.
Figure S2: Liver pathology. Liver tissue obtained before transplant during liver resection and at time of liver transplantation for patient 1 (A–F) and for patient 2 (G–L), stained with hematoxyolin eosin (A, B, G, H), Sirius red (C, D, I, J) and CK19 (E, F, K, L).
Acknowledgments
This study was supported by grants from the Research Strategy Committee Karolinska Institute and the Stockholm County Council (Forskar ST) and the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institute and the Swedish Research Council (VR) “Treatments of the future” and the Torsten och Ragnar Södeberg Stiftelse. We thank Pontus Blomgren and the staff at VECURA for continuous support and access to the good manufacturing practice facility. We thank the members of the Nordic Liver Transplantion Group for providing deceased donor liver tissue for hepatocyte isolation. We thank Gunilla Fahlström for performing immunocytochemistry staining.
Jorns C, Nowak G, Nemeth A, Zemack H, Mörk L‐M, Johansson H, Gramignoli R, Watanabe M, Karadagi A, Alheim M, Hauzenberger D, van Dijk R, Bosma PJ, Ebbesen F, Szakos A, Fischler B, Strom S, Ellis E & Ericzon B‐G. De Novo Donor‐Specific HLA Antibody Formation in Two Patients With Crigler‐Najjar Syndrome Type I Following Human Hepatocyte Transplantation With Partial Hepatectomy Preconditioning. Am J Transplant 2016; 16: 1021–1030.
[The copyright line for this article was changed on 22 September, 2016 after original online publication.]
References
- 1. Strauss KA, Robinson DL, Vreman HJ, Puffenberger EG, Hart G, Morton DH. Management of hyperbilirubinemia and prevention of kernicterus in 20 patients with Crigler‐Najjar disease. Eur J Pediatr 2006; 165: 306–319. [DOI] [PubMed] [Google Scholar]
- 2. Jorns C, Ellis EC, Nowak G, et al. Hepatocyte transplantation for inherited metabolic diseases of the liver. J Intern Med 2012; 272: 201–223. [DOI] [PubMed] [Google Scholar]
- 3. Gupta S, Johnstone R, Darby H, Selden C, Price Y, Hodgson HJ. Transplanted isolated hepatocytes: Effect of partial hepatectomy on proliferation of long‐term syngeneic implants in rat spleen. Pathology 1987; 19: 28–30. [DOI] [PubMed] [Google Scholar]
- 4. Taner T, Stegall MD, Heimbach JK. Antibody‐mediated rejection in liver transplantation: Current controversies and future directions. Liver Transpl 2014; 20: 514–527. [DOI] [PubMed] [Google Scholar]
- 5. Campbell PM, Senior PA, Salam A, et al. High risk of sensitization after failed islet transplantation. Am J Transplant 2007; 7: 2311–2317. [DOI] [PubMed] [Google Scholar]
- 6. Gramignoli R, Tahan V, Dorko K, et al. Rapid and sensitive assessment of human hepatocyte functions. Cell Transplant 2014; 23: 1545–1556. [DOI] [PubMed] [Google Scholar]
- 7. Alheim M, AlMahri A, Nilsson J, Tyden G, Holgersson J. The outcome of the endothelial precursor cell crossmatch test in lymphocyte crossmatch positive and negative patients evaluated for living donor kidney transplantation. Hum Immunol 2013; 74: 1437–1444. [DOI] [PubMed] [Google Scholar]
- 8. Seppen J, Bakker C, de Jong B, et al. Adeno‐associated virus vector serotypes mediate sustained correction of bilirubin UDP glucuronosyltransferase deficiency in rats. Mol Ther 2006; 13: 1085–1092. [DOI] [PubMed] [Google Scholar]
- 9. Puppi J, Strom SC, Hughes RD, et al. Improving the techniques for human hepatocyte transplantation: Report from a consensus meeting in London. Cell Transplant 2012; 21: 1–10. [DOI] [PubMed] [Google Scholar]
- 10. Grossman M. A pilot study of ex vivo gene therapy for homozygous familial hypercholesterolaemia. Nat Med 1995; 1: 1148–1154. [DOI] [PubMed] [Google Scholar]
- 11. Baccarani U, Adani GL, Sanna A, et al. Portal vein thrombosis after intraportal hepatocytes transplantation in a liver transplant recipient. Transpl Int 2005; 18: 750–754. [DOI] [PubMed] [Google Scholar]
- 12. Meyburg J, Hoerster F, Schmidt J, Poeschl J, Hoffmann GF, Schenk JP. Monitoring of intraportal liver cell application in children. Cell Transplant 2010; 19: 629–638. [DOI] [PubMed] [Google Scholar]
- 13. Clavien PA, Camargo CA Jr, Croxford R, Langer B, Levy GA, Greig PD. Definition and classification of negative outcomes in solid organ transplantation. Application in liver transplantation. Ann Surg 1994; 220: 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Debonera F, Krasinkas AM, Gelman AE, et al. Dexamethasone inhibits early regenerative response of rat liver after cold preservation and transplantation. Hepatology 2003; 38: 1563–1572. [DOI] [PubMed] [Google Scholar]
- 15. Hayashi H, Beppu T, Sugita H, et al. Serum HGF and TGF‐beta1 levels after right portal vein embolization. Hepatol Res 2010; 40: 311–317. [DOI] [PubMed] [Google Scholar]
- 16. Fox IJ, Chowdhury JR, Kaufman SS, et al. Treatment of the Crigler‐Najjar syndrome type I with hepatocyte transplantation. N Engl J Med 1998; 338: 1422–1426. [DOI] [PubMed] [Google Scholar]
- 17. Picard N, Ratanasavanh D, Premaud A, Le Meur Y, Marquet P. Identification of the UDP‐glucuronosyltransferase isoforms involved in mycophenolic acid phase II metabolism. Drug Metab Dispos 2005; 33: 139–146. [DOI] [PubMed] [Google Scholar]
- 18. Erps LT, Ritter JK, Hersh JH, Blossom D, Martin NC, Owens IS. Identification of two single base substitutions in the UGT1 gene locus which abolish bilirubin uridine diphosphate glucuronosyltransferase activity in vitro. J Clin Invest 1994; 93: 564–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Allen KJ, Mifsud NA, Williamson R, Bertolino P, Hardikar W. Cell‐mediated rejection results in allograft loss after liver cell transplantation. Liver Transpl 2008; 14: 688–694. [DOI] [PubMed] [Google Scholar]
- 20. Bumgardner GL, Li J, Heininger M, Ferguson RM, Orosz CG. In vivo immunogenicity of purified allogeneic hepatocytes in a murine hepatocyte transplant model. Transplantation 1998; 65: 47–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Immunocytochemistry. Cell smears from three representative hepatocyte isolations were stained for (A) hepatocyte marker CK18, (B) macrophage/Kupffer cell marker CD68, (C) hematopoetic marker CD45 and (D) endothelial marker CD31. Negative control (E) was treated without primary antibody. (F) Percentage of positively stained cells.
Figure S2: Liver pathology. Liver tissue obtained before transplant during liver resection and at time of liver transplantation for patient 1 (A–F) and for patient 2 (G–L), stained with hematoxyolin eosin (A, B, G, H), Sirius red (C, D, I, J) and CK19 (E, F, K, L).


