Abstract
Aim
To compare the effectiveness of dulaglutide 1.5 and 0.75 mg with active comparators and placebo with regard to a composite endpoint of glycated haemoglobin (HbA1c), weight and hypoglycaemia, using post hoc analyses.
Methods
A logistic regression analysis was performed on the intention‐to‐treat population, using data from the last observation carried forward, and the composite endpoint of HbA1c <7.0% (53 mmol/mol), no weight gain (≤0 kg) and no hypoglycaemia (glucose <3.0 mmol/l or severe hypoglycaemia) after 26 weeks for each trial in the AWARD programme separately.
Results
At 26 weeks, within each study, 37–58% of patients on dulaglutide 1.5 mg, 27–49% of patients on dulaglutide 0.75 mg, and 9–61% of patients on active comparators achieved the composite endpoint. Significantly more patients reached the composite endpoint with dulaglutide 1.5 mg than with metformin, sitagliptin, exenatide twice daily or insulin glargine: odds ratio (OR) 1.5 [95% confidence interval (CI) 1.0, 2.2; p < 0.05], OR 4.5 (95% CI 3.0, 6.6; p < 0.001), OR 2.6 (95% CI 1.8, 3.7; p < 0.001) and OR 7.4 (95% CI 4.4, 12.6; p < 0.001), respectively, with no difference between dulaglutide 1.5 mg and liraglutide 1.8 mg. In addition, significantly more patients reached the composite endpoint with dulaglutide 0.75 mg than with sitagliptin or insulin glargine: OR 3.3 (95% CI 2.2, 4.8; p < 0.001) and OR 4.5 (95% CI 2.7, 7.8; p < 0.001), respectively.
Conclusions
Dulaglutide is an effective treatment option, resulting in a similar or greater proportion of patients reaching the HbA1c target of <7.0% (53 mmol/mol), without weight gain or hypoglycaemia compared with active comparators.
Keywords: composite endpoint, dulaglutide, GLP‐1 analogue
Introduction
Type 2 diabetes (T2D) is a chronic metabolic disorder with increasing worldwide prevalence. The effective management of patients with T2D involves a multifactorial approach, including the maintenance of glycaemic control, lifestyle adjustment, weight and blood pressure control, and lipid management 1, 2. Personalized, patient‐centred diabetes management must balance the benefits of glycaemic control and potential weight effects against the risk of adverse effects, particularly hypoglycaemia 2. Adverse effects such as weight gain and hypoglycaemia may also affect adherence to therapy 3, 4, 5, 6; thus, a composite endpoint to simultaneously assess glycaemic control, weight gain and hypoglycaemia risk is a clinically relevant and patient‐centred approach that is increasingly being used to evaluate treatment options within T2D management 7. Similarly, to address multiple desired outcomes, composite endpoint measures are increasingly being implemented in a variety of other clinical areas, such as cardiovascular diseases 8, 9, 10, infectious diseases 11, anaesthesia 12 and urology 13.
The American Diabetes Association and the European Association for the Study of Diabetes recommend glucagon‐like peptide‐1 (GLP‐1) receptor agonists as a potential second‐line therapy in combination with metformin or as a third‐line therapy within more complex treatment regimens for the treatment of T2D 1, 2. Dulaglutide, a once‐weekly GLP‐1 receptor agonist, is approved for the treatment of T2D. Dulaglutide is a fusion protein that combines two identical human GLP‐1 receptor analogues modified to resist dipeptidyl peptidase‐4 inactivation with soluble human IgG4‐Fc domains. It has prolonged pharmacological activity, allowing once‐weekly administration in a solution formulation that does not require reconstitution.
In the published AWARD trials, the efficacy and safety of dulaglutide 1.5 and 0.75 mg was explored across the diabetes treatment continuum (i.e. as monotherapy, combination therapy with one or two oral antihyperglycaemic medications, or combination therapy with meal‐time insulin lispro) 14, 15, 16, 17, 18, 19. At the primary endpoint, both dulaglutide doses resulted in superior glycaemic control to metformin 14, sitagliptin 15, exenatide twice daily 17 and insulin glargine (except for the comparison with dulaglutide 0.75 mg on metformin plus glimepiride background which was non‐inferior) 18, 19, and non‐inferiority to liraglutide (dulaglutide 1.5 mg only) 16. In addition, weight reduction was observed with dulaglutide that is consistent with the GLP‐1 receptor agonist class, and the incidence of total hypoglycaemia in dulaglutide‐treated patients was similar to, or lower than, that for other comparators 14, 15, 16, 17, 18, 19.
The present post hoc analysis focuses on the comparison of dulaglutide 1.5 and 0.75 mg with active comparator therapies and placebo in the AWARD trial programme for achieving the composite endpoint of glycated haemoglobin (HbA1c) <7.0% (53 mmol/mol), no weight gain, and no hypoglycaemia after 26 weeks of treatment.
Research Design and Methods
Design of Clinical Trial Programme
The designs and HbA1c results with regard to the primary endpoint of the five clinical trials included in the present analysis are shown in Table 1. Composite analyses of the AWARD‐4 trial results have previously been published and therefore were not included 18. These AWARD clinical trials included randomized, controlled clinical studies ranging from 26 to 104 weeks' duration with 4287 patients. All of the trials were designed to evaluate the safety and efficacy of dulaglutide in adult patients with T2D, with primary endpoints of 26 or 52 weeks, depending on the individual study. All five of the trials included the dulaglutide 1.5 mg dose, and four of the trials also evaluated the dulaglutide 0.75 mg dose. The trials were designed to evaluate the superiority of HbA1c reduction from baseline compared with placebo and/or non‐inferiority relative to active comparators, with type 1 error controlled gatekeeping to then test for superiority of active comparators.
Table 1.
Overview of AWARD trials included in the analysis.
| Trial | Concomitant medication | Active comparator | Primary endpoint (weeks) | HbA1c change at primary endpoint | |
|---|---|---|---|---|---|
| Dulaglutide 1.5 mg | Dulaglutide 0.75 mg | ||||
| AWARD‐3 | None | Metformin | 26 | Superior | Superior |
| AWARD‐5 | Metformin | Sitagliptin | 52 | Superior | Superior |
| AWARD‐6 | Metformin | Liraglutide | 26 | Non‐inferior | — |
| AWARD‐1 | Metformin + Pioglitazone | Exenatide twice daily | 26 | Superior | Superior |
| AWARD‐2 | Metformin + Glimepiride | Insulin glargine | 52 | Superior | Non‐inferior |
Superiority and non‐inferiority comparisons with active comparators with regard to HbA1c change from baseline. HbA1c, glycated haemoglobin; ITT, intent to treat.
Statistical Analyses
The five phase III clinical trials were analysed separately. The predefined composite endpoint of HbA1c <7.0% (53 mmol/mol), no weight gain and no hypoglycaemia after 26 weeks of treatment was analysed using a logistic regression analysis of the intention‐to‐treat population, comprising all randomized patients who received at least one dose of study treatment. The logistic regression models included baseline covariates for HbA1c and weight, a factor for geographic region, and a factor for any stratification variables used to account for variation in background medication (as applicable based on the individual study). Missing post‐baseline data were imputed using the last observation carried forward method. Similar analyses were performed on either HbA1c <7.0% (53 mmol/mol) and no weight gain or HbA1c <7.0% (53 mmol/mol) and no hypoglycaemia composite endpoints.
No weight gain was defined as ≤0 kg of weight change for an individual patient at 26 weeks. Hypoglycaemic episodes were defined as any blood glucose value <3.0 mmol/l and/or any event that required the assistance of another individual (i.e. a severe hypoglycaemic event) 7. The composite analysis was also performed at 52 weeks for those studies with a 52‐week endpoint (AWARD‐3, ‐5, ‐1 and ‐2).
Results
A total of 4287 patients with T2D were enrolled in the five trials, of whom 1424 patients received dulaglutide 1.5 mg and 1124 patients received dulaglutide 0.75 mg treatment. The baseline characteristics and demographics of these patients are shown in Table 2. Within each study, baseline characteristics were similar across the individual treatments.
Table 2.
Baseline characteristics and demographics.
| Trial | Monotherapy (AWARD‐3) | Add‐on to MET (AWARD‐5) | Add‐on to MET (AWARD‐6) | Add‐on to MET + PIO (AWARD‐1) | Add‐on to MET + GLIM (AWARD‐2) |
|---|---|---|---|---|---|
| N (ITT) | 807 | 1098 | 599 | 976 | 807 |
| Sex (%) | |||||
| Male | 43.7 | 47.4 | 47.9 | 58.4 | 51.3 |
| Female | 56.3 | 52.6 | 52.1 | 41.6 | 48.7 |
| Age (years) | 55.6 | 54.1 | 56.7 | 55.6 | 56.7 |
| Ethnicity (%) | |||||
| Hispanic or Latino | 33.7 | 19.1 | 24.5 | 33.9 | 36.1 |
| Not Hispanic or Latino | 66.3 | 80.8 | 74.1 | 66.0 | 63.9 |
| Race (%) | |||||
| American Indian | 10.5 | 0.1 | 7.2 | 13.8 | 11.0 |
| Asian | 7.6 | 24.9 | 0.2 | 2.5 | 17.0 |
| Black | 6.6 | 4.0 | 6.2 | 7.8 | 0.5 |
| Multiple | 0.9 | 19.2 | 0.5 | 1.2 | 0.9 |
| Native Hawaiian | 0.1 | 0.1 | 0 | 0.3 | 0 |
| White | 74.3 | 51.7 | 86.0 | 74.4 | 70.6 |
| Weight (kg) | 92.3 | 86.4 | 94.1 | 96.0 | 86.3 |
| BMI (kg/m2) | 33.3 | 31.2 | 33.6 | 33.2 | 31.5 |
| Diabetes duration (years) | 2.6 | 7.1 | 7.2 | 8.8 | 9.1 |
| HbA1c (%) | 7.6 | 8.1 | 8.1 | 8.1 | 8.1 |
| FBG (mmol/l) | 9.0 | 9.7 | 9.2 | 9.0 | 9.1 |
BMI, body mass index; FBG, fasting blood glucose; GLIM, glimepiride; HbA1c, glycated haemoglobin; ITT, intention to treat; MET, metformin; PIO, pioglitazone.
Glycated Haemoglobin <7.0%
The proportions of patients who achieved HbA1c <7.0% (53 mmol/mol) at 26 weeks were significantly greater for both dulaglutide doses versus metformin, sitagliptin, exenatide twice daily and insulin glargine (Table 3). HbA1c and weight changes from baseline and incidence of hypoglycaemia at week 26 are shown in Table 3 for all analysed trials.
Table 3.
Glycated haemoglobin, weight, hypoglycaemia and composite endpoint at week 26.
| Trial | Treatment | ΔHbA1c (%) | HbA1c <7.0% (% of patients) | Δ weight (kg) | Hypoglycaemia incidencea (% of patients) | Achieving composite endpoint (% of patients) | |
|---|---|---|---|---|---|---|---|
| Monotherapy (AWARD‐3 ) | DU 1.5 mg | N = 269 | −0.78# | 61.5# | −2.29 | 0 | 51.3# |
| DU 0.75 mg | N = 270 | −0.71# | 62.6# | −1.36# | 0.4 | 44.2 | |
| MET | N = 268 | −0.56 | 53.6 | −2.22 | 1.1 | 43.4 | |
| Add‐on to MET (AWARD‐5) | DU 1.5 mg | N = 304 | −1.22‡ | 60.9##, ** | −3.18##, ** | 0.7 | 55.3##, ** |
| DU 0.75 mg | N = 302 | −1.01‡ | 55.2##, ** | −2.63##, ** | 0.7 | 48.5##, ** | |
| SITA | N = 315 | −0.61 | 37.8** | −1.46 | 1.3 | 28.5* | |
| Placebo | N = 177 | 0.03 | 21.0 | −1.47 | 0 | 17.0 | |
| Add‐on to MET (AWARD‐6) | DU 1.5 mg | N = 299 | −1.42† | 68.3 | −2.90# | 0 | 58.0 |
| LIRA 1.8 mg | N = 300 | −1.36 | 67.9 | −3.61 | 1.7 | 61.1 | |
| Add‐on to MET + PIO (AWARD‐1) | DU 1.5 mg | N = 279 | −1.51##, ** | 78.2##, ** | −1.30** | 0 | 52.4##, ** |
| DU 0.75 mg | N = 280 | −1.30##, ** | 65.8##, ** | 0.20 ##, * | 2.5 | 33.8** | |
| EX twice daily | N = 276 | −0.99** | 52.3* | −1.07** | 5.4 | 33.1** | |
| Placebo | N = 141 | −0.46 | 42.9 | 1.24 | 0 | 17.6 | |
| Add‐on to MET + GLIM (AWARD‐2) | DU 1.5 mg | N = 273 | −1.16## | 58.2## | −1.82## | 13.6 | 36.5## |
| DU 0.75 mg | N = 272 | −0.89## | 45.9## | −1.47## | 12.5 | 27.1## | |
| GLAR | N = 262 | −0.65 | 32.6 | 1.01 | 16.8 | 8.9 | |
Data presented are least squares means, intention to treat (ITT), LOCF, analysis of covariance for ΔHbA1c and Δweight (except MMRM for AWARD‐6 ΔHbA1c); ITT, LOCF logistic regression analysis for HbA1c <7.0%, hypoglycaemia incidence and achieving composite endpoint. DU, dulaglutide; GLIM, glimepiride; HbA1c, glycated haemoglobin; LIRA, liraglutide; MET, metformin; PIO, pioglitazone; SIT, sitagliptin.
p < 0.05,
p < 0.001 vs active comparator.
Multiplicity adjusted one‐sided p < 0.001 superiority vs placebo.
p < 0.05.
p < 0.001 vs placebo.
p < 0.001 non‐inferiority vs comparator, non‐inferiority margin = 0.4%.
Hypoglycaemia with blood glucose <3.0 mmol/l and/or severe hypoglycaemia.
Glycated Haemoglobin <7.0% and No Weight Gain
At 26 weeks, within each study, 45–58% of patients on dulaglutide 1.5 mg, 34–49% of patients on dulaglutide 0.75 mg, and 12–63% of patients on active comparators achieved the HbA1c <7.0% (53 mmol/mol) and no weight gain target (Figure 1A). Significantly more patients reached this endpoint with dulaglutide 1.5 mg than with sitagliptin, exenatide twice daily or insulin glargine [odds ratios (ORs) 4.4 (p < 0.001), 2.3 (p < 0.001) and 7.9 (p < 0.001), respectively (Table 4)]; with no difference between dulaglutide 1.5 mg and metformin or liraglutide 1.8 mg. In addition, significantly more patients reached the endpoint with dulaglutide 0.75 mg compared with sitagliptin or insulin glargine [ORs 3.1 (p < 0.001) and 4.5 (p < 0.001), respectively (Table 4)], with no difference between dulaglutide 0.75 mg and metformin, or exenatide.
Figure 1.
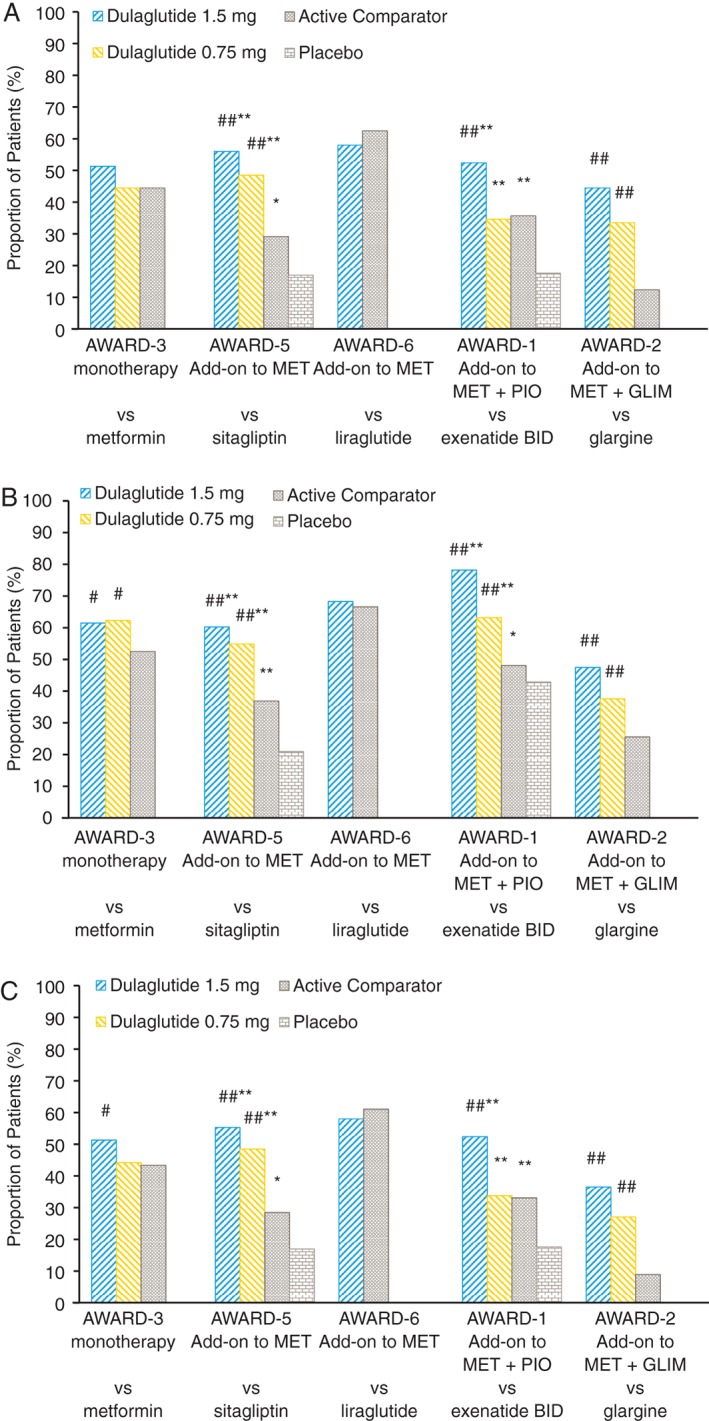
Proportion of patients achieving the composite outcome of (A) glycated haemoglobin (HbA1c) <7.0% and no weight gain, (B) HbA1c <7.0% and no hypoglycaemia and (C) HbA1c <7.0%, no weight gain, and no hypoglycaemia at week 26. #p < 0.05 and ##p < 0.001 vs active comparator; *p < 0.05 and **p < 0.001 vs placebo. GLIM, glimepiride; MET, metformin; PIO, pioglitazone.
Table 4.
Odds ratios for dulaglutide and active comparators on percentage of patients achieving composite endpoints.
| Active comparator | Monotherapy (AWARD‐3) | Add‐on to MET (AWARD‐5) | Add‐on to MET (AWARD‐6) | Add‐on to MET + PIO (AWARD‐1) | Add‐on to MET + GLIM (AWARD‐2) | |
|---|---|---|---|---|---|---|
| Metformin | Sitagliptin | Liraglutide | Exenatide twice daily | Insulin glargine | ||
| HbA1c <7.0% (53 mmol/mol) and no weight gain | ||||||
| DU 1.5 mg | OR (95% CI) | 1.4 (1.0, 2.0) | 4.4 (3.0, 6.5) | 0.8 (0.6, 1.2) | 2.3 (1.6, 3.3) | 7.9 (4.8, 12.9) |
| p = 0.087 | p < 0.001 | p = 0.309 | p < 0.001 | p < 0.001 | ||
| DU 0.75 mg | OR (95% CI) | 1.0 (0.7, 1.4) | 3.1 (2.1, 4.6) | — | 0.9 (0.7, 1.4) | 4.5 (2.7, 7.3) |
| p = 0.931 | p < 0.001 | p = 0.773 | p < 0.001 | |||
| HbA1c <7.0% (53 mmol/mol) and no hypoglycaemia | ||||||
| DU 1.5 mg | OR (95% CI) | 1.7 (1.1, 2.6) | 3.9 (2.6, 5.7) | 1.1 (0.8, 1.6) | 6.6 (4.2, 10.4) | 3.5 (2.3, 5.3) |
| p < 0.05 | p < 0.001 | p = 0.546 | p < 0.001 | p < 0.001 | ||
| DU 0.75 mg | OR (95% CI) | 1.8 (1.2, 2.7) | 3.0 (2.0, 4.4) | — | 2.3 (1.5, 3.4) | 2.1 (1.4, 3.1) |
| p < 0.05 | p < 0.001 | p < 0.001 | p < 0.001 | |||
| HbA1c <7.0% (53 mmol/mol), no weight gain and no hypoglycaemia | ||||||
| DU 1.5 mg | OR (95% CI) | 1.5 (1.0, 2.2) | 4.5 (3.0, 6.6) | 0.9 (0.6, 1.3) | 2.6 (1.8, 3.7) | 7.4 (4.4, 12.6) |
| p < 0.05 | p < 0.001 | p = 0.511 | p < 0.001 | p < 0.001 | ||
| DU 0.75 mg | OR (95% CI) | 1.0 (0.7, 1.5) | 3.3 (2.2, 4.8) | — | 1.0 (0.7, 1.5) | 4.5 (2.7, 7.8) |
| p = 0.920 | p < 0.001 | p = 0.832 | p < 0.001 | |||
CI, confidence interval; DU, dulaglutide; GLIM, glimepiride; MET, metformin; OR, odds ratio; PIO, pioglitazone.
Glycated Haemoglobin <7.0% and No Hypoglycaemia
At 26 weeks, within each study, 48–78% of patients on dulaglutide 1.5 mg, 38–63% of patients on dulaglutide 0.75 mg, and 26–67% on active comparators achieved the HbA1c <7.0% (53 mmol/mol) and no hypoglycaemia endpoint (Figure 1B). Significantly more patients reached the composite endpoint with dulaglutide 1.5 and 0.75 mg than with metformin [ORs 1.7 (p < 0.05) and 1.8 (p < 0.05), respectively (Table 4)], sitagliptin [ORs 3.9 (p < 0.001) and 3.0, (p < 0.001), respectively], exenatide twice daily [ORs 6.6 (p < 0.001) and 2.3 (p < 0.001), respectively], and insulin glargine [ORs 3.5 (p < 0.001) and 2.1 (p < 0.001), respectively], with no difference between dulaglutide 1.5 mg and liraglutide 1.8 mg.
Glycated Haemoglobin <7.0%, No Weight Gain and No Hypoglycaemia
At 26 weeks, within each study, 37–58% of patients on dulaglutide 1.5 mg, 27–49% of patients on dulaglutide 0.75 mg, and 9–61% on active comparators achieved the composite endpoint (Figure 1C). Significantly more patients reached the composite endpoint with dulaglutide 1.5 mg than with metformin, sitagliptin, exenatide twice daily and insulin glargine [ORs 1.5 (p < 0.05), 4.5 (p < 0.001), 2.6 (p < 0.001) and 7.4 (p < 0.001), respectively (Table 4)], with no difference between dulaglutide 1.5 mg and liraglutide 1.8 mg. In addition, significantly more patients reached the composite endpoint with dulaglutide 0.75 mg than with sitagliptin or insulin glargine [ORs 3.3 (p < 0.001) and 4.5 (p < 0.001), respectively (Table 4)]. At 52 weeks, results similar to those at 26 weeks were observed with dulaglutide compared with sitagliptin, exenatide twice daily and insulin glargine, while patients who reached the composite endpoint with dulaglutide 1.5 mg and metformin were not significantly different (Figure S1).
Discussion
Treatment with dulaglutide 1.5 mg, compared with metformin, sitagliptin, exenatide twice daily and insulin glargine, significantly increased the proportion of patients achieving the composite endpoint. Across the programme, 37–58% of patients treated with dulaglutide 1.5 mg achieved the composite endpoint. Notably, there was no difference observed in the percentages of patients achieving the composite with dulaglutide 1.5 mg compared with liraglutide 1.8 mg. Patients receiving dulaglutide 1.5 mg had a 1.5–7.4 times greater odds of achieving the composite endpoint as compared with those receiving four commonly used agents for diabetes treatment. In addition, 27–49% of patients achieved the composite endpoint with dulaglutide 0.75 mg, with significantly greater proportions compared with sitagliptin and insulin glargine.
While a large number of patients reached individual targets, the composite endpoint is arguably more clinically relevant and was achieved in far fewer patients. The advantage of using a composite endpoint is that it allows one to define efficacy and safety more comprehensively, particularly when more than one difference in response to therapy is important. For comparisons with metformin and exenatide, the composite outcome appears to be driven largely by HbA1c, while in the comparisons with sitagliptin and glargine, it appears to be driven by both HbA1c and weight. None of the within‐study comparisons of composite endpoints were significantly influenced by hypoglycaemia in this analysis. AWARD‐2 (comparison with glargine), in particular, was the only study with a comparator that would be likely to show more hypoglycaemia than dulaglutide in this analysis; however, reported between‐group differences in hypoglycaemia in AWARD‐2 observed at 52 weeks for the 3.9 mmol/l threshold of hypoglycaemia did not appear to have influenced the composite. This was not unexpected, given that hypoglycaemia was generally mild and would not be captured with the definition of hypoglycaemia used in the present analysis 19. It is possible that the concomitant high dose of background glimepiride may have masked subtle differences in hypoglycaemia between groups. Nevertheless, the proportion of patients in all treatment arms within AWARD‐2 who achieved the composite endpoint was numerically lower compared with the other trials (Table 3), and this is probably influenced by background glimepiride‐induced hypoglycaemia.
Previously, a meta‐analysis of the LEAD studies reported that 40 and 32% of subjects receiving liraglutide 1.8 and 1.2 mg, respectively, achieved a similar composite outcome at 26 weeks, as opposed to 6–25% of subjects receiving comparators 7. Liraglutide was superior to rosiglitazone, glimepiride, glargine, exenatide and sitagliptin in this regard. In the present analysis, patients receiving liraglutide and dulaglutide were equally likely to achieve the composite outcome, despite small differences in weight. By comparison, 48% of patients receiving exenatide once weekly achieved a similar composite endpoint of all three goals 20. Outcomes for individual exenatide studies were not reported, however, and data may have been limited by inclusion of non‐randomized studies. Direct comparisons between these previous analyses and the present results are impossible because of significant differences in background therapies, in allowance for titration of sulphonylureas and in definitions of hypoglycaemia.
The present composite endpoint analysis was modelled after previous reports 7, 20. The 26‐week time point for evaluation was selected because this was a common time point across all five studies. The most important difference between the present analysis and previous reports is that data were not pooled in the present analysis. There are several reasons why pooling the data was not considered appropriate. First, as is shown in Table 2, patients were on similar background therapy within each study, but this varied greatly across the AWARD programme and may have influenced outcomes of interest for this particular composite. In AWARD‐1, background therapy consisted of maximum tolerated doses of metformin and pioglitazone. This led to an attenuation of weight reduction observed for both GLP‐1 receptor agonists in the trial. Similarly, in AWARD‐2, in addition to concomitant metformin, patients were on maximum tolerated doses of glimepiride, which affected weight reduction and increased the risk of hypoglycaemia. Second, the line of therapy, i.e. first‐line drug therapy versus intensification of therapy, can affect the HbA1c reduction in pooled data 21. This may have attenuated the HbA1c reduction observed in AWARD‐2, where either dulaglutide or glargine were added on to metformin plus glimepiride as third‐line therapy. Third, baseline values, such as HbA1c are also strong predictors of change in pooled data 21, and varied across the studies, as noted by the smaller HbA1c reduction in AWARD‐3, which had a lower baseline HbA1c than other AWARD trials (7.6 vs 8.1%). Given the confounding effects of these factors on the individual components of this specific composite, it was therefore chosen not to integrate the data for this analysis 21; hence, the composite index was applied to each study separately.
The safety of dulaglutide and active comparators has been characterized in each of the individual studies 14, 15, 16, 17, 19. The safety and tolerability profile of dulaglutide is similar to that of other agents in the GLP‐1 receptor agonist class. The most common side effects are gastrointestinal‐related, including nausea, vomiting and diarrhoea. Also consistent with the class, gastrointestinal side effects are mostly mild to moderate, occur early in the course of treatment, and are transient. Cases of pancreatitis have been rarely reported and there is no increase in calcitonin levels with dulaglutide treatment. A low incidence of antidrug antibody formation, with very few potentially immune‐mediated injection site adverse events or systemic hypersensitivity reactions have been observed.
Notably, one further phase III dulaglutide trial, AWARD‐4, has been published 18. The AWARD‐4 study was the first to evaluate the combination of prandial insulin with a GLP‐1 receptor agonist in patients with inadequate control on a conventional insulin regimen. The study demonstrated superior glycaemic control with the combination of dulaglutide and three times daily prandial insulin compared with basal‐bolus therapy with insulin glargine and insulin lispro. Patients in the dulaglutide arms had prandial insulin doses of >90 units, while patients in the basal‐bolus arm had mean insulin doses of 130 units at the 26‐week primary endpoint. As expected, weight loss was attenuated in the study and the incidence of hypoglycaemia was similar to that observed in other basal‐bolus studies 22. Within the study, composites more appropriate for this particular patient population and these particular regimens were prespecified and have been previously reported 18. As such, this study was not included in the present analysis.
It is important to note that, while the composite endpoint was prespecified, the present study was a post hoc analysis; however, all studies were prospective randomized controlled trials. Comparisons with some other available therapies, such as sulphonylureas and sodium‐glucose cotransporter‐2 inhibitors were not available, nor were data from all background therapies. The present analysis does, however, include comparison of the proportion of patients achieving the composite outcome with a long‐acting GLP‐1 receptor agonist versus metformin, which has not been reported previously. Finally, the relationship between this composite endpoint and long‐term outcomes, such as mortality or cardiovascular events, is unknown. Nor is it understood how this composite relates to adherence or patient‐reported outcomes. It is possible that additional or alternative measures, such as blood pressure and lipids, would be of use in these studies. Additionally, the weighting of individual elements may need to be optimized to provide the best risk–benefit prediction. Analyses of long‐term composite outcomes will be possible as data from long‐term cardiovascular studies become available. In the meantime, the present composite endpoint may be more relevant for informing immediate treatment decisions.
In summary, the present post hoc analysis shows that dulaglutide is an effective treatment option, resulting in a similar or greater proportion of patients who reached the HbA1c target of <7.0% (53 mmol/mol), without weight gain or hypoglycaemia, compared with active comparators.
Conflict of Interest
K. D. is a consultant to Eli Lilly and Company and GlaxoSmithKline plc, and receives research support from Merck & Co., Inc., Novo Nordisk Inc., AstraZeneca plc and Mylan N.V.
I. R. is on the advisory panel of AstraZeneca/Bristol‐Myers Squibb, Eli Lilly and Company, Medscape, LLC, Merck Sharp & Dohme Ltd, Novo Nordisk Inc., Sanofi, Orgenesis, SmartZyme Innovation Ltd and Labstyle Innovations Ltd. I. R. works as a consultant to AstraZeneca/Bristol‐Meyers Squibb, Insuline Medical, Gili Medical, Kamada Ltd and FuturRx Ltd. I. R. serves on the speaker's bureau of AstraZeneca/Bristol‐Meyers Squibb, Eli Lilly and Company, Johnson & Johnson, Merck Sharp & Dohme Ltd., Novartis Pharma AG, Novo Nordisk, Inc., Sanofi and Teva. I. R. owns stock/shares in Insuline Medical, Labstyle Innovations, SmartZyme Innovation Ltd, Orgenesis and Glucome Ltd. Z. S. and J. F. are full‐time employees of and own stock or stock options in Eli Lilly and Company. W.S. is a full‐time employee of Eli Lilly and Company.
K. D. contributed to design, conduct/data collection, analysis and writing of the paper. I. R. contributed to design and analysis of the paper. Z. S. contributed to conduct/data collection and analysis of the paper. W. S. contributed to conduct/data collection and analysis of the paper. J. F. contributed to design, conduct/data collection, analysis and writing of the paper.
Supporting information
Figure S1. Proportion of patients achieving the composite outcome of glycated haemoglobin (HbA1c) <7.0% and no weight gain and no hypoglycaemia at week 52. #p < 0.05 and ##p ≤ 0.001 vs active comparator. MET, metformin; PIO, pioglitazone; GLIM, glimepiride.
Acknowledgements
The authors thank Nan Zhang Ph.D. for writing assistance. This work was sponsored by Eli Lilly and Company.
References
- 1. Inzucchi SE, Bergenstal RM, Buse JB et al. Management of hyperglycemia in type 2 diabetes: a patient‐centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35: 1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inzucchi SE, Bergenstal RM, Buse JB et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38: 140–149. [DOI] [PubMed] [Google Scholar]
- 3. Ross SA, Tildesley HD, Ashkenas J. Barriers to effective insulin treatment: the persistence of poor glycemic control in type 2 diabetes. Curr Med Res Opin 2011; 27(Suppl. 3): 13–20. [DOI] [PubMed] [Google Scholar]
- 4. Russell‐Jones D, Khan R. Insulin‐associated weight gain in diabetes–causes, effects and coping strategies. Diabetes Obes Metab 2007; 9: 799–812. [DOI] [PubMed] [Google Scholar]
- 5. Bennett WL, Maruthur NM, Singh S et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2‐drug combinations. Ann Intern Med 2011; 154: 602–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garber AJ. Hypoglycaemia: a therapeutic concern in type 2 diabetes. Lancet 2012; 379: 2215–2216. [DOI] [PubMed] [Google Scholar]
- 7. Zinman B, Schmidt WE, Moses A, Lund N, Gough S. Achieving a clinically relevant composite outcome of an HbA1c of <7% without weight gain or hypoglycaemia in type 2 diabetes: a meta‐analysis of the liraglutide clinical trial programme. Diabetes Obes Metab 2012; 14: 77–82. [DOI] [PubMed] [Google Scholar]
- 8. Tong BC, Huber JC, Ascheim DD et al. Weighting composite endpoints in clinical trials: essential evidence for the heart team. Ann Thorac Surg 2012; 94: 1908–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stamler J, Neaton JD, Cohen JD et al. Multiple risk factor intervention trial revisited: a new perspective based on nonfatal and fatal composite endpoints, coronary and cardiovascular, during the trial. J Am Heart Assoc 2012; 1: e003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Banerjee A, Fauchier L, Bernard‐Brunet A, Clementy N, Lip GY. Composite risk scores and composite endpoints in the risk prediction of outcomes in anticoagulated patients with atrial fibrillation. The Loire Valley Atrial Fibrillation Project. Thromb Haemost 2014; 111: 549–556. [DOI] [PubMed] [Google Scholar]
- 11. Wittkop L, Smith C, Fox Z et al. Methodological issues in the use of composite endpoints in clinical trials: examples from the HIV field. Clin Trials 2010; 7: 19–35. [DOI] [PubMed] [Google Scholar]
- 12. Myles PS, Devereaux PJ. Pros and cons of composite endpoints in anesthesia trials. Anesthesiology 2010; 113: 776–778. [DOI] [PubMed] [Google Scholar]
- 13. Lavallee LT, Dahm P, Breau RH. Evidence‐based urology in practice: composite endpoints. BJU Int 2010; 106: 610–612. [DOI] [PubMed] [Google Scholar]
- 14. Umpierrez G, Tofe Povedano S, Perez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD‐3). Diabetes Care 2014; 37: 2168–2176. [DOI] [PubMed] [Google Scholar]
- 15. Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD‐5). Diabetes Care 2014; 37: 2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dungan KM, Povedano ST, Forst T et al. Once‐weekly dulaglutide versus once‐daily liraglutide in metformin‐treated patients with type 2 diabetes (AWARD‐6): a randomised, open‐label, phase 3, non‐inferiority trial. Lancet 2014; 384: 1349–1357. [DOI] [PubMed] [Google Scholar]
- 17. Wysham C, Blevins T, Arakaki R et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD‐1). Diabetes Care 2014; 37: 2159–2167. [DOI] [PubMed] [Google Scholar]
- 18. Blonde L, Jendle J, Gross J et al. Once‐weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD‐4): a randomised, open‐label, phase 3, non‐inferiority study. Lancet 2015; 385: 2057–2066. [DOI] [PubMed] [Google Scholar]
- 19. Giorgino F, Benroubi M, Sun JH, Zimmermann AG, Pechtner V. Efficacy and safety of once‐weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD‐2). Diabetes Care 2015. Jun 18. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20. Bergenstal RM, Li Y, Porter TK, Weaver C, Han J. Exenatide once weekly improved glycaemic control, cardiometabolic risk factors and a composite index of an HbA1c < 7%, without weight gain or hypoglycaemia, over 52 weeks. Diabetes Obes Metab 2013; 15: 264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Einarson TR, Garg M, Kaur V, Hemels ME. Composite endpoints in trials of type‐2 diabetes. Diabetes Obes Metab 2014; 16: 492–499. [DOI] [PubMed] [Google Scholar]
- 22. Rosenstock J, Ahmann AJ, Colon G, Scism‐Bacon J, Jiang H, Martin S. Advancing insulin therapy in type 2 diabetes previously treated with glargine plus oral agents: prandial premixed (insulin lispro protamine suspension/lispro) versus basal/bolus (glargine/lispro) therapy. Diabetes Care 2008; 31: 20–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Proportion of patients achieving the composite outcome of glycated haemoglobin (HbA1c) <7.0% and no weight gain and no hypoglycaemia at week 52. #p < 0.05 and ##p ≤ 0.001 vs active comparator. MET, metformin; PIO, pioglitazone; GLIM, glimepiride.


