Abstract
Objective
To determine whether an anabolic drug (sprifermin) is capable of reducing cartilage loss wherever it occurs in a given knee, using a subject‐specific, location‐independent analysis of cartilage change in patients with knee osteoarthritis (OA).
Methods
Study participants (n = 168; ages ≥40 years, 69% women) had symptomatic femorotibial OA not confined to the medial compartment. Sprifermin (10, 30, or 100 μg) or placebo was injected intraarticularly 3 times over 3 weeks, both after randomization (baseline) and 3 months later. Coronal magnetic resonance images were acquired at baseline and 3, 6, and 12 months after treatment. The femorotibial cartilage of each subject was segmented, and changes in cartilage thickness were computed across 16 subregions. Location‐independent post hoc analysis was used to compute summary scores of negative and positive changes in the subregions, summarized as the total cartilage thinning sum score (ThCTnS) and the total cartilage thickening sum score (ThCTkS), capturing change in either direction in each knee. Ordered values of the magnitude of subject‐specific subregional changes in thickness were determined. The ThCTnS and ThCTkS in each sprifermin dose group at 12 months of followup were compared with the values in the matched placebo groups, using the Wilcoxon‐Mann‐Whitney test.
Results
The mean ± SD ThCTnS was −591 ± 617 μm (median −360 μm, Q1/Q3 = −820/−200 μm) in patients treated with 100 μg sprifermin (n = 57), and −921 ± 777 μm (median −745 μm, Q1/Q3 = −1,190/−380 μm) in patients given placebo (n = 18). The mean difference in the ThCTnS between the 100‐μg sprifermin group and the placebo group was 331 μm (95% confidence interval [95% CI] 24, 685), a difference that was statistically significant (P = 0.03). The mean difference in the ThCTkS in the 100‐μg sprifermin group compared with the placebo group was 237 μm (95% CI 34, 440), also a statistically significant difference (P = 0.028).
Conclusion
Sprifermin not only increases cartilage thickness, but also reduces cartilage loss. Subject‐specific, location‐independent analysis of both cartilage thinning and thickening represents a sensitive and informative approach for studying the effects of disease‐modifying OA drugs.
Osteoarthritis (OA) is the most common form of arthritis, and the lifetime risk of symptomatic knee OA is 45% 1. Current treatments focus on symptom management 2 or joint surgery 3; no disease‐modifying OA drug (DMOAD) aimed at modifying structural pathologic progression in the synovial tissue has been approved by regulatory agencies 2. In the US, 1 of every 2 patients in whom knee OA is diagnosed eventually receives surgical knee replacement, and the average age at which patients undergo knee replacement has been decreasing 3. Therapies that could delay structural pathologic development and reverse symptoms and functional limitations, thereby preventing joint replacement, would thus represent an important, unmet medical need.
Most DMOAD studies have evaluated structural progression as a reduction in radiographic joint space width (JSW) 4, 5, 6, an end point that is accepted by regulatory agencies. However, accurate measurement of minimum JSW is technically challenging, is limited to progression in the medial compartment, and requires large sample sizes and long observation periods 6. Recent DMOAD trials used magnetic resonance imaging (MRI) and cartilage thickness or volume as structural outcome measures 6, 7, 8, 9. Many of these trials demonstrated significant DMOAD effects in the lateral, but not the medial, femorotibial compartment, albeit the studies were conducted in patients with predominantly medial disease 7, 8, 9. In a recent study by Lohmander et al, patients with knee OA (not specifically selected for medial compartment disease) were evaluated in a randomized controlled trial (RCT) of intraarticular sprifermin (recombinant human growth factor 18), and the results indicated that treatment with sprifermin had statistically significant, dose‐dependent effects on total and lateral femorotibial cartilage thickness 10. With the highest dose of sprifermin (100 μg), cartilage thickness and JSW in the lateral compartment were shown to increase over 12 months, but no statistically significant dose response could be ascertained with regard to changes in central medial femorotibial cartilage thickness 10.
These observations have raised questions as to whether the effects of DMOADs are greater in joint regions where mechanical challenges are potentially small and cartilage thickness may be static, and whether drugs can also be effective in regions where mechanical challenges are high and cartilage loss is supposedly most progressive. These considerations apply between compartments (i.e., medial versus lateral), but also within these compartments (i.e., peripheral versus central). In several studies, subregional cartilage change was shown to be highly variable between knees 11, 12, and often occurred in opposite directions (loss versus gain), thereby canceling each other out when the mean change across different knees from different participants 11 was reported. Therefore, analyses to ascertain the average cartilage change across compartments, plates, or subregions of the cartilage among patients are unable to conclusively answer whether, at an individual level, cartilage loss is reduced by a DMOAD.
The objective of this post hoc analysis of the results from the recent RCT evaluating sprifermin for the treatment of knee OA 10 was to use subject‐specific, location‐independent analysis of cartilage change, in order to test whether an anabolic drug such as sprifermin increases cartilage thickness at some locations in the joint, and whether the treatment is also capable of reducing cartilage loss wherever it occurs in a given knee.
PATIENTS AND METHODS
Study design
A multicenter, randomized, double‐blind, placebo‐controlled trial evaluating the effects of sprifermin was conducted in patients with knee OA across 30 sites on multiple continents. The inclusion/exclusion criteria for this RCT have been previously published 10. Trial investigators selected patients with radiographic knee OA in the femorotibial compartment, whether in the medial or lateral region or both.
The efficacy of intraarticular sprifermin was evaluated in 168 participants, using multiple ascending‐dose regimens of 10, 30, and 100 μg 10. Eligible patients were randomized 3:1 within each cohort to receive sprifermin or placebo (Table 1). An intraarticular injection was administered once per week over 3 weeks to one or both knees of the participants after randomization and baseline MRI, and was again administered 3 months later, once per week over 3 weeks. The results reported herein are from those patients in the sprifermin dose groups (100 μg n = 57, 30 μg n = 40, and 10 μg n = 18) and the matched placebo groups (100 μg n = 18, 30 μg n = 12, and 10 μg n = 7) who had MRI data available at baseline and at the 12‐month followup. The dropout rate observed in this study has been reported previously 10.
Table 1.
Baseline characteristics and subregional cartilage thickness among patients treated with 100 μg sprifermin and matched placebo‐treated patientsa
| 100 μg sprifermin (n = 57) | Matched placebo (n = 18) | |||
|---|---|---|---|---|
| Characteristic | ||||
| Age, mean ± SD years | 61.2 ± 9.1 | 60.9 ± 6.9 | ||
| Female, no. (%) | 39 (68.4) | 12 (66.7) | ||
| BMI, mean ± SD kg/m2 | 30.5 ± 5.0 | 31.5 ± 5.3 | ||
| K/L grade 3, no. (%) | 29 (50.9) | 11 (61.1) | ||
| Time since OA onset, mean ± SD years | 7.1 ± 5.4 | 7.4 ± 3.7 | ||
| Cartilage subregion thickness, mean ± SD μm | ||||
| cMT | 2,189.5 ± 556.1 | 2,236.1 ± 560.5 | ||
| eMT | 1,194.4 ± 378.9 | 1,168.9 ± 427.1 | ||
| iMT | 1,858.6 ± 329.6 | 1,871.1 ± 269.3 | ||
| aMT | 1,487.2 ± 251.8 | 1,453.9 ± 345.9 | ||
| pMT | 1,408.6 ± 223.4 | 1,478.3 ± 263.9 | ||
| ccMF | 1,920.4 ± 658.4 | 1,800.0 ± 738.1 | ||
| ecMF | 1,281.8 ± 393.0 | 1,265.0 ± 367.3 | ||
| icMF | 1,772.6 ± 383.5 | 1,740.0 ± 419.1 | ||
| cLT | 2,775.3 ± 869.6 | 2,593.9 ± 813.4 | ||
| eLT | 1,431.1 ± 393.9 | 1,370.6 ± 542.3 | ||
| iLT | 1,790.0 ± 438.7 | 1,758.3 ± 280.6 | ||
| aLT | 1,585.6 ± 305.0 | 1,628.3 ± 319.3 | ||
| pLT | 1,653.3 ± 427.9 | 1,484.4 ± 316.3 | ||
| ccLF | 2,157.0 ± 416.5 | 2,205.6 ± 546.1 | ||
| ecLF | 1,563.5 ± 348.1 | 1,550.0 ± 456.8 | ||
| icLF | 1,681.4 ± 308.9 | 1,759.4 ± 400.3 | ||
Thickness of cartilage from the medial tibia (MT), central part of the medial weight‐bearing femur (cMF), lateral tibia (LT), and central part of the lateral weight‐bearing femur (cLF) was determined in the central (c), external (e), internal (i), anterior (a), and posterior (p) subregions. BMI = body mass index; K/L = Kellgren/Lawrence; OA = osteoarthritis.
MRI acquisition and analysis
MRIs were acquired using 1.5T or 3T magnets and knee coils 10. Coronal spoiled gradient‐recalled sequences, known to be accurate and robust with regard to delineating cartilage in multicenter trials 6, were acquired at baseline and at 3, 6, and 12 months of followup 6, 10. Images were acquired with a contiguous 1.5‐mm slice thickness and with in‐plane resolutions ranging from 0.23 × 0.23 mm to 0.32 × 0.32 mm (repetition time 18–50 msec, echo time 6.5–14 msec, flip angle 15–20°) depending on the magnet and on the fat saturation/water excitation status. Identical parameters were used at the baseline and followup assessments in all cases. No test–retest MRI acquisitions to assess the reproducibility of the study were planned in the protocol.
Segmentation of the femorotibial cartilage was performed at a single center (Chondrometrics GmbH, Ainring, Germany). The subchondral bone and cartilage surface area were traced manually in the medial and lateral femorotibial plates, excluding osteophyte cartilage 10. Baseline and followup images were processed in one session, and all images were blinded with regard to acquisition order and active treatment/placebo status. All segmentations were checked by 1 of 2 experts (SM and FE). Measures of compartment, plate, and subregion cartilage thickness 13, 14 were computed using software from Chondrometrics (provided by WW). The specific implementation for determining the 16 femorotibial subregions, and the reliability (test–retest reproducibility with repositioning of the joint between acquisitions) have been published previously 13, with the root mean square coefficient of variation and standard deviation for subregion cartilage thickness ranging from 1.5% and 19 μm to 4.7% and 84 μm, respectively, across regions. The RCT results with regard to compartment measures and safety, symptom, and radiographic outcomes have been reported previously 10. The current analysis focused on the 12‐month cartilage change in 16 femorotibial subregions (results in the cohort receiving 100 μg sprifermin are reported herein; results in the 10‐μg and 30‐μg cohorts are reported in Supplementary Tables 1 and 2, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39265/abstract). Three‐ and 6‐month data on cartilage change were not included, in order to limit multiple comparisons and because the 12‐month observation interval was shown to provide a robust and persistent treatment effect in the RCT 10.
Location‐independent analysis of cartilage change
To derive subject‐specific, location‐independent measures of cartilage change, all subregions with negative changes were summarized (cartilage thinning sum score [ThCTnS]) over as many of the 16 subregions that displayed cartilage thickness loss in each patient. Furthermore, all subregions with positive changes were summarized (cartilage thickening sum score [ThCTkS]) over as many of the 16 subregions that displayed cartilage thickness gain. Moreover, to demonstrate the relationship between loss and gain (in different subregions) occurring within the same knee, the ratio of median ThCTnS to median ThCTkS was determined in each patient. Finally, a previously described ordered value (OV) approach 14 was applied, in which subject‐specific subregional change in cartilage thickness was sorted from most negative (OV1) to least negative or most positive (OV16).
Statistical analysis
Statistical testing was performed using SAS software version 9.1 (SAS Institute). Analyses were conducted in a subset of patients in the modified intent‐to‐treat population, as described by Lohmander et al 10, for whom efficacy data were available from the baseline and 12‐month followup assessments. No imputations of missing data were applied.
The ThCTnS of cartilage thinning was defined as the primary focus, while the ThCTkS of cartilage thickening and the ThCTnS:ThCTkS ratio were secondary analytic end points of this post hoc exploratory analysis. Subregions 13 and OVs 14 were used as exploratory and explanatory outcomes. For each of these structural measures of the cartilage, the mean ± SD and median values (with Q1 and Q3 of the interquartile range) for the change in thickness were analyzed. Differences between treatment groups are reported as the mean difference with 95% confidence intervals (95% CIs). Given the non‐normal distribution of change and the small sample size, differences between the sprifermin‐ and placebo‐treated patients were tested for statistical significance using the Wilcoxon‐Mann‐Whitney test. P values less than 0.05 were considered significant. The P values were not adjusted for multiple testing.
RESULTS
Baseline characteristics of the patients
Baseline demographic and clinical characteristics and the baseline cartilage thickness values across subregions of the femorotibial cartilage of patients in the 100‐μg sprifermin group and matching placebo group are shown in Table 1. The baseline demographic and clinical characteristics of patients in the 10‐μg and 30‐μg sprifermin groups and matching placebo cohorts are listed in Supplementary Table 1.
Cartilage thinning and thickening summary scores in patients treated with 100 μg sprifermin versus placebo
One‐year changes from baseline and differences in cartilage thickness change in patients treated with sprifermin compared with the matched placebo groups are shown in Table 2. The mean ± SD ThCTnS was −591 ± 617 μm (median −360 μm, Q1/Q3 = −820/−200 μm) in knees treated with 100 μg sprifermin, and −921 ± 777 μm (median −745 μm, Q1/Q3 = −1,190/−380 μm) in knees treated with placebo. The mean difference in CThTnS change between the groups was 331 μm (95% CI −24, 685 μm); this was a statistically significant difference (P = 0.03).
Table 2.
One‐year changes from baseline in cartilage thickness in patients treated with 100 μg sprifermin compared with matched placebo‐treated patients, according to thinning and thickening scores, OVs of the magnitude of change, and anatomic subregionsa
| One‐year change from baseline | Between‐group difference, mean (95% CI) μm | |||
|---|---|---|---|---|
| 100 μg sprifermin (n = 57) | Matched placebo (n = 18) | P | ||
| ThCTnS | −590.5 ± 616.8 (−360) | −921.1 ± 776.8 (−745) | 330.6 (−23.7, 684.9) | 0.0304 |
| ThCTkS | 744.2 ± 360.4 (710) | 507.2 ± 429.0 (380) | 237.0 (33.6, 440.4) | 0.0286 |
| ThCTnS:ThCTkS ratio | −3.1 ± 9.0 (−0.55) | −9.4 ± 16.1 (−2.1) | 6.3 (0.3, 12.3) | 0.0143 |
| OVs | ||||
| OV1 | −178.6 ± 151.1 | −217.2 ± 158.1 | 38.6 (−43.7, 121.0) | 0.1208 |
| OV2 | −122.8 ± 121.6 | −153.3 ± 114.7 | 30.5 (−34.2, 95.2) | 0.1758 |
| OV3 | −85.6 ± 92.9 | −121.1 ± 88.2 | 35.5 (−14.0, 85.0) | 0.0699 |
| OV4 | −53.5 ± 64.7 | −97.2 ± 77.4 | 43.7 (7.1, 80.3) | 0.0297 |
| OV5 | −36.0 ± 61.0 | −81.1 ± 67.6 | 45.1 (11.4, 78.9) | 0.0086 |
| OV6 | −22.3 ± 55.8 | −66.7 ± 63.2 | 44.4 (13.4, 75.4) | 0.0074 |
| OV7 | −8.1 ± 54.7 | −46.1 ± 57.7 | 38.0 (8.2, 67.9) | 0.0226 |
| OV8 | 5.4 ± 50.2 | −27.2 ± 59.8 | 32.7 (4.3, 61.0) | 0.0429 |
| OV9 | 17.5 ± 49.5 | −8.3 ± 60.1 | 25.9 (−2.2, 54.0) | 0.0786 |
| OV10 | 31.8 ± 46.7 | 6.7 ± 63.8 | 25.1 (−2.5, 52.7) | 0.0747 |
| OV11 | 47.0 ± 45.6 | 15.0 ± 62.9 | 32.0 (5.0, 59.1) | 0.0343 |
| OV12 | 63.3 ± 42.0 | 25.0 ± 64.6 | 38.3 (12.4, 64.3) | 0.0280 |
| OV13 | 81.8 ± 46.2 | 43.3 ± 63.5 | 38.4 (11.1, 65.8) | 0.0332 |
| OV14 | 103.2 ± 49.9 | 68.3 ± 71.0 | 34.8 (4.9, 64.7) | 0.0450 |
| OV15 | 131.4 ± 59.1 | 93.9 ± 74.1 | 37.5 (3.6, 71.4) | 0.0274 |
| OV16 | 179.1 ± 70.9 | 152.2 ± 101.5 | 26.9 (−15.7, 69.5) | 0.1644 |
| Cartilage subregion thicknessb | ||||
| cMT | −2.6 ± 144.9 | −22.8 ± 193.3 | 20.1 (−64.7, 105.0) | 0.6595 |
| eMT | −10.9 ± 129.5 | −25.0 ± 101.3 | 14.1 (−52.4, 80.7) | 0.1925 |
| iMT | 10.5 ± 82.0 | −22.8 ± 92.7 | 33.3 (−12.3, 78.9) | 0.2587 |
| aMT | 18.2 ± 103.2 | 22.8 ± 110.4 | −4.5 (−61.1, 52.0) | 0.9950 |
| pMT | 27.0 ± 85.8 | −21.7 ± 85.0 | 48.7 (2.5, 94.8) | 0.0463 |
| ccMF | −29.6 ± 176.2 | −86.1 ± 145.8 | 56.5 (−34.9, 147.8) | 0.0750 |
| ecMF | 4.4 ± 146.3 | −28.3 ± 115.6 | 32.7 (−42.6, 108.0) | 0.4128 |
| icMF | −5.6 ± 110.1 | −57.8 ± 106.2 | 52.2 (−6.7, 111.0) | 0.0463 |
| cLT | −0.4 ± 129.7 | −53.9 ± 184.8 | 53.5 (−24.3, 131.4) | 0.1646 |
| eLT | 21.8 ± 103.3 | −26.7 ± 86.1 | 48.4 (−5.2, 102.1) | 0.0205 |
| iLT | 3.5 ± 73.2 | −15.0 ± 98.5 | 18.5 (−24.5, 61.5) | 0.5267 |
| aLT | 8.4 ± 91.6 | −7.8 ± 102.4 | 16.2 (−34.6, 67.0) | 0.4641 |
| pLT | 22.6 ± 108.5 | −14.4 ± 91.5 | 37.1 (−19.4, 93.6) | 0.2940 |
| ccLF | 32.3 ± 116.4 | −12.2 ± 172.7 | 44.5 (−26.5, 115.5) | 0.2287 |
| ecLF | 43.5 ± 89.7 | −29.4 ± 108.0 | 73.0 (22.1, 123.8) | 0.0088 |
| icLF | 10.5 ± 109.1 | −12.8 ± 105.7 | 23.3 (−35.0, 81.7) | 0.2310 |
Values are the mean ± SD (median) μm. ThCTnS = total femorotibial subregional cartilage thinning summary score of negative change; ThCTkS = total femorotibial subregional cartilage thinning summary score of positive change; OVs = ordered values of subregional change in cartilage thickness (ranging from OV1, representing greatest cartilage loss, to OV16, representing greatest cartilage thickening).
Thickness of cartilage from the medial tibia (MT), central part of the medial weight‐bearing femur (cMF), lateral tibia (LT), and central part of the lateral weight‐bearing femur (cLF) was determined in the central (c), external (e), internal (i), anterior (a), and posterior (p) subregions.
Knees treated with 100 μg sprifermin also displayed significantly greater cartilage thickening than did knees treated with placebo (P = 0.029), with a mean ± SD ThCTkS of 744 ± 360 μm (median 710 μm, Q1/Q3 = 540/950 μm) in the 100‐μg sprifermin group compared with 507 ± 429 μm (median 380 μm, Q1/Q3 = 140/750 μm) in the placebo group (mean difference 237 μm, 95% CI 34, 440). The individual ThCTnS:ThCTkS ratio medians were −0.6 (Q1/Q3 = −1.0/−0.3) in the sprifermin‐treated patients and −2.1 (Q1/Q3 = −9.8/−0.4 μm) in the placebo group (P = 0.014).
Observational results in femorotibial subregions and ordered values
Compared with placebo‐treated patients, those treated with 100 μg sprifermin gained more cartilage thickness over 12 months in 11 of the 16 subregions and lost less cartilage in 5 of the 16 subregions. However, the difference between the sprifermin‐ and placebo‐treated patients reached significance (P < 0.05) in only 4 of the subregions (absolute mean change from baseline ranging from 48.4 μm to 73.0 μm) (Table 2 and Figure 1A).
Figure 1.
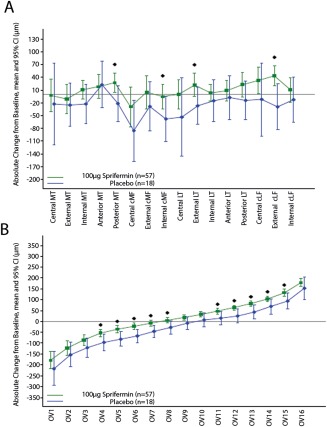
Change from baseline in subregional cartilage thickness at 12 months of followup, according to A, subregions of the cartilage and B, ordered values (OVs) of the magnitude of change in cartilage thickness (ranging from OV1, representing greatest cartilage loss, to OV16, representing greatest cartilage thickening). Significant treatment effects (unadjusted P < 0.05) were detected in 4 of 16 subregions and in 10 of 16 OVs. Bars show the mean and 95% confidence interval (95% CI). Symbols indicate individual change values that differed significantly between the 100 μg sprifermin–treated and placebo‐treated patients. MT = medial tibia; cMF = central part of the medial weight‐bearing femur; LT = lateral tibia; cLF = central part of the lateral weight‐bearing femur.
Sprifermin‐treated patients either gained more or lost less cartilage thickness than placebo‐treated patients in all 16 OVs, with the difference reaching significance (P < 0.05) in 10 of the 16 OVs (absolute mean change from baseline ranging from 32.0 μm to 45.1 μm) (Table 2 and Figure 1B). These 10 OVs included 5 (OV4–OV8) for which greater cartilage loss was observed in the placebo‐treated patients as compared with the sprifermin‐treated patients, and 5 (OV11–OV15) for which greater cartilage gain was observed in the sprifermin‐treated patients as compared with the placebo‐treated patients (Figure 1B).
Results in the 10‐μg and 30‐μg sprifermin cohorts
Results in patients treated with 10 μg or 30 μg sprifermin are provided in Supplementary Table 2. Differences in thinning and thickening of the cartilage, subregions of the cartilage, and OVs did not reach statistical significance between the sprifermin‐ and placebo‐treated patients in these dose groups.
DISCUSSION
This is the first study to apply a location‐independent approach to the analysis of cartilage change in a DMOAD trial, the first to use summary scores of cartilage thinning and thickening as exploratory end points, and the first to show that an anabolic drug such as sprifermin has the potential to reduce cartilage loss.
Limitations of the study include the small sample size, in particular in the sprifermin cohorts receiving the lower doses. Furthermore, we used only matched placebo‐treated patients, rather than all placebo‐treated patients, for comparison with the 100‐μg sprifermin cohort, because this was a dose escalation safety study, and therefore the 10‐μg, 30‐μg, and 100‐μg cohorts were randomized and matched with placebo controls at different times throughout the study. Moreover, the current analysis did not account for missing data and did not adjust for baseline values and covariance.
Another limitation is that this study was a post hoc analysis, being completed after the RCT; although the ThCTnS was a prespecified primary focus of this analysis, it was not a prespecified end point in the original study protocol. Furthermore, it has to be noted that, like the subregion analysis, the OV approach 14 is subject to multiplicity issues and needs to be formally adjusted for multiple parallel testing, when all 16 OVs are evaluated. In contrast, a strength of using thinning and thickening summary scores is the reduction in statistical multiplicity (2 versus 16).
Finally, the spatial resolution of MRI is limited and relatively coarse, in view of the changes in cartilage thickness seen in knee OA during typical trial durations of ≤1 year. Nevertheless, the mean thickness measurements are averaged over >2,000 single measurements per subregion 13, and OV1 (the subregion with the greatest loss) assumed a mean ± SD cartilage thickness change of −217 ± 158 μm in the placebo‐treated patients (those matched to the 100‐μg sprifermin cohort), which compares very favorably with the test–retest error (with repositioning) of 19–84 μm reported for subregions 13. The thinning and thickening scores provide a further advantage over OVs in this context, representing averages across several subregions. Averaging results of cartilage thickness change over defined anatomic regions, however, has inherent limitations; this is because knee OA is spatially heterogeneous, and local change in cartilage thickness occurs at different, largely unpredictable joint locations 11, owing, at least in part, to the biomechanical (micro)environment 12. Averaging results over regions in DMOAD trials would be similar to limiting observations to single vertebrae (e.g., T10) in osteoporotic fracture trials, whereas the subregional summary score equates to taking all vertebral fractures into account, independent of the thoracolumbar level at which they occur. With regional approaches, DMOAD effects may be obscured and cancel out each other, when occurring in different directions across different knees from different patients, in the same anatomic location 11. Thus, the subject‐specific, location‐independent approach of determining cartilage thinning and thickening summary scores is more informative and sensitive in detecting efficacy when compared with subregion analysis, because “noise” from the spatial heterogeneity of cartilage change is reduced.
Sprifermin is known to stabilize an anabolic chondrocyte phenotype, stimulate chondrogenesis in vitro, and induce cartilage repair in OA animal models 15. The current results suggest that, when compared with placebo, treatment with sprifermin not only adds cartilage in some locations in the joint (to increase cartilage volume and thickness globally, or at regions where cartilage thickness can be expected to be static), but also reduces cartilage loss, if it occurs. As demonstrated by ThCTnS:ThCTkS ratio medians of −0.6 μm in the 100‐μg sprifermin–treated patients and 2.1 μm in the placebo‐treated patients, on a location‐independent subject level, the gain in cartilage thickness in sprifermin‐treated patients was greater than the loss, whereas in placebo‐treated patients, the loss was greater than the gain. These findings are generally consistent with the results from the RCT reported previously 10.
Therefore, when administered intraarticularly at a 100‐μg dose in 2 cycles of 3 once‐weekly injections, sprifermin appears to be effective at locations of cartilage loss. To what extent these disease‐modifying effects translate into clinical benefit, and at what point in time they occur, are beyond the scope of this report and will have to be explored in future studies.
In conclusion, this study shows that sprifermin not only increases cartilage thickness, but also reduces cartilage loss. Subject‐specific, location‐independent analysis of cartilage thinning and thickening represents a sensitive and informative approach to studying disease‐modifying drugs for the treatment of knee OA.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Eckstein had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Eckstein, Guermazi, Aydemir.
Acquisition of data
Eckstein, Guermazi.
Analysis and interpretation of data
Eckstein, Wirth, Guermazi, Maschek, Aydemir.
ROLE OF THE STUDY SPONSOR
This study was sponsored by Merck Serono S.A. (Geneva, Switzerland), a subsidiary of Merck KGaA (Darmstadt, Germany). Merck Serono was involved in the study design, collection, analysis, and interpretation of the data, and manuscript preparation, as well as the decision to publish. Statistical analyses were completed at the Department of Biostatistics at EMD Serono. Publication of this article was not contingent on the approval of Merck Serono.
ADDITIONAL DISCLOSURES
Dr. Eckstein is chief executive officer/chief medical officer and co‐owner of Chondrometrics GmbH, a company providing MRI reading services to academic researchers and to industry, and Drs. Wirth and Maschek are part‐time employees and co‐owners of Chondrometrics GmbH. Dr. Guermazi is president of Boston Imaging Core Lab, LLC, a company providing MRI reading services to academic researchers and to industry. Ms Aydemir is an employee of EMD Serono.
Supporting information
Supplementary Table 1. Baseline characteristics of the 10 μg and 30 μg sprifermin and matched placebo study populations
Supplementary Table 2. One‐year changes from baseline in the 10 μg and 30 μg sprifermin and matched placebo cohorts, and differences from matching placebo in cartilage thickness (μm), including ordered values (OVs) and anatomic subregion comparisons
ACKNOWLEDGMENTS
We thank the principal study investigators of the sprifermin RCT, the readers at Chondrometrics who performed the quantitative cartilage analysis, and Scarlett Hellot for help with the statistical analysis. We thank Claudia Pena Rossi, Norma Muurahainen, and Yong Li of EMD Serono for assistance in the development of the manuscript. We also appreciate the editorial assistance provided by Sharon Cato (Discovery London Ltd., London, UK), whose work was funded by Merck Serono.
Clinicaltrials.gov identifier: NCT01033994.
REFERENCES
- 1. Losina E, Weinstein AM, Reichmann WM, Burbine SA, Solomon DH, Daigle ME, et al. Lifetime risk and age at diagnosis of symptomatic knee osteoarthritis in the US. Arthritis Care Res (Hoboken) 2013;65:703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence‐based, expert consensus guidelines. Osteoarthritis Cartilage 2008;16:137–62. [DOI] [PubMed] [Google Scholar]
- 3. Weinstein AM, Rome BN, Reichmann WM, Collins JE, Burbine SA, Thornhill TS, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am 2013;95:385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reginster JY, Badurski J, Bellamy N, Bensen W, Chapurlat R, Chevalier X, et al. Efficacy and safety of strontium ranelate in the treatment of knee osteoarthritis: results of a double‐blind, randomised placebo‐controlled trial. Ann Rheum Dis 2013;72:179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hellio Le Graverand MP, Clemmer RS, Redifer P, Brunell RM, Hayes CW, Brandt KD, et al. A 2‐year randomised, double‐blind, placebo‐controlled, multicentre study of oral selective iNOS inhibitor, cindunistat (SD‐6010), in patients with symptomatic osteoarthritis of the knee. Ann Rheum Dis 2013;72:187–95. [DOI] [PubMed] [Google Scholar]
- 6. Eckstein F, Guermazi A, Gold G, Duryea J, Hellio Le Graverand MP, Wirth W, et al. Imaging of cartilage and bone: promises and pitfalls in clinical trials of osteoarthritis. Osteoarthritis Cartilage 2014;22:1516–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Raynauld JP, Martel‐Pelletier J, Bias P, Laufer S, Haraoui B, Choquette D, et al. Protective effects of licofelone, a 5‐lipoxygenase and cyclo‐oxygenase inhibitor, versus naproxen on cartilage loss in knee osteoarthritis: a first multicentre clinical trial using quantitative MRI. Ann Rheum Dis 2009;68:938–47. [DOI] [PubMed] [Google Scholar]
- 8. Wildi LM, Raynauld JP, Martel‐Pelletier J, Beaulieu A, Bessette L, Morin F, et al. Chondroitin sulphate reduces both cartilage volume loss and bone marrow lesions in knee osteoarthritis patients starting as early as 6 months after initiation of therapy: a randomised, double‐blind, placebo‐controlled pilot study using MRI. Ann Rheum Dis 2011;70:982–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pelletier JP, Roubille C, Raynauld JP, Abram F, Dorais M, Delorme P, et al. Disease‐modifying effect of strontium ranelate in a subset of patients from the Phase III knee osteoarthritis study SEKOIA using quantitative MRI: reduction in bone marrow lesions protects against cartilage loss. Ann Rheum Dis 2015;74:422–9. [DOI] [PubMed] [Google Scholar]
- 10. Lohmander LS, Hellot S, Dreher D, Krantz EF, Kruger DS, Guermazi A, et al. Intraarticular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: randomized, double‐blind, placebo‐controlled trial. Arthritis Rheumatol 2014;66:1820–31. [DOI] [PubMed] [Google Scholar]
- 11. Buck RJ, Wirth W, Dreher D, Nevitt M, Eckstein F. Frequency and spatial distribution of cartilage thickness change in knee osteoarthritis and its relation to clinical and radiographic covariates: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2013;21:102–9. [DOI] [PubMed] [Google Scholar]
- 12. Chang A, Moisio K, Chmiel JS, Eckstein F, Guermazi A, Almagor O, et al. Subregional effects of meniscal tears on cartilage loss over 2 years in knee osteoarthritis. Ann Rheum Dis 2011;70:74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wirth W, Eckstein F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging 2008;27:737–44. [DOI] [PubMed] [Google Scholar]
- 14. Wirth W, Buck R, Nevitt M, Le Graverand MP, Benichou O, Dreher D et al. MRI‐based extended ordered values more efficiently differentiate cartilage loss in knees with and without joint space narrowing than region‐specific approaches using MRI or radiography: data from the OA Initiative. Osteoarthritis Cartilage 2011;19:689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moore EE, Bendele AM, Thompson DL, Littau A, Waggie KS, Reardon B et al. Fibroblast growth factor‐18 stimulates chondrogenesis and cartilage repair in a rat model of injury‐induced osteoarthritis. Osteoarthritis Cartilage 2005;13:623–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Baseline characteristics of the 10 μg and 30 μg sprifermin and matched placebo study populations
Supplementary Table 2. One‐year changes from baseline in the 10 μg and 30 μg sprifermin and matched placebo cohorts, and differences from matching placebo in cartilage thickness (μm), including ordered values (OVs) and anatomic subregion comparisons


