Abstract
Ill or immunosuppressed hospital patients are at increased risk for herpes simplex and herpes zoster virus infections, with high potential morbidity and mortality. Here, we present two cases of reactivation of herpes virus infections with delay in diagnosis, with ultimately fatal results. Since these infections are treatable, it is important to keep a high index of suspicion to facilitate early diagnosis and treatment.
Abbreviations: ARDS, acute respiratory distress syndrome; BMT, bone marrow transplant; CLL, chronic lymphocytic leukemia; CT, computed tomography; ECMO, extracorporeal membrane oxygenation; HSV, herpes simplex virus; PCR, polymerase chain reaction; TEN, toxic epidermal necrolysis; VZV, varicella zoster virus
Keywords: Varicella zoster virus, Herpes simplex virus, Immunosuppressed, Autopsy
Introduction
Herpesviruses (family Herpesviridae) are linear, double stranded DNA viruses that can establish latency in humans, and viral reactivation may cause substantial morbidity and mortality. In this report, we present two examples of cases of reactivation of herpes virus infections in immunosuppressed or ill patients, with ultimately fatal results. The first case was diagnosed after several days of hospitalization, and the second was only recognized postmortem. Since these infections are treatable, a high index of suspicion for herpes virus infections in hospitalized patients is crucial for early diagnosis and treatment.
Case reports
Case 1
A 77-year-old woman with multiple medical problems, including chronic lymphocytic leukemia (CLL), was admitted with severe abdominal pain and emesis. Endoscopy revealed gastritis with gastric ulcerations. A computed tomography (CT) scan showed diffuse abdominal lymphadenopathy. The patient was leukopenic (white blood cell count 2600/μL) and thrombocytopenic (platelet count 78,000/μL) after recent ofatumumab chemotherapy, which targets cells expressing CD20. While hospitalized, she became febrile and hypotensive and was treated for an E. coli urinary tract infection. Several days after admission, she developed a rash consisting of small erythematous macules and papules, involving the face, trunk, upper arms, and upper thighs, with biopsy consistent with varicella zoster. At that point, her clinical picture was considered suspicious for disseminated varicella zoster virus (VZV), and acyclovir therapy was initiated. During hospitalization, her creatinine increased, she had a worsening transaminitis, and her amylase and lipase were mildly elevated. She became persistently febrile, diaphoretic, and dyspneic, and died. Permission for autopsy was obtained.
At autopsy, the patient had innumerable erythematous macules and papules on the skin of her face, trunk, and proximal limbs. Her liver displayed numerous small hemorrhagic lesions of the capsule and parenchyma (Fig. 1). Histologically, foci of necrosis were present. Multifocal shallow ulcerations and mucosal erythema were seen in the stomach. Patchy hemorrhage and necrosis were present in the pancreas, with inflammation of peripancreatic nerves. The liver, stomach, pancreas, and peripancreatic nerves all showed strong positivity with immunohistochemical staining for VZV, consistent with disseminated VZV with visceral involvement (Fig. 2). Other autopsy findings included diffuse lymphadenopathy, bone marrow lymphocytic aggregates (consistent with her known CLL), and bladder erythema with mucosal hemorrhage.
Fig. 1.
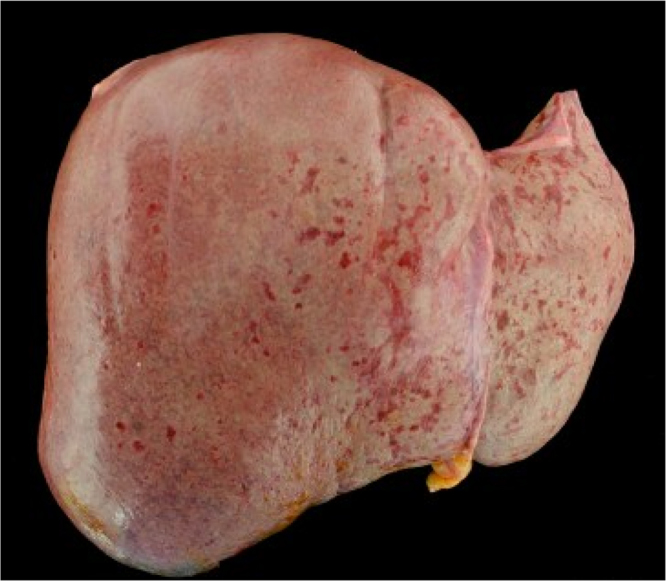
External surface of liver, Case 1.
Fig. 2.

Positive varicella zoster virus stain on section of peripancreatic nerves, Case 1.
Case 2
A 32-year-old woman with a history of type 1 diabetes and stage IV chronic kidney disease was admitted for toxic epidermal necrolysis syndrome. She had been treated with trimethoprim/sulfamethoxazole a few weeks prior for dermatitis after a tick bite, and presented to the hospital with fever and myalgias. After treatment with doxycycline, she developed skin sloughing and rash. She then developed multi-drug resistant Pseudomonas necrotizing pneumonia, complicated by bronchopulmonary fistula. She underwent right anterior thoracotomy with lung resection and debridement, but eventually developed progressive multi-organ failure including the kidneys and lungs, as well as loss of skin barrier function. She remained intubated, was treated with multiple antimicrobials and vasopressors, and received high-dose steroids. Despite being placed on extracorporeal membrane oxygenation (ECMO), her pulmonary status did not improve, and she died a few days later.
At autopsy, the patient had bilateral pulmonary consolidation with diffuse parenchymal nodularity. There was right pleural exudate and adhesions with residual necrotic parenchyma adjacent to the site of her recent debridement and resection. Histologically, the lungs showed diffuse necrotizing bronchopneumonia with multifocal necrotic and inflammatory infarcts with cells displaying viral cytopathic effect suspicious for herpes simplex virus (HSV). Bacterial cultures of the lungs grew Pseudomonas aeruginosa. Lung tissue sent for molecular analysis using polymerase chain reaction (PCR) was positive for HSV2 and negative for HSV1.
The liver displayed numerous small foci of acute hepatocyte necrosis with hemorrhage and apparent viral cytopathic effect. PCR testing performed on liver tissue was positive for HSV2 and negative for HSV1. The skin demonstrated generalized epidermal desquamation consistent with toxic epidermal necrolysis (TEN); however, microscopic examination showed viral cytopathic effect consistent with HSV. Additionally, inflammation, necrosis, and viral cytopathic effect were present in the trachea. Therefore, the patient was proven to have HSV2 infection of the lungs and liver, with likely involvement of the skin and trachea, in addition to Pseudomonas pneumonia.
Discussion
Reactivation of herpes virus infections in immunosuppressed or critically ill patients may cause substantial morbidity and mortality. These two cases are examples with ultimately fatal results. In the first case, VZV was not diagnosed for several days until a characteristic skin rash appeared, although visceral zoster likely caused the patient’s presenting symptom of severe abdominal pain. In the second case, infection with HSV2 was not recognized until autopsy. Since these infections are treatable, earlier recognition may have had impacted the clinical course.
Prior to varicella vaccination, over 90% of adults had been exposed to VZV by their 20s and thus harbor latent infection in their dorsal root ganglia [1], with risk for reactivation. Disseminated cutaneous zoster has been defined as more than 20 vesicles outside the area of the primary and adjacent dermatomes [2], or involvement of 3 or more dermatomes. Visceral zoster can be defined as histologic or culture evidence of VZV and clinical evidence of internal organ involvement in the setting of cutaneous zoster [3]. Laboratory evidence can take the form of immunohistochemical, direct immunofluorescence, genetic, or culture positivity for VZV [4]. Internal organs affected may include the liver, stomach, lungs, brain, and pancreas [4], [5]. In case 1, the cause of death was disseminated VZV infection with visceral involvement, including hepatitis, gastritis, pancreatitis, peripancreatic neuritis, and disseminated cutaneous disease.
Disseminated and visceral zoster are more common in immunosuppressed individuals, cancer patients (especially those with lymphoma and leukemia) [6], and transplant recipients [1]. Disseminated zoster can occasionally present in the absence of known immunosuppression, and age-related declines in cellular immunity may play a role [7]. This patient was at high risk due to her hematologic malignancy, recent chemotherapy, and leukopenia. In combination with her age and other comorbidities, reactivated zoster infection resulted in multiorgan failure and death. Had clinical suspicion for zoster been present at admission, prior to development of the characteristic rash, earlier initiation of acyclovir therapy may have been helpful.
Similar cases have been previously reported in the literature. Schiller et al. reported 3 cases of visceral VZV preceding cutaneous symptoms in allogeneic bone marrow transplant (BMT) patients, with life-threatening complications including hepatitis and pancreatitis [3]. Stratman reported a case with gastric dissemination of VZV as the presenting symptom, and suggested that “unexplained hepatitis, pancreatitis, gastritis, or complaints of abdominal pain in immunocompromised patients…should prompt a high degree of suspicion for visceral zoster” [4]. Rusthoven et al. studied several hundred cases of VZV infection in cancer patients, and found the highest risk of infection in patients with leukemia and lymphoma [6]. They reported disseminated disease in 12% of cases [6]. Rarely, disseminated zoster has been reported even in immunocompetent patients [7]. Disseminated visceral zoster is associated with a high mortality rate; for example, 55% of those affected died in one 1985 study of BMT patients [8]; in contrast, pure cutaneous dissemination is rarely fatal.
In case 2, the patient had toxic epidermal necrolysis (TEN), presumably associated with antimicrobial use, then developed a necrotizing pneumonia during her hospitalization. Her antemortem cultures repeatedly grew multi-drug resistant Pseudomonas aeruginosa, despite treatment with several broad-spectrum antimicrobials, with cavitary disease requiring debridement. Pseudomonas pneumonia is unusual in otherwise healthy individuals, but is a common nosocomial pathogen in ill and immunosuppressed patients [9], [10]. The patient had received high-dose steroids for her TEN, which likely contributed to immunosuppression.
At autopsy, the presence of viral cytopathic effect consistent with HSV in the patient’s lungs, liver, trachea, and skin was an unexpected finding. Postmortem lung and liver tissue tested positive for HSV2 by PCR. HSV1 has a seroprevalence of 50 to 80% among adults, with HSV2 seroprevalence of 15 to 50% depending on age, gender, and race [1], and 22% overall in US adults [11]. Hence, reactivation disease is possible in the majority of patients. HSV can cause pneumonia, hepatitis, and disseminated visceral disease, especially in immunosuppressed patients. It can be difficult to diagnose in the absence of cutaneous lesions, and is often fatal [5].
In the literature, Kusne et al. reported 12 cases of HSV hepatitis, where 8 patients died and 7 of those were not diagnosed with HSV until autopsy or within 24–48 h of death [12]. Fatal HSV2 hepatitis has also been reported in a heart transplant recipient [13]. Luyt et al. studied non-immunocompromised patients receiving mechanical ventilation for >5 days, and found that 21% of those who deteriorated clinically had HSV bronchopneumonitis [14]. Additional studies have shown HSV positivity in the upper respiratory tract of intensive care patients is associated with worse outcomes, including increased length of stay, acute respiratory distress syndrome (ARDS), and reduced survival [15], [16].
In our patient, untreated visceral HSV infection superimposed on her Pseudomonas pneumonia could explain a progressive decline despite appropriate antimicrobial treatment. Of note, HSV can be associated with TEN [17], [18]; however, antimicrobials are a more likely etiology based on the history in this case, especially since viral cytopathic effect was not noted on an early skin biopsy. Premortem recognition of this infection, and initiation of appropriate antiviral therapy, may have been clinically beneficial.
Conclusion
Given the protean manifestations of herpes simplex and herpes zoster infections, their potential morbidity and mortality, and the increased risk in ill or immunosuppressed hospital patients, it is important to keep a high index of suspicion to facilitate early diagnosis and treatment.
Competing interests
None declared.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Not required.
Acknowledgements
None.
Contributor Information
Christine Bookhout, Email: Christine.Bookhout@unchealth.unc.edu.
Vincent Moylan, Email: Vincent.Moylan@unchealth.unc.edu.
Leigh B. Thorne, Email: Leigh.Thorne@unchealth.unc.edu.
References
- 1.Jenkins F.J., Rowe D.T., Rinaldo C.R. Herpesvirus infections in organ transplant recipients. Clin Diagn Lab Immunol. 2003;10:1–7. doi: 10.1128/CDLI.10.1.1-7.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCrary M.L., Severson J., Tyring S.K. Varicella zoster virus. J Am Acad Dermatol. 1999;41(1):1–14. doi: 10.1016/s0190-9622(99)70398-1. [DOI] [PubMed] [Google Scholar]
- 3.Schiller G.J., Nimer S.D., Gajewski J.L., Golde D.W. Abdominal presentation of varicella-zoster infection in recipients of allogeneic bone marrow transplantation. Bone Marrow Transplant. 1991;7(6):489–491. [PubMed] [Google Scholar]
- 4.Stratman E. Visceral zoster as the presenting feature of disseminated herpes zoster. J Am Acad Dermatol. 2002;46(5):771–774. doi: 10.1067/mjd.2002.119091. [DOI] [PubMed] [Google Scholar]
- 5.Miller G.G., Dummer J.S. Herpes simplex and varicella zoster viruses: forgotten but not gone. Am J Transplant. 2007;7(4):741–747. doi: 10.1111/j.1600-6143.2006.01718.x. [DOI] [PubMed] [Google Scholar]
- 6.Rusthoven J.J., Ahlgren P., Elhakim T., Pinfold P., Reid J., Stewart L., Feld R. Varicella-zoster infection in adult cancer patients: a population study. Arch Intern Med. 1988;148(7):1561–1566. [PubMed] [Google Scholar]
- 7.Gupta S., Jain A., Gardiner C., Tyring S.K. A rare case of disseminated cutaneous zoster in an immunocompetent patient. BMC Fam Pract. 2005;14(6):50. doi: 10.1186/1471-2296-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Locksley R.M., Flournoy N., Sullivan K.M., Meyers J.D. Infection with varicella-zoster virus after marrow transplantation. J Infect Dis. 1985;152(6):1172–1181. doi: 10.1093/infdis/152.6.1172. [DOI] [PubMed] [Google Scholar]
- 9.Kumar P.D., Ravakhah K., West B. Disseminated Pseudomonas aeruginosa and necrotizing pneumonia with complete recovery. South Med J. 2001;94:229–232. [PubMed] [Google Scholar]
- 10.Zavascki A.P., Barth A.L., Fernandes J.F., Moro A.L., Goncalves A.L., Goldani L.Z. Reappraisal of Pseudomonas aeruginosa hospital-acquired pneumonia mortality in the era of metallo-beta-lactamase-mediated multidrug resistance: a prospective observational study. Crit Care. 2006;10(4):R114. doi: 10.1186/cc5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleming D.T., McQuillan G.M., Johnson R.E., Nahmias A.J., Aral S.O., Lee F.K. Herpes simplex virus type 2 in the United States, 1976 to 1994. N Engl J Med. 1997;337:1105–1111. doi: 10.1056/NEJM199710163371601. [DOI] [PubMed] [Google Scholar]
- 12.Kusne S., Schwartz M., Breinig M.K. Herpes simplex virus hepatitis after solid organ transplantation in adults. J Infect Dis. 1999;163:1001–1007. doi: 10.1093/infdis/163.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pietrucha-Dilanchian P., Tanawuttiwat T., Abbo L., Regatieri A., Chaparro S., Ruiz P., Morris M.I. Fatal herpes simplex virus type 2 hepatitis in a heart transplant recipient: a case report and review of the literature. Transpl Infect Dis. 2013;15(3):E87–96. doi: 10.1111/tid.12077. [DOI] [PubMed] [Google Scholar]
- 14.Luyt C.E., Combes A., Debacket C., Aubriot-Lorton M.H., Nieszkowska A., Trouillet J.L. Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am J Respir Crit Care Med. 2007;175:935–942. doi: 10.1164/rccm.200609-1322OC. [DOI] [PubMed] [Google Scholar]
- 15.Bruynseels P., Jorens P.G., Demey H.E., Goossens H., Pattyn S.R., Elseviers M.M. Herpes simplex virus in the respiratory tract of critical care patients: a prospective study. Lancet. 2003;362:1536–1541. doi: 10.1016/S0140-6736(03)14740-X. [DOI] [PubMed] [Google Scholar]
- 16.Ong G.M., Lowry K., Mahajan S., Wyatt D.E., Simpson C., O’Neill H.J., et al Herpes simplex type 1 shedding is associated with reduced hospital survival in patients receiving assisted ventilation in a tertiary referral intensive care unit. J Med Virol. 2004;72:121–125. doi: 10.1002/jmv.10524. [DOI] [PubMed] [Google Scholar]
- 17.Harr T., French L.E. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;16:5–39. doi: 10.1186/1750-1172-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forman R., Koren G., Shear N.H. Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis in children: a review of 10 years’ experience. Drug Saf. 2002;25:965–972. doi: 10.2165/00002018-200225130-00006. [DOI] [PubMed] [Google Scholar]


