Competition for prioritization between basic motivational states, such as pain and thirst, is thought to inhibit the currently nonprioritized state. We show that thirst can reduce pain in humans, whereas at the same time enhance pain through increased negative mood.
Keywords: Pain, Motivation, Mood, Thirst, Emotion, Motivation-decision model
Abstract
Introduction: Although the motivation to avoid injury and pain is central to human and animal behavior, this goal compete priority with other homeostatic goals. Animal studies have shown that competing motivational states, such as thirst, reduce pain. However, such states may also induce negative mood, which in humans has been found to increase pain. These opposing effects complicate study of the effects of motivational states in humans.
Objectives: To evaluate concurrent effects of motivational state competition and mood on pain ratings.
Methods: We compared a thirst challenge against a control group and measured thirst and mood as potential mediators. Pain induced through contact heat stimulation on the left forearm and was tested at 3 time points: before group randomization, after thirst induction, and after rehydration.
Results: Overall, the thirst group reported more pain when thirsty compared with baseline and controls. Mediation analyses showed evidence for two opposing effects. First, the thirst challenge increased negative mood and thirstiness, which was related to increased pain. Second, the thirst challenge produced a direct, pain-reducing effect.
Conclusion: Competing motivational states reduce pain but also induce concurrent mood changes that can mask motivational state-related effects.
1. Introduction
In humans as in other organisms, physiological needs induce powerful motivational states that organize behavior to fulfill the goals imposed by these states. Such states include hunger, thirst, pain, social contact, and others.3,5,13,18,49 When multiple motivational states are present at the same time, they are thought to compete for priority, resulting in the inhibition of brain pathways that mediate low priority motivational states.1,18,20
Pain can be thought of as a motivational drive state related to potential tissue damage, which motivates behavior to minimizing present and future injury.9,20,49 Accordingly, the intensity and quality of pain is thought to be shaped by competing motivational states. Particularly, pain reduction due to competition among motivational states is linked to the activation of descending modulatory pathways and modulation at cerebral and spinal levels.20–22,26,31 Such interactions of motivational states are described in the Motivation-Decision model of pain20 and in more recent models.9,45
These models provide a set of underlying principles that explain how competing motivational states shape pain experience and thus pain behavior observable in animals. Consistent with this model, animal research has shown that several motivational states reduce pain behaviors, including thirst13 or the hedonic component of chocolate consumption.22 In humans, motivation to obtain secondary reinforcers, ie, monetary rewards, has been used to study motivational effects on pain perception. Gambles resulting in monetary gains or losses affected pain in opposite directions, with wins reducing, and losses increasing pain.4 Rewarding participants for good task performance also reduces pain.46
In addition to these direct effects on pain experience and pain behavior, motivational states can also induce changes in mood and emotions, especially when the strength of the competing state is increasing over time and the associated goal cannot be fulfilled, eg, becoming more and more thirsty during a long day.9,45 A number of studies have demonstrated that negative mood and emotions affects pain perception.25,34,36,37,51 In those studies, negative mood enhanced pain and positive mood reduced it. Thus, competing motivational states associated with positive mood are expected to reduce pain becausse of both the effects of mood and the motivational demand itself, rendering it impossible to distinguish the 2 effects. By contrast, experimentally inducing aversive motivational states could help to disentangle the effects of mood and the motivational challenge, because they are expected to have opposite effects. In doing so, it is necessary to induce an aversive motivational state without concurrent cognitive demand (which is also expected to reduce pain7,42), and measure negative mood (which is expected to increase pain) as a potential suppressor of motivational challenge effects.29
In this study, we manipulated thirst, a basic motivational state, by randomizing participants into a thirst or a control (no-thirst) group. We induced thirst through water deprivation and the consumption of salty food. Pain was tested at baseline, during the thirsty state, and after rehydration along with measures of mood and thirst. According to the Motivation-Decision model, the thirst challenge should reduce pain.9,20,21 Conversely, we expected the negative mood accompanying the challenge to increase pain.36,37 Mediation analyses28 were used to disentangle the direct motivational effect from effects mediated by mood.
2. Materials and methods
2.1. Sample
Forty-two healthy participants (20 female) aged between 18 and 53 years of age (mean age: 27.2 years) participated in this study. Half of the participants were randomized into the thirst group (12 female, mean age: 26.6 years) and the other half served as control group (8 female, mean age: 27.9 years). We chose a sample size of at least 42 on the basis of expected effect sizes based on experience on pain modulatory studies from our lab. Sample sizes of about 42 provide approximately 80% power to detect an effect size (partial correlation) of 0.4 or larger (G*Power 319). Participants reported no history of neurological, psychiatric, or dermatologic conditions, and had not taken any medication during the 48 hours before the experiment. Also, participants reporting acute and chronic pain conditions in an initial online screening were excluded from participation. We recruited participants from the university and local community through flyers and online ads. The Institutional Review Board of the University of Colorado Boulder approved the protocol.
2.2. Procedures
Participants first provided informed consent. Participants then rated their current thirst on a visual analog scale (VAS), anchored at the extreme ends with “no thirst at all” and “extremely thirsty.” They also reported their current mood on three dimensions (pleasant-unpleasant, awake-sleepy, and calm-restless) using the Multidimensional Mood Questionnaire (MDMQ43). Participants then completed the first of three pain tests.
Each pain test included 16 heat pain trials, totaling 48 trials over the course of the experiment. Within each block, 8 different temperatures ranging from 45°C to 49.5°C in steps of 0.5°C were repeated 2 times each. Stimulus duration was 11.5 seconds (including 2 seconds ramp up and down to a 32°C baseline) followed by a delay of 5 to 7 seconds before the pain VAS appeared on the screen. During an intertrial interval (ITI) of 12 to 16 seconds, a fixation dot was presented centrally on the screen. The fixation dot remained unchanged on the screen for the duration of the cutaneous heat stimulation (Fig. 1B). A Peltier thermode (1.5 × 1.5 cm surface, PATHWAY ATS; Medoc, Inc, Israel) delivered heat to the left volar forearm of the participant. Thermode position was changed in each block to prevent sensitization. Participants were instructed how to use the VAS scale and how to interpret the anchors. The VAS had 3 anchors, “no pain,” “pain threshold,” and “unbearable pain” positioned at the 0, 25, and 100 marks of the scale, respectively. The order of temperatures was randomized across participants, but the same order was used across the three blocks for a single participant. Stimulus presentation and response logging were controlled by MATLAB (R2015a; The MathWorks Inc, Natick, MA) using the Psychophysics Toolbox v3 (http://psychtoolbox.org/).
Figure 1.

Experimental design. (A) Timeline of the experiment. The experiment started with a thirst (visual analog scale) and mood rating (Multidimensional Mood Questionnaire). This rating was repeated 7 times during the experiment. A baseline pain test was administered before participants were randomized into either thirst or control group. After a waiting period, a second pain test was completed. All participants were then provided with water ad libitum to eliminate their thirst. After 30 mins, a last pain test was completed. (B) Trial structure. Each trial started with a variable intertrial interval (ITI). A heat pain stimulus was then applied while the fixation crosshair remained unchanged on the screen. After a variable delay, participants had 10 seconds to provide their pain rating on a visual analog scale.
After completing the first pain block, participants were randomized into either the thirst or the control group. Participants then moved to a separate room, where they spent a 4-hour waiting period. At the beginning of this waiting period, participants completed a set of questionnaires, including demographic information, the state-trait anxiety inventory (STAI),41 the pain catastrophizing scale (PCS),44 the life orientation test (LOT-R),38 the social desirability scale (SDS),10 the fear of pain (FoP30), and the personal distress subscale of the interpersonal reactivity index (IRI).12 Every hour during the waiting period participants rated their thirst and completed the MDMQ using the same scales as detailed above.
All participants ate 1 package of salty pretzels (100 g) and 1 package of salty goldfish (75 g) while waiting. The thirst group was not allowed to drink until the completion of the second pain test (Fig. 1A), whereas the control group was provided with water ad libitum during the waiting period.
Five hours after arrival, participants completed their sixth thirst and mood survey before undergoing the second pain block. Participants were then given as much water as they wanted to drink, regardless of which group they had been randomized into, and water intake was recorded. Thirty minutes later, participants completed the last thirst and mood questionnaire and a third pain block. Each pain block lasted 15 minutes and the entire procedure took approximately 6 hours per participant.
2.3. Analyses
Visual analog scale ratings were converted to numeric values ranging from 0 to 100 for both pain and thirst ratings. Pain ratings were averaged within each block for each participant for subsequent analyzes. We used the pleasant-unpleasant subscale of the MDMQ as a measure of negative mood in the following analyses. All results also hold for a summary score of the 3 MDMQ subscales. A 2 (control vs thirst group) × 7 (time) mixed analysis of variance (ANOVA) was conducted on the thirst ratings and pleasantness–unpleasantness MDMQ subscale. To explore the significant group effect in the thirst ratings, we conducted post hoc 2-sample t tests for each time point.
We hypothesized that the thirst group would show a change in pain perception only when thirsty (block 2), but not during baseline (block 1) or after rehydration (block 3). We therefore computed an a priori contrast testing the effects of thirst vs non-thirst blocks, ie, comparing block 2 (thirst) against the other conditions (pre- and post-thirst) using a multilevel general linear model (implemented in glmfit_multilevel.m27). This contrast assesses whether either of the two proposed mechanisms is strong enough to produce an overall change in mean pain ratings. Including participant sex as did not change the results and the sex effect was not significant (t39 = 0.76, P = 0.45). Stimulus intensity did not interact with group or the planned contrast of interest (all t39 < 1.22, all P > 0.22), suggesting stable effects across temperatures. Subsequent mediation analyses tested for effects controlling mood.
To disentangle opposing effects of the motivational challenge from the thirst and mood changes on pain, we used mediation analyses28 implemented in the Multilevel Mediation and Moderation (M3) Toolbox for MATLAB (http://wagerlab.colorado.edu/tools50). For the mediation analysis, we focused on the second pain test, because this was the critical test condition when half of the participants were thirsty. We first tested whether the effect of the motivational challenge (group) on pain was mediated by reported thirst. We then tested a second model to investigate whether the relationship between subjective thirst ratings and pain was in turn mediated by mood (note that higher values indicate more negative mood).
Mediation analysis tests whether the covariance between 2 variables X and Y (group and pain) can be explained by a third variable M (thirst). The following equations capture the mediation model:
Here y, x, and m represent the outcome (y, the reported pain), the predictor (x, group), and data from a potential mediator (m, thirst or mood).  ,
,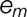 , and
, and 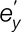 denote residual errors for the outcome and mediator controlling for x and the outcome controlling for x and m, respectively. Path a is the slope of m regressed onto x. Path b is the slope of the mediator-outcome relationship controlling for x. The paths c and c′ describe the linear relationship of x and y with and without the mediator, respectively. The mediation effect is obtained by the combined path ab. We report correlation coefficients and partial correlations as effect size measures for path coefficients a, b, and c′.28 For the indirect effect, we report
denote residual errors for the outcome and mediator controlling for x and the outcome controlling for x and m, respectively. Path a is the slope of m regressed onto x. Path b is the slope of the mediator-outcome relationship controlling for x. The paths c and c′ describe the linear relationship of x and y with and without the mediator, respectively. The mediation effect is obtained by the combined path ab. We report correlation coefficients and partial correlations as effect size measures for path coefficients a, b, and c′.28 For the indirect effect, we report 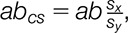 , in which the indirect effect is standardized by the SD of X and Y.33 This effect size indicates that Y increases by abcs SDs for every 1 SD increase in X indirectly through M.33 As is common practice,40,50 we used bias-corrected, accelerated bootstrap tests14 to assess statistical significance, using 50,000 bootstrap samples.
, in which the indirect effect is standardized by the SD of X and Y.33 This effect size indicates that Y increases by abcs SDs for every 1 SD increase in X indirectly through M.33 As is common practice,40,50 we used bias-corrected, accelerated bootstrap tests14 to assess statistical significance, using 50,000 bootstrap samples.
3. Results
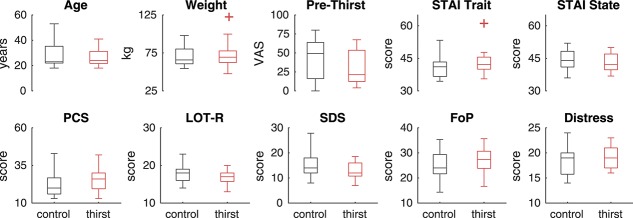
Thirst and control groups did not differ in age (t40 = 0.53, P = 0.59), male/female proportions (X2 = 0.875, P = 0.34), weight (t40 = 0.3, P = 0.77), baseline thirst (t40 = 1.21, P = 0.24), or psychological measures (all t40 < 1.8, all P > 0.07; Fig. 2 and Table 1).
Figure 2.
Group comparisons. Thirst and control groups did not differ in terms of age or psychological profile. None of the pairwise comparisons were significant at P < 0.05, uncorrected. Box plots show medians within the box and boxes extend from 25th to 75th percentiles. Outliers more than 2 interquartile ranges outside the boxes are represented by plus symbols. Prethirst ratings were obtained at the start of the experiment before block 1. STAI, state-trait anxiety inventory; PCS, pain catastrophizing scale; LOT-R, life orientation test–revised; SDS, social desirability scale; FoP, fear of pain; Distress, personal distress subscale.
Table 1.
Group statistics.
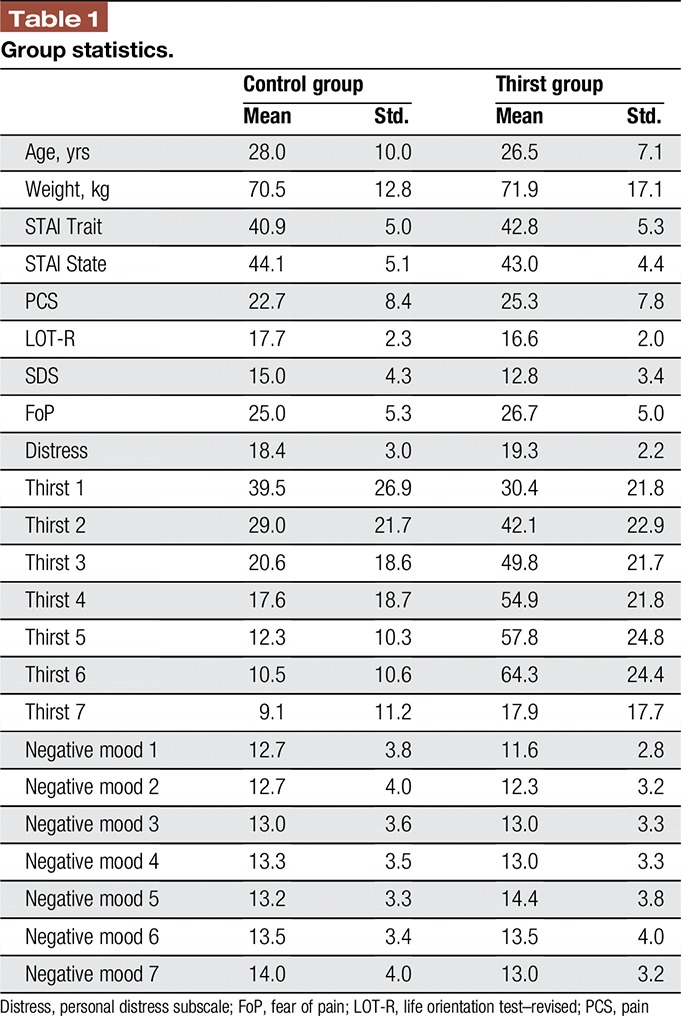
Testing the success of our thirst manipulation on the thirst ratings with an ANOVA revealed a significant main effect of group (F1,40 = 26.0, P < 0.001), a main effect of time (F6,240 = 17.7, P < 0.001), and a significant group × time interaction (F6,240 = 30.9, P < 0.001). Post-hoc t tests revealed that the thirst group was thirstier at time points 3 to 6, that is from 2 hours after the start of the experiment until the rehydration period (all t40 > 18.8, all P < 0.001, Fig. 3A). Furthermore, participants in the thirst group drank more than participants in the control group during the rehydration period (MThirst = 667 mL, MControl = 205 mL, t40 = 4.64, P < 0.001). An ANOVA on the mood ratings revealed a significant main effect of time (F6,240 = 15.2, P < 0.001). No significant effects were observed for group or the group × time interaction (all P > 0.11). Plotting the mood ratings against time revealed an increase in negative mood over the course of the experiment (Fig. 3B).
Figure 3.

Thirst and mood ratings. (A) Thirst ratings over the course of the experiment (see Fig. 1A) for control (black) and thirst (red) groups. Thirst ratings differed significantly between groups at time points 3 to 6 (all P < 0.001). (B) Mood ratings (pleasantness–unpleasantness subscale) for both groups using the same color coding as in A. The analysis of variance main effect of time was significant (P < 0.001), but the group effect was not. Note that higher mood values indicate negative mood. Gray bars depict pain tests in both groups (see Fig. 1A). VAS, visual analog scale.
We next investigated group and thirst effects on pain perception. The planned contrast testing the block × group interaction was significant (b = 1.08, Z = 2.03, P = 0.02) indicating that the pain increase during block 2 was stronger in the thirst group than in the control group (Fig. 4A). If motivational competition was the only relevant processes here, we would expect a decrease in pain ratings at this time point. Figure 4B–D shows pairwise relationships between the variables of interest: pain, mood, and thirst. Inspection of these scatterplots confirmed that no outliers were present.
Figure 4.

Pain ratings and bivariate relationships. (A) Mean pain ratings for control and thirst groups for the three pain tests. The a priori contrast testing for greater change at pain test 2 in the pain group than in the control group was significant (P = 0.02). Errors bars are between participant SEM. (B) Mean pain ratings at pain test 2 plotted against thirst ratings immediately before the pain test. Thirst and pain correlate positively in both groups, but the intercept is larger in the control group than in the thirst group. (C) Negative mood ratings plotted against thirst at the same time as in B. Stronger thirst correlates with negative feelings. (D) Pain ratings from pain test 2 plotted against the negative mood ratings. Negative mood correlates positively with pain. *P < 0.05. VAS, visual analog scale.
The first mediation model tested whether subjective thirst ratings (M) mediate effects of group (X) on pain (Y). All paths of the mediation model (Fig. 5A) were significant. As expected, group had a negative direct effect on pain, controlling for thirst (path c′ = −27.7, P = 0.002, rXY,M = −0.39). This demonstrates an analgesic effect of the thirst challenge as predicted by the Motivation-Decision model.20 Furthermore, participants in the thirst group were thirstier than control group participants as shown by the positive relationship between group and thirst (path a = 53.9, P < 0.001, rXM = 0.83). In addition, greater thirst predicted increased pain, controlling for group (path b = 0.63, P = 0.001, rMY,X = 0.54). The indirect, mediated pathway from group to pain through thirst was positive (path ab = 34.3, P = 0.002, abcs = 0.81). The latter 2 path coefficients indicate a pain-enhancing effect of subjective thirst experiences, instead of a pain reduction. The motivational challenge thus affected pain through two opposing processes, a direct analgesic effect (path c′), and another hyperalgesic process mediated by subjective thirst (path ab).
Figure 5.

Mediation analyses. (A) Mediation analysis of group onto pain perception by thirst. A significant mediation (path ab) by thirst was observed indicating that more thirst was related to more pain. The significant direct effect of group on pain (c') controlling for thirst, showed that the motivational challenge reduced pain. (B) Mediation analysis of thirst on pain by mood. The effect of thirst on pain was partially mediated by reduction in mood (path ab). The significant path c' indicates that mood does not explain the entire effect of thirst on pain. The same data as in Figure 4 are used in the mediation analyses. Note that higher values of mood indicate more negative mood to ease interpretability of the mediation effect. All path coefficients are unstandardized, standard errors are shown in parentheses. Orange arrows depict positive paths; blue arrows represent negative path coefficients. *P < 0.05; **P < 0.01; ***P < 0.001.
To test whether the hyperalgesic thirst rating effect was related to pain modulation by negative mood (M), we conducted a second mediation analysis. Again, all path coefficients were significant (Fig. 5B). A significant, direct effect of thirst on pain controlling for mood (path c′ = 0.21, P = 0.017, rXY,M = 0.34), confirmed the positive relationship between thirst and pain that is partially independent of the mediator mood. Path a demonstrated a positive relationship between thirst and negative mood (path a = 0.03, P = 0.03, rXM = 0.31). Negative mood was in turn positively related to pain (path b = 1.89, P = 0.021, rMY,X = 0.34) and negative mood mediated effects of thirst on pain (path ab = 0.07, P = 0.037, abcs = 0.1). The second mediation model demonstrated that negative mood contributed positively to pain and that mood mediated parts of the hyperalgesic thirst effect.
In summary, the mediation models indicated that the motivational challenge (group) had a direct analgesic effect, consistent with the Motivation-Decision model. This effect was opposed by a hyperalgesic effect of negative mood and subjective thirst.
To investigate personal differences contributing to pain, we explored relationships between personality traits and the pain. In this exploratory post-hoc analysis, only personal distress correlated with the increase in pain during thirst (r = 0.47, P = 0.001) across both groups.
4. Discussion
Using a primary motivational challenge in humans in combination with mediation analysis, we identified 2 opposing effects of basic motivational states on pain: first, a direct analgesic effect of the thirst challenge and, second, a hyperalgesic effect of subjective thirst mediated by mood. These results translate analgesic effects of primary motivational states previously observed in rodents to humans,13,20–22 and at the same time demonstrate the importance of opposing secondary processes, such as concurrent changes in mood.
Of these 2 component processes, the direct, analgesic effect of the motivational thirst challenge on pain supports theories of motivational competition.9,20,45 This analgesic process is reflected by the lower intercept of the thirst group in Figure 4B and is evidenced by the significant direct path c′ from group to pain (Fig. 5A). Perceived pain is thus lower in the thirst group than in the control group after controlling for subjective thirst reports in both groups. As indicated by the partial mediation effect, the thirst ratings capture only part of the changes induced by the motivational challenge. However, the subjective ratings were positively related to pain, whereas the motivational challenge (group) was negatively related to pain.
Participants exposed to the motivational challenge were in greater need of rehydration, a basic need. Current theories of pain processing suggest that information unrelated to the currently prioritized goal and underlying motivational state are suppressed, so pain should be reduced in this case,9,20 possibly through descending pain regulatory systems, which include the periaqueductal gray and the rostral ventromedial medulla6,21,26,31. The degree to which 1 state is prioritized over the other may depend on personal predispositions.2,8 For instance, some people may tend to prioritize thirst processing over pain processing. However, as thirst was manipulated between groups, and participants had no option to forgo painful stimulation, we reasoned that personal preference effects should be small compared with the group manipulation. Patients with chronic pain, however, may prioritize pain over competing motivational states. Recent rat studies have reported reduced reward pursuit in experimental models of chronic pain. These motivational impairments have been linked to changes in the nucleus accumbens,35,39 long known to be a key player in motivational drives.5 Our results suggest that a competing motivational state can reduce pain, if it is not accompanied by negative mood. Engaging in the pursuit of goals in situations that minimize frustration and negative mood could thus help patients with pain to cope with their pain, and possibly even reduce pain through descending neuromodulation.
One alternative hypothesis would be that participants in the thirst group shifted their attention away from pain toward the thirst as the water becomes more “salient.” Distraction and attentional shifts are known to reduce to reduce pain,7,32 in some cases through descending modulation.42 Furthermore, attention and mood can act independently on pain perception,47,48 which we also observe here. However, we would expect distraction and attentional shifts to be stronger with more subjective thirst, resulting in less pain. Instead, we observed that higher subjective thirst ratings were associated with increased pain (see below). It is thus unlikely that the analgesic effect is purely attentional. Another explanation is based on research dissociating consciously accessible and precognitive responses to threats and basic physiological challenges.23 For example, a recent study found that subjective expectation ratings were positively related to higher pain ratings, after a cue was associated with a high pain condition.24 Interestingly, in the same study, anticipatory skin conductance responses, as a measure of precognitive processing, were negatively correlated with pain ratings, suggesting that precognitive processes underlying autonomic threat responses can decrease pain. Pain perception is also susceptible to subliminally presented cues,23 suggesting that at least part of the pain modulatory system can operate without conscious processing. Further support for this idea comes from research on physiological responses in the anticipation of food and water consumption16,52. Here, anticipated motivationally relevant events, such as expecting water when thirsty, can induce compensatory reactions that oppose the normal responses to the anticipated event (eg, insulin release in anticipation of food, which aids sugar clearance from the bloodstream). In addition, the opioidergic system, which is critical for endogenous pain regulation, seems to be particularly sensitive to exhibit such opposing, compensatory responses.15
The second, hyperalgesic processes identified by the mediation analyses linked stronger thirst and more negative mood reports to increased pain. Choosing an aversive motivational state in this study ensured that the emotional reaction to this state would be negative, and in this study, an increase in negative mood led to increased pain ratings. This effect is in line with previous reports of thirst leading to negative mood11,17 and of increased pain after negative mood.25,51 For example, previous studies have reported increased pain perception when participants viewed unpleasant pictures.36,37 Here, thirsty participants were eager to drink water, but we prevented them from reaching this goal until the rehydration period after the second pain test. This goal interference induces negative affect.9 Participants who felt most thirsty experienced this most strongly, leading to more negative effect, and consequently to increased pain. This finding is also in line with the idea that emotions provide valuable feedback about motivational states,2 thus helping humans to fulfill their needs. However, an interesting hypothesis for future studies suggested by Baumeister's framework2 is that motivational states may be able to affect perception, emotion, and cognition even in the absence of motivational competition. Although, we expected the thirst ratings to be related to the analgesic motivational process, their positive relationship with negative mood suggests that they are at least partially dissociable from the underlying motivational state.
Overall, the mood-related, hyperalgesic effect was stronger than the analgesic effect of the motivational challenge. The balance between these two processes may shift depending on the strength of the competing states. For example, water deprivation in rats led to an analgesic behavioral response.13 In this case, the motivational state competition dominates the pain perception, whereas in our study the opposing (hyperalgesic) mood effect dominates pain experience. We have 3 explanations for this: first, the strength of the effects of competing states (eg, thirst) may change with their survival relevance. In rats, without any knowledge of the experimental context and in the absence of behavioral control, water deprivation represents a strong threat to survival. By contrast, participants in our study knew that they would receive water later. When participants know they are safe, the mood effect dominates pain ratings. Second, the change in mood may be specific for humans, and rats might simply not undergo a similar shift in mood after a thirst induction. Third, we measured perceived pain intensity, whereas rat studies measure pain-related changes in behavior. Although pain perception is thought to be reduced by competing states,20,21 pain behavior might be a more sensitive measure in the context of motivational demands.8
A limitation of this study is that we did not independently manipulate mood. This is clearly a logical next step. One potential problem is that such a mood manipulation would either work in the same direction as the naturally occurring mood changes because of the thirst, or the mood manipulation would have to be so strong as to overcome the mood effects of thirst. We thus decided to leverage the individual differences in mood in response to our thirst challenge rather than to attempt multiple simultaneous mood manipulations. Furthermore, measures of attention, stress, and choice behavior should be included in future studies to elucidate psychological processes related to the analgesic effect of the motivational challenge in more detail.
In summary, the present results demonstrate 2 important effects of motivational competition in human pain processing. A direct, analgesic effect occurs in response to a motivational challenge and an indirect, hyperalgesic process occurs at the same time that is mediated by the participants' mood. The balance between these opposing processes will most likely depend on the strength of the competing motivational states. More broadly, studies of the effects of motivational states in humans must jointly consider the effects of the motivational challenge itself, and mood.
Conflict of interest statement
The authors have no conflicts of interest to declare.
S. Geuter was supported by the DFG (GE 2774/1-1). T. D. Wager was supported by NIH grants 2R01MH076136 and R01DA035484.
Acknowledgements
Author Contributions: S. Geuter and T. D. Wager conceived the study. S. Geuter designed the paradigm. S. Geuter and J. T. Cunningham performed experiments. S. Geuter performed the data analysis. S. Geuter drafted the manuscript, and J. T. Cunningham and T. D. Wager provided critical revisions. All authors approved the final version of the article for submission.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Bahlmann J, Aarts E, D'Esposito M. Influence of motivation on control hierarchy in the human frontal cortex. J Neurosci 2015;35:3207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Baumeister RF. Toward a general theory of motivation: problems, challenges, opportunities, and the big picture. Motiv Emot 2016;40:1–10. [Google Scholar]
- [3].Bear T, Philipp M, Hill S, Mündel T. A preliminary study on how hypohydration affects pain perception. Psychophysiology 2016;53:605–10. [DOI] [PubMed] [Google Scholar]
- [4].Becker S, Gandhi W, Elfassy NM, Schweinhardt P. The role of dopamine in the perceptual modulation of nociceptive stimuli by monetary wins or losses. Eur J Neurosci 2013;38:3080–8. [DOI] [PubMed] [Google Scholar]
- [5].Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav 2004;81:179–209. [DOI] [PubMed] [Google Scholar]
- [6].Bingel U, Tracey I. Imaging CNS modulation of pain in humans. Physiol (Bethesda) 2008;23:371–80. [DOI] [PubMed] [Google Scholar]
- [7].Buhle J, Wager TD. Performance-dependent inhibition of pain by an executive working memory task. PAIN 2010;149:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Claes N, Crombez G, Vlaeyen JWS. Pain-avoidance versus reward-seeking: an experimental investigation. PAIN 2015;156:1449–57. [DOI] [PubMed] [Google Scholar]
- [9].Crombez G, Eccleston C, Van Damme S, Vlaeyen JWS, Karoly P. Fear-avoidance model of chronic pain: the next generation. Clin J Pain 2012;28:475–83. [DOI] [PubMed] [Google Scholar]
- [10].Crowne DP, Marlowe D. A new scale of social desirability independent of psychopathology. J Consult Psychol 1960;24:349–54. [DOI] [PubMed] [Google Scholar]
- [11].D'anci KE, Vibhakar A, Kanter JH, Mahoney CR, Taylor HA. Voluntary dehydration and cognitive performance in trained college athletes. Percept Mot Skills 2009;109:251–69. [DOI] [PubMed] [Google Scholar]
- [12].Davis MH. Measuring individual differences in empathy: evidence for a multidimensional approach. J Pers Soc Psychol 1983;44:113–26. [Google Scholar]
- [13].Dum J, Herz A. Endorphinergic modulation of neural reward systems indicated by behavioral changes. Pharmacol Biochem Behav 1984;21:259–66. [DOI] [PubMed] [Google Scholar]
- [14].Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- [15].Eikelboom R, Stewart J. Conditioned temperature effects using morphine as the unconditioned stimulus. Psychopharmacology (Berl) 1979;61:31–8. [DOI] [PubMed] [Google Scholar]
- [16].Eikelboom R, Stewart J. Conditioning of drug-induced physiological responses. Psychol Rev 1982;89:507–28. [PubMed] [Google Scholar]
- [17].Ely BR, Sollanek KJ, Cheuvront SN, Lieberman HR, Kenefick RW. Hypohydration and acute thermal stress affect mood state but not cognition or dynamic postural balance. Eur J Appl Physiol 2013;113:1027–34. [DOI] [PubMed] [Google Scholar]
- [18].Farmer MA, Leja A, Foxen-Craft E, Chan L, MacIntyre LC, Niaki T, Chen M, Mapplebeck JCS, Tabry V, Topham L, Sukosd M, Binik YM, Pfaus JG, Mogil JS. Pain reduces sexual motivation in female but not male mice. J Neurosci 2014;34:5747–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009;41:1149–60. [DOI] [PubMed] [Google Scholar]
- [20].Fields HL. A motivation-decision model of pain: the role of opioids. In: Flor H, Kalso E, Dostrovsky JO, eds. Proceedings of the 11th World Congress on Pain. Seattle, WA: IASP Press; 2006:449–59. [Google Scholar]
- [21].Fields HL. State-dependent opioid control of pain. Nat Rev Neurosci 2004;5:565–75. [DOI] [PubMed] [Google Scholar]
- [22].Foo H, Mason P. Analgesia accompanying food consumption requires ingestion of hedonic foods. J Neurosci 2009;29:13053–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jensen KB, Kaptchuk TJ, Kirsch I, Raicek J, Lindstrom KM, Berna C, Gollub RL, Ingvar M, Kong J. Nonconscious activation of placebo and nocebo pain responses. Proc Natl Acad Sci U S A 2012;109:15959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jepma M, Wager TD. Conceptual conditioning: mechanisms mediating conditioning effects on pain. Psychol Sci 2015;26:1728–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kenntner-Mabiala R, Pauli P. Affective modulation of brain potentials to painful and nonpainful stimuli. Psychophysiology 2005;42:559–67. [DOI] [PubMed] [Google Scholar]
- [26].Leknes S, Berna C, Lee MC, Snyder GD, Biele G, Tracey I. The importance of context: when relative relief renders pain pleasant. PAIN 2013;154:402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lindquist MA, Spicer J, Asllani I, Wager TD. Estimating and testing variance components in a multi-level GLM. Neuroimage 2012;59:490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol 2007;58:593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prev Sci 2000;1:173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McNeil DW, Rainwater AJ. Development of the fear of pain Questionnaire-III. J Behav Med 1998;21:389–410. [DOI] [PubMed] [Google Scholar]
- [31].Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest 2010;120:3779–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Petrovic P, Ingvar M. Imaging cognitive modulation of pain processing. PAIN 2002;95:1–5. [DOI] [PubMed] [Google Scholar]
- [33].Preacher KJ, Kelley K. Effect size measures for mediation models: quantitative strategies for communicating indirect effects. Psychol Methods 2011;16:93–115. [DOI] [PubMed] [Google Scholar]
- [34].Reicherts P, Gerdes ABM, Pauli P, Wieser MJ. On the mutual effects of pain and emotion: facial pain expressions enhance pain perception and vice versa are perceived as more arousing when feeling pain. PAIN 2013;154:793–800. [DOI] [PubMed] [Google Scholar]
- [35].Ren W, Centeno MV, Berger S, Wu Y, Na X, Liu X, Kondapalli J, Apkarian AV, Martina M, Surmeier DJ. The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain. Nat Neurosci 2016;19:220–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rhudy JL, Meagher MW. The role of emotion in pain modulation. Curr Opin Psychiatry 2001;14:241–5. [Google Scholar]
- [37].Roy M, Piché M, Chen J-I, Peretz I, Rainville P. Cerebral and spinal modulation of pain by emotions. Proc Natl Acad Sci U S A 2009;106:20900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the life orientation test. J Pers Soc Psychol 1994;67:1063–78. [DOI] [PubMed] [Google Scholar]
- [39].Schwartz N, Temkin P, Jurado S, Lim BK, Heifets BD, Polepalli JS, Malenka RC. Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science 2014;345:535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychol Methods 2002;7:422–45. [PubMed] [Google Scholar]
- [41].Spielberger C. Manual for the state-trait anxiety inventory (rev. ed.). Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- [42].Sprenger C, Eippert F, Finsterbusch J, Bingel U, Rose M, Büchel C. Attention modulates spinal cord responses to pain. Curr Biol 2012;22:1019–22. [DOI] [PubMed] [Google Scholar]
- [43].Steyer R, Schwenkmetzger P, Notz P, Eid M. Der Mehrdimensionale Befindlichkeitsfragebogen. Göttingen: Hogrefe; 1997. [Google Scholar]
- [44].Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess 1995;7:524–32. [Google Scholar]
- [45].Van Damme S, Crombez G, Eccleston C. Coping with pain: a motivational perspective. PAIN 2008;139:1–4. [DOI] [PubMed] [Google Scholar]
- [46].Verhoeven K, Crombez G, Eccleston C, Van Ryckeghem DML, Morley S, Van Damme S. The role of motivation in distracting attention away from pain: an experimental study. PAIN 2010;149:229–34. [DOI] [PubMed] [Google Scholar]
- [47].Villemure C, Bushnell MC. Mood influences supraspinal pain processing separately from attention. J Neurosci 2009;29:705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Villemure C, Slotnick BM, Bushnell MC. Effects of odors on pain perception: deciphering the roles of emotion and attention. PAIN 2003;106:101–8. [DOI] [PubMed] [Google Scholar]
- [49].Vlaeyen JWS. Learning to predict and control harmful events: chronic pain and conditioning. PAIN 2015;156:S86–93. [DOI] [PubMed] [Google Scholar]
- [50].Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat: part I: reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage 2009;47:821–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wiech K, Tracey I. The influence of negative emotions on pain: behavioral effects and neural mechanisms. Neuroimage 2009;47:987–94. [DOI] [PubMed] [Google Scholar]
- [52].Woods SC, Ramsay DS. Pavlovian influences over food and drug intake. Behav Brain Res 2000;110:175–82. [DOI] [PubMed] [Google Scholar]