Highlights
-
•
Chest wall sarcomas most commonly present as painless slow growing masses.
-
•
Resection is often precarious due to involvement of important structures.
-
•
The multidisciplinary approach is crucial for the optimal management of this tumor.
Keywords: Thoracic surgery, Soft tissue sarcoma, Chest wall tumor, Case report
Abstract
Introduction
Soft tissue sarcomas of the chest wall are exceptionally rare entities that present as painless slow growing masses. Resection is often precarious due to involvement of vital structures, and patients are left with large chest wall defects postoperatively requiring extensive reconstruction.
Presentation of case
We present a case report of a 29 year-old man who presented with a giant soft tissue sarcoma of the chest that had been growing slowly for one year prior to presentation. The patient had a biopsy that was positive for sarcoma, and PET CT demonstrated a large lobulated mass in the left chest wall with an SUV of 6.7. He received 50 Gy of radiation therapy; however, the mass continued to grow in size. He subsequently underwent an en-bloc resection of the mass with latissimus and serratus muscle primary reconstruction. Final pathology showed a 27 cm high-grade fibrosarcoma with prominent myxoid component. To our knowledge, this is the largest soft tissue sarcoma of the chest wall reported in the literature. Postoperatively, the patient received 6 cycles of adjuvant chemotherapy.
Discussion
Surgery is the mainstay of treatment, and chemotherapy and radiation are used in specific circumstances. Risk of recurrence is dependent on many factors, including histologic subtype, grade, and size of tumor. Long term surveillance with physical exam and imaging is recommended.
Conclusion
We feel that the multidisciplinary approach is crucial for optimal management of large soft tissue sarcomas. We recommend this approach to all patients with chest wall sarcomas.
1. Introduction
Most soft tissue sarcomas (STS) of the chest wall present as painless slow growing masses [1], [2], [3], [4] and approximately 0.1–0.15% of all adult malignancy is chest wall STS [5]. Chest wall tumors have a 50% malignancy rate and comprise <5% of all thoracic malignancies [6]. Chest wall tumors may represent primary or metastatic lesions or may be due to direct invasion of by an intrathoracic mass [6]. These tumors pose a challenge to treating physicians, as surgery is the mainstay of treatment; however, resection is often precarious due to involvement of vital structures [2], [6]. Additionally, patients are left with large chest wall defects post operatively, which require extensive reconstruction [1], [4], [5], [7]. We report management of a giant STS of chest wall.
2. Case
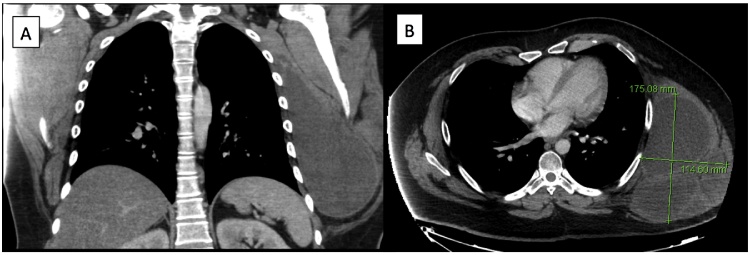
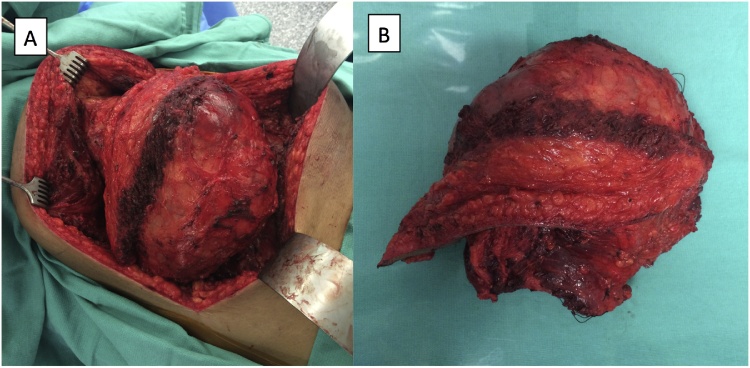
A 29 year old male presented to our thoracic surgery clinic with a left chest wall mass which he reported to be growing over the past year. The patient decided to seek medical treatment when he developed a mild pain at the site of the mass one-week prior to his presentation. On exam, the patient had a large chest wall mass. A computed tomography (CT) scan of the chest showed a 17.5 cm × 11.5 cm large lobulated mass, and a positron emission tomograph (PET)-CT showed uptake at the mass with an standardized uptake value (SUV) of 6.7 with no evidence of metastatic disease. He underwent a biopsy that was positive for sarcoma. The case was presented at the multidisciplinary thoracic tumor board, and given the size of the mass, he was recommended to undergo induction radiation therapy followed by surgery and subsequent chemotherapy. The mass continued to grow in size despite undergoing 50 Gy of radiation therapy. Patient then underwent an en-bloc resection of the mass with a 2 cm margin (R0 resection) arising from the serratus muscle with partial resection of lattisimus muscle. The ribs were not involved, and thus no chest wall resection was performed. A latissimus and serratus muscle primary reconstruction was performed by plastic surgery to cover the large defect. Final pathology showed a 27 cm × 16 cm × 16 cm high-grade (grade 3) fibrosarcoma with prominent myxoid component. To our knowledge, this is the largest STS of the chest wall reported in the literature. Six weeks later, the patient received 6 cycles of ifosfamide and adriamycin. The patient had disease free survival for 12 months, after which he developed metastatic disease in the lung and the pleura without evidence of local recurrence (Fig. 1, Fig. 2).
Fig. 1.
Preoperative CT scan demonstrates large chest wall mass on (a) coronal and (b) axial views.
Fig. 2.
Large chest wall sarcoma measuring 27 cm × 16 cm × 16 cm demonstrated (a) during and (b) after excision.
3. Discussion
The largest published review on STS by McMillan et al. comprised of 192 patients and found the most common histologic subtype to be desmoid tumor [3]. Five and ten-year survival rates were 73% and 61%, respectively, with a median survival of 14 years. Twenty-three percent of patients presented with recurrence of disease at a median of 11.6 months for local recurrence versus 13.5 months for distant recurrence. According to the study, tumors >5 cm and high-grade tumors had the highest risk for recurrence, and the histologic subtype associated with the greatest recurrence rate was an undifferentiated pleomorphic sarcoma. Five and ten-year survival rates after recurrence were 30% and 18%, respectively, with a median survival of 19.4 months.
CT scan of the chest is usually the first diagnostic test for work up of a chest wall mass. Since the lungs are the most common site of metastasis, they are included in the initial survey [4], [7], [8]. MRI of the chest may also be performed to assess any anatomic challenges in planning for resection, such as proximity of adjacent neurovascular structures [4], [7], [8], [9]. PET-CT may also be used in certain instances, especially more high-risk patients, such as those with large, high-grade tumors, for metastatic work up [10].
Prior to definitive treatment, biopsy is performed to determine treatment planning. While lesions <5 cm that are amenable to resection may undergo excisional biopsy, lesions >5 cm or in anatomically challenging locations should typically undergo Tru-cut needle biopsy or incisional biopsy for tissue diagnosis [6].
Surgical resection is the treatment of choice for STS of the chest wall [2], [3], [5], [9]. R0 resection can be obtained in up to 80% of patients and is critical for risk reduction of disease recurrence [5]. Neoadjuvant/adjuvant therapies are used in specific cases: radiation is used in patients with positive margins or high risk of recurrence [8]; however, radiation has not been shown to improve disease-specific survival [4], [5]. Neoadjuvant radiation may be used in patients with large, high-grade tumors if wound complications are not anticipated and it is advantageous to adjuvant radiotherapy with lower rates of late morbidity including fibrosis and bone fracture [11]. In our patient, radiation therapy had very little impact on the size of the tumor. Chemotherapy is used sparingly as most sarcomas are minimally responsive, but it may be considered for large (> 5 cm) and high grade STS [11].
Finally, many patients require complicated chest wall reconstruction after resection of their sarcoma, especially when multiple ribs have to be resected [6], [7]. Surgeries are most often successful with a multidisciplinary approach including plastic surgery. Large defects necessitate mesh and/or musculocutaneous flaps for chest wall stabilization and avoidance of paradoxical respiratory movements [7], [9]. Most commonly, a latissimus dorsi flap is used, however, bilateral pectoral flaps are also useful, especially in the case of sternal resection [1].
Patients must have routine follow-up with physical examination and be counseled on self-examination for detection of recurrence [3]. Additionally, patients should undergo CT screening for surveillance of their tumor [3].
4. Conclusion
This case represents an example of the complexity of treating patient with giant STS. We feel that a multidisciplinary approach including thoracic surgery, plastic surgery, medical oncology, and radiation oncology is crucial to optimal management of this difficult disease process. We recommend this approach to all patients with chest wall sarcomas.
Ethical approval
Study has been approved by IRB at Houston Methodist Research Institute. Study has been reported in line with SCARE criteria [12].
Conflicts of interest
None.
Sources of funding
None.
Consent
Approved by IRB at Houston Methodist Hospital. Pro00013298.
Author contributions
Catherine H. Davis and Halim Yammine – study concept, data collection, data analysis, writing the paper.
Min P. Kim, Puja G. Khaitan, Edward Y. Chan – study concept, data analysis and writing the paper.
Guarantor
Min P. Kim.
Acknowledgement
None.
References
- 1.Bagheri R., Haghi S.Z., Kalantari M.R. Primary malignant chest wall tumors: analysis of 40 patients. J. Cardiothorac. Surg. 2014;9:106. doi: 10.1186/1749-8090-9-106. PMC4079176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z., Wu X.H., Li B. CyberKnife radiotherapy for malignant fibrous histiocytoma of the chest wall: a case report and review of the literature. Oncol. Lett. 2014;7:1877–1880. doi: 10.3892/ol.2014.1995. PMC4049717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMillan R.R., Sima C.S., Moraco N.H. Recurrence patterns after resection of soft tissue sarcomas of the chest wall. Ann. Thorac. Surg. 2013;96:1223–1228. doi: 10.1016/j.athoracsur.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Wilder F., D’Angelo S., Crago A.M. Soft tissue tumors of the trunk: management of local disease in the breast and chest and abdominal walls. J. Surg. Oncol. 2015;111:546–552. doi: 10.1002/jso.23843. [DOI] [PubMed] [Google Scholar]
- 5.Wouters M.W., van Geel A.N., Nieuwenhuis L. Outcome after surgical resections of recurrent chest wall sarcomas. J. Clin. Oncol. 2008;26:5113–5118. doi: 10.1200/JCO.2008.17.4631. [DOI] [PubMed] [Google Scholar]
- 6.Lin G.Q., Li Y.Q., Huang L.J. Chest wall tumors: diagnosis, treatment and reconstruction. Exp. Ther. Med. 2015;9:1807–1812. doi: 10.3892/etm.2015.2353. PMC4471685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friesenbichler J., Leithner A., Maurer-Ertl W. Surgical therapy of primary malignant bone tumours and soft tissue sarcomas of the chest wall: a two-institutional experience. Int. Orthop. 2014;38:1235–1240. doi: 10.1007/s00264-014-2304-3. PMC4037511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De A.B., Delaney T.F. Role of radiation therapy for non-extremity soft tissue sarcomas. J. Surg. Oncol. 2015;111:604–614. doi: 10.1002/jso.23863. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee K., Pal M., Saha E. Giant chondrosarcoma of chest wall. Indian J. Chest Dis. Allied Sci. 2013;55:229–231. [PubMed] [Google Scholar]
- 10.Chadaz T., Hobbs S.K., Son H. Chest wall sarcoma: 18F-FDG PET/CT in a patient with Li-Fraumeni syndrome. Clin. Nucl. Med. 2013;38:818–820. doi: 10.1097/RLU.0b013e3182a20033. [DOI] [PubMed] [Google Scholar]
- 11.ESMO/European Sarcoma Network Working Group Soft tissue and visceral sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014;25(Suppl. 3) doi: 10.1093/annonc/mdu254. iii102-12. [DOI] [PubMed] [Google Scholar]
- 12.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P., SCARE Group The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016 doi: 10.1016/j.ijsu.2016.08.014. (in press) [DOI] [PubMed] [Google Scholar]