Abstract
In Olmsted County, Minn., USA, reliable, population-based epidemiologic research studies can be performed because of a unique medical records linkage system, the Rochester Epidemiology Project (REP). Our objective was to summarize the epidemiologic data describing the prevalence of skin and skin-related diseases derived from the REP and to compare the findings with those from other studies worldwide. Retrospectively, we reviewed the results of population-based REP studies reporting the prevalence of skin and skin-related diseases over more than 4 decades and compared them to other published prevalences globally. Prevalences from the REP reported per 100,000 persons were as follows: hidradenitis suppurativa, 130.0; psoriasis, 700.0; psoriatic arthritis in 1992, 100.0, and in 2000, 160.0; Behçet disease, 5.2; scleroderma, 13.8; dermatomyositis, 21.42; systemic lupus erythematosus (SLE), from 30.5 to 122.0 suspected SLE, 32.8; combined SLE, 41.8; discoid lupus erythematosus, 27.6, and cutaneous lupus erythematosus, 70.4 and 73.2 (from 2 studies). Many of the population-based prevalences of specific skin and skin-related diseases derived from the REP are different from those estimated globally. Suggested reasons for disparity in the prevalences globally may include differences in the type of reported prevalence, study methodology, geographic areas, ethnic groups, age distribution, and socioeconomic status.
Keywords: Epidemiology, Prevalence, Skin diseases, Skin-related diseases
Introduction
Skin and skin-related diseases cause a global health burden, and the epidemiology of disease must be understood in order to plan for the allocation of health care resources. Epidemiologic data include measures of incidence and prevalence. We previously summarized the incidence (a measure of new cases in a population over a given period) of skin and skin-related diseases in Olmsted County, Minn., USA, from the Rochester Epidemiology Project (REP) data [1].
Prevalence is defined as the proportion of a population found to have a disease over a specified period (period prevalence) or at a specific point in time (point prevalence). The prevalence of skin and skin-related diseases may change over time and vary depending on geographic areas, age distributions, and ethnic groups.
In Olmsted County, in southeastern Minnesota, population-based epidemiologic studies can be derived from the REP, a unique records linkage research infrastructure that has existed since 1966. The REP permits access to the medical records of virtually all persons living in this geographically isolated population [2, 3]. The population of Olmsted County is relatively small (146,000 persons according to 2011 census data) and mostly white (i.e. less racially diverse than the USA as a whole).
Among patients seeking health care in Olmsted County, skin disorders are reported as the most prevalent, followed by osteoarthritis, joint disorders, and back problems [4]. Since almost half the Olmsted County population has received a diagnosis of a skin disorder, we decided to gather all available published prevalence data on skin and skin-related diseases from the REP data published over the past 4 decades. In addition, we compared the reported prevalence data from the REP with other reported prevalence data.
Methods
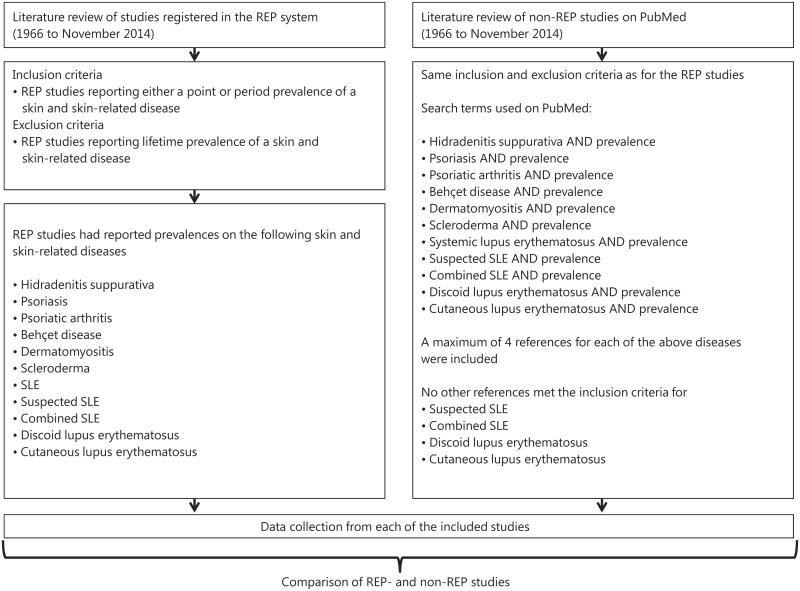
Over 2,100 papers have been published from the REP. All studies using the source of the REP are approved and registered in their system. In conjunction with the REP team, we abstracted a complete list of all published studies from 1966 (starting date of REP) to November 2014. We reviewed each article and identified those that described prevalences of skin and skin-related diseases. We included all REP studies that reported either a point or period prevalence of a certain skin or skin-related disease. Studies reporting lifetime prevalence were excluded. The REP studies had reported prevalences on the following skin and skin-related diseases: hidradenitis suppurativa, psoriasis, psoriatic arthritis, Behçet disease, dermatomyositis, scleroderma, systemic lupus erythematosus (SLE), suspected SLE, combined SLE, discoid lupus erythematosus, and cutaneous lupus erythematosus. The following data were abstracted from each of the REP studies: reference, disease, age of studied population, and date/period of prevalence estimate with corresponding 95% CIs, when available. If sex-specific prevalences were reported in the REP studies, these were abstracted too. All measured prevalence data were per 100,000 persons.
For comparison, we also reviewed the English-language literature to identify additional studies reporting overall prevalences using the electronic database PubMed. We searched PubMed for prevalence studies within the same period as the REP studies (1966 to November 2014). The following search terms were used on PubMed: hidradenitis suppurativa OR psoriasis OR psoriatic arthritis OR Behçet disease OR dermatomyositis OR scleroderma OR systemic lupus erythematosus (SLE) OR suspected SLE OR combined SLE OR discoid lupus erythematosus OR cutaneous lupus erythematosus AND prevalence. To complete the literature seach, reference lists of relevant articles were reviewed to identify possible additional studies not retrieved by the electronic search on PubMed. Only original articles were included. However, if multiple non-REP studies on prevalence were available, a maximum of 4 references for each skin and skin-related disease were included for comparison. We could not identify any non-REP prevalence studies that met our inclusion criteria on suspected SLE, combined SLE, discoid lupus erythematosus, or cutaneous lupus erythematosus. For each included non-REP study, we abstracted the following data: reference, geographic area, study methodology, age of studied population, and date/period of prevalence estimate with corresponding 95% CIs, when available. Measured prevalence data were per 100,000 persons. In a limited number of the included non-REP studies, sex-specific prevalences were reported, and these were also abstracted.
Results
A total of 11 REP studies met the study inclusion criteria. The majority of the REP data were reported as point prevalence and were as follows (prevalences are expressed per 100,000 persons): hidradenitis suppurativa (HS), 130.0; psoriasis, 700.0; psoriatic arthritis in 1992, 100.0, and in 2000, 160.0; Behçet disease (BD), 5.2; scleroderma, 13.8; dermatomyositis (DM), 21.42; systemic lupus erythematosus (SLE) in 1968, 48.0; SLE in 1980, 40.0; SLE in 1993, 122.0; SLE in 2006, 30.5; suspected SLE, 32.8; combined SLE, 41.8; discoid lupus erythematosus, 27.6; and cutaneous lupus erythematosus, 70.4 and 73.2 (prevalences were reported from 2 studies). Table 1 summarizes the abstracted data for each REP study [5–15].
Table 1.
Studies of prevalence rate of skin and skin-related disease using the REP, Olmsted County, Minn., USA
| Reference | Disease | Age, years | Date/period of prevalence estimate | Prevalence |
|---|---|---|---|---|
| Shahi et al. [5] | HS | All | Jan 1, 2009 | 127.8 (108.9–146.8) |
| Shbeeb et al. [6] | Psoriasis | ≥18 | Jan 1, 1992 | 700.0 (650.0–750.0) |
| Shbeeb et al. [6] | Psoriatic arthritis | ≥18 | Jan 1, 1992 | 101.0 (80.0–120.0) |
| Wilson et al. [7] | Psoriatic arthritis | All | Jan 1, 2000 | 158.0 (132.0–185.0) |
| Calamia et al. [8] | BD | ≥18 | 2000 | 5.2 (0.64–9.84) |
| Bendewald et al. [9] | DM | All | Jan 1, 2007 | 21.42 (13.07–29.77) |
| Michet et al. [10] | Scleroderma | All | Jan 1, 1980 | 13.8 (4.2–23.4) |
| Kurland et al. [11] | SLE | All | Jan 1, 1968 | 48.0 |
| Michet et al. [10] | SLE | All | Jan 1, 1980 | 40.0 (23.5–57.5) |
| Uramoto et al. [12] | SLE | All | Jan 1, 1993 | 122.0 (97.0–147.0) |
| Jarukitsopa et al. [13] | SLE | All | Jan 1, 2006 | 30.5 (21.1–39.9) |
| Michet et al. [10] | Suspected SLE | All | Jan 1, 1980 | 32.8 (18.1–47.5) |
| Nobrega et al. [14] | Combined SLE | All | Jan 1, 1966 | 41.8 |
| Michet et al. [10] | Discoid lupus erythematosus | All | Jan 1, 1980 | 27.6 (14.1–41.1) |
| Durosaro et al. [15] | CLE | All | Jan 1, 2006 | 73.2 (58.3–88.2) |
| Jarukitsopa et al. [13] | CLE | All | Jan 1, 2006 | 70.4 (55.9–84.8) |
CLE = Cutaneous lupus erythematosus. Figures in parentheses indicate 95% confidence intervals. Prevalence estimate: the prevalence is a point prevalence if it was reported for a specific point in time (i.e. a specific day and year), but the prevalence is a period prevalence if it was reported for a specified period (e.g. months or years). In Nobrega et al. [14], combined SLE is defined as classic SLE (positive LE cell test and ≥3 major systemic manifestations of LE) plus rheumatoid arthritis with LE (previous rheumatoid arthritis with positive LE cell test and ≥3 major systemic manifestations of LE). As for Durosaro et al. [15] and Jarukitsopa et al. [13], both studies reported the point prevalence of cutaneous lupus erythematosus for the Olmsted County population in January 2006. Differences in these prevalences are due to differences in the methodology used in defining the denominator and in adjusting the population. Moreover, these prevalence estimates for cutaneous lupus erythematosus are underestimates since they were derived from incident cases in Olmsted County from 1965 to 2002.
Table 2 summarizes data abstracted from each of the included non-REP studies [16–43]. The prevalences of HS in Denmark and France were significantly higher than in Olmsted County [5, 16, 17]. The prevalence of HS in South Wales was similar to the prevalence in Olmsted County [5, 18]. The prevalences of psoriasis in Europe were higher than the prevalence reported for Olmsted County [6, 21–23]. For psoriatic arthritis, the prevalences were reported to be lower in the Czech Republic [24] and Northwest Greece [25] compared to Olmsted County [6] but much higher both in Norway [26] and Italy [27]. The prevalence of BD was significantly higher in Turkey [31] compared to Olmsted County [8], other European countries and Taiwan [28–30]. The prevalence of DM in central Greece [35] was relatively high compared to the prevalences in the USA, Australia, and Argentina [9, 32–34]. The prevalences of scleroderma in Detroit, Mich., USA [36], areas of Canada [37, 38], and South Australia [39] were higher than the prevalence reported in Olmsted County [10]. The prevalence of SLE varied considerably over the years in Olmsted County, but was similar to other white populations in Europe and the USA [10–13, 40–42].
Table 2. Prevalence of skin and skin-related diseases globally.
| Disease | Reference | Geographic location | Study methodology | Ages of persons in population, years | Date of prevalence estimate | Prevalence per 100,000 | 95% CI |
|---|---|---|---|---|---|---|---|
| HS | Jemec et al. [16] | Denmark | Random sample (n = 793) was invited for a general health examination by a standard letter; 599 persons (75.5%) responded and were examined | ≥15–69 | 1992 | 1,000.0 | 400.0–2,200.0 |
| Consecutive series of patients at a sexually transmitted diseases clinic; 507 patients were examined | All | Two 6-month periods (1992–1995) | 4,100.0 | 3,000.0–6,000.0 | |||
| Revuz et al. [17] | France | Survey was mailed to a representative sample of the French population (n = 10,000) and was returned by 68.9% (6,887 of 10,000) | ≥15 | 2002 | 970.0 | 790.0–1,250.0 | |
| Harrison et al. [18] | South Wales | Retrospective analysis of all registered patients at 2 general practices; practice A (n = 5,652) was an industrial valley community and practice B (n = 6,919) an urban practice | All | Not reported | Practice A: 140.0 Practice B: 190.0 |
||
| Cosmatos et al. [19] | USA | Retrospective analysis of a large health insurance claims database (n = 7,929) | All | 2007 | 53.0 | 51.0–54.0 | |
| Psoriasis | Gelfand et al. [20] | USA | Random sample (n = 27,220) was asked standard demographic questions; if a physician-confirmed diagnosis of psoriasis was reported, additional questions were asked | All | Not reported | Whites: 2,500.0 African-Americans: 1,300.0 |
2,200.0–2,700.0 700.0–1,800.0 |
| Nevitt and Hutchinson [21] | Leicester, UK | Letter with a short description of psoriasis was sent to 5,395 patients in a general medical practice; patients replying positively were invited for an examination | All | Not reported | 1,480.0 | 1,200.0–1,800.0 | |
| Augustin et al. [22] | Germany | Retrospective analysis of data from a database of about 1.3 million nonselected persons enlisted in a German statutory health insurance organization covering all regions in Germany | All <18 |
2005 | 2,530.0 710.0 |
2,500.0–2,560.0 | |
| Saraceno et al. [23] | Italy | Survey was mailed to a representative sample of the Italian population (n = 3,500 families) | All | Feb 2006 | 2,900.0 | ||
| Psoriatic arthritis | Hanova et al. [24] | Czech Republic: City of Ceske Budejovice (urban) and district of Cheb (rural) | Retrospective analysis of a population-based cohort identified from 2 regions (total n = 154,374); diagnosis was confirmed by rheumatologists or dermatologists | ≥16 | Mar 1, 2002 | City of Ceske Budejovice: 36.7 District of Cheb: 63.0 |
25.6–50.9 47.6–81.8 |
| Alamanos et al. [25] | Northwest Greece | Retrospective analysis of records for inpatients and outpatients referred to rheumatology clinics (private and hospitals) in an area with 500,000 inhabitants | ≥16 | Dec 31, 2001 | 57.0 | 50.0–63.0 | |
| Madland et al. [26] | Bergen, Norway | Retrospective analysis of data from rheumatology centers and 2 private rheumatologists in a county with 442,000 inhabitants | ≥20 | Jan 1, 2003 | 195.0 | 180.0–210.0 | |
| Salaffi et al. [27] | Italy | Questionnaires were sent to a random sample of 3,664 patients selected from the practice lists of 16 general practitioners | ≥18 | 2004 | 420.0 | 310.0–610.0 | |
| BD | Salvarani et al. [28] | Reggio Emilia, Northern Italy | Retrospective analysis of a population-based cohort; data were derived from multiple sources | ≥18 | Jan 1, 2005 | 3.8 | 2.0–5.8 |
| Mahr et al. [29] | Paris metropolitan area, France | Questionnaire was mailed to community-based general physicians, rheumatologists, dermatologists, and ophthalmologists, and asked whether they were aware of any patients with BD (metropolitan area is home for 1,094,412 adults of whom 26% are of non-European ancestry) | ≥15 | 2003 | All ethnic groups: 7.1 European: 2.4 North African: 34.6 Asian (incl. Turkish): 17.5 Sub-Saharan African: 5.1 Noncontinental French: 6.2 |
3.5–14.4 0.6–7.2 24.4–47.5 10.7–27.2 2.2–11. 2.8–13.1 |
|
| Yu et al. [30] | Taiwan | Retrospective analysis of a population-based cohort; cases registered with Taiwan National Health Insurance (comprising 1,000,000 beneficiaries) | All | 2000 | 1.4 | 0.4–2.3 | |
| Azizlerli et al. [31] | Istanbul, Turkey | A 2-stage study: identified people with recurrent oral ulcers by visiting homes | ≥12 | Not reported | 420.0 | 3,40.0–510.0 | |
| DM | Furst et al. [32] | USA | Retrospective analysis of medical records in a large managed-care database (35 million insured members) | ≥18 | Jan 1, 2008 | 5.9 | 5.3–6.5 |
| Tan et al. [33] | South Australia | All muscle biopsy reports from the Neuropathology Laboratory, Hanson Institute, were reviewed; patient medical records were reviewed for clinical correlation | All | 1980–2009 | 1.97 | ||
| Rosa et al. [34] | Buenos Aires, Argentina | Cases registered with the Hospital Italiano Medical Care Program (n = 140,000 members) | All | Jun 1, 2009 | 10.22 | 4.9–18.8 | |
| Anagnostopoulos et al. [35] | Central Greece | Mailed questionnaire was followed by confirmation with clinical examination and tests of persons with a positive reply (n = 3,528) | Adults (ages not specified) | Apr 2007 to Jun 2008 | 58.0 | 50.0–180.0 | |
| Scleroderma | Mayes et al. [36] | Detroit, Mich., USA | Retrospective analysis of the population of the Detroit Tri-County Area; data were derived from multiple sources | ≥18 | 1989–1991 | All ethnic groups: 24.2 White: 22.5 Black: 31.5 |
21.3–27.4 19.7–25.6 28.2–35.2 |
| Bernatsky et al [37] | Quebec, Canada | Retrospective analysis of Quebec physician billing and hospitalization databases (covering 7.5 million people) | All | 2003 | 44.3 | 41.1–47.6 | |
| Thompson and Pope [38] | Windsor, Woodstock, Sarnia, Ont., Canada | Case-control study of patients with scleroderma and 2 age- and sex-matched controls from the same rheumatologist's practice; a questionnaire was mailed to both groups | All | Not reported | Windsor: 7.1 Woodstock: 28.0 Sarnia: 9.6 |
3.4–10.8 9.7–46.4 2.5–16.8 |
|
| Roberts-Thomson et al. [39] | South Australia | Analysis of the database of the South Australian Scleroderma Register | All | 1993–2002 | 21.4 | 20.2–22.6 | |
| SLE | Somers et al. [40] | Southeastern Michigan, USA | Analysis of multiple case-finding sources in Wayne and Washtenaw counties (population 2.4 million) | All | 2002–2004 | All ethnic groups: 72.8 Black: 111.6 White: 47.5 Asian/Pacific Islander: 4.4 Hispanic: 42.1 |
70.8–74.8 107.7–115. 45.5–49.7 1.4–10.4 35.0–50.2 |
| Arnaud et al. [41] | France | Cases from the French national administrative databases (86% of the French population) | All <19 | 2010 | 47.0 3.75 |
46.5–47.6 | |
| Lerang et al. [42] | Oslo, Norway | Cases from 5 hospitals (population of 580,000, of whom 20% are immigrants of non-European origin) | ≥16 | Jan 1, 2008 | Ethnic Norwegian population: 52.8 European descent immigrant: 35.5 Asian population: 59.7 In foreign country adopted population: 307.0 |
45.2–58.4 17.6–53.5 38.0–81.5 75.4–538.7 |
|
| Johnson et al. [43] | Birmingham, UK | Attending physicians (n = 204) were contacted twice by mail with requests about their SLE patients (population a broad ethnic mix) | ≥18 | Jan 1, 1992 | All ethnic groups: 27.7 Afro-Caribbean: 111.8 Asian: 46.7 Caucasian: 20. |
24.2–31.2 80.8–142.8 31.5–61.9 17.5–24.0 |
Prevalence estimate: the prevalence is a point prevalence if it was reported for a specific point in time (i.e. a specific day and year), but it is a period prevalence if it was reported for a specified period (e.g. months or years).
Discussion
Skin and skin-related diseases accounted for a high percentage of all medical visits both in Olmsted County [4] and around the world [44]. The REP has been used to study the prevalence of certain specific skin diseases. For these specific skin diseases, we found the highest prevalences among patients with HS, psoriasis, and psoriatic arthritis, whereas the SLE and its subtypes, scleroderma, and DM were rare.
Estimates of prevalence are critically dependent on the study methodology used. All REP studies were based on population-based cohorts where the diagnosis of a disease had been confirmed by a physician. We compared these data to studies from other population-based cohorts in which data were drawn from other hospital registries, general practice, or established registries such as insurance data. Other prevalence studies were based on data gathered from self-reported patients and/or questionnaires. We noted that registry data mostly provided the lowest estimates of prevalences, particularly when compared to studies in which estimated data were based on self-reported patients.
As expected, the prevalence of skin and skin-related diseases varied between Olmsted County and other countries, states, or areas, suggesting a role for environmental and/or genetic factors in the pathogenesis. For example, the prevalence of BD was higher in Turkey [31] compared to Olmsted County [8], other European countries [28, 29], and Taiwan [30]. The prevalence of HS in South Wales did vary between areas, with the highest prevalence in an urban practice compared to a practice in an industrial valley [18]. Between Italian regions, a 2.8-times higher prevalence of psoriasis was reported in the central regions compared to Sardinia and the Southern region (Calabria, Apulia, and Basilicata), also suggesting a possible association with sunlight exposure and weather [23]. Between two areas in the Czech Republic, the highest prevalence was reported in the district of Cheb (a rural area) compared to the City of Ceske Budejovice (an urban area) [24]. In southwestern Ontario, Canada, the prevalences of scleroderma also varied between the areas of Windsor, Sarnia, and Woodstock. Interestingly, it was noted that scleroderma patients in these areas were more likely to drink alcohol [38]. In southern Australia, a lower prevalence of DM was reported [33] compared to other countries as well as Olmsted County [9, 32, 34, 35]. This study found a possible association between DM and a higher socioeconomic status [33].
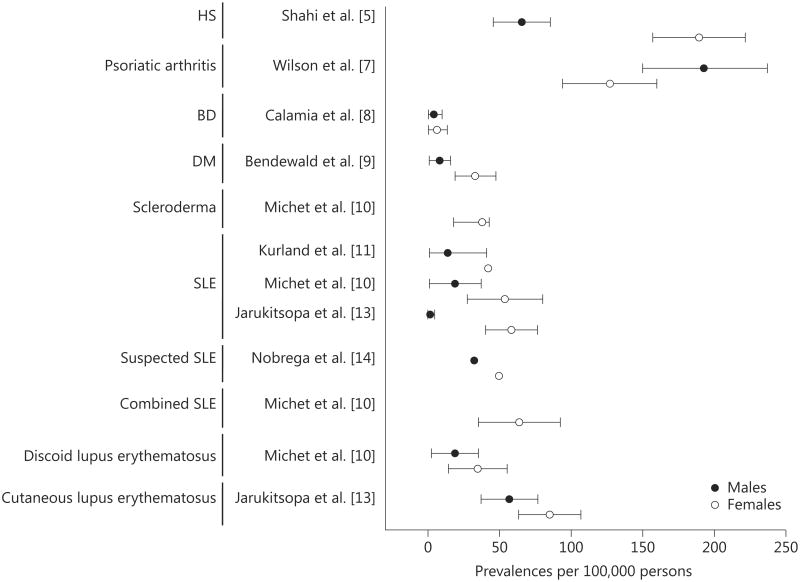
Most of the skin and skin-related diseases studied in Olmsted County were more common in females compared to males (fig. 2) [5, 7–11, 13, 14]. Similar differences were reported elsewhere (fig. 3) [19, 24–26, 29, 30, 34, 36, 37, 40–43], showing that differences in skin layers, physiology, and sex hormones also affect the pathogenesis.
Fig. 2.
Sex-specific prevalences of skin and skin-related diseases in Olmsted County, Minn., USA.
Fig. 3.
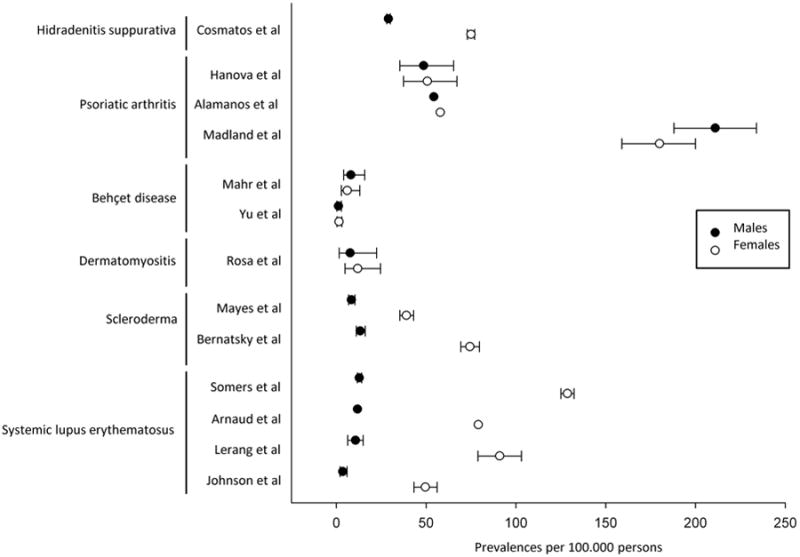
Sex-specific prevalences of skin and skin-related diseases globally.
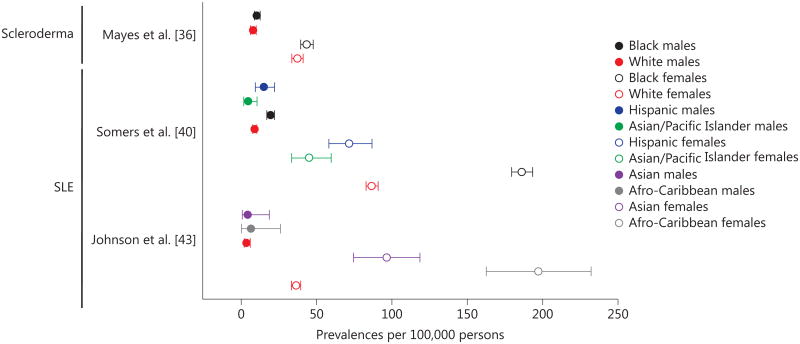
The population of Olmsted County is a predominantly white US population. Other prevalence studies have demonstrated that certain skin diseases are more prevalent in black populations. For example, in the Detroit Tri-County Area in the USA, the black population had a higher prevalence of scleroderma when compared with the white population [36]. In southeastern Michigan, USA, the prevalence of SLE was highest among the black female population, followed by the white, Hispanic, and Asian/pacific Islander female populations [40]. Similar differences between ethnic groups were found for SLE prevalences in Birmingham, UK, with the highest prevalence reported among Afro-Caribbean females followed by Asian and white females, irrespective of place of birth (fig. 4) [36, 40, 43]. For BD, when looking at people living in metropolitan areas in Paris, France, the prevalence was highest among people of North African origin, followed by Asian (incl. Turkish), noncontinental French, sub-Saharan African, and European nationality [29], irrespective of place of birth. Also shown were disease susceptibility differences among ethnic groups. On the other hand, the prevalence of psoriasis was found to be more common among the white population compared to African-Americans in the USA [20].
Fig. 4.
Sex-specific prevalences of skin and skin-related diseases by ethnic groups.
In summary, the results of the above prevalence studies were determined by many factors, such as type of prevalence, study methodology, geographic areas, ethnic group, age distribution and socioeconomic status.
Limitation
In addition, there are various possible issues when comparing prevalence data: (1) the definition of the prevalence – the prevalence data were either reported as a point prevalence or period prevalence; (2) different study methodologies – data were either drawn from a population-based source such as hospital, general practice, or established registers such as insurance data or from self-reported patients and/or questionnaires; (3) different dates/years of prevalences – the prevalences were reported from a number of different dates and years; (4) geographical areas – the reported prevalence comes from different countries or areas; (5) different ethnic groups – the prevalences may reflect a certain ethnic group (e.g. black or white population) more accurately and may not represent the entire population of a country; (6) different categorization of a skin and skin-related disease – certain studies have used the International Classification of Diseases categories or other classification criteria to determine if the patient had the disease, while others were not confirmed by a physician, but by the patients; (7) age groups – the reported prevalences may represent a certain age group (e.g. adults only or children only) and therefore not represent the overall prevalence in the entire population across all ages.
Conclusion
Skin and skin-related diseases are an important public health concern. Describing public health issues from an epidemiologic perspective can increase an understanding of the potential impact and provide a basis for developing and prioritizing public health programs. The prevalence of skin and skin-related disease varies from study to study, and many factors contribute to these differences.
Fig. 1.
Flowchart. SLE = Systemic lupus erythematosus.
Acknowledgments
We acknowledge Jill M. Killian and Amy L. Weaver for their assistance with statistical review and analysis of the data.
This study was made possible using the resources of the REP, which is supported by the National Institute on Aging of the National Institutes of Health under Award No. R01AG034676.
Footnotes
Disclosure Statement: The authors have no conflicts of interest to disclose.
References
- 1.Andersen LK, Davis MD. The epidemiology of skin and skin-related diseases: a review of population-based studies performed by using the Rochester Epidemiology Project. Mayo Clin Proc. 2013;88:1462–1467. doi: 10.1016/j.mayocp.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 3.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.St Sauver JL, Warner DO, Yawn BP, Jacobson DJ, McGree ME, Pankratz JJ, Melton LJ, 3rd, Roger VL, Ebbert JO, Rocca WA. Why patients visit their doctors: assessing the most prevalent conditions in a defined American population. Mayo Clin Proc. 2013;88:56–67. doi: 10.1016/j.mayocp.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shahi V, Alikhan A, Vazquez BG, Weaver AL, Davis MD. Prevalence of hidradenitis suppurativa: a population-based study in Olmsted County, Minnesota. Dermatology. 2014;229:154–158. doi: 10.1159/000363381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shbeeb M, Uramoto KM, Gibson LE, O'Fallon WM, Gabriel SE. The epidemiology of psoriatic arthritis in Olmsted County, Minnesota, USA, 1982-1991. J Rheumatol. 2000;27:1247–1250. [PubMed] [Google Scholar]
- 7.Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers HM. Time trends in epidemiology and characteristics of psoriatic arthritis over 3 decades: a population-based study. J Rheumatol. 2009;36:361–367. doi: 10.3899/jrheum.080691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calamia KT, Wilson FC, Icen M, Crowson CS, Gabriel SE, Kremers HM. Epidemiology and clinical characteristics of Behçet's disease in the US: a population-based study. Arthritis Rheum. 2009;61:600–604. doi: 10.1002/art.24423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendewald MJ, Wetter DA, Li X, Davis MD. Incidence of dermatomyositis and clinically amyopathic dermatomyositis: a population-based study in Olmsted County, Minnesota. Arch Dermatol. 2010;146:26–30. doi: 10.1001/archdermatol.2009.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michet CJ, Jr, McKenna CH, Elveback LR, Kaslow RA, Kurland LT. Epidemiology of systemic lupus erythematosus and other connective tissue diseases in Rochester, Minnesota, 1950 through 1979. Mayo Clin Proc. 1985;60:105–113. doi: 10.1016/s0025-6196(12)60294-8. [DOI] [PubMed] [Google Scholar]
- 11.Kurland LT, Hauser WA, Ferguson RH, Holley KE. Epidemiologic features of diffuse connective tissue disorders in Rochester, Minn., 1951 through 1967, with special reference to systemic lupus erythematosus. Mayo Clin Proc. 1969;44:649–663. [PubMed] [Google Scholar]
- 12.Uramoto KM, Michet CJ, Jr, Thumboo J, Sunku J, O'Fallon WM, Gabriel SE. Trends in the incidence and mortality of systemic lupus erythematosus, 1950–1992. Arthritis Rheum. 1999;42:46–50. doi: 10.1002/1529-0131(199901)42:1<46::AID-ANR6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Jarukitsopa S, Hoganson DD, Crowson CS, Sokumbi O, Davis MD, Michet CJ, Jr, Matteson EL, Maradit Kremers H, Chowdhary VR. Epidemiology of systemic lupus erythematosus and cutaneous lupus erythematosus in a predominantly white population in the United States. Arthritis Care Res (Hoboken) 2015;67:817–828. doi: 10.1002/acr.22502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nobrega F, Ferguson RH, Kurland LT, Hargraves MM. Proceedings of the Third International Symposium on Population Studies of the Rheumatic Diseases Excerpta Medica International Congress Series No 148. New York: Excerpta Medica; 1966. Lupus erythematosus in Rochester, Minnesota, 1950–1965: a preliminary study; pp. 259–266. [Google Scholar]
- 15.Durosaro O, Davis MD, Reed KB, Rohlinger AL. Incidence of cutaneous lupus erythematosus, 1965–2005: a population-based study. Arch Dermatol. 2009;145:249–253. doi: 10.1001/archdermatol.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jemec GB, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol. 1996;35:191–194. doi: 10.1016/s0190-9622(96)90321-7. [DOI] [PubMed] [Google Scholar]
- 17.Revuz JE, Canoui-Poitrine F, Wolkenstein P, Viallette C, Gabison G, Pouget F, Poli F, Faye O, Roujeau JC, Bonnelye G, Grob JJ, Bastuji-Garin S. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59:596–601. doi: 10.1016/j.jaad.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Harrison BJ, Mudge M, Hughes LE. The prevalence of hidradenitis in South Wales. In: Marks R, Plewig G, editors. Acne and Related Disorders. London: Dunitz; 1991. pp. 365–366. [Google Scholar]
- 19.Cosmatos I, Matcho A, Weinstein R, Montgomery MO, Stang P. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412–419. doi: 10.1016/j.jaad.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 20.Gelfand JM, Stern RS, Nijsten T. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23–26. doi: 10.1016/j.jaad.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 21.Nevitt GJ, Hutchinson PE. Psoriasis in the community: prevalence, severity and patients' beliefs and attitudes towards the disease. Br J Dermatol. 1996;135:533–537. [PubMed] [Google Scholar]
- 22.Augustin M, Glaeske G, Radtke MA, Christophers E, Reich K, Schafer I. Epidemiology and comorbidity of psoriasis in children. Br J Dermatol. 2010;162:633–636. doi: 10.1111/j.1365-2133.2009.09593.x. [DOI] [PubMed] [Google Scholar]
- 23.Saraceno R, Mannheimer R, Chimenti S. Regional distribution of psoriasis in Italy. J Eur Acad Dermatol Venereol. 2008;22:324–329. doi: 10.1111/j.1468-3083.2007.02423.x. [DOI] [PubMed] [Google Scholar]
- 24.Hanova P, Pavelka K, Holcatova I, Pikhart H. Incidence and prevalence of psoriatic arthritis, ankylosing spondylitis, and reactive arthritis in the first descriptive population-based study in the Czech Republic. Scand J Rheumatol. 2010;39:310–317. doi: 10.3109/03009740903544212. [DOI] [PubMed] [Google Scholar]
- 25.Alamanos Y, Papadopoulos NG, Voulgari PV, Siozos C, Psychos DN, Tympanidou M, Drosos AA. Epidemiology of psoriatic arthritis in northwest Greece, 1982–2001. J Rheumatol. 2003;30:2641–2644. [PubMed] [Google Scholar]
- 26.Madland TM, Apalset EM, Johannessen AE, Rossebo B, Brun JG. Prevalence, disease manifestations, and treatment of psoriatic arthritis in western Norway. J Rheumatol. 2005;32:1918–1922. [PubMed] [Google Scholar]
- 27.Salaffi F, De Angelis R, Grassi W. Prevalence of musculoskeletal conditions in an Italian population sample: results of a regional community-based study. I. The mapping study. Clin Exp Rheumatol. 2005;23:819–828. [PubMed] [Google Scholar]
- 28.Salvarani C, Pipitone N, Catanoso MG, Cimino L, Tumiati B, Macchioni P, Bajocchi G, Oli vieri I, Boiardi L. Epidemiology and clinical course of Behçet's disease in the Reggio Emilia area of northern Italy: a seventeen-year population-based study. Arthritis Rheum. 2007;57:171–178. doi: 10.1002/art.22500. [DOI] [PubMed] [Google Scholar]
- 29.Mahr A, Belarbi L, Wechsler B, Jeanneret D, Dhote R, Fain O, Lhote F, Ramanoelina J, Coste J, Guillevin L. Population-based prevalence study of Behcet's disease: differences by ethnic origin and low variation by age at immigration. Arthritis Rheum. 2008;58:3951–3959. doi: 10.1002/art.24149. [DOI] [PubMed] [Google Scholar]
- 30.Yu KH, See LC, Kuo CF, Chou IJ, Chou MJ. Prevalence and incidence in patients with autoimmune rheumatic diseases: a nationwide population-based study in Taiwan. Arthritis Care Res (Hoboken) 2013;65:244–250. doi: 10.1002/acr.21820. [DOI] [PubMed] [Google Scholar]
- 31.Azizlerli G, Kose AA, Sarica R, Gul A, Tutkun IT, Kulac M, Tunc R, Urgancioglu M, Disci R. Prevalence of Behcet's disease in Istanbul, Turkey. Int J Dermatol. 2003;42:803–806. doi: 10.1046/j.1365-4362.2003.01893.x. [DOI] [PubMed] [Google Scholar]
- 32.Furst DE, Amato AA, Iorga SR, Gajria K, Fernandes AW. Epidemiology of adult idiopathic inflammatory myopathies in a US managed care plan. Muscle Nerve. 2012;45:676–683. doi: 10.1002/mus.23302. [DOI] [PubMed] [Google Scholar]
- 33.Tan JA, Roberts-Thomson PJ, Blumberg P, Hakendorf P, Cox SR, Limaye V. Incidence and prevalence of idiopathic inflammatory myopathies in south Australia: a 30-year epidemiologic study of histology-proven cases. Int J Rheum Dis. 2013;16:331–338. doi: 10.1111/j.1756-185X.2011.01669.x. [DOI] [PubMed] [Google Scholar]
- 34.Rosa J, Garrot LF, Navarta DA, Saucedo C, Scolnik M, Bedran Z, Garcia MV, Sabelli M, Catoggio LJ, Soriano ER. Incidence and prevalence of polymyositis and dermatomyositis in a health management organization in Buenos Aires. J Clin Rheumatol. 2013;19:303–307. doi: 10.1097/RHU.0b013e3182a21ba8. [DOI] [PubMed] [Google Scholar]
- 35.Anagnostopoulos I, Zinzaras E, Alexiou I, Papathanasiou AA, Davas E, Koutroumpas A, Barouta G, Sakkas LI. The prevalence of rheumatic diseases in central Greece: a population survey. BMC Musculoskelet Disord. 2010;11:98. doi: 10.1186/1471-2474-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayes MD, Lacey JV, Jr, Beebe-Dimmer J, Gillespie BW, Cooper B, Laing TJ, Schottenfeld D. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48:2246–2255. doi: 10.1002/art.11073. [DOI] [PubMed] [Google Scholar]
- 37.Bernatsky S, Joseph L, Pineau CA, Belisle P, Hudson M, Clarke AE. Scleroderma prevalence: demographic variations in a population-based sample. Arthritis Rheum. 2009;61:400–404. doi: 10.1002/art.24339. [DOI] [PubMed] [Google Scholar]
- 38.Thompson AE, Pope JE. Increased prevalence of scleroderma in southwestern Ontario: a cluster analysis. J Rheumatol. 2002;29:1867–1873. [PubMed] [Google Scholar]
- 39.Roberts-Thomson PJ, Walker JG, Lu TY, Esterman A, Hakendorf P, Smith MD, Ahern MJ. Scleroderma in south Australia: further epidemiological observations supporting a stochastic explanation. Intern Med J. 2006;36:489–497. doi: 10.1111/j.1445-5994.2006.01125.x. [DOI] [PubMed] [Google Scholar]
- 40.Somers EC, Marder W, Cagnoli P, Lewis EE, DeGuire P, Gordon C, Helmick CG, Wang L, Wing JJ, Dhar JP, Leisen J, Shaltis D, McCune WJ. Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance Program. Arthritis Rheum. 2014;66:369–378. doi: 10.1002/art.38238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arnaud L, Fagot JP, Mathian A, Paita M, Fagot-Campagna A, Amoura Z. Prevalence and incidence of systemic lupus erythematosus in France: a 2010 nation-wide population-based study. Autoimmun Rev. 2014;13:1082–1089. doi: 10.1016/j.autrev.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 42.Lerang K, Gilboe I, Garen T, Thelle DS, Gran JT. High incidence and prevalence of systemic lupus erythematosus in Norway. Lupus. 2012;21:1362–1369. doi: 10.1177/0961203312458168. [DOI] [PubMed] [Google Scholar]
- 43.Johnson AE, Gordon C, Palmer RG, Bacon PA. The prevalence and incidence of systemic lupus erythematosus in Birmingham, England. Relationship to ethnicity and country of birth. Arthritis Rheum. 1995;38:551–558. doi: 10.1002/art.1780380415. [DOI] [PubMed] [Google Scholar]
- 44.Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, Margolis DJ. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527–1534. doi: 10.1038/jid.2013.446. [DOI] [PubMed] [Google Scholar]