Introduction
A majority of cancer patients suffer from severe pain leading to poor quality of life.1 However, pain remains undertreated in more than 20% of patients.2 Opioids remain the mainstay for treating cancer patients with chronic pain and during pre-, intra- and postoperative pain management.3 Opioids alleviate pain by their anti-nociceptive activity mediated via the central nervous system (CNS). On the other hand opioids can cause side-effects including constipation, respiratory depression and mast cell activation involving peripheral and central mechanisms, and the possibility of addiction.4 Of significance to the tumor microenvironment and inflammation, opioids interact with endothelium, tumor cells, and inflammatory cells via opioid receptor (OP-R) and non-OP-R mediated mechanisms.5,6 In the peri-operative setting replete with inflammation, opioids can thus modulate the process of metastasis, which is dependent upon angiogenesis, tumor cell survival and inflammation.
Morphine has been shown to stimulate growth- and survival-promoting signaling and cell migration in endothelial and tumor cells.7–10 These cellular processes are integral to cancer progression and metastasis. Indeed, morphine has been demonstrated to promote breast and lung cancer progression and metastasis in mice.8,11,12 Additionally, the use of morphine with general anesthesia during surgery has been implicated in increasing the risk of recurrence of cancer.13–15
In a retrospective analysis of prostate cancer patients, we found that higher opioid requirement was associated with poorer outcomes including shorter time to progression and shorter survival.16 Several subsequent studies from our and other centers have since observed similar association of morphine requirement with poorer outcomes including increased cancer progression and metastases and reduced survival in lung cancer patients (Table 1).17–20 Since opioids are commonly used for pain management in cancer in a peri-operative setting as well as for chronic pain, a clear understanding of the underlying biology and outcomes is required to develop strategies to prevent their inadvertent effect on tumor progression and metastasis. In this review, we describe the current understanding of opioids’ effects on cancer progression and metastasis which prompt the need for clinical trials to prospectively test the critical questions raised by pre-clinical and retrospective studies.
Table 1.
Association of opioids and/or opioid receptors with cancer progression, metastases and patient survival
| Cancer type and progression | Pain measurement | Opioid requirement | Outcomes |
|---|---|---|---|
| Metastatic prostate cancer | No | As a variable of 5 mg OME/day increments after diagnosis | Higher MOP-R expression and opioid requirement are independently associated with reduced progression free survival and overall survival.16 |
| Stage IIIB/IV NSCLC | Yes | High: ≥ 5 mg OME/day Low: < 5 mg OME/day |
Severity of pain and higher opioid requirement are independently associated with shorter survival.17 |
| Stage I and IIa NSCLC | No | Post-operative initial 96 hour dose – 124 OME in 5 yr recurrence free patients and 232 mg OME in patients with recurrence within 5 yr | Recurrence rate of NSCLC was associated with increased opioid dosage during initial 96 hour postoperative period after video-assisted thoracoscopic surgery lobectomy procedures.19 |
| Stage I-IIIa NSCLC | No | Intraoperative median of 10.15 μg/kg fentanyl equivalent, 233 minutes | Intraoperative opioid usage was found to be associated with decreased overall survival in stage I patients, while in stage II-IIIa patients no such association was found.31 |
| NSCLC | No | N/A | Significantly increased MOP-R expression was observed in cancerous lung tissue compared to adjacent control tissue; while metastatic lung tissue showed two-fold increase in MOP-R expression compared to the total cohort.18 |
Abbreviations: oral morphine equivalent (OME), non-small cell lung cancer (NSCLC) and mu-opioid receptor (MOP-R).
Opioid receptors
OP-Rs are 7 transmembrane domain G protein-coupled receptors (GPCRs), coupled to heteromeric Gi/o proteins.21 Four major OP-Rs are mu OP-R (MOP-R), kappa OP-R (KOP-R), delta OP-R (DOP-R) and nociceptin/orphanin FQ receptor (NOP-R). The activation of these receptors following ligand binding initiates dissociation of the α and βγ subunits of the heterotrimeric G protein followed by Gα translocation and subsequent inhibition of adenyl cyclase activity. Simultaneously, inhibition of voltage gated calcium channels and activation of potassium channels is initiated by the separated Gβγ subunit. This leads to changes in calcium influx in cells. Subsequent changes in intracellular effectors lead to hydrolysis of guanosine triphosphate (GTP) to guanosine diphosphate (GDP), causing inactivation of the α subunit and conjugation with the βγ subunit, leading to receptor inactivation and endocytosis.21 As an exception, MOP-R can remain activated for prolonged periods following the removal of the ligand, due to a high receptor activation versus endocytosis (RAVE) value.4 Chronic activation of MOP-R leads to the super-activation of adenyl cyclase resulting in increased cyclic adenosine monophosphate (cAMP), which promotes cell survival and proliferation.5 Since opioid analgesics have high affinity for MOP-Rs, their use has the potential to stimulate mitogenic signaling via cAMP, in part due to the high RAVE value leading to sustained activation of MOP-R.
Association of MOP-R with cancer progression and metastasis
Morphine and its congeners act through MOP-R in the CNS to induce analgesia. However, the expression of MOP-R is not limited to the CNS. MOP-R is expressed in non-neuronal tissues including immune cells, endothelial cells and tumor cells.21 Opioid-induced cell proliferation, growth and migration have been observed in different cell types.6,8,9,12,22–26 In human lung, prostate and colon cancer tissues, high expression of MOP-R has been observed as compared to healthy tissue.9,18,27–29 We and others demonstrated that MOP-R expression is associated with tumor progression (but not tumor initiation) in lung9,12 and breast cancer6 in mice. Additionally, in a retrospective clinical study of patients with advanced prostate cancer receiving androgen deprivation therapy, we found that greater opioid requirement or higher levels of MOP-R expression in the tumor are independently associated with shorter progression-free survival and overall survival (Table 1).29 In another retrospective study, we showed that higher opioid requirement and severity of cancer pain is associated with shorter survival in advanced non-small cell lung cancer (NSCLC).17 Furthermore, pain itself may increase the levels of circulating endogenous opioids including endorphins.30 It is, therefore, possible that even in patients not receiving pharmacological opioid medications, elevated levels of endogenous opioids may themselves activate peripheral MOP-Rs and cross-activate signaling pathways that influence tumor progression.
Signaling pathways contributing to opioid-induced angiogenesis and tumor growth
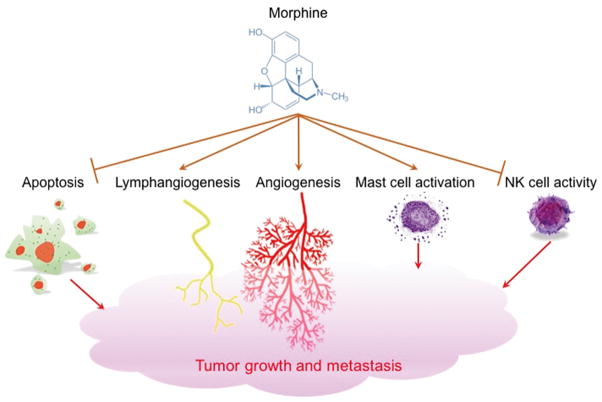
We found that physiologic doses of morphine stimulate nitric oxide (NO)-dependent mitogen-activated protein kinase (MAPK) and anti-apoptotic protein kinase B (PKB)/Akt phosphorylation and cyclin D1 in human dermal microvascular endothelial cells (HDMEC) in vitro.8 This mitogenic signaling of morphine led to cell cycle progression and increased survival of HDMEC, which translated into promotion of angiogenesis and breast tumor growth in mice in vivo. Thus, morphine induces vascular endothelium growth factor (VEGF)-like NO-dependent mitogenic signaling.8 Furthermore, morphine has been demonstrated to promote angiogenesis, lymphangiogenesis and tumor growth in several mouse models of breast and lung cancer [Figure 1].7,8,11,18,22,32–34
Figure 1.
Morphine promotes tumor growth and metastasis via induction of angiogenesis and lymphangiogenesis. Opioids can also have pro-cancer progression effect via dysregulation of apoptosis and inhibition of natural killer (NK) cells leading to the reduction in immune response. Opioid-induced mast cell activation can lead to enhanced tumor growth via action of substance P and other mediators on endothelial cells and tumor cells.
Endothelial cell proliferation and migration induced by morphine is further accompanied by transactivation of VEGF receptor 2 (VEGFR2)22,35 and/or platelet-derived growth factor beta receptor (PDGFR-β).35 The activation of Ras homolog family protein A (RhoA) and proto-oncogene tyrosine-protein kinase (Src kinase) downstream of VEGF-signaling is also required for morphine-induced endothelial proliferation and migration.22 Additionally, VEGF-induced tyrosine phosphatase inhibition promotes Src-mediated activation of Akt and mammalian target of rapamycin (mTOR) during angiogenesis.25 Inhibition of angiogenesis was observed by using the peripherally acting OP-R antagonist methylnaltrexone (MNTX) with high affinity for MOP-R, or by silencing Src, Akt or mTOR complex components, suggestive of the involvement of MOP-R in VEGF-like angiogenesis.25
As described above, MOP-R-dependent signaling may contribute to morphine/opioid-induced tumor progression and angiogenesis. Our group has shown that MOP-Rs and receptor tyrosine kinases (RTKs) are expressed and co-localized in advanced lung cancer, and that morphine induces RTK activation and signaling, leading to growth and spread of lung cancer cells.9 Concurrently, it was demonstrated that MOP-R silencing of Lewis lung carcinoma cells (LLC) led to decreased metastasis in mice, and that MOP-R knockout mice did not develop significant LLC tumor growth as compared to wild type mice.12 Moreover, overexpression of MOP-R in human lung cancers promotes tumor growth and metastases in xenografts in mice via Akt and mTOR activation.32 In transgenic mice with breast cancer, we found that tumor growth is associated with increased MOP-R expression, and morphine stimulates angiogenesis, lymphangiogenesis and tumor growth in this mouse model.6 Therefore, MOP-R-mediated signaling is important in the promotion of tumor growth and metastasis.
Vascular permeability characterized by leaky microvasculature is another important feature of cancer.36 Morphine and MOR-specific agonist [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) induced increased permeability in human pulmonary microvascular endothelial cells mediated via sphingosine-1 phosphate receptor (S1P3R).34 This barrier dysfunction was inhibited by the OP-R antagonist MNTX, suggesting that MOP-R activation may be linked to morphine-induced vascular permeability.34 Morphine stimulated platelet-derived growth factor (PDGF)-BB expression in endothelial cells in vitro and co-activation of PDGFR-β on pericytes recruited to the tumor vasculature in a transgenic breast cancer mouse model.37 Increased PDGFR-β in the tumor vasculature may contribute to increased vascular permeability via morphine-induced PDGF-BB release.38 These findings demonstrate that increased MOP-R expression in the tumor microenvironment, coupled with morphine-induced signaling, can promote tumor progression and growth through increased vascular permeability, in addition to promotion of angiogenesis. Thus morphine induces mitogenic-signaling directly via GPCRs and/or by co-activating RTKs for growth factors, thus contributing to angiogenesis, tumor growth and metastasis, and possibly leading to chemotherapy resistance. Indeed, a recent study in a nasopharyngeal carcinoma (NPC) model demonstrated that low dose morphine can contribute to resistance to chemotherapy via inhibition of apoptotic and anti-angiogenic effects of cisplatin.39 Thus strategies to inhibit the mitogenic activity of morphine without antagonizing its analgesic activity are required.
Chronic administration of morphine also results in in cyclooxygenase-2 (COX-2) in the tumor microenvironment.40 NO-induced COX-2 contributes to production of prostaglandin E2 (PGE2),41 and PGE2 is known to promote angiogenesis.42–44 We found that co-treatment with morphine and the COX-2 inhibitor celecoxib inhibits angiogenesis, tumor growth, metastasis and mortality in a mouse model of breast cancer.11 This inhibition is a result of decreased morphine-induced expression of COX-2 and PGE2. Co-treatment of mice with morphine and celecoxib also led to sustained analgesia over a period of time, suggestive of prevention of analgesic tolerance.
On the other hand, some reports suggest that morphine inhibits angiogenesis and tumor growth.45,46 Koodie et al found that morphine administration in a murine Lewis lung carcinoma model resulted in inhibition of angiogenesis modulated via a reduction in the effect of hypoxia inducible factor 1α (HIF1α) on the MAPK pathway.46 In this study, the investigators implanted a 75 mg morphine pellet subcutaneously in each mouse. Due to the dose dumping effect, about 57% of the morphine in this pellet is released in the circulation within the first 36 hours.47 This is estimated to be equivalent to 4.2 grams of morphine/kg mouse in this study, which is several thousand fold the dose administered to cancer patients with severe pain. Such high doses of morphine can be cytotoxic and even lethal, and therefore are not relevant to clinical use.
Role of the inflammatory system in morphine-induced cancer progression
Another possible mechanism by which opioids indirectly promote cancer progression is via the modulation of immune response [Figure 1]. Decreased activity of natural killer (NK) cells in post-operative patients receiving morphine has been observed.48 Morphine-mediated suppression of NK cell activity resulted in promotion of tumor growth in rodent models of breast cancer.49,50 These effects could be inhibited in mice treated with the OP-R antagonists naloxone and naltrexone.51 Morphine-induced immune suppression was not observed in MOP-R-deficient mice, suggestive of involvement of MOP-R in the inhibition of NK cells via morphine.52
Mast cells are tissue-resident immune cells that release a large number of mediators including growth factors, neuropeptides, cytokines, chemokines and other molecules, which can influence tumor progression and metastasis.53 Mast cells have been implicated in angiogenesis and tumor progression via their recruitment and the effects of mediators released into the tumor microenvironment.54 Morphine is known to stimulate mast cell degranulation.55 Upon activation, mast cells release, among other mediators, the neuropeptide substance P (SP).53 The activity of SP is mediated via tachykinin 1 (NK-1) receptor.56 Tumor cells overexpress NK-1 receptors, and SP has been shown to enhance tumor cell proliferation in different types of cancer cell lines and cancer cell migration in pancreatic cancer via NK-1 receptor.57 Additionally, SP induces endothelial cell proliferation in vitro and angiogenesis in vivo.58 SP is also known to increase vascular permeability in tumors, which can further enhance cancer progression.59 Morphine-induced activation of mast cells leads to release of tryptase, which can activate peripheral nerve endings via protease activated receptor 2 (PAR2) to cause further SP release. Thus, a feed-forward cycle of SP from both morphine-induced mast cell activation and subsequent activation of peripheral nerve endings coupled with mast cell infiltration and NK-1 receptor expression in the tumor microenvironment can lead to altered vascular permeability, increased blood flow and neovascularization promoting tumor growth and metastases. Indeed, inhibition of NK-1 receptor ameliorated human epidermal growth factor receptor 2 (HER2) and epidermal growth factor receptor (EGFR)-expressing tumors in a breast cancer mouse model.60 SP transactivated HER2 and inhibited responsiveness to the EGFR and HER2 tyrosine kinase inhibitors in human breast cancer cells in vitro, and co-treatment with a NK-1 receptor inhibitor and a EGFR/HER2 inhibitor resulted in improved response to treatment.60 We have recently found that morphine induces mast cell activation in breast tumors of a transgenic mouse model, which was associated with increased tumor growth and reduced survival compared to the control group.6 In this study, morphine treated mice also showed significantly increased mast cell dependent and independent expression of SP as compared to vehicle treated mice. Therefore, morphine-induced SP release may contribute to pain as well as tumor progression and survival.
SP is also released from nerve fibers and is a critical mediator of chronic pain. An elegant study by Magnon et al., showed that nerve fibers permeating and around the prostate cancer may play a role in promoting tumor growth and metastases.61 In this study, higher densities of sympathetic and parasympathetic nerve fibers in and around the tumor were associated with poorer outcomes. Prostate cancer is also associated with severe pain, and it is likely that pain due to the activation of nerve fibers in and around the tumor leads to further release of SP, which in turn leads to a feed-forward cycle of intractable pain and poorer outcomes. While opioids relieve pain on one hand, they might promote tumor growth and metastasis directly and by stimulating the release of SP from intratumoral mast cells. Surgical procedures may further enhance the release of SP from the nerve endings surrounding the tumor and may influence clinical outcomes.
Role of opioids in cancer progression, metastasis and recurrence in a peri-operative setting
Dissemination of tumor cells to distant sites through the circulation involves mechanisms supporting tumor cell survival, migration and vascular permeability, in addition to factors that support homing, and provide a supportive niche for tumor cells to survive, proliferate and form a tumor. Solid tumors are removed surgically as a treatment strategy, but a significant proportion of cancers recur following surgery.20 Opioids, volatile anesthetics and the neuroendocrine stress response to surgery are suggested to be critical factors underlying dissemination of cancer cells during and following surgery.62 This is perhaps initiated with the escape of tumor cells during surgery and a supportive environment provided by the release of endogenous endorphins due to stress and opioids used for analgesia. The signaling mechanisms described above suggest a pro-survival, pro-migration and permeability-promoting role for opioids. Additionally, as described above, pain and the CNS may contribute by inhibiting the protective NK cell activity and increasing release of angiogenic and growth promoting cytokines. Increased MOP-R expression shown by us in human prostate tumors and in advanced mouse breast cancers, may further contribute to the increased activity of endogenous opioids released and those used pharmacologically for analgesia.6,16 Therefore, use of opioid analgesics during surgery and post-operatively may promote the establishment of micrometastases that subsequently lead to clinically evident loco-regional recurrence and/or distant metastases. A major challenge in determining the impact of opioids in a peri-operative setting is finding the small number of tumor cells that may escape from the primary tumor and identifying their homing site, which could vary from individual to individual. In spite of major challenges, significant clinical data underscore the influence of anesthetic techniques with and without opioids on clinical outcomes including cancer progression and recurrence.
It is likely that paravertebral block, a technique of regional anesthesia, prevents the inputs from primary afferents to the CNS, thus preventing the subsequent influence on cellular and humoral immunity, and reducing requirement for systemic opioids. This may in turn reduce the promotion of micrometastases as compared to general anesthesia (GA), which also requires systemic opioids for analgesia. The first landmark study, reported in 2006, showed improved outcomes using regional anesthesia (paravertebral block) as compared to GA and systemic opioids combined.14 In this retrospective study, surgery using paravertebral anesthesia showed a significant improvement in recurrence- and metastasis-free survival as compared to GA after 24 and 36 months following mastectomy. Notably, in this study GA was induced with 1.5–3.0 mg/Kg propofol with 0.5 μg/Kg fentanyl and boluses of 0.5 mg/Kg morphine intra-operatively, whereas paravertebral anesthesia did not utilize any opioids. These data support the mitogenic role of opioids suggested in cellular and preclinical studies.
A single exposure of morphine at the cellular level can alter the expression of a wide variety of genes including those of cytoskeletal proteins, which are associated with cell migration, a critical requirement for the spread of cancer.63 Therefore, even short-term administration of opioids, such as that occurs during anesthesia, can alter cellular function. A single gene alteration in tumor cells can stimulate the metastatic process.64 One such gene is NET1, which shows increased expression in the lymph nodes of patients with increased risk of metastases.65 NET1 leads to actin reorganization and migration of cancer cells.66 Morphine has been shown to stimulate NET1-dependent migration of MCF-7 and MDA-MB-231 breast cancer cells in a dose-dependent manner.10 In addition to tumor cells, the tumor microenvironment including angiogenesis discussed above, components of the cellular matrix and cytokines are also critical for tumor progression and metastasis. In an elegantly designed prospective analysis of estrogen receptor (ER) positive and HER-2-positive breast cancer patients undergoing mastectomy or wide local tumor excision, comparison between paravertebral and sevoflurane/opioid anesthetics showed a significant difference in cytokine and matrix metalloprotease (MMP) levels as well as visual analog scale (VAS) scores for pain following surgery.67 In this study significantly improved analgesia was observed with paravertebral anesthesia as compared to sevoflurane/opioids after 1 and 2 hours of surgery, but not 24 hours post-surgery. Additionally, a significant decrease was observed in the blood levels of pro-inflammatory cytokine interleukin (IL)1-β, MMP-3, and MMP-9 with a concomitant increase in IL-10 24 hours post-operation in patients who received paravertebral anesthesia as compared to those who received sevoflurane/opioid anesthesia. In another similar study of women undergoing breast cancer surgery, the GA/opioid anesthesia group showed significantly increased serum levels of pro-angiogenic cytokine VEGF but decreased TGF-β levels post-surgery when compared to paravertebral block.68 In allthese prospective studies paravertebral anesthesia showed a mechanistic advantage by reducing the inflammatory, angiogenic and invasive tumor microenvironment while improving early analgesic outcomes post-surgery as compared to GA/opioid anesthesia. Improved analgesic outcomes up to 2 hours post-surgery in patients receiving paravertebral anesthesia as compared to GA/opioids was further validated by a sub-study of an ongoing large prospective clinical trial on women undergoing breast cancer surgery.69 As discussed above, pain may influence cancer progression and metastasis, thus contributing to poorer outcomes. Moreover, it is likely that opioids used with GA may underlie the possible promotion of minimal residual disease following surgery. Thus, anesthetic technique-related outcomes may play a critical role in cancer recurrence and metastases.
Strategies to ameliorate the adverse effects of opioids with “peripherally acting mu-opioid receptor antagonists” (PAMORAs)
Although morphine is commonly used for management of severe pain in cancer, the unwanted enhancement of tumor progression and other deleterious multi-organ side effects have prompted investigation on strategies to prevent the inadvertent effects of morphine without compromising analgesia. To this end, investigations have focused on peripherally acting mu-opioid receptor antagonists (PAMORAs), which were primarily developed for opioid-induced constipation (OIC). Methylnaltrexone, naloxegol (NKTR-118), and alvimopan are in clinical use, and several other oral PAMORAs (TD-1211, ADL-7445, and ADL-5945) are being studied.70,71 These antagonists do not cross the blood brain barrier, but selectively reverse opioid actions mediated by receptors outside the central nervous system, and thus do not influence centrally mediated opioid analgesia. Methylnaltrexone and naloxone do not reverse opioid analgesia in non-cancer pain patients, although naloxone, but not methylnaltrexone, triggers central opioid withdrawal.72–74 Phase III trials in patients with moderate to severe chronic, non-malignant pain demonstrated that oxycodone in combination with naloxone improved bowel function, and relieved pain as effectively as oxycodone.75,76
Recently Singleton et al showed that morphine, acting via MOP-R, upregulates multi-drug resistance to chemotherapeutic agents in human NSCLC cells; this effect is blocked by methylnaltrexone.33 Suzuki et al have shown that methylnaltrexone augments the antineoplastic activity of the chemotherapeutic agent docetaxel and improves survival in a mouse xenograft model of human gastric cancer.77 Furthermore, MOP-R activation in primary afferent CGRP nociceptive fiber free nerve endings in mice was inihibited using a PAMORA, HS-731, which produced analgesia primarily through ‘big conductance’ Ca(2+)-activated K(+) channels.78 More recently, in a randomized placebo-controlled trial on a cohort of 370 patients, methylnaltrexone slowed down the progression of advanced illness in patients who responded to methylnaltrexone for OIC79, suggestive of effectiveness of PAMORAs in treating cancer. Thus PAMORAs may provide additional beneficial effects in cancer patients beyond alleviating OIC.
Future directions
In the early 2000s we showed that morphine stimulates pro-angiogenic signaling in vitro which translates into angiogenesis and tumor growth in vivo. The first clinical study showing the possible association of morphine with breast cancer recurrence and metastases following surgery under paravertebral anesthesia compared to GA/opioids, was reported in 2006.14. However, the translational clinical relevance of these critical findings is limited to recent retrospective analyses listed in Table 1. Therefore, well-designed clinical studies are required to prospectively test strategies that ameliorate the unwanted effects of systemic opioids administered in conjunction with anesthesia in the peri-operative setting.
Acknowledgments
**Sources of Support: Institute for Engineering in Medicine, University of Minnesota and NIH grants, RO1 103773 and UO1 HL117664 to K.G.
Authors would like to thank Yann Lamarre, PhD for assistance with the artistic illustration of Figure 1. We also thank the Institute for Engineering in Medicine, University of Minnesota and NIH grants, RO1 103773 and UO1 HL117664 to K.G for funding support. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Anupam Aich, Vascular Biology Center, Division of Hematology, Oncology and Transplantation, Department of Medicine, University of Minnesota, Minneapolis, MN, USA.
Pankaj Gupta, Hematology/Oncology Section, Department of Medicine, Minneapolis VA Health Care System, Minneapolis, MN, USA; Division of Hematology, Oncology and Transplantation, Department of Medicine, University of Minnesota, Minneapolis, MN, USA.
Kalpna Gupta, Vascular Biology Center, Division of Hematology, Oncology and Transplantation, Department of Medicine, University of Minnesota, Minneapolis, MN, USA.
References
- 1.Ripamonti CI, Bandieri E, Roila F Group, O. behalf of the E. G. W. Management of cancer pain: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22:vi69–vi77. doi: 10.1093/annonc/mdr390. [DOI] [PubMed] [Google Scholar]
- 2.Apolone G, Corli O, Caraceni A, et al. Pattern and quality of care of cancer pain management. Results from the Cancer Pain Outcome Research Study Group. Br J Cancer. 2009;100:1566–1574. doi: 10.1038/sj.bjc.6605053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plante GE, VanItallie TB. Opioids for cancer pain: the challenge of optimizing treatment. Metabolism. 2010;59(Suppl 1):S47–52. doi: 10.1016/j.metabol.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Gupta M, Msambichaka L, Ballas SK, Gupta K. Morphine for the Treatment of Pain in Sickle Cell Disease. Sci World J. 2015;2015:540154. doi: 10.1155/2015/540154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta K. Morphine and Metastasis. Springer; 2013. pp. 63–78. [Google Scholar]
- 6.Nguyen J, Luk K, Vang D, et al. Morphine stimulates cancer progression and mast cell activation and impairs survival in transgenic mice with breast cancer. Br J Anaesth. 2014;113 doi: 10.1093/bja/aeu090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lennon FE, Mirzapoiazova T, Mambetsariev B, et al. The Mu Opioid Receptor Promotes Opioid and Growth Factor-Induced Proliferation, Migration and Epithelial Mesenchymal Transition (EMT) in Human Lung Cancer. PLoS One. 2014;9:e91577. doi: 10.1371/journal.pone.0091577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta K, Kshirsagar S, Chang L, et al. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62:4491–4498. [PubMed] [Google Scholar]
- 9.Fujioka N, Nguyen J, Chen C, et al. Morphine-induced epidermal growth factor pathway activation in non-small cell lung cancer. Anesth Analg. 2011;113:1353–1364. doi: 10.1213/ANE.0b013e318232b35a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ecimovic P, Murray D, Doran P, et al. Direct effect of morphine on breast cancer cell function in vitro: role of the NET1 gene. Br J Anaesth. 2011;107:916–923. doi: 10.1093/bja/aer259. [DOI] [PubMed] [Google Scholar]
- 11.Farooqui M, Li Y, Rogers T, et al. COX-2 inhibitor celecoxib prevents chronic morphine-induced promotion of angiogenesis, tumour growth, metastasis and mortality, without compromising analgesia. Br J Cancer. 2007;97:1523–31. doi: 10.1038/sj.bjc.6604057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathew B, Lennon FE, Siegler J, et al. The novel role of the mu opioid receptor in lung cancer progression: A laboratory investigation. Anesth Analg. 2011;112:558–567. doi: 10.1213/ANE.0b013e31820568af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee BM, Singh Ghotra V, Karam JA, et al. Regional anesthesia/analgesia and the risk of cancer recurrence and mortality after prostatectomy: a meta-analysis. Pain Manag. 2015;5:387–395. doi: 10.2217/pmt.15.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? J Am Soc Anesthesiol. 2006;105:660–664. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singleton PA, Moss J. Effect of perioperative opioids on cancer recurrence: a hypothesis. Futur Oncol. 2010;6:1237–1242. doi: 10.2217/fon.10.99. [DOI] [PubMed] [Google Scholar]
- 16.Zylla D, Gourley BL, Vang D, et al. Opioid requirement, opioid receptor expression, and clinical outcomes in patients with advanced prostate cancer. Cancer. 2013;119:4103–4110. doi: 10.1002/cncr.28345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zylla D, Kuskowski Ma, Gupta K, Gupta P. Association of opioid requirement and cancer pain with survival in advanced non-small cell lung cancer. Br J Anaesth. 2014:1–8. doi: 10.1093/bja/aeu351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singleton PA, Mirzapoiazova T, Hasina R, Salgia R, Moss J. Increased mu-opioid receptor expression in metastatic lung cancer. Br J Anaesth. 2014;113 doi: 10.1093/bja/aeu165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maher DP, Wong W, White PF, et al. Association of increased postoperative opioid administration with non-small-cell lung cancer recurrence: a retrospective analysis. Br J Anaesth. 2014:aeu192. doi: 10.1093/bja/aeu192. [DOI] [PubMed] [Google Scholar]
- 20.Cata JP, Keerty V, Keerty D, et al. A retrospective analysis of the effect of intraoperative opioid dose on cancer recurrence after non-small cell lung cancer resection. Cancer Med. 2014;3:900–8. doi: 10.1002/cam4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Hasani R, Bruchas MR. Molecular Mechanisms of Opioid Receptor-dependent Signaling and Behavior. Anesthesiology. 2011 doi: 10.1097/ALN.0b013e318238bba6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singleton PA, Lingen MW, Fekete MJ, Garcia JGN, Moss J. Methylnaltrexone inhibits opiate and VEGF-induced angiogenesis: Role of receptor transactivation. Microvasc Res. 2006;72:3–11. doi: 10.1016/j.mvr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Singleton PA, Garcia JG, Moss J. Synergistic effects of methylnaltrexone with 5-fluorouracil and bevacizumab on inhibition of vascular endothelial growth factor-induced angiogenesis. Mol Cancer Ther. 2008;7:1669–1679. doi: 10.1158/1535-7163.MCT-07-2217. [DOI] [PubMed] [Google Scholar]
- 24.Tegeder I, Geisslinger G. Opioids as modulators of cell death and survival--unraveling mechanisms and revealing new indications. Pharmacol Rev. 2004;56:351–369. doi: 10.1124/pr.56.3.2. [DOI] [PubMed] [Google Scholar]
- 25.Singleton Pa, Mambetsariev N, Lennon FE, et al. Methylnaltrexone potentiates the anti-angiogenic effects of mTOR inhibitors. J Angiogenes Res. 2010;2:5. doi: 10.1186/2040-2384-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber ML, Farooqui M, Nguyen J, et al. Morphine induces mesangial cell proliferation and glomerulopathy via kappa-opioid receptors. Am J Physiol Renal Physiol. 2008;294:F1388–97. doi: 10.1152/ajprenal.00389.2007. [DOI] [PubMed] [Google Scholar]
- 27.Madar I, Bencherif B, Bencherif B, et al. Imaging delta- and mu-opioid receptors by PET in lung carcinoma patients. J Nucl Med. 2007;48:207–13. [PubMed] [Google Scholar]
- 28.Nylund G, Pettersson A, Bengtsson C, et al. Functional expression of mu-opioid receptors in the human colon cancer cell line, HT-29, and their localization in human colon. Dig Dis Sci. 2008;53:461–6. doi: 10.1007/s10620-007-9897-y. [DOI] [PubMed] [Google Scholar]
- 29.Zylla D, Gourley BL, Vang D, et al. Opioid requirement, opioid receptor expression, and clinical outcomes in patients with advanced prostate cancer. Cancer. 2013;119:4103–4110. doi: 10.1002/cncr.28345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bodnar RJ. Endogenous opiates and behavior: 2014. Peptides. 2016;75:18–70. doi: 10.1016/j.peptides.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Cata JP, Keerty V, Keerty D, et al. A retrospective analysis of the effect of intraoperative opioid dose on cancer recurrence after non-small cell lung cancer resection. Cancer Med. 2014;3:900–8. doi: 10.1002/cam4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lennon FE, Mirzapoiazova T, Mambetsariev B, et al. Overexpression of the μ-opioid receptor in human non-small cell lung cancer promotes Akt and mTOR activation, tumor growth, and metastasis. Anesthesiology. 2012;116:857–67. doi: 10.1097/ALN.0b013e31824babe2. [DOI] [PubMed] [Google Scholar]
- 33.Singleton PA, Mirzapoiazova T, Salgia R, Moss J. THORACIC CANCERS: PATHOGENESIS AND NOVEL TARGETS. American Thoracic Society; 2015. p. A110. [DOI] [Google Scholar]
- 34.Singleton PA, Moreno-Vinasco L, Sammani S, et al. Attenuation of vascular permeability by methylnaltrexone: role of mOP-R and S1P3 transactivation. Am J Respir Cell Mol Biol. 2007;37:222–31. doi: 10.1165/rcmb.2006-0327OC. [DOI] [PubMed] [Google Scholar]
- 35.Chen C, Farooqui M, Gupta K. Morphine stimulates vascular endothelial growth factor-like signaling in mouse retinal endothelial cells. Curr Neurovasc Res. 2006;3:171–180. doi: 10.2174/156720206778018767. [DOI] [PubMed] [Google Scholar]
- 36.Nagy JA, Dvorak AM, Dvorak HF. Vascular Hyperpermeability, Angiogenesis, and Stroma Generation. Cold Spring Harb Perspect Med. 2012;2:a006544–a006544. doi: 10.1101/cshperspect.a006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luk K, Boatman S, Johnson KN, et al. Influence of morphine on pericyte-endothelial interaction: Implications for antiangiogenic therapy. J Oncol. 2012 doi: 10.1155/2012/458385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wen H, Lu Y, Yao H, Buch S. Morphine induces expression of platelet-derived growth factor in human brain microvascular endothelial cells: Implication for vascular permeability. PLoS One. 2011;6 doi: 10.1371/journal.pone.0021707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao LH, Li HT, Lin WQ, et al. Morphine, a potential antagonist of cisplatin cytotoxicity, inhibits cisplatin-induced apoptosis and suppression of tumor growth in nasopharyngeal carcinoma xenografts. Sci Rep. 2016;6:18706. doi: 10.1038/srep18706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arerangaiah R, Chalasani N, Udager AM, et al. Opioids Induce Renal Abnormalities in Tumor-Bearing Mice. Nephron Exp Nephrol. 2007;105:e80–e89. doi: 10.1159/000098564. [DOI] [PubMed] [Google Scholar]
- 41.Salvemini D, Seibert K, Masferrer JL, et al. Endogenous nitric oxide enhances prostaglandin production in a model of renal inflammation. J Clin Invest. 1994;93:1940. doi: 10.1172/JCI117185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang SH, Liu CH, Conway R, et al. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci U S A. 2004;101:591–596. doi: 10.1073/pnas.2535911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Griffin RJ, Williams BW, Wild R, et al. Simultaneous inhibition of the receptor kinase activity of vascular endothelial, fibroblast, and platelet-derived growth factors suppresses tumor growth and enhances tumor radiation response. Cancer Res. 2002;62:1702–1706. [PubMed] [Google Scholar]
- 44.Leahy KM, Ornberg RL, Wang Y, et al. Cyclooxygenase-2 inhibition by celecoxib reduces proliferation and induces apoptosis in angiogenic endothelial cells in vivo. Cancer Res. 2002;62:625–631. [PubMed] [Google Scholar]
- 45.Koodie L, Yuan H, Pumper JA, et al. Morphine inhibits migration of tumor-infiltrating leukocytes and suppresses angiogenesis associated with tumor growth in mice. Am J Pathol. 2014;184:1073–1084. doi: 10.1016/j.ajpath.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koodie L, Ramakrishnan S, Roy S. Morphine suppresses tumor angiogenesis through a HIF-1α/p38MAPK pathway. Am J Pathol. 2010;177:984–997. doi: 10.2353/ajpath.2010.090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoburn BC, Chen J, Huang T, Inturrisi CE. Pharmacokinetics and pharmacodynamics of subcutaneous morphine pellets in the rat. J Pharmacol Exp Ther. 1985;235:282–286. [PubMed] [Google Scholar]
- 48.Yokota T, Uehara K, Nomoto Y. Addition of noradrenaline to intrathecal morphine augments the postoperative suppression of natural killer cell activity. J Anesth. 2004;18:190–195. doi: 10.1007/s00540-004-0247-3. [DOI] [PubMed] [Google Scholar]
- 49.Page GG, Blakely WP, Ben-Eliyahu S. Evidence that postoperative pain is a mediator of the tumor-promoting effects of surgery in rats. Pain. 2001;90:191–199. doi: 10.1016/s0304-3959(00)00403-6. [DOI] [PubMed] [Google Scholar]
- 50.Page GG, Ben-Eliyahu S, Yirmiya R, Liebeskind JC. Morphine attenuates surgery-induced enhancement of metastatic colonization in rats. Pain. 1993;54:21–28. doi: 10.1016/0304-3959(93)90095-7. [DOI] [PubMed] [Google Scholar]
- 51.Freier DO, Fuchs BA. A mechanism of action for morphine-induced immunosuppression: corticosterone mediates morphine-induced suppression of natural killer cell activity. J Pharmacol Exp Ther. 1994;270:1127–1133. [PubMed] [Google Scholar]
- 52.Gavériaux-Ruff C, Matthes HWD, Peluso J, Kieffer BL. Abolition of morphine-immunosuppression in mice lacking the μ-opioid receptor gene. Proc Natl Acad Sci. 1998;95:6326–6330. doi: 10.1073/pnas.95.11.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aich A, Afrin LB, Gupta K. Mast cell-mediated mechanisms of nociception. International Journal of Molecular Sciences. 2015;16:29069–29092. doi: 10.3390/ijms161226151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ribatti D, Crivellato E. Mast cells, angiogenesis, and tumour growth. Biochimica et Biophysica Acta - Molecular Basis of Disease. 2012;1822:2–8. doi: 10.1016/j.bbadis.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 55.Vincent L, Vang D, Nguyen J, et al. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood. 2013;122:1853–1862. doi: 10.1182/blood-2013-04-498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mantyh PW. Neurobiology of substance P and the NK1 receptor. Journal of Clinical Psychiatry. 2002;63:6–10. [PubMed] [Google Scholar]
- 57.Muñoz M, Coveñas R. Involvement of substance P and the NK-1 receptor in cancer progression. Peptides. 2013;48:1–9. doi: 10.1016/j.peptides.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 58.Ziche M, Morbidelli L, Pacini M, et al. Substance P stimulates neovascularization in vivo and proliferation of cultured endothelial cells. Microvasc Res. 1990;40:264–78. doi: 10.1016/0026-2862(90)90024-l. [DOI] [PubMed] [Google Scholar]
- 59.Zhou XR, Pogue BW, Hoopes PJ, Hasan T. Substance P enhances photosensitizer uptake in MAT-LyLu prostate tumors. Cancer Res. 2006;66:1216–1216. [Google Scholar]
- 60.Garcia-Recio S, Fuster G, Fernandez-Nogueira P, et al. Substance P autocrine signaling contributes to persistent HER2 activation that drives malignant progression and drug resistance in breast cancer. Cancer Res. 2013;73:6424–34. doi: 10.1158/0008-5472.CAN-12-4573. [DOI] [PubMed] [Google Scholar]
- 61.Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science (80-) 2013;341:1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 62.Sessler DI, Ben-Eliyahu S, Mascha EJ, Parat MO, Buggy DJ. Can regional analgesia reduce the risk of recurrence after breast cancer?: Methodology of a multicenter randomized trial. Contemp Clin Trials. 2008;29:517–526. doi: 10.1016/j.cct.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 63.Wandel C, Kim R, Wood M, Wood A. Interaction of morphine, fentanyl, sufentanil, alfentanil, and loperamide with the efflux drug transporter P-glycoprotein. J Am Soc Anesthesiol. 2002;96:913–920. doi: 10.1097/00000542-200204000-00019. [DOI] [PubMed] [Google Scholar]
- 64.Weigelt B, Glas AM, Wessels LFA, et al. Gene expression profiles of primary breast tumors maintained in distant metastases. Proc Natl Acad Sci. 2003;100:15901–15905. doi: 10.1073/pnas.2634067100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gilcrease MZ, Kilpatrick SK, Woodward WA, et al. Coexpression of α6β4 integrin and guanine nucleotide exchange factor Net1 identifies node-positive breast cancer patients at high risk for distant metastasis. Cancer Epidemiol Biomarkers Prev. 2009;18:80–86. doi: 10.1158/1055-9965.EPI-08-0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen X, Li J, Hu PP, et al. The activity of guanine exchange factor NET1 is essential for transforming growth factor-β-mediated stress fiber formation. J Biol Chem. 2001;276:15362–15368. doi: 10.1074/jbc.M009534200. [DOI] [PubMed] [Google Scholar]
- 67.Deegan CA, Murray D, Doran P, et al. Anesthetic technique and the cytokine and matrix metalloproteinase response to primary breast cancer surgery. Reg Anesth Pain Med. 2010;35:490–495. doi: 10.1097/AAP.0b013e3181ef4d05. [DOI] [PubMed] [Google Scholar]
- 68.Looney M, Doran P, Buggy DJ. Effect of anesthetic technique on serum vascular endothelial growth factor C and transforming growth factor β in women undergoing anesthesia and surgery for breast cancer. J Am Soc Anesthesiol. 2010;113:1118–1125. doi: 10.1097/ALN.0b013e3181f79a69. [DOI] [PubMed] [Google Scholar]
- 69.Wu J, Buggy D, Fleischmann E, et al. Thoracic paravertebral regional anesthesia improves analgesia after breast cancer surgery: a randomized controlled multicentre clinical trial. Can J Anesth Can d’anesthésie. 2015;62:241–251. doi: 10.1007/s12630-014-0285-8. [DOI] [PubMed] [Google Scholar]
- 70.Diego L, Atayee R, Helmons P, Hsiao G, von Gunten CF. Novel opioid antagonists for opioid-induced bowel dysfunction. Expert Opin Investig Drugs. 2011;20:1047–1056. doi: 10.1517/13543784.2011.592830. [DOI] [PubMed] [Google Scholar]
- 71.Moss J, Rosow CE. Development of peripheral opioid antagonists: new insights into opioid effects. Mayo Clinic Proceedings Elsevier. 2008;83:1116–1130. doi: 10.4065/83.10.1116. [DOI] [PubMed] [Google Scholar]
- 72.Corsetti M, Tack J. Naloxegol, a new drug for the treatment of opioid-induced constipation. Expert Opin Pharmacother. 2015;16:399–406. doi: 10.1517/14656566.2015.991306. [DOI] [PubMed] [Google Scholar]
- 73.Garnock-Jones KP. Naloxegol: a review of its use in patients with opioid-induced constipation. Drugs. 2015;75:419–425. doi: 10.1007/s40265-015-0357-2. [DOI] [PubMed] [Google Scholar]
- 74.Weber HC. Opioid-induced constipation in chronic noncancer pain. Curr Opin Endocrinol Diabetes Obes. 2016;23 doi: 10.1097/MED.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 75.Burness CB, Keating GM. Oxycodone/Naloxone prolonged-release: a review of its use in the management of chronic pain while counteracting opioid-induced constipation. Drugs. 2014;74:353–375. doi: 10.1007/s40265-014-0177-9. [DOI] [PubMed] [Google Scholar]
- 76.Li Z, Pergolizzi JV, Huttner RP, et al. Management of opioid-induced constipation in pregnancy: a concise review with emphasis on the PAMORAs. J Clin Pharm Ther. 2015;40:615–9. doi: 10.1111/jcpt.12331. [DOI] [PubMed] [Google Scholar]
- 77.Suzuki M, Chiwaki F, Sawada Y, et al. Peripheral opioid antagonist enhances the effect of anti-tumor drug by blocking a cell growth-suppressive pathway in vivo. PLoS One. 2015;10:e0123407. doi: 10.1371/journal.pone.0123407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baillie LD, Schmidhammer H, Mulligan SJ. Peripheral μ-opioid receptor mediated inhibition of calcium signaling and action potential-evoked calcium fluorescent transients in primary afferent CGRP nociceptive terminals. Neuropharmacology. 2015;93:267–273. doi: 10.1016/j.neuropharm.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 79.Moss J, Singleton PA, Barrett AJL. Effect of methylnaltrexone on disease progression rate in advanced illness patients with cancer. Annual Meeting of the American Society of Anesthesiologists; 2015; Abstract 4032. [Google Scholar]