Abstract
Sponge metagenomes are a useful platform to mine cryptic biosynthetic gene clusters responsible for production of natural products involved in the sponge-microbe association. Since numerous sponge-derived bioactive metabolites are biosynthesized by the symbiotic bacteria, this strategy may concurrently reveal sponge-symbiont produced compounds. Accordingly, a metagenomic analysis of the Japanese marine sponge Discodermia calyx has resulted in the identification of a hybrid type I polyketide synthase-nonribosomal peptide synthetase gene (kas). Bioinformatic analysis of the gene product suggested its involvement in the biosynthesis of kasumigamide, a tetrapeptide originally isolated from freshwater free-living cyanobacterium Microcystis aeruginosa NIES-87. Subsequent investigation of the sponge metabolic profile revealed the presence of kasumigamide in the sponge extract. The kasumigamide producing bacterium was identified as an ‘Entotheonella’ sp. Moreover, an in silico analysis of kas gene homologs uncovered the presence of kas family genes in two additional bacteria from different phyla. The production of kasumigamide by distantly related multiple bacterial strains implicates horizontal gene transfer and raises the potential for a wider distribution across other bacterial groups.
Introduction
Metagenome mining strategies [1] are applicable for the discovery of unknown compounds, particularly polyketides and nonribosomal peptides, from uncultured bacteria. Based on the colinearity rule of domain and module organization of modular polyketide synthase (PKS) and nonribosomal peptide synthetases (NRPS) [2–3], it is possible to predict the chemical structures of products derived from orphan gene clusters comprising PKS and/or NRPS genes. As many polyketides and nonribosomal peptides have been isolated from marine sponges, and there is strong evidence that those natural products are actually produced by their microbial symbionts, sponge metagenomes have become a useful source for identifying the real producers of those natural products. Among the many different sponge symbionts, e.g., Proteobacteria, Actinobacteria, Acidobacteria, Cyanobacteria [4], the ‘Enthotheonella’ group belonging to the candidate phylum ‘Tectomicrobia’ has recently been described as a highly prolific bacterial symbiont phylotype capable of producing structurally complex bioactive molecules [5–6]. This as-yet-uncultured group of bacteria has been frequently detected in several marine sponges. In the marine sponges Theonella swinhoei Y and T. swinhoei WA, ‘Entotheonella’ was identified as the true producer of a number of natural products [7–8]. The Japanese marine sponge Discodermia calyx (Fig 1A) [9] is also known as a rich source of bioactive compounds, such as calyculins and calyxamides [10–12]. Through extensive screening of the metagenomic library of D. calyx, we recently identified the calyculin biosynthetic gene cluster and linked this cluster to the symbiotic bacterium, ‘Entotheonella’ sp. by employing single cell analysis [13].
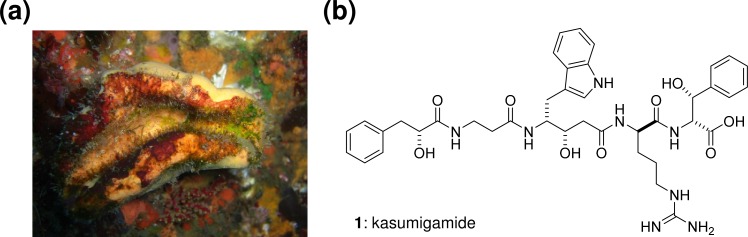
Fig 1. Kasumigamide from the marine sponge Discodermia calyx.
(a) The marine sponge Discodermia calyx. (b) Structure of kasumigamide.
Furthermore, we employed degenerate PCR to target gene within the D. calyx metagenome that encode ketosynthase (KS) domains [14–18] and found a range of distinct KS domain sequences. Of these, several were associated with trans-acyl transferase (AT) type KS domains and characterized as components of the calyculin biosynthetic gene cluster. Other KS domains, however, could not be attributed to a previously characterized biosynthetic pathway. Therefore, to search for new biosynthetic gene clusters and their products, extensive characterization of the diverse KS domains present in the D. calyx metagenomic library is warranted. Here, we present such a study, targeting KS domains affiliated with the cis-AT KS group and subsequently characterizing the production of the cyanobacterial natural product kasumigamide by a sponge symbiont.
Results
Annotation and predicted product of kas gene cluster
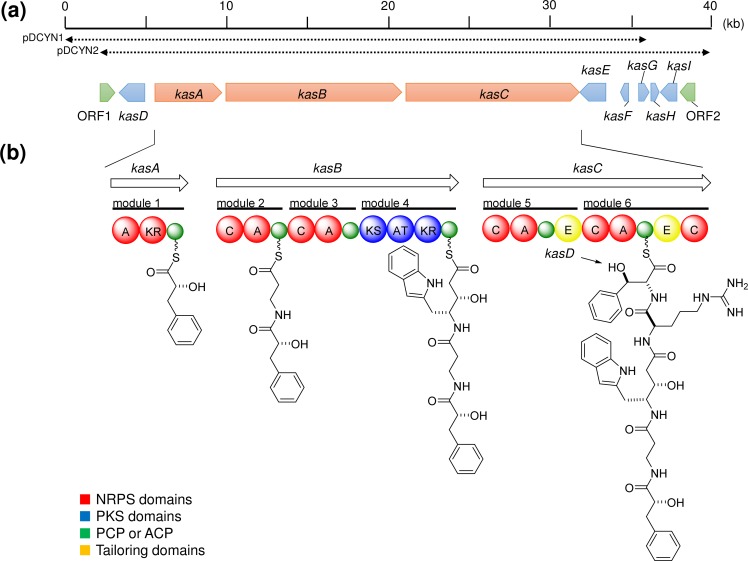
Screening of the D. calyx fosmid library using specific primers for the cis-AT KS domain resulted in the identification of two clones pDCYN1 and pDCYN2. Sequencing of the fosmid clones revealed the existence of a hybrid PKS-NRPS biosynthetic gene cluster (kasA-I, ~37 kb, LC160290) (Table 1, Fig 2), composed of 9 open reading frames (ORFs), with kasA-C forming a PKS-NRPS core. KasA is comprised of an adenylation (A) domain (KasA-A1), a ketoreductase (KR) domain (KasA-KR), and a peptidyl carrier protein (PCP) domain. KasB consists of three modules (modules 2–4): the first two modules encode NRPSs, and the latter encodes a PKS. The two following NRPS modules (modules 5–6) are encoded in kasC. The substrates for the five A domains (KasA-A1, KasB-A1, A2, KasC-A1, and A2) were predicted from the NRPS codes [19] with the exception of KasA-A1, which was unmatched to known data, but annotated to the A domain recruiting phenyl pyruvic acid (PP) based on the close resemblance to AerA of aeruginosin biosynthetic gene cluster (S2 Table) [20]. Collectively, the chemical structure of the PKS and NRPS hybrid compound was predicted to be kasumigamide, which had previously been isolated from the freshwater cyanobacterium Microcystis aeruginosa NIES-87 (Fig 1B) [21]. However there have been no reports of the isolation of this compound from marine sponges.
Table 1. Putative ORFs of kasumigamide NRPS-PKS gene cluster derived from “Entotheonella” sp.
| ORFs | Protein size (aa) | Proposed function | Sequence similarity, protein (origin) | Identity/Similarity (%) | Accession number |
|---|---|---|---|---|---|
| ORF1 | 305 | Transposase | Transposase (Rhodopirellula europaea) | 27/47 | WP_008672175 |
| kasD | 529 | Hydroxylase | CmlA (Streptomyces Venezuelae) | 39/57 | WP_015032127 |
| kasA | 1381 | NRPS (A, KR, PCP) | |||
| kasB | 3694 | PKS/NRPS (C, A, PCP, C, A, PCP, KS, AT, KR, ACP) | |||
| kasC | 3560 | NRPS (C, A, PCP, E, C, A, PCP, E, C) | |||
| kasE | 540 | Transporter | Cyclic peptide transporter (Methylobacter tundripaludum) | 38/59 | WP_006892918 |
| kasF | 168 | Transcriptional regulator | Transcriptional regulator (Desulfurivibrio alkaliphilus) | 36/54 | WP_013162517 |
| kasG | 215 | Methyltransferase | Methyltransferase (Rhodococcus hoagie) | 38/55 | WP_022594278 |
| kasH | 173 | Chorismate synthase | Chorismate synthase (Kribbella catacumbae) | 39/50 | WP_020385871 |
| kasI | 341 | Hypothetical protein | Hypothetical protein ETSY1_01160 (Candidatus “Entotheonella” sp. TSY1) | 92/94 | ETX03129 |
| ORF2 | 305 | Transposase | Transposase (Rhodopirellula europaea) | 27/47 | WP_008672175 |
Fig 2. The biosynthetic gene cluster and proposed biosynthetic pathway to kasumigamide.
(a) ORFs encoded in the putative kasumigamide biosynthetic gene cluster, kasA-I. Double-headed arrows show the location of pDCYN1-2. The ORFs related to PKS-NRPS are highlighted in red. Putative transposases are colored in green. (b) The domain organization and proposed biosynthetic pathway to kasumigamide.
Isolation of kasumigamide from marine sponge D. calyx
In order to search for kasumigamide and related compounds, the MeOH extract of the frozen sponge D. calyx was fractionated by column chromatography, based on LC-MS monitoring, to yield the putative kas gene product 1.
The molecular formula of 1 was established by ESI-TOFMS to be C40H50N8O9 [m/z 809.3801 (M+Na)+ for C40H50N8NaO9], which is consistent with that of kasumigamide (S1 Fig). Furthermore, 1D and 2D NMR data of 1, including 1H-1H COSY, HMQC, HMBC, and NOESY spectra in DMSO-d6+TFA, suggested the presence of β-Ala and Arg (S3 Table) (S4 Table) (S2 Fig) (S3 Fig) (S4 Fig) (S5 Fig) (S6 Fig) [21]. The four aromatic protons (δH = 6.91, 7.00, 7.28, and 7.47) and an exchangeable proton (δH = 10.74) coupled to another aromatic proton (δH = 7.00) were consistent with an indole ring, supported by the UV absorption maxima at 280 nm. In addition, the other ten aromatic protons at δH = 7.10–7.23 overlapped, which suggested the presence of two mono-substituted benzene rings. The HMBC correlation between aromatic protons (δH = 7.23) and an oxymethine carbon (δC 75.9) indicated the presence of 3-phenylserine. In contrast, other aromatic protons (δH = 7.16) showed HMBC correlation with a methylene carbon (δC 41.2), which further correlated with an oxymethine proton at δH = 4.00, supporting the presence of phenyl lactic acid. The amino acid sequence of 1 was confirmed by NOESY and HMBC correlations. The ODS HPLC analysis of L-FDAA derivatives of the hydrolysate of 1 revealed the presence D-Arg and D-erythro-3-phenylserine (D-erythro-PS). Therefore, the gross structure was concluded to be 1, which is coincident with the predicted product of the PKS and NRPS hybrid gene cluster, kasA-I.
Kasumigamide producer in the marine sponge
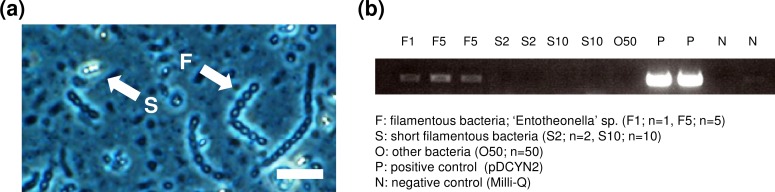
To identify the true producer of kasumigamide in D. calyx, we used the laser microdissection (LMD) method to isolate the symbiont cells for PCR analysis. As the candidates, two types of cells designated as “F” and “S” filamentous morphologies (Fig 3A) were isolated from the sponge material. Of these two, only cells with the “F” morphology (Fig 3A) were returned positive when the genomes were amplified using the kas specific primer pair (Fig 3B). This filamentous bacterium was previously reported to be ‘Entotheonella’ sp. as the producer of calyculins in D. calyx (Fig 3A) [13].
Fig 3. Symbiont bacteria bearing kas genes.
(a) Phase contrast image of D. calyx homogenate. A filamentous bacterium ‘Entotheonella’ sp. is designated as “F”. A small filamentous bacterium with bright color is designated as “S”. Scale bars was 20 μm. (b) PCR analysis of dissected cells with the kas-specific primers pair, DCKS10F/DCKS10R (S1 Table), using dissected cells (“F” or “S”) as templates.
Kasumigamide biosynthetic gene cluster in cyanobacterium
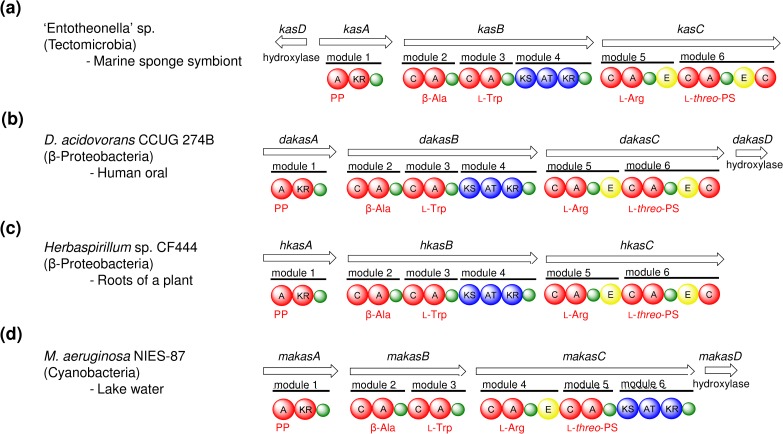
Kasumigamide was first identified in the free-living cyanobacterium M. aeruginosa NIES-87 [21]; however, its biosynthetic pathway in this organism was unknown. To compare the corresponding gene clusters between the two different bacterial species, we set out to identify a kasumigamide biosynthetic gene cluster in M. aeruginosa NIES-87. First, the ability of M. aeruginosa NIES-87 to produce kasumigamide was confirmed by LC-MS analysis (S7 Fig). One of the M. aeruginosa metabolites showed the same retention time and molecular mass as those of kasumigamide isolated from D. calyx. These results corroborated the previous report of kasumigamide production in M. aeruginosa NIES-87 [21]. The genome was then sequenced and assembled (see Materials and Methods). A homology-based search revealed the existence of the PKS-NRPS biosynthetic gene cluster, named makasA-D (S5 Table, LC160291) (S8C Fig). The makasA-C genes encode five NRPS modules and one PKS module (Fig 4D).
Fig 4. Comparative analysis of domain organizations of the putative kasumigamide biosynthetic gene clusters.
Each gene cluster derived from (a) D. acidovorans CCUG 274B (b) Herbaspirillum sp. CF444 (c) ‘Entotheonella’ sp. (d) M. aeruginosa NIES-87.
Substrate specificity of the adenylation domains
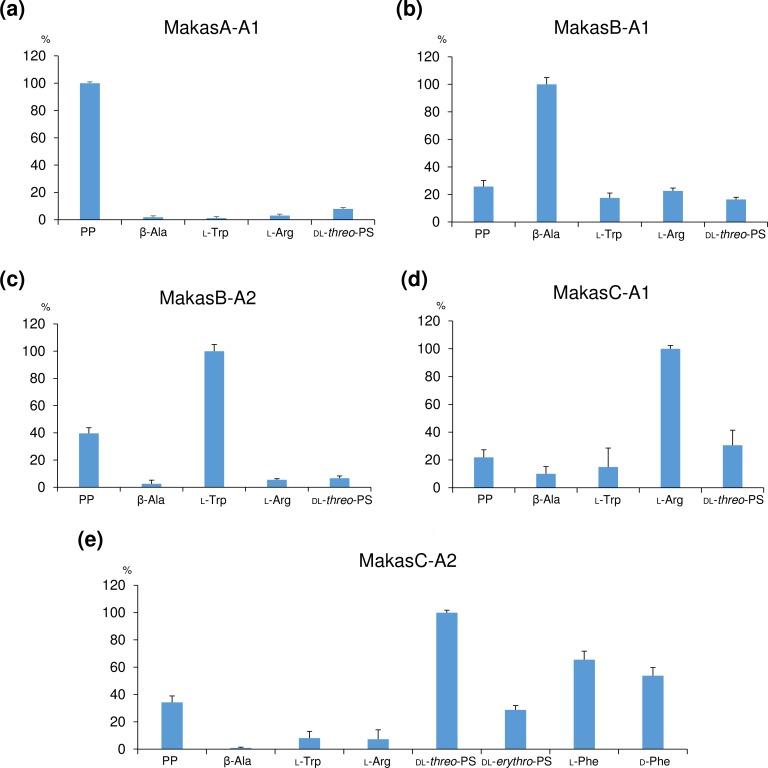
In order to confirm the predicted substrates for the A domains in MakasA-C, an in vitro analysis was performed using the Biomol Green assay strategy [22–23]. The His6-tagged recombinant proteins of the five A domains, MakasA-A1, MakasB-A1, MakasB-A2, MakasC-A1, and MakasC-A2, were produced in Escherichia coli and purified by nickel-nitrilotriacetic acid (Ni-NTA) affinity chromatography (S9 Fig). Maltose binding protein (MBP) was used to improve the solubility of MakasB2 and MakasC1. In the case of MakasB-A1 and MakasC-A2, the C and PCP domains were coexpressed. MakasA-A1, MakasB-A1, MakasB-A2, and MakasC-A1 exhibited catalytic activities selective for PP, β-Ala, L-Trp, and L-Arg, respectively (Fig 5). In addition, MakasC-A2 showed higher selectivity to DL-threo-3-phenylserine (DL-threo-PS) than L-Phe, in accord to the substrate binding prediction (S2 Table). However, we have so far not succeeded in the heterologous expression of each A domain of KasA-C as well as the entire gene cluster, kasA-I using E. coli, Streptomyces lividans, or Streptomyces avermitilis as a host.
Fig 5. Substrate selectivity of A domains.
The relative adenylation activity was estimated by the malachite green phosphate assay. Error bars represent SEM (n = 3).
Kasumigamide biosynthetic gene cluster in phylogenetically diverse bacteria
The above studies revealed that kasumigamide is produced by two phylogenetically distant bacterial species, ‘Entotheonella’ sp. and M. aeruginosa NIES-87. This observation suggests a horizontal transfer of kasumigamide biosynthetic gene cluster can occur between different bacterial species. Therefore, we searched for additional kas biosynthetic gene clusters using BLASTP, with KasA-D as a query. As a result, two candidate clusters were found in the β-proteobacteria, Delftia acidovorans CCUG 274B (WP_016445283-WP_016445295) and Herbaspirillum sp. CF444 (EJL94052-EJL94061), and the putative kasumigamide biosynthetic gene clusters were annotated and named dakasA-J and hkasA-H, respectively (S6 Table) (S7 Table) (S8A and S8B Fig) [24]. The GC contents of kasA-I, makasA-D, dakasA-J, and hkasA-H are 57.5%, 52.9%, 70.6%, and 64.7% respectively. The domain organizations of PKS-NRPS modules of dakasA-J and hkasA-H were identical to those of kasA-C (Fig 4A–4C). The A domain binding sites exhibit 77~100% identity to the corresponding domains of KasA-C and/or MakasA-C (S2 Table). Finally, phylogenetic analysis of the KS domains (S10 Fig) shows their close relationships to each other despite that they are originated from phylogenetically distinct bacteria (S11 Fig).
Discussion
The strategy for isolating kasumigamide from D. calyx exemplifies the effectiveness of the metagenome mining approach in identifying cryptic biosynthetic gene clusters as well as their products. The involvement of the kas cluster in kasumigamide biosynthesis was predicted based on its domain organization (Fig 2). Whereas the NRPS code of KasA-A1 did not provide any information about its natural substrate (S2 Table), KasA-KR resembles the KR domain of AerA (~41% identity), which reportedly reduces PP to generate D-phenyl lactic acid [20]. KasD was annotated as a β-hydroxylase, according to its similarity to CmlA [25] (~39% identity). CmlA catalyzes β-hydroxylation of L-p-aminophenylalanine (L-PAPA) to L-p-aminophenylserine (L-PAPS), and the metal ion binding site of CmlA are also well conserved in KasD (S12 Fig). Since L-Phe is structurally similar to L-PAPA, KasD was proposed to catalyze the β-hydroxylation of L-Phe, to generate L-threo-3-phenylserine (L-threo-PS). The epimerase (E) domain, encoded in module 6, was predicted to epimerize L-threo-PS to D-erythro-PS.
The kasumigamide producer in D. calyx was identified as an ‘Entotheonella’ sp. by a single cell analysis in conjunction with PCR. This bacterial phylotype has been reported as the symbiotic producer of not only secondary metabolites in T. swinhoei [7], but also calyculin A in D. calyx [13], highlighting the fact that ‘Entotheonella’ is responsible for the production of multiple natural products in Theonellidae sponges. In addition, four kasumigamide gene clusters were detected in very different bacterial species, namely ‘Entotheonella’ sp. (a marine sponge symbiont), the free-living cyanobacterium M. aruginosa NIES-87, the human oral bacterium D. acidovorans CCUG 274B, and Herbaspirillum sp. CF444 from the endosphere of the tree Populus deltoids (Fig 4). Although we did not confirm the production of kasumigamide in the latter two bacterial species, the phylogenetic tree analysis of KS domains illuminate close relationships among the kas-related gene clusters (S10 Fig).
On the other hand, some peculiar features of the domain or module organization can be found in the kas family gene clusters. One of the remarkable points is the shift of PKS module in the M. aeruginosa NIES-87 kas-cluster (makasA-D), while the positions of the PKS module in the other kas genes were located on module 4, in agreement with the order of the biosynthetic reactions. To rule out the possibility that this inconsistency is due to misassembly, the contiguous domain organization between modules 5 and 6 in makasC was confirmed by cloning of the corresponding region. To obtain further evidence for the biosynthetic mechanism of makasA-D, we conducted substrate specificity assays with all five A domains, which have the substrate binding sites closely related to their counterpart domains in KasA-C with 77%~89% identity (S2 Table). Four of them accepted the substrates expected from the amino acid sequences of their substrate binding sites. Although the putative substrate of MakasC-A2 was L-Phe, this A domain exhibited specificity for DL-threo-PS. Considering the fact that D-erythro-PS is the C-terminal residue of kasumigamide, the biosynthetic mechanism of this step was proposed, as follows. First, L-Phe is hydroxylated by MakasD to generate L-threo-PS. Subsequently, MakasC-A2 loads the L-threo-PS onto the PCP domain. Finally, L-threo-PS is epimerized to D-erythro-PS by MakasC-E1, which is encoded between module 4 and 5 (S13 Fig) [26–27].
Other unusual features of the kas family genes are the absence of a thioesterase (TE) domain and the likely termination of the chain by a condensation (C) domain. Since some C domains have been reported to function as a TE domain [28], we expect that the C-terminal C domain encoded in kasC, dakasC, and hkasC can serve as a thioesterase. However, since the C-terminal C domain is missing in makasA-D, the release mechanism of the makas pathway remains unclear.
The presence of putative kasumigamide biosynthetic gene clusters among different kinds of bacteria living in various environments implies horizontal gene transfer between different bacterial species. The pair of long terminal repeats flanking the kas gene was annotated as putative transposases (Table 1, S14 Fig), suggesting the role of transposons in interspecies transfer of kas gene clusters. Sponge-associated bacteria reportedly contain high numbers of transposable insertion elements, expected to take part in the evolution of symbiont bacteria genomes [29]. Examples of hypothetical horizontal transmission have been suggested for gene clusters encoding synthesis of actin-binding macrolides, such as luminolide, tolytoxin, and misakinolide [8]. Although macrolide compounds are produced by different bacterial species, including ‘Entotheonella serta’ (which is associated with the marine sponge T. swinhoei WA), the gene clusters encoding some transposases exhibit high relationship between the corresponding PKS domains [8]. The makas gene is also flanked by two putative transposases, ORFM1 and ORFM2, which are widely conserved in several M. aeruginosa strains. Notably, makasA-D sequences were only found in M. aeruginosa NIES-87 strain (S8C Fig) among sixteen different strains whose genomes are available in National Center for Biotechnology Information (NCBI) data bank. As in the case for M. aeruginosa strains, only one of four sequenced D. acidovorans strains contains the kas family genes, though we could not observe a putative transposase region in the kas-related genes encoded in the other two kinds of bacterial species, the β-proteobacteria, D. acidovorans CCUG 274B and Herbaspirillum sp. CF444.
It is known that the same or similar secondary metabolites were identified from different kinds of bacteria, even across phyla. Lyngbyatoxins, which are potent skin irritants, were originally isolated from the marine cyanobacterium Moorea producens (formerly Lyngbya majuscule) [30–31]. On the other hand, structurally and pharmacologically related compounds, teleocidin and olivoretin, were isolated from the marine Streptomyces spp [32–35]. Saxitoxin, which is produced by marine dinoflagellates, is also made by some freshwater cyanobacteria [36]. The gene clusters were assembled independently in the distantly related bacteria [37]. Thus, the study presented here suggests that other bacteria may also have the ability to produce kasumigamide. Although the biological activity of kasumigamide had been reported to be antialgae against Chlamydomonas neglecta NIES-439 [21], further investigations are required to decipher its advantageous role in the survival competition among taxonomically distant bacterial species.
Materials and Methods
Specimen collection
The marine sponge D. calyx was collected by hand, at a depth of 10 m, during scuba diving at Shikine-jima Island, Tokyo, on May 18, 2011. The specimens were kept frozen at −30°C and used for the construction of the clone library and isolation of kasumigamide. The single cell isolation was performed with the specimen collected at a depth of 10 m in the ocean near Nakagi, Shizuoka, Japan in December 4, 2013. Samples were transported to the laboratory (4 h) in a cooling box and immediately processed for single cell analysis. Permits and approval for the collections at Shikine-jima and Nakagi were obtained from the local governments. Both specimens contained calyculins, calyxamides as well as kasumigamide, which was confirmed by LC-MS analysis.
Fosmid clone library screening
The D. calyx metagenomic DNA fosmid library is composed of 250,000 colonies, each contains approximately 40 kb of insert DNA, as previously reported [38]. We focused on the DCKS10, which shares homology with the cis-AT-type-KS domains (the KS region of JamM [39], 59% identity), among 19 different partial sequences of KS domains obtained in our previous PCR analysis with KS degenerate primers from D. calyx metagenomic DNA [10]. The following primer pair was used: DCKS10F/DCKS10R (S1 Table) for screening positive fosmids. The library was screened by the Piel pooling strategy [40]. The screening yielded two positive fosmids, pDCYN1-2.
Genome sequencing and assembly
The sequencing of pDCYN1-2 was performed on an Ion PGM sequencer (Life Technologies), with a total number of 48,198 sequence reads (~300 bp). These sequences were assembled using the de novo assembler MIRA (v3.4.2.0) [41], and 17 contigs were obtained. Further assembly to produce larger contigs was achieved with the Genious assembler (Biomatters), with the default medium sensitivity. This assembly provided a contig of 40 kb. Putative protein-coding sequences (CDSs) were determined by a combination of FramePlot [42] and the Glimmer 3.02 [43]. The domain organizations were assessed by BLASTP and PKS/NRPS analysis [44].
Isolation and structure elucidation of kasumigamide
To isolate the putative kasA-I product from the frozen sponge, D. calyx (2.0 kg, wet weight), the methanol extract was partitioned between hexane and H2O. The aqueous layer was then partitioned between EtOAc and H2O. The aqueous layer was further partitioned between n-BuOH and H2O. The n-BuOH-soluble material was fractionated by gel-filtration column chromatography (Sephadex LH-20; 2.5 × 75 cm) with MeOH. The fraction was further separated by HPLC (Cosmosil MS-II C18; 10 × 250 mm, Nacalai Tesque, flow rate 4.0 mL/min; 0−100% CH3CN/H2O over 30 min; UV detection at 280 nm) to obtain pure kasumigamide (2.3 mg). The LC-MS data for monitoring were obtained from an Agilent 1100 series HPLC-micro TOF mass spectrometer (Bruker Daltonics), using electrospray ionization with a Cosmosil 5C18 MS-II column (2.0 × 75 mm), 5–100% MeOH/H2O in 0.1% AcOH over 20 min, 0.2 mL/min, positive ESI mode. To monitor an (M + H)+ ion peak of kasumigamide at m/z 787.38, the mass range between m/z 787.3 and 787.5 was selected for the extracted ion chromatogram.
PCR analysis of dissected filamentous bacterial cells
Aliquots of the calcium/magnesium-free artificial seawater suspension of minced sponge tissues were spread onto Membrane slides (PEN-Membrane 2.0 μm, Leica), dehydrated by sequential incubations in 50%, 70%, and 90% aqueous ethanol for 3 min at each step, and air dried. Two or ten portions of autofluorescent cells and single filament or five filaments of filamentous bacteria (‘Entotheonella’ sp.) were directly isolated into a PCR tube by laser microdissection (Leica LMD7000). As other bacteria in Fig 3B, the membrane area containing other bacterial cells, except for filamentous bacterial cells, was concurrently dissected. The template DNA for the dissection PCR was adjusted according to a previously published procedure [13]. The PCR was performed in a volume of 10 μl, containing 1.75 mM MgCl2, 0.4 μM of each primers, 0.3 mM dNTPs and 0.25 U of KAPATaq Extra DNA polymerase (Nippon Genetics). The specific primers DCKS10F/DCKS10R were used for detection of the kasumigamide biosynthetic gene cluster.
Metabolic and genomic analysis of M. aeruginosa NIES-87
M. aeruginosa NIES-87 was obtained from the National Institute for Environmental Studies collection, and cultured in MA medium [45]. The methanol extract (9.6 mg) of freeze-dried bacteria was analyzed on LC-MS, as described above. The M. aeruginosa NIES-87 genomic DNA was isolated according to the previously published method [46]. Sequencing of the genomic DNA was performed by an Ion PGM sequencer, with a total number of 414,749 sequence reads (~300 bp). These sequences were assembled de novo into contigs by using the Genious assembler, with the default medium sensitivity. Putative CDSs were determined by combining the prediction results from the FramePlot [42] and the Glimmer 3.02 [43] programs into one large contig (26 kb). The domain organizations were assessed by BLASTP and PKS/NRPS analysis [44]. In order to confirm the DNA sequence of makasC, the cloning of the corresponding region was performed with the primer pair, MA5F/MA6R (S1 Table). The PCR products amplified from the M. aeruginosa NIES-87 genomic DNA were introduced into pT7Blue T-vector (Novagen). The constructed plasmid pMAYN1 was subjected to the sequence analysis by Eurofins Genomics K. K.
Heterologous expression of adenylation domains
To examine the substrate specificity of the A domains, we performed the heterologous expression of five A domains: MakasA-A1, MakasB-A1, MakasB-A2, MakasC-A1, and MakasC-A2. Although MakasA-A1, MakasB-A2, and MakasC-A1 were expressed as the single A domain, MakasB-A1 and MakasC-A2 were coexpressed with the corresponding C domain and PCP domain in E. coli. The DNA fragments encoding makasA-A1, makasB-A1, and makasC-A2 were amplified by PCR and cloned into pCold II plasmid (Takara). The DNA fragments encoding makasB-A2, makasC-A1 were amplified by PCR and cloned into NhisMBP-pET28b(+). In NhisMBP-pET28b(+), MBP was introduced into the N-terminal region of the pET28b(+) (Novagen) multicloning site. The makasA-A1, makasB-A1, makasB-A2, makasC-A1, and makasC-A2 sequences were amplified with the MakasA-A1F/MakasA-A1R, MakasB-A1F/MakasB-A1R, MakasB-A2F/MakasB-A2R, MakasC-A1F/MakasC-A1R, and MakasC-A2F/MakasC-A2R primers, respectively (S1 Table). The E. coli BLR(DE3) cells harboring the plasmids pCold II makasA-A1/makasB-A1/makasC-A2 and NhisMBP-pET28b(+) makasB-A2/makasC-A1 were cultured at 37°C to an OD600 of 0.6–0.8, in LB medium, containing 100 μg/ml ampicillin or 50 μg/ml kanamycin. To induce protein expression, 0.5 mM isopropyl-1-thio-β-D-galactopyranoside was added to the cooled cultures. Expression was performed at 15°C for 16 h. All of the following procedures were performed at 4°C. For MakasA-A1, MakasB-A1, and MakasC-A2, the cells were collected by centrifugation at 10,000 g and resuspended in 50 mM Tris-HCl buffer (pH 8.0), containing 300 mM NaCl, 10% (v/v) glycerol and 5 mM imidazole (buffer A). The cells were disrupted by sonication, and the lysate was centrifuged at 10,000 g for 20 min. The supernatant was loaded onto a COSMOGEL His-Accept (Nacalai Tesque) column equilibrated with buffer A. After washing the resin with buffer A containing 10 mM imidazole, Nhis-MakasA-A1, Nhis-MakasB-A1, and Nhis-MakasC-A2 were eluted with buffer A containing 300 mM imidazole. For MakasB-A2 and MakasC-A1, the cells were collected by centrifugation at 10,000 g and resuspended in 50 mM Tris-HCl buffer (pH 8.0), containing 300 mM NaCl, 10% (v/v) glycerol (buffer B). The cells were disrupted by sonication, and the lysate was centrifuged at 10,000 g for 20 min. The supernatant was loaded onto an amylose resin (BioLabs) column equilibrated with buffer B. After washing the resin with buffer B, NhisMBP-MakasB-A2 and NhisMBP-MakasC-A1 were eluted with buffer B containing 20 mM maltose. These eluate were filtered, using an Amicon Ultra 10 K device (Merck Millipore), to remove the imidazole and to concentrate the protein.
Functional analysis of adenylation domains
Each purified protein (1 μM) was incubated with 1 mM substrate in 50 μl of buffer, containing 32 mM hydroxylamine, 1 mM dithiothreitol, 0.4 U/ml pyrophosphatase (Sigma), 0.5 mM ATP, 10 mM MgCl2, and 50 mM Tris-HCl buffer (pH7.5). The reaction was incubated for 10 min at room temperature, and then quenched by adding 50 μl of the working reagent from the malachite green phosphate assay kit (Enzo). After a 10 min incubation at room temperature, the absorption at 620 nm (A620) was measured. The control A620 value was subtracted from the A620 value of the reaction mixture, and then the relative adenylation activity was calculated.
Supporting Information
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
Extracted ion chromatogram (EIC) of m/z 787.3–787.5, derived from (a) M. aeruginosa NIES-87 extract and (c) kasumigamide isolated from D. calyx. (b) and (d) are scan mass spectra of the peaks labeled in (a) and (c), respectively.
(TIFF)
Each gene cluster is derived from (a) D. acidovorans CCUG 274B (b) Herbaspirillum sp. CF444 (c) M. aeruginosa NIES-87. The ORFs related to PKS-NRPS are highlighted in red. The ORFs and regions, widely conserved in M. aeruginosa strains, are highlighted in green allow. Red arrow indicates the specific regions, detected only in the M. aeruginosa NIES-87 genomic DNA.
(TIFF)
10% SDS-PAGE of (a) MakasA-A1 (61.8 kDa), (b) MakasB-CA1P (118.6 kDa), (c) MakasB-A2 (101.2 kDa), (d) MakasC-A1 (109.8 kDa), and (e) MakasC-CA2P (119.1 kDa), purified by Ni-NTA affinity chromatography.
(TIFF)
KS domains derived from kas family genes are highlighted in red. BarE: AAN32979, CurA: AAT70096, CurF: AAT70101, CurG: AAT70102, CurH: AAT70103, CurI: AAT70104, CurJ: AAT70105, CurK: AAT70106, CurL: AAT70107, CurM: AAT70108, EpoA: ABB92690, EpoC: ABB92692, EpoD: ABB92693, EpoE: ABB92694, EpoF: ABB92695, JamE: AAS98777, JamJ: AAS98781, JamK: AAS98782, JamL: AAS98783, JamM: AAS98784, JamP: AAS98787, MmxB: ABA29782, MmxC: ABA29781, MscA: AHB82051, MscB: AHB82052, MscC: AHB82053, MscD: AHB82054, MscG: AHB82057, MscI: AHB82059, MxaB: AAK57186, MxaC: AAK57187, MxaD: AAK57188, MxaE: AAK57189, MxaF: AAK57190, NpnA: AEU11005, NpnB: AEU11006, StiA: CAD19085, StiB: CAD19086, StiC: CAD19087, StiD: CAD19088, StiE: CAD19089, StiF: CAD19090, StiG: CAD19091, StiH: CAD19092, StiJ: CAD19093, TlmVIII: ABL74938, TtcA: AGC65513, ZmaA: ACM79805, ZmaK: AAR87760.
(TIFF)
The bacteria, possessing kas family genes, are highlighted in red.
(TIFF)
# Residues involved in metal ion binding.
(TIFF)
(TIFF)
Underlined DNA sequence region shows ORF1 or ORF2 encoding putative transposase.
(TIFF)
(TIFF)
The putative substrates of A domains were predicted by the NRPS codes except for the first A domain (KasA-A1 and its homologs). The substrates of MakasA-C are indicated based on the in vitro assay.
(TIFF)
(TIFF)
Each NMR data for kasumigamide derived from the original literature (X) in DMSO-d6 and our data (Y) in DMSO-d6 + TFA.
(TIFF)
(TIFF)
(TIFF)
CF444.
(TIFF)
Acknowledgments
We thank Prof. J. Piel (ETH Zurich), Dr. M. F. Freeman (University of Minnesota), and Prof. Taifo Mahmud (Oregon State University) for valuable discussions, Prof. S. Matsunaga and Dr. K. Takada (University of Tokyo) for assistance with the sponge collection. This work was carried out in part as a joint-research in Japanese Association for Marine Biology (JAMBIO) and partly supported by the Takeda Science Foundation, the Sumitmo Foundation, the Naito Foundation, the Bilateral Program between Japan and Switzerland from the Japan Society for the Promotion of Science (JSPS), and Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan (JSPS KAKENHI Grant Numbers JP16H06443, JP16H06448, JP16K13084, JP15H01836, JP15H03112, JP15K12740, and JP26303005). Y.N. is a recipient of the JSPS Fellowship for Young Scientist.
Data Availability
Sequence data for the kas- and makas-genes have been deposited at the DNA DataBank of Japan (DDBJ) under accession numbers LC160290 and LC160291.
Funding Statement
This work was partly supported by the Takeda Science Foundation, the Sumitmo Foundation, the Naito Foundation, the Bilateral Program between Japan and Switzerland from the Japan Society for the Promotion of Science (JSPS), and Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan (JSPS KAKENHI Grant Numbers JP16H06443, JP16H06448, JP16K13084, JP15H01836, JP15H03112, JP15K12740, and JP26303005). Y.N. is a recipient of the JSPS Fellowship for Young Scientist. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Donia MS, Cimermancic P, Schulze CJ, Wieland Brown LC, Martin J, Mitreva M, et al. A systematic analysis of biosynthetic gene clusters in the human microbiome reveals a common family of antibiotics. Cell. 2014; 158: 1402–1414. 10.1016/j.cell.2014.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischbach MA, Walsh CT. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: logic, machinery, and mechanisms. Chem Rev. 2006; 106: 3468–3496. 10.1021/cr0503097 [DOI] [PubMed] [Google Scholar]
- 3.Kampa A, Gagunashvili AN, Gulder TA, Morinaka BI, Daolio C, Godejohann M, et al. Metagenomic natural product discovery in lichen provides evidence for a family of biosynthetic pathways in diverse symbioses. Proc Natl Acad Sci U S A. 2013; 110: 3129–3137. 10.1073/pnas.1305867110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hentschel U, Piel J, Degnan SM, Taylor MW. Genomic insights into the marine sponge microbiome. Nat Rev Microbiol. 2012; 10: 641–654. 10.1038/nrmicro2839 [DOI] [PubMed] [Google Scholar]
- 5.Piel J, Hui D, Wen G, Butzke D, Platzer M, Fusetani N, et al. Antitumor polyketide biosynthesis by an uncultivated bacterial symbiont of the marine sponge Theonella swinhoei. Proc Natl Acad Sci U S A. 2004; 101: 16222–16227. 10.1073/pnas.0405976101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman MF, Gurgui C, Helf MJ, Morinaka BI, Uria AR, Oldham NJ, et al. Metagenome mining reveals polytheonamides as posttranslationally modified ribosomal peptides. Science. 2012; 338: 387–390. 10.1126/science.1226121 [DOI] [PubMed] [Google Scholar]
- 7.Wilson MC, Mori T, Rückert C, Uria AR, Helf MJ, Takada K, et al. An environmental bacterial taxon with a large and distinct metabolic repertoire. Nature. 2014; 506: 58–62. 10.1038/nature12959 [DOI] [PubMed] [Google Scholar]
- 8.Ueoka R, Uria AR, Reiter S, Mori T, Karbaum P, Peters EE, et al. Metabolic and evolutionary origin of actin-binding polyketides from diverse organisms. Nat Chem Biol. 2015; 11; 705–712. 10.1038/nchembio.1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Döderlein L. Studien an japanischen Lithistiden. Zeitschr. f. wiss. Zool. 1883; 40: 62–104. [Google Scholar]
- 10.Kato Y, Fusetani N, Matsunaga S, Hashimoto K, Fujita S, Furuya T. Bioactive marine metabolites. Part 16. Calyculin A. A novel antitumor metabolite from the marine sponge Discodermia calyx. J Am Chem Soc. 1986; 108: 2780–2781. 10.1021/ja00270a061 [DOI] [Google Scholar]
- 11.Egami Y, Wakimoto T, Abe I. Phosphocalyculin C as a pyrophosphate protoxin of calyculin C in the marine sponge Discodermia calyx. Bioorg Med Chem Lett. 2014; 24: 5150–5153. 10.1016/j.bmcl.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 12.Kimura M, Wakimoto T, Egami Y, Tan KC, Ise Y, Abe I. Calyxamides A and B, cytotoxic cyclic peptides from the marine sponge Discodermia calyx. J Nat Prod. 2012; 75: 290–294. 10.1021/np2009187 [DOI] [PubMed] [Google Scholar]
- 13.Wakimoto T, Egami Y, Nakashima Y, Wakimoto Y, Mori T, Awakawa T, et al. Calyculin biogenesis from a pyrophosphate protoxin produced by a sponge symbiont. Nat Chem Biol. 2014; 10: 648–655. 10.1038/nchembio.1573 [DOI] [PubMed] [Google Scholar]
- 14.Piel J, Hui D, Fusetani N, Matsunaga S. Targeting modular polyketide synthases with iteratively acting acyltransferases from metagenomes of uncultured bacterial consortia. Environ Microbiol. 2004; 8: 921–927. 10.1111/j.1462-2920.2004.00531.x [DOI] [PubMed] [Google Scholar]
- 15.Beyer S, Kunze B, Silakowski B, Muller R. Metabolic diversity in myxobacteria: identification of the myxalamid and the stigmatellin biosynthetic gene cluster of Stigmatella aurantiaca Sg a15 and a combined polyketide-(poly)peptide gene cluster from the epothilone producing strain Sorangium cellulosum So ce90. Biochim Biophys Acta. 1999; 1445: 185–195. 10.1016/s0167-4781(99)00041-x [DOI] [PubMed] [Google Scholar]
- 16.Moffitt MC, Neilan BA. Evolutionary affiliations within the superfamily of ketosynthases reflect complex pathway associations. J Mol Evol. 2003; 56: 446–457. 10.1007/s00239-002-2415-0 [DOI] [PubMed] [Google Scholar]
- 17.Schirmer A, Gadkari R, Reeves CD, Ibrahim F, DeLong EF, Hutchinson CR. Metagenomic analysis reveals diverse polyketide synthase gene clusters in microorganisms associated with the marine sponge Discodermia dissoluta. Appl Environ Microbiol. 2005; 71: 4840–4849. 10.1128/AEM.71.8.4840-4849.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ginolhac A, Jarrin C, Gillet B, Robe P, Pujic P, Tuphile K, et al. Phylogenetic analysis of polyketide synthase I domains from soil metagenomic libraries allows selection of promising clones. Appl Environ Microbiol. 2004; 70: 5522–5527. 10.1128/AEM.70.9.5522-5527.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stachelhaus T, Mootz HD, Marahiel MA. The specificity-conferring code of adenylation domains in nonribosomal peptide synthetases. Chem Biol. 1999; 6: 493–505. 10.1016/S1074-5521(99)80082-9 [DOI] [PubMed] [Google Scholar]
- 20.Ishida K, Welker M, Christiansen G, Cadel-Six S, Bouchier C, Dittmann E, et al. Plasticity and evolution of aeruginosin biosynthesis in cyanobacteria. Appl Environ Microbiol. 2009; 75: 2017–2026. 10.1128/AEM.02258-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishida K, Murakami M. Kasumigamide, an antialgal peptide from the cyanobacterium Microcystis aeruginosa. J Org Chem. 2000; 65: 5898–5900. 10.1021/jo991918f [DOI] [PubMed] [Google Scholar]
- 22.McQuade TJ, Shallop AD, Sheoran A, Delproposto JE, Tsodikov OV, Garneau-Tsodikova S. A nonradioactive high-throughput assay for screening and characterization of adenylation domains for nonribosomal peptide combinatorial biosynthesis. Anal Biochem. 2009; 386: 244–250. 10.1016/j.ab.2008.12.014 [DOI] [PubMed] [Google Scholar]
- 23.Kadi N, Challis GL. Chapter 17. Siderophore biosynthesis a substrate specificity assay for nonribosomal peptide synthetase-independent siderophore synthetases involving trapping of acyl-adenylate intermediates with hydroxylamine. Methods Enzymol. 2009; 458: 431–457. 10.1016/S0076-6879(09)04817-4 [DOI] [PubMed] [Google Scholar]
- 24.Brown SD, Utturkar SM, Klingeman DM, Johnson CM, Martin SL, Land ML, et al. Twenty-one genome sequences from Pseudomonas species and 19 genome sequences from diverse bacteria isolated from the rhizosphere and endosphere of Populus deltoides. J Bacteriol. 2012; 194: 5991–5993. 10.1128/JB.01243-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makris TM, Knoot CJ, Wilmot CM, Lipscomb JD. Structure of a dinuclear iron cluster-containing β-hydroxylase active in antibiotic biosynthesis. Biochemistry. 2013; 52: 6662–6671. 10.1021/bi400845b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross AC, Xu Y, Lu L, Kersten RD, Shao Z, Al-Suwailem AM, et al. Biosynthetic multitasking facilitates thalassospiramide structural diversity in marine bacteria. J Am Chem Soc. 2015; 135: 1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kevany BM, Rasko DA, Thomas MG. Characterization of the complete zwittermicin A biosynthetic gene cluster from Bacillus cereus. Appl Environ Microbiol. 2009; 75: 1144–1155. 10.1128/AEM.02518-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du L, Lou L. PKS and NRPS release mechanisms. Nat Prod Rep. 2010; 27: 255–278. 10.1039/b912037h [DOI] [PubMed] [Google Scholar]
- 29.Thomas T, Rusch D, DeMaere MZ, Yung PY, Lewis M, Halpern A, et al. Functional genomic signatures of sponge bacteria reveal unique and shared features of symbiosis. ISME J. 2010; 4: 1557–1567. 10.1038/ismej.2010.74 [DOI] [PubMed] [Google Scholar]
- 30.Cardellina JH II, Marner FJ, Moore RE. Seaweed dermatitis: structure of lyngbyatoxin A. Science. 1979; 2014: 193–195. 10.1126/science.107586 [DOI] [PubMed] [Google Scholar]
- 31.Aimi N, Odaka H, Sakai S, Fujiki H, Suganuma M, Moore RE, et al. Lyngbyatoxins B and C, two new irritants from Lyngbya majuscule. J Nat Prod. 1990; 53: 1593–1596. 10.1021/np50072a035 [DOI] [PubMed] [Google Scholar]
- 32.Fujiki H, Mori M, Nakayasu M, Terada M, Sugimura T, Moore RE. Indole alkaloids: dihydroteleocidin B, teleocidin, and lyngbyatoxin A as members of a new class of tumor promoters. Proc Natl Acad Sci U S A. 1981; 78: 3872–3876. 10.1073/pnas.78.6.3872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hitotsuyanagi Y, Fujiki H, Suganuma M, Aimi N, Sakai S, Endo Y, et al. Isolation and structure elucidation of teleocidin B-1, B-2, B-3, and B-4. Chem Pharm Bull (Tokyo). 1984; 32: 4233–4236. 10.1248/cpb.32.4233 [DOI] [PubMed] [Google Scholar]
- 34.Hitotsuyanagi Y, Yamaguchi K, Ogata K, Aimi N, Sakai S, Koyama Y, et al. Elucidation of the structures of olivoretin B and C. Chem Pharm Bull (Tokyo). 1984; 32: 3774–3778. 10.1248/cpb.32.3774 [DOI] [PubMed] [Google Scholar]
- 35.Ninomiya M, Fujiki H, Paik NS, Hakii H, Suganuma M, Hitotsuyanagi Y, et al. Des-O-methylolivoretin C is a new member of the teleocidin class of tumor promoters. Jpn J Cancer Res. 1986; 77: 222–225. [PubMed] [Google Scholar]
- 36.Wiese M, D-Agostino PM, Mihali TK, Moffitt MC, Neilan BA. Neurotoxic alkaloids: saxitoxin and its analogs. Mar Drugs. 2010; 8: 2185–2211. 10.3390/md8072185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hackett JD, Wisecaver JH, Brosnahan ML, Kulis DM, Anderson DM, Bhattacharya D, et al. Evolution of saxitoxin synthesis in cyanobacteria and dinoflagellates. Mol Biol Evol. 2013; 30: 70–78. 10.1093/molbev/mss142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He R, Wakimoto T, Takeshige Y, Egami Y, Kenmoku H, Ito T, et al. Porphyrins a metagenomic library of the marine sponge Discodermia calyx. Mol Biosyst. 2012; 8: 2334–2338. 10.1039/c2mb25169h [DOI] [PubMed] [Google Scholar]
- 39.Edwards DJ, Marquez BL, Nogle LM, McPhail K, Goeger DE, Roberts MA, et al. Structure and biosynthesis of the jamaicamides, new mixed polyketide-peptide neurotoxins from the marine cyanobacterium Lyngbya majuscula. Chem Biol. 2004; 11: 817–833. 10.1016/j.chembiol.2004.03.030 [DOI] [PubMed] [Google Scholar]
- 40.Hrvatin S, Piel J. Rapid isolation of rare clones from highly complex DNA libraries by PCR analysis of liquid gel pools. J Microbiol Methods. 2007; 68: 434–436. 10.1016/j.mimet.2006.09.009 [DOI] [PubMed] [Google Scholar]
- 41.Chevreux B, Wetter T, Suhai S. Genome sequence assembly using trace signals and additional sequence information. In: Computer Science and Biology: Proceedings of the German Conference on Bioinformatics. Braunschweig (Germany): GBF Braunschweig Department of Bioinformatics. 1999; 45–56.
- 42.Ishikawa J, Hotta K. FramePlot: a new implementation of the Frame analysis for predicting protein-coding regions in bacterial DNA with a high G+C content. FEMS Microbiol Lett. 1999; 174: 251–253. 10.1111/j.1574-6968.1999.tb13576.x [DOI] [PubMed] [Google Scholar]
- 43.Delcher AL, Harmon D, Kasif S, White O, Salzberg SL. Improved microbial gene identification with GLIMMER. Nucleic Acids Res. 1999; 27: 4636–4641. 10.1093/nar/27.23.4636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bachmann BO, Ravel J. Methods for in silico prediction of microbial polyketide and nonribosomal peptide biosynthetic pathways from DNA sequence data. Methods Enzymol. 2009; 458: 181–217. 10.1016/S0076-6879(09)04808-3 [DOI] [PubMed] [Google Scholar]
- 45.Ichimura T. In: Nishizawa K, Chihara M (Eds.). 2. Isolation and culture methods of algae. 2.5.B. Freshwater algae [2. Sôrui no bunri to baiyôhô. 2.5.B. Tansui sôrui]. In: Methods in Phycological Studies [Sôrui Kenkyûhô]. Kyoritu Syuppan. 1979; 294–305, (in Japanese).
- 46.Morin N, Vallaeys T, Hendrickx L, Natalie L, Wilmotte A. An efficient DNA isolation protocol for filamentous cyanobacteria of the genus Arthrospira. J Microbiol. 2010; Methods 80: 148–154. 10.1016/j.mimet.2009.11.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
Extracted ion chromatogram (EIC) of m/z 787.3–787.5, derived from (a) M. aeruginosa NIES-87 extract and (c) kasumigamide isolated from D. calyx. (b) and (d) are scan mass spectra of the peaks labeled in (a) and (c), respectively.
(TIFF)
Each gene cluster is derived from (a) D. acidovorans CCUG 274B (b) Herbaspirillum sp. CF444 (c) M. aeruginosa NIES-87. The ORFs related to PKS-NRPS are highlighted in red. The ORFs and regions, widely conserved in M. aeruginosa strains, are highlighted in green allow. Red arrow indicates the specific regions, detected only in the M. aeruginosa NIES-87 genomic DNA.
(TIFF)
10% SDS-PAGE of (a) MakasA-A1 (61.8 kDa), (b) MakasB-CA1P (118.6 kDa), (c) MakasB-A2 (101.2 kDa), (d) MakasC-A1 (109.8 kDa), and (e) MakasC-CA2P (119.1 kDa), purified by Ni-NTA affinity chromatography.
(TIFF)
KS domains derived from kas family genes are highlighted in red. BarE: AAN32979, CurA: AAT70096, CurF: AAT70101, CurG: AAT70102, CurH: AAT70103, CurI: AAT70104, CurJ: AAT70105, CurK: AAT70106, CurL: AAT70107, CurM: AAT70108, EpoA: ABB92690, EpoC: ABB92692, EpoD: ABB92693, EpoE: ABB92694, EpoF: ABB92695, JamE: AAS98777, JamJ: AAS98781, JamK: AAS98782, JamL: AAS98783, JamM: AAS98784, JamP: AAS98787, MmxB: ABA29782, MmxC: ABA29781, MscA: AHB82051, MscB: AHB82052, MscC: AHB82053, MscD: AHB82054, MscG: AHB82057, MscI: AHB82059, MxaB: AAK57186, MxaC: AAK57187, MxaD: AAK57188, MxaE: AAK57189, MxaF: AAK57190, NpnA: AEU11005, NpnB: AEU11006, StiA: CAD19085, StiB: CAD19086, StiC: CAD19087, StiD: CAD19088, StiE: CAD19089, StiF: CAD19090, StiG: CAD19091, StiH: CAD19092, StiJ: CAD19093, TlmVIII: ABL74938, TtcA: AGC65513, ZmaA: ACM79805, ZmaK: AAR87760.
(TIFF)
The bacteria, possessing kas family genes, are highlighted in red.
(TIFF)
# Residues involved in metal ion binding.
(TIFF)
(TIFF)
Underlined DNA sequence region shows ORF1 or ORF2 encoding putative transposase.
(TIFF)
(TIFF)
The putative substrates of A domains were predicted by the NRPS codes except for the first A domain (KasA-A1 and its homologs). The substrates of MakasA-C are indicated based on the in vitro assay.
(TIFF)
(TIFF)
Each NMR data for kasumigamide derived from the original literature (X) in DMSO-d6 and our data (Y) in DMSO-d6 + TFA.
(TIFF)
(TIFF)
(TIFF)
CF444.
(TIFF)
Data Availability Statement
Sequence data for the kas- and makas-genes have been deposited at the DNA DataBank of Japan (DDBJ) under accession numbers LC160290 and LC160291.