Abstract
CD4 T cell-dependent antibody responses are essential for limiting Plasmodium parasite replication and the severity of malaria; however, the factors that regulate humoral immunity during highly inflammatory, Th1-biased systemic infections are poorly understood. Using genetic and biochemical approaches, we show that Plasmodium infection-induced type I interferons limit T follicular helper accumulation and constrain anti-malarial humoral immunity. Mechanistically we show that CD4 T cell-intrinsic type I interferon signaling induces T-bet and Blimp-1 expression, thereby promoting T regulatory 1 responses. We further show that the secreted effector cytokines of T regulatory 1 cells, IL-10 and IFN-γ, collaborate to restrict T follicular helper accumulation, limit parasite-specific antibody responses, and diminish parasite control. This circuit of interferon-mediated Blimp-1 induction is also operational during chronic virus infection and can occur independently of IL-2 signaling. Thus, type I interferon-mediated induction of Blimp-1 and subsequent expansion of T regulatory 1 cells represent generalizable features of systemic, inflammatory Th1-biased viral and parasitic infections that are associated with suppression of humoral immunity.
Author Summary
Humoral immunity is essential for host resistance to pathogens that trigger highly inflammatory immune responses, including Plasmodium parasites, the causative agents of malaria. Long-lived, secreted antibody responses depend on a specialized subset of CD4 T cells called T follicular helper (Tfh) cells. However, anti-Plasmodium humoral immunity is often short-lived, non-sterilizing, and immunity rapidly wanes, leaving individuals susceptible to repeated bouts of malaria. Here we explored the relationship between inflammatory type I interferons, the regulation of pathogen-specific CD4 T cell responses, and humoral immunity using models of experimental malaria and systemic virus infection. We identified that type I interferons promote the formation and accumulation of pathogen-specific CD4 T regulatory 1 cells that co-express interferon-gamma and interleukin-10. Moreover, we show that the combined activity of interferon-gamma and interleukin-10 limits the magnitude of infection-induced Tfh responses, the secretion of parasite-specific secreted antibody, and parasite control. Our study provides new insight into the regulation of T regulatory 1 responses and humoral immunity during inflammatory immune reactions against systemic infections.
Introduction
Malaria, caused by mosquito-borne Plasmodium parasites, remains a significant burden on public health that is responsible for over 400,000 deaths annually [1]. Immunological studies in humans and mice have identified parasite-specific antibodies as critical for Plasmodium control and parasite clearance [2]. However, an abundance of data show that antibody responses generated against Plasmodium parasites are relatively short-lived and dominated by antibodies of low affinity [3–6], which leaves individuals susceptible to repeated infection [2, 7]. Despite these long-standing observations, the infection-induced, host-specific factors that limit the acquisition of long-lived anti-Plasmodium antibody responses following single or repeated Plasmodium infection remain poorly defined.
T follicular helper (Tfh) cells are essential for the generation of memory B cells and plasma cells that produce high-affinity antibodies, two B cell subsets that comprise long-lived humoral immunity against pathogen reinfection [8, 9]. Tfh cells functionally orchestrate germinal center (GC) B cell reactions through ligand-receptor interactions and cytokine secretion [10]. The importance of Tfh cells in promoting antibody-mediated control of numerous acute and chronic infections is well established [10–12]. However, less is known about Plasmodium-specific Tfh cell differentiation, maintenance, and function, despite the critical role of Plasmodium-specific secreted antibodies in limiting disease severity and promoting parasite clearance. Indeed, numerically or functionally skewed pathogen-specific Tfh responses represent an emerging hypothesis to explain the defects or delays in the acquisition of antibody-mediated immunity following infection [13].
In support of this hypothesis, a recent survey of Tfh cell responses in Plasmodium falciparum-exposed children revealed that a phenotypically and functionally distinct T helper 1 (Th1)-like Tfh cell subset (CXCR3+ CXCR5+PD-1+) is preferentially expanded during human malaria [14]. Strikingly, these Th1-like Tfh cells exhibit a markedly reduced capacity to provide B cell help in vitro and the expansion of this subset was further linked to Th1-associated, Plasmodium infection-induced inflammation [14]. In agreement with the later observation, we originally reported that excessive type II IFN (IFN-γ-associated inflammation impairs Tfh activity and humoral immunity during experimental malaria [15], a finding recently confirmed by others [16]. Together, these data support that Tfh responses generated during malaria may be suboptimal, and that the inflammatory environment or cytokine milieu induced by Plasmodium blood-stage infection can impact the quantity or quality of anti-Plasmodium Tfh cell responses with subsequent impacts on humoral immunity.
In addition to Th1-associated inflammation and systemic production of IFN-γ, type I interferons (IFNα/β) are also highly induced during human and experimental blood-stage Plasmodium infection [17–22]. Type I IFNs are pleiotropic cytokines with reported variable effects on Tfh development and function. During acute viral infection, type I IFNs suppress the Tfh developmental program [23]. On the other hand, in vitro studies show STAT1-dependent, type I IFN receptor (IFNAR) signaling can promote Tfh cell differentiation [24]. To date, the functional roles of type I IFNs during Plasmodium infection have mainly focused on acutely lethal, experimental cerebral malaria (ECM) models. In this context, IFNAR signaling suppressed Th1 development and activity, which led to elevated parasite burdens and exacerbated malaria-induced neurological disease [17, 25]. However, the contribution of type I IFNs in regulating Plasmodium-specific Th1 and/or Tfh activity and parasite-specific antibody responses during non-lethal experimental malaria have not been extensively investigated.
Here we used complementary genetic and biochemical approaches to test the hypothesis that IFNAR signaling represents an additional inflammatory signaling cascade that limits the quantity and quality of Plasmodium-specific Tfh cell responses and the subsequent generation of protective humoral immunity. Using both chronic viral and parasitic infection models, we uncovered a molecular circuit in which type I IFNs directly induce Blimp-1 expression in pathogen-specific CD4 T cells and promote T regulatory 1 responses. Furthermore, we identified that type I IFN-mediated induction of Tr1- associated cytokines IL-10 and IFN-γ collaborate to limit the generation of protective humoral immunity during experimental malaria.
Results
Type I IFNs limit parasite control, germinal center B cell and secreted antibody responses during experimental Plasmodium infection
To begin to dissect the biological effects of type I IFNs during Plasmodium blood-stage infection [17–22], we employed reagents to block IFNAR signaling in a model of non-lethal experimental P. yoelii malaria. We administered to P. yoelii-infected mice either an irrelevant rat IgG (clone MOPC) or an extensively characterized monoclonal antibody (mAb, clone MAR-15A3) that blocks signaling from the type I IFN receptor (IFNAR) [26–28] (Fig 1A). Blocking IFNAR signaling through day 4 p.i. resulted in a 25–50% decrease in P. yoelii parasitema after day 16 p.i. (Fig 1B). By contrast, blocking IFNAR signaling during the second week of infection (day 10, 12, 14 p.i.) had no impact on parasite control (S1A Fig). These data demonstrate that early type I IFN responses impede parasite control during experimental Plasmodium blood-stage infection.
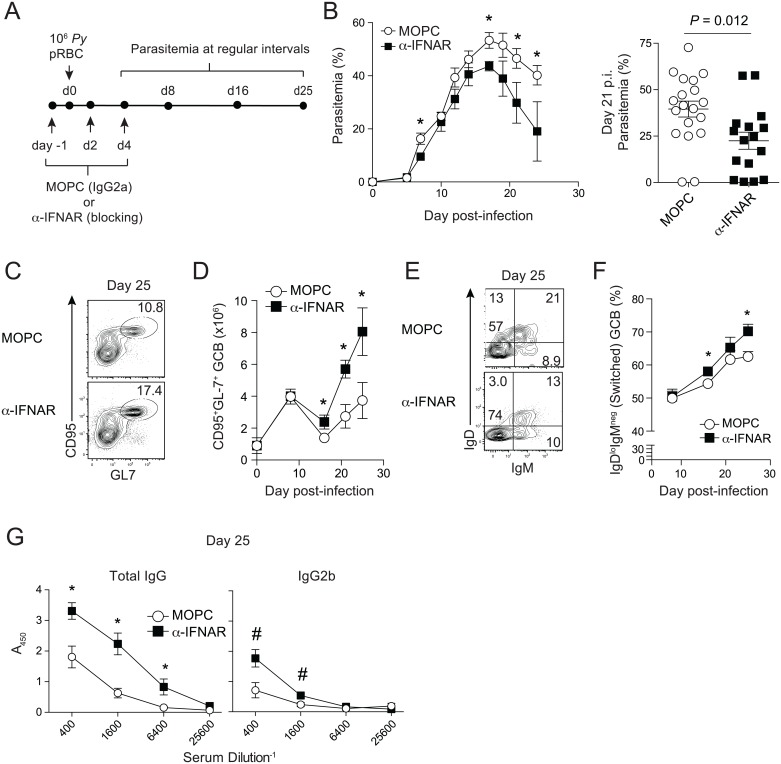
Fig 1. Blockade of IFNAR signaling improves parasite control and enhances humoral immunity during P. yoelli infection.
(A) Experimental design. Mice were administered either MOPC (isotype) or α-IFNAR antibodies at the indicated time points and were infected with 106 P. yoelii infected red blood cells (pRBCs). (B) Parasitemia (% of RBCs infected) kinetics (left) and cumulative data from 4 independent experiments displaying parasitemia on day 21 p.i. (right). (C) Representative flow plots from MOPC (top) or α-IFNAR-treated mice (bottom) depicting the proportion of CD95+GL7+CD19+ splenic germinal center (GC) B cells on day 25 p.i. (D) Summary kinetics displaying the total numbers of GC B cells. (E,F) Representative flow plots (E) and summary data (F) showing the proportion of class-switched (IgD-IgM-) GC B cells (G) Summary graphs displaying the relative titers of MSP119-specific total IgG (left) and IgG2b (right) on day 25 p.i. Data (Mean +/- SEM) in (B,D,F,G) are pooled from 2–3 independent experiments per time point (3–5 mice/group for each experiment) and were analyzed using Mann-Whitney (non-parametric) tests of statistical significance. Data in (B-G) are representative of >3 independent experiments. *P<0.05, #P = 0.05.
Notably, blocking IFNAR signaling through day 4 p.i. largely did not impact parasite replication until the second week of infection (Fig 1B), which is consistent with our hypothesis that type I IFNs negatively impact the development of humoral immunity. Thus, we next examined the magnitude and kinetics of Plasmodium infection-induced GC B cell responses in MOPC- and α-IFNAR-treated mice. By the third week of experimental malaria, α-IFNAR treatment resulted in 2 to 4-fold increases in the number of P. yoelii infection-induced (S1B Fig) splenic GL7+CD95+ CD19+ GC B cells (Fig 1C and 1D). IFNAR signaling blockade was also associated with enhanced class-switching in GC B cells (Fig 1E and 1F). The quantitative and qualitative changes in responding B cells in α-IFNAR-treated mice were additionally linked to 2-fold higher serum titers of T cell-dependent, merozoite surface protein 1 (MSP119)-specific antibody (Fig 1G). Collectively, these results show that early type I IFN signaling negatively regulates parasite control and the quantity and quality of humoral immunity during experimental Plasmodium infection.
Type I IFNs limit CD4+ T follicular helper cell accumulation and promote T regulatory 1 responses during experimental malaria
IFNAR signaling is reported to regulate the proliferation, survival and differentiation of multiple effector subsets [29], including T follicular helper (Tfh) cell responses in vitro [24] and in vivo following immunization with model antigens [30]. Type I IFNs have also been shown to indirectly regulate Th1 CD4 T cell activity during acutely lethal experimental cerebral malaria and acute virus infection [25, 27, 28]. Thus, we next explored whether IFNAR signaling blockade differentially impacts the differentiation of Tfh or Th1 cells during prolonged P. yoelii blood stage malaria. Of note, the total Plasmodium infection-induced effector CD4 T cell compartment can be distinguished from irrelevant (naïve) CD4 T cells via published surrogate marker approaches that monitor conformational changes in CD11a and upregulation of CD44 on CD4 T cells following infection or vaccination [15, 31–36]. Consistent with enhanced humoral immunity, we found α-IFNAR treatment resulted in 2 to 3-fold expansions in the frequency and total number of Plasmodium-infection-induced (S1C Fig) splenic PD-1+CXCR5+Bcl-6+ Tfh cells (Fig 2A–2D). We also observed qualitative changes in the Tfh compartment following IFNAR signaling blockade, including a 25% increase in Tfh expression of ICOS (Fig 2C and 2E), a co-stimulatory molecule that is essential for Tfh commitment, migration and function [37, 38]. These data support that Plasmodium infection-induced type I IFNs limit Tfh accumulation.
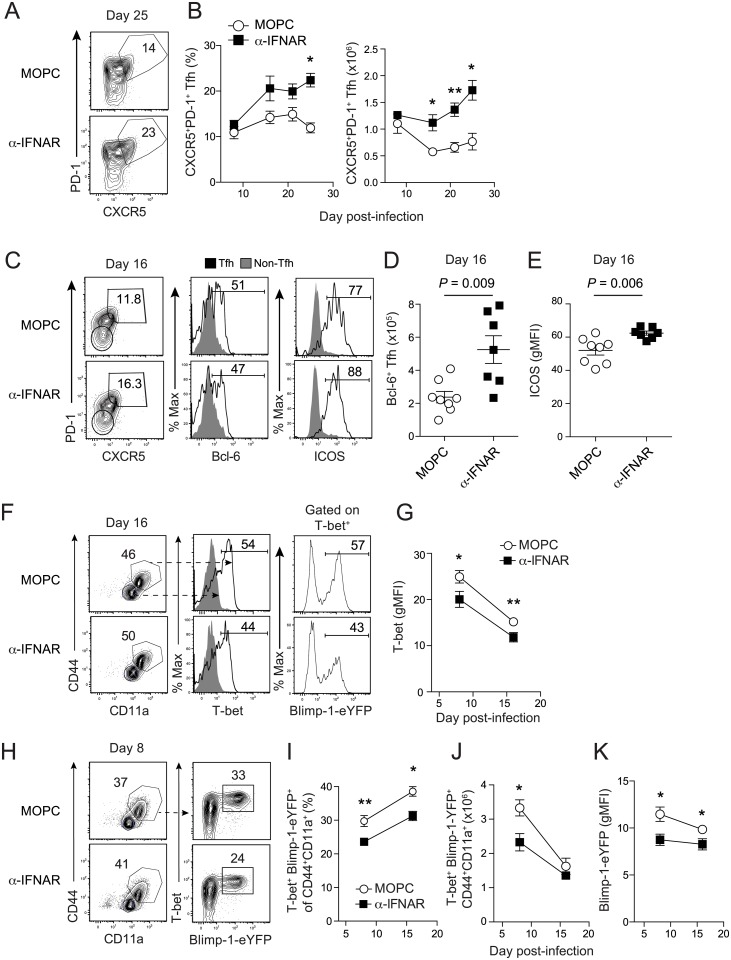
Fig 2. Blockade of IFNAR signaling enhances Tfh cell accumulation and limits T regulatory 1 responses during experimental malaria.
(A-K) Wild type (WT) mice were administered either MOPC or α-IFNAR antibodies and were infected with 106 pRBCs. (A) Representative flow plots from day 25 p.i. depicting the percentage of CXCR5+PD-1+ Tfh cells among splenic CD44+ CD4 T cells from MOPC and α-IFNAR-treated mice. (B) Summary kinetics displaying the proportion (left) and total number (right) of Tfh cells from MOPC and α-IFNAR-treated mice. Representative flow plots (C) and summary data depicting total numbers of CXCR5+PD-1+Bcl-6+ Tfh (D) and ICOS (E) expression (geometric MFI) on Tfh cells from MOPC and α-IFNAR-treated mice on day 16 p.i. (F-K) Blimp-1-eYFP reporter mice were administered either MOPC or α-IFNAR antibodies and were infected with 106 pRBCs. (F) Representative dot plots (left) depicting the proportion of splenic CD44+CD11ahi CD4 T cells in MOPC and α-IFNAR-treated mice expressing either T-bet (middle) or Blimp-1-eYFP in T-bet+ CD4 T cells (right). (G) Summary kinetics depicting the relative expression of T-bet (gMFI) among Plasmodium infection-induced CD44+CD11a+ CD4 T cells. (H) Representative flow plots from MOPC and α-IFNAR-treated mice on day 8 p.i showing the proportion of splenic CD44+CD11ahi CD4 T cells simultaneously expressing both T-bet and Blimp-1-eYFP. (I-J) Summary kinetics depicting the frequency (I) and total number (J) of T-bet+Blimp1-eYFP+ splenic CD44+CD11ahi CD4 T cells in MOPC and α-IFNAR-treated mice. (K) Summary kinetics of Blimp-1-eYFP expression (gMFI) in splenic CD44+CD11ahi CD4 T cells in MOPC and α-IFNAR-treated mice. Data (Mean +/- SEM) in (A,D,G,J-K) are pooled from 2–3 independent experiments per time point (3–5 mice/group for each time point) and were analyzed using Mann-Whitney (non-parametric) tests of statistical significance. N.S. = not statistically significant. *P<0.05, **P<0.01.
In addition to alterations in the Tfh compartment, α-IFNAR treatment also resulted in 20% decreases in both the amount of CD4 T cell-expressed T-bet and the proportion of T-bet+ Th1 effector CD4 T cells, compared to control MOPC treatment (Fig 2F and 2G). Notably, ectopic expression of T-bet can induce expression of Blimp-1 (34), a transcriptional repressor that regulates terminal differentiation of effector T cells (35) and limits Tfh and T cell effector function (36). Moreover, pathogen-specific T-bet+ effector CD4 T cells responding to prolonged or chronic infections often co-express Blimp-1 (37) and are functionally categorized as T regulatory 1 (Tr1) CD4 T cells. Thus, we further reasoned that type I IFNs also promote the expansion or accumulation of T-bet+Blimp-1+ Tr1 cells during experimental malaria. To formally test this, we infected Blimp-1-eYFP reporter mice with P. yoelii and examined the kinetics of simultaneous T-bet and Blimp-1-eYFP expression in Plasmodium infection-induced (S2A Fig) effector CD4 T cells. We found that ~50% of T-bet+ effector CD4 T cells co-expressed Blimp-1-eYFP in both MOPC-control and α-IFNAR-treated mice (Fig 2F). Notably, α-IFNAR treatment reduced the proportion and number of infection-induced effector CD4 T cells co-expressing both T-bet and Blimp-1-eYFP by >30% (Fig 2H–2J). Moreover, compared to effector CD4 T cells recovered from MOPC-treated mice, expression of Blimp-1-eYFP signal was reduced by >30% on days 8 and 16 p.i. in Plasmodium infection-induced effector CD4 T cells recovered from α-IFNAR-treated mice (Fig 2K).
To gain further insight into the link between type I IFN signaling and Blimp-1 induction, and determine whether the IFNα/β-Blimp-1 circuit is unique to Plasmodium infection, we next initiated comparative studies using mice infected with LCMV clone 13, which also triggers a highly inflammatory, Th1-biased response. On day 14 p.i., total virus infection-induced (CD44hiCD11ahi) and epitope-specific (GP61-80) effector CD4 T cells showed marked reductions in both the proportion of Blimp-1-eYFP+ cells (S2B Fig) and per-cell expression of Blimp-1-eYFP (S2C Fig) following administration of α-IFNAR to LCMV clone 13-infected mice. As a composite, these data show that type I IFNs suppress the development or accumulation of Tfh cells and promote the expansion of T-bet+Blimp-1+ effector CD4 T cells during blood stage Plasmodium infection and that the IFNα/β-Blimp-1 circuit is a generalizable feature of systemic, Th1-biased infections.
Type I IFN signaling promotes the development and function of T regulatory 1 cells during experimental malaria
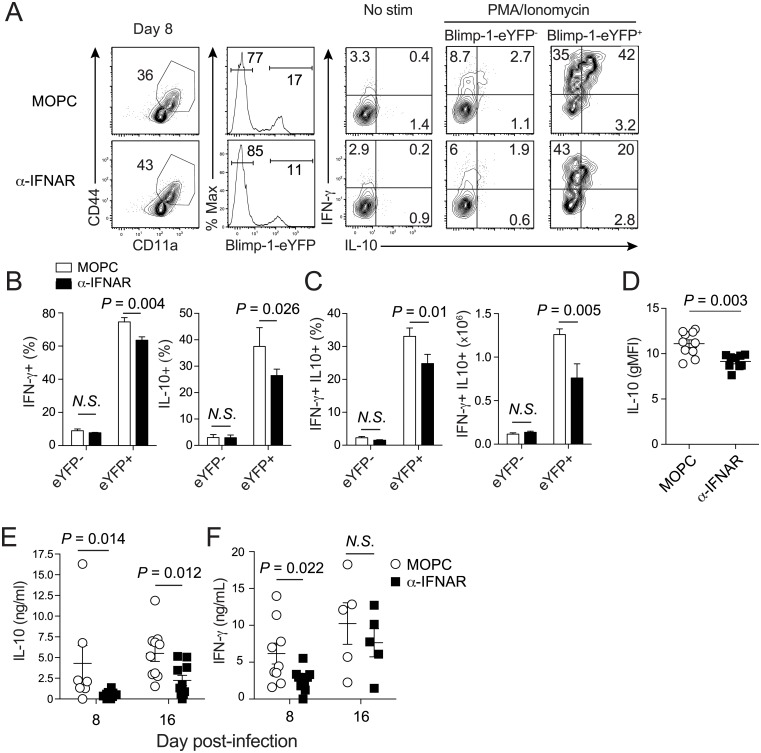
To explore the biological consequences of reduced CD4 T cell co-expression of T-bet and Blimp-1 following α-IFNAR treatment we next examined cytokine production by Plasmodium infection-induced effector CD4+ Tr1 cells. Tr1 cells simultaneously express T-bet, Blimp-1, IFN-γ and IL-10 during chronic viral and protozoan infections and have been shown to sharply limit pathogen control [39–41]. Moreover, Foxp3-negative Tr1 CD4 T cells are the major source of IL-10 in these scenarios [39, 42], and Tr1 activity and expression of IL-10 is Blimp-1-dependent [42–44]. As predicted, co-expression of IFN-γ and IL-10 was largely restricted to Blimp-1-eYFP+ Plasmodium infection-induced effector CD4 T cells (Fig 3A), and α-IFNAR treatment significantly decreased the frequency and number of Blimp-1-eYFP+ IFN-γ+IL-10+ effector cells on day 8 p.i. (Fig 3A–3C) and the amount of IL-10 expressed per cell (Fig 3D). Consistent with alterations in T-bet+Blimp-1-eYFP+ Tr1 numbers (Fig 2J) and cytokine expression, we also observed 3-4-fold reductions in serum IL-10 levels and 2-fold reductions in serum IFN-γ between days 8–16 p.i. in α-IFNAR-treated Plasmodium-infected mice compared to MOPC-treated mice (Fig 3E and 3F). Of note, simultaneous expression of CD49b and LAG-3 is reported to identify Tr1 cells in both mice and humans [45]. Although the majority (>70%) of Plasmodium infection-induced Blimp-1-eYFP cells expressed LAG-3 (S3A and S3B Fig), CD49b expression was largely restricted to NK cells (S3C Fig). Collectively, these data show that early, Plasmodium-induced type I IFNs promote the development of effector Tr1 responses and Tr1 co-expression of IL-10 and IFN-γ during experimental malaria.
Fig 3. Type I interferons promote co-production of IFN-γ and IL-10 by Plasmodium infection-induced Tr1 cells.
(A-F) Blimp-1-eYFP reporter mice were administered either MOPC or α-IFNAR antibodies and were infected with 106 pRBCs. (A) Representative flow plots depicting the frequency of Blimp-1-eYFP expression and subsequent IFN-γ and IL-10 cytokine production in Blimp-1-eYFP- and Blimp-1-eYFP+ splenic CD44+CD11ahi CD4 T cells from MOPC and α-IFNAR-treated mice after ex vivo stimulation with PMA/Ionomycin. (B) Summary data displaying the proportion of Blimp-1-eYFP- and Blimp-1-eYFP+ effector CD44+CD11ahi CD4 T cells that are positive for either IFN-γ+ (left) or IL-10+ (right) after ex vivo stimulation. (C) Summary data displaying the proportion (left) and total number (right) of IFN-γ+ IL-10+ cells among Blimp-1-eYFP- and Blimp-1-eYFP+ Plasmodium infection-induced CD4 T cells from MOPC and α-IFNAR-treated mice on day 8 p.i. (D) Summary data showing the gMFI of IL-10 in Plasmodium infection-induced CD4 T cells on day 8 p.i. (E-F) Summary data (Mean +/- SEM) showing the levels of circulating IL-10 (E) and IFN-γ (F) in sera from MOPC and α-IFNAR-treated mice. Data (Mean +/- SEM) in (B-F) are pooled from 2–3 independent experiments (3–4 mice/group per experiment) and were analyzed using Mann-Whitney non-parametric tests. Data in (A-F) are representative of 3 independent experiments. N.S. = not statistically significant.
IFN-γ and IL-10 collaborate to limit Tfh accumulation, humoral immunity and parasite control during Plasmodium blood-stage infection
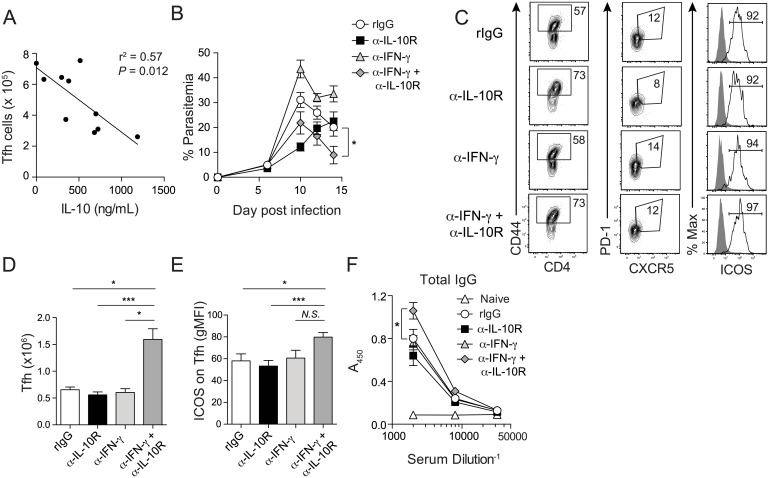
Excessive IFN-γ production by T cells limits Tfh and GC B cell activity and constrains anti-Plasmodium humoral immunity [15], supporting that IFN-γ can negatively regulate Tfh function. However, whether IL-10 can additionally or independently inhibit Tfh responses during Plasmodium infection has not been addressed. Indeed, the consistent improvements in parasite control and humoral immunity (Fig 1) and marked reductions in circulating serum and Tr1-expressed IL-10 and IFN-γ (Fig 3) following α-IFNAR treatment raised the possibility that together these cytokines may limit Tfh responses and humoral immunity during Plasmodium infection. In support of this notion, both serum IFN-γ [15] and IL-10 levels in experimental mice strongly and inversely correlated with the magnitude of the Tfh response on day 16 p.i (Fig 4A). Because α-IFNAR treatment significantly reduced the number of IL-10+IFN-γ+ Plasmodium infection-induced Tr1 cells and serum IFN-γ and IL-10 levels, we hypothesized that combinatorial IL-10R signaling blockade and IFN-γ neutralization would phenocopy α-IFNAR treatment, resulting in increased Tfh accumulation, improved humoral immunity, and enhanced parasite control during experimental malaria. We found that simultaneously blocking the activity of these Tr1-derived cytokines potently enhanced parasite control (Fig 4B), which was associated with expanded numbers of ICOS+ Tfh cells (Fig 4C–4E). Of note, GC B cell reactions were not significantly elevated by day 14 p.i. following simultaneous neutralization of IFN-γ and blockade of IL-10 signaling (S4A and S4B Fig), consistent with marginally altered GC B cell responses following α-IFNAR treatment at this early time point (Fig 1D) Nevertheless, combined targeting of both IFN-γ and IL-10 elevated titers of parasite-specific secreted antibody by day 14 p.i. (Fig 4F). Solely blocking IL-10 signaling transiently limited parasite replication (Fig 4B), but parasite control was eventually lost after day 14 p.i., which was associated with reduced GC B cell reactions (S4A and S4B Fig) and diminished secretion of parasite-specific antibody (Fig 4F). These data support that IL-10 insulates the host against the reported pathological effects of excess IFN-γ [15, 16, 43]. While neutralization of IFN-γ modestly enhanced GC B cell frequencies (S4B Fig), parasite control was also eventually impaired (Fig 4B). Indeed, improvements in both anti-Plasmodium humoral immunity and parasite control only occurred following simultaneous neutralization of IFN-γ and blockade of IL-10 signaling. As a composite, our data support that a primary effect of Plasmodium infection-induced type I IFN is the expansion of effector Tr1 cells and that Tr1-associated cytokines IL-10 and IFN-γ act together to limit Tfh accumulation, anti-malarial humoral immunity and parasite control via release of IL-10 and IFN-γ.
Fig 4. IFN-γ and IL-10 cooperate to limit Tfh accumulation and secreted antibody responses during Plasmodium blood-stage infection.
(A) Negative correlation between levels of circulating IL-10 and the total number of splenic Tfh cells in MOPC and α-IFNAR-treated mice on day 16 p.i. Data were analyzed using linear regression. (B-F) Groups of mice (n = 5/group) were treated with either rIgG, α-IL-10R, α-IFN-γ, or α-IFN-γ+α-IL-10R. (B) Parasitemia kinetics. Statistical results for comparisons between rIgG- and α-IFN-γ+α-IL-10R-treated mice are displayed. Data were analyzed using Mann-Whitney non-parametric tests. (C) Representative flow plots depicting the proportion of PD-1+CXCR5+ Tfh cells among CD44hi splenic CD4+ T cells in rIgG-, α-IL-10R-, α-IFN-γ or α-IFN-γ+α-IL-10R-treated mice. (D) Summary data showing the total number of splenic Tfh cells in rIgG-, α-IL-10R-, or α-IFN-γ+α-IL-10R-treated mice on day 14 p.i. (E) Summary data showing ICOS expression (gMFI) on Tfh cells from rIgG-, α-IL-10R-, α-IFN-γ or α-IFN-γ+α-IL-10R-treated mice. (F) Summary graph displaying the relative serum titers of MSP119-specific total IgG in rIgG-, α-IL-10R-, α-IFN-γ, or α-IFN-γ+α-IL-10R-treated mice on d14 p.i. Statistical results for comparisons between rIgG- and α-IFN-γ+α-IL-10R-treated mice are displayed and data were analyzed using Mann-Whitney non-parametric tests. Summary data (Mean +/- SEM) in (D-E) were analyzed using Kruskal-Wallis non-parametric tests. Data in (B-F) are representative of two independent experiments (5 mice/group per experiment). N.S. = not statistically significant. *P<0.05, ***P<0.0001.
Type I IFNs directly induce T-bet and Blimp-1 and co-expression of IL-10 and IFN-γ in Plasmodium infection-induced effector CD4 T cells during Plasmodium blood-stage infection
Our data show that neutralizing either type I IFN or Tr1 effector cytokines IFN-γ and IL-10 promotes the expansion of Tfh cells and enhances humoral immunity and parasite control. However, to formally test whether type I IFN-mediated suppression of Tfh accumulation and/or induction of the T-bet-Blimp-1 axis in responding CD4 T cells is CD4 T cell-intrinsic, we seeded naive tcrα -/- mice with equivalent numbers of naïve, congenically marked wild type (WT) and ifnar1 -/- CD4 T cells one day before P. yoelii challenge (Fig 5A). At defined intervals after challenge, we assessed Tfh Bcl-6 expression, as well as T-bet and Blimp-1 co-expression in effector WT and ifnar1 -/- CD4 T cells recovered from the same host. Although ifnar1 -/- CD4 T cells did not expand or accumulate to the same degree as WT CD4 T cells (S5A and S5B Fig) we found no difference in the proportion of WT and ifnar1 -/- donor-derived cells that adopted the canonical CXCR5+PD-1hiBcl-6+ Tfh phenotype (S5A–S5D Fig), suggesting that direct IFNAR signaling on CD4 T cells does not intrinsically impair Tfh differentiation during experimental malaria. In line with this, expression of IFNAR was negligible on effector Tfh cells, compared to effector Th1/Tr1 effector CD4 T cells (not depicted). Decoupling of IFNAR signaling in expanding Tfh cells would also be consistent with reports showing that Tfh cells become refractory to specific cytokine signaling networks very early after their initial priming [46]. By contrast, we found markedly lower proportions of T-bet+Blimp-1+ ifnar1 -/- CD4 T cells (Fig 5B and 5C), as well as 20–40% less T-bet and Blimp-1 in ifnar1 -/- CD4 T cells, compared to WT CD4 T cells (Fig 5D and 5E). Notably, the proportionate decreases in T-bet and Blimp-1 expression in ifnar -/- CD4 T cells mirror those observed following anti-IFNAR-treatment (Fig 2H and 2I) and co-adoptive transfer studies also revealed sharp reductions in IFN-γ, and IL-10 expression by ifnar1 -/- effector CD4 T cells, compared to WT CD4 T cells recovered from the same host (Fig 5F–5I), confirming that the impact of IFNα/β signaling on IL-10 and IFN-γ expression are CD4 T cell-intrinsic. These data support that type I IFN-mediated induction of the Tr1 program during experimental malaria is primarily regulated by CD4 T cell-intrinsic IFNAR signaling. To determine the impact of CD4 T cell-intrinsic ifnar1-deficiency on anti-Plasmodium humoral immunity, we undertook two complementary chimeric approaches. First, transfer of WT or ifnar1 -/- naïve CD4 T cells into separate groups of tcrα -/- mice (e.g. Fig 5A) revealed that both Plasmodium infection-induced GC B cell responses and parasite control were significantly enhanced in mice seeded with ifnar1 -/- CD4 T cells (Fig 5J and 5K). Second, and consistent with our chimeric transfer studies, infection of mixed bone marrow chimeras in which ifnar1 deficiency was restricted to the T cell compartment (Fig 5L) revealed 2-fold greater parasite-specific secreted IgG (Fig 5M), as well as elevated Tfh (Fig 5N and S5E Fig), and GC B cell responses (S5F and S5G Fig). Collectively, these data support that type I IFNs indirectly limit the accumulation of Plasmodium-specific Tfh cells and impede humoral immunity and parasite control during experimental malaria via the activity of Tr1 effector cytokines. Mechanistically, although Plasmodium infection-induced type I IFNs have no appreciable direct effects on modulating Bcl-6+ Tfh development during malaria, they directly promote the accumulation and function of Plasmodium infection-induced T-bet+Blimp-1+ Tr1 cells that limit humoral immunity and parasite control via secretion of effector cytokines IL-10 and IFN-γ.
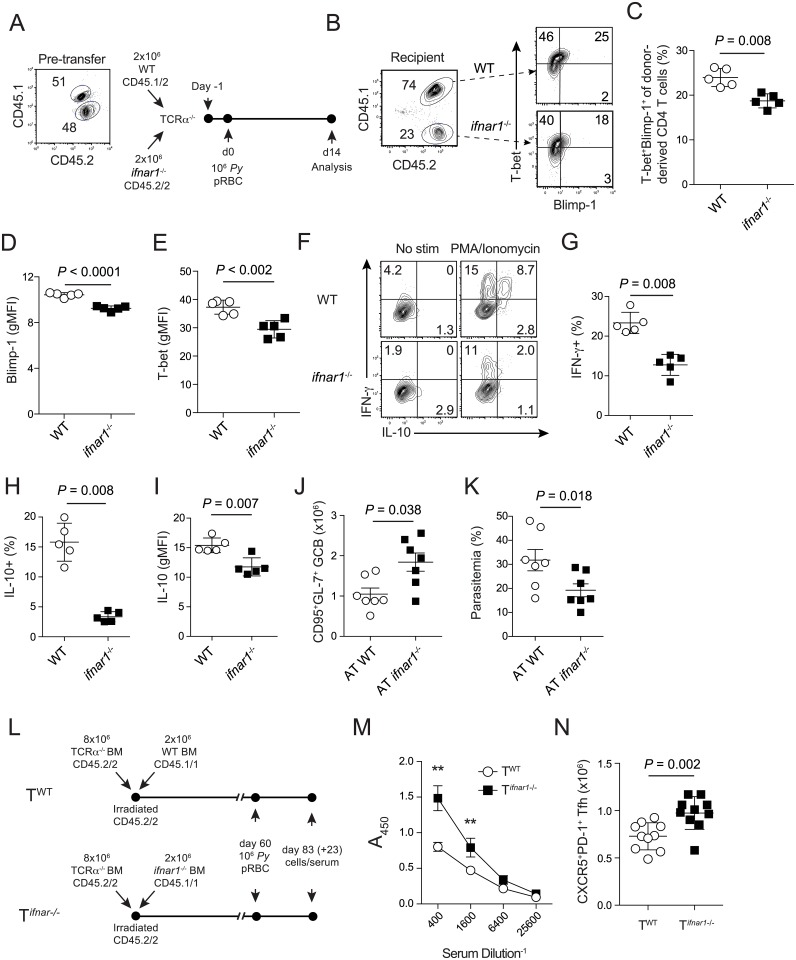
Fig 5. CD4 T cell intrinsic IFNAR signaling limits humoral immunity and parasite control via induction of Tr1 cell expression of T-bet and Blimp-1 and co-production of IFN-γ and IL-10.
(A) Experimental design. Tcrα -/- mice were seeded with equivalent numbers of CD45.1/2 WT and CD45.2/2 (ifnar1 -/-) naïve CD4 T cells and infected with 106 pRBCs one day post-transfer. Cellular reactions were analyzed 2 weeks p.i. (B) Representative flow plots showing the proportion of WT and ifnar1 -/- cells among recovered activated CD44hi CD4+ T cells and their simultaneous expression of T-bet by Blimp-1 on day 14 p.i. (C) Summary graph depicting the proportion of activated WT and ifnar1 -/- CD4 T cells simultaneously expressing both T-bet and Blimp-1. (D-E) Summary graphs displaying the relative expression (gMFI) of Blimp-1 (D) and T-bet (E) in activated WT and ifnar1 -/- CD4 T cells. (F) Representative flow plots depicting the proportion of activated WT and ifnar1 -/- CD4 T cells competent to produce IFN-γ and IL-10 after ex vivo stimulation. (G-I) Summary data displaying the frequency of activated WT and ifnar1 -/- CD4 T cells producing either IFN-γ (G) or IL-10 (H) on day 14 p.i. after ex vivo stimulation. (J-K) Separate groups of tcrα -/- mice were seeded with equivalent numbers of WT (CD45.1/2) and ifnar1 -/- (CD45.2/2) naïve CD4 T cells and infected with 106 pRBCs once day post-transfer. Parasite burdens and cellular reactions were analyzed on day 16 p.i. (L) Experimental design for generating mixed bone marrow chimeras in which the T cell compartment is either WT (TWT) or ifnar-deficient (Tifnar-/-). (M) Secreted parasite-specific IgG was evaluated by ELISA in chimeric mice on day 23 p.i.. (N) Summary data depicting the total number of Tfh cells in chimeric mice. Data (Mean +/- SD) in (C-E, G-I, and M,N) were analyzed using Mann-Whitney non-parametric tests and are representative of two independent experiments with 5 mice per group per experiment. Data (Mean +/- SEM) in (J,K) were pooled from two independent experiments with 3–4 mice/group per experiment and were analyzed using Mann-Whitney non-parametric tests.
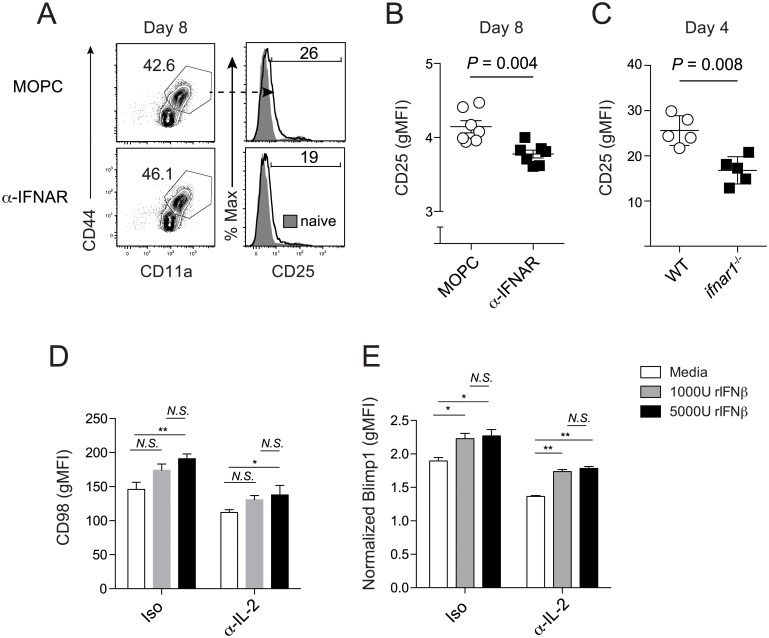
Type I IFN induction of Blimp-1 can occur independently of IL-2 signaling
CD4 T cell-intrinsic IFNAR signaling can induce expression of the high-affinity IL-2 receptor (CD25), sensitizing CD4 T cells to IL-2 signaling [47] and activation of STAT5 [23] a known inducer of prdm1, which encodes for Blimp-1. To begin to explore the mechanistic role of IL-2 in the IFNα/β-Blimp-1 circuit, we examined the expression kinetics of CD25 on Plasmodium infection-induced and LCMV GP61-80-specific CD4 T cells in MOPC- and α-IFNAR-treated Plasmodium parasite- or virus-infected mice, respectively. We found that α-IFNAR treatment significantly reduced CD25 expression on CD4 T cells responding to either Plasmodium (Fig 6A and 6B) or LCMV clone 13 infection (S6A and S6B Fig). Notably, reduced Blimp-1 expression in co-transferred ifnar1 -/- CD4 T cells (Fig 5B–5D) also correlated with a 30% decrease in CD25 surface expression compared to WT effector CD4 T cells on day 4 p.i. (Fig 6C), suggesting that type I IFNs may function to elevate or sustain CD25 expression, thereby enhancing STAT5 activity and Blimp-1 induction. To formally test this, we next performed in vitro CD4 T cell culture experiments. We observed dose-dependent effects of exogenous IFNβ-mediated phosphatidylinositol 3-kinase (PI3K) activation and Blimp-1 induction, as measured by CD98 expression [48] and Blimp-1-eYFP expression, respectively, in CD4 T cells (Fig 6D and 6E). Strikingly, IFNβ-mediated induction of Blimp-1 and PI3K activation occurred even when IL-2 was neutralized (Fig 6D and 6E). These results support that type I IFNs may directly induce Blimp-1 expression in CD4 T cells independently of IL-2 signaling, likely via activation of the PI3K pathway. Collectively, our results show that CD4 T cell intrinsic type I IFN signaling directly induces T-bet and Blimp-1 expression in Plasmodium infection-induced effector CD4 T cells, thereby promoting Tr1 cell development and function. Moreover, Tr1 effector cytokines IL-10 and IFN-γ collaborate to suppress Tfh accumulation, anti-Plasmodium humoral immunity, and parasite control.
Fig 6. Type I IFN-mediated induction of Blimp-1 in CD4 T cells can occur independently of IL-2 signaling.
(A-B) Blimp-1 reporter mice were administered either MOPC or α-IFNAR antibodies and were infected with either106 pRBCs. (A) Representative flow plots depicting the expression of CD25 on Plasmodium infection-induced effector CD4 T cells from MOPC and α-IFNAR-treated mice on day 8 p.i. (B) Cumulative data showing surface CD25 expression (gMFI) on Plasmodium infection-induced splenic CD44hiCD11ahi CD4+ T cells. Data (Mean +/- SEM) in (B) are pooled from 2 independent experiments (3–4 mice/group). (C) Summary data (Mean +/- SD) showing CD25 expression (gMFI) on WT and ifnar1 -/- CD4 T cells recovered from tcrα -/- on day 4 post-Plasmodium infection. Data in (B,C) were analyzed using Mann-Whitney non-parametric tests of statistical significance. (D,E) Naïve CD4 T cells from Blimp-1-eYFP reporter mice were stimulated with α-CD3/α-CD28 and treated with or without rIFNβ or anti-IL-2 blocking antibodies. (D) Summary data (Mean +/- SD) depicting surface expression of PI3K-dependent CD98 on CD44hi CD4+ T cells cultured under the various treatment conditions. (E) Summary data (Mean +/- SEM) showing Blimp-1-eYFP expression (gMFI) in CD44hi CD4+ T cells under the various treatment conditions. Data in (E) are normalized to Blimp-1 expression (MFI) in non-stimulated CD4 T cells and are pooled from 3 independent experiments. Data in (D,E) are representative of 3 independent experiments and were analyzed using Kruskal-Wallis non-parametric tests of statistical significance. N.S. = not statistically significant. *P<0.05, **P<0.01.
Discussion
The importance of IL-10-secreting Tr1 cells in limiting immunopathology during highly inflammatory Th1-biased infection is well appreciated [40, 41, 43, 49]. However, the pathways that induce or support Tr1 differentiation are not well understood. Here we show that early type I IFN responses during inflammatory Th1-biased infections promote the differentiation of T-bet+Blimp-1+ Tr1 cells and limit humoral immunity and parasite control during experimental malaria. Mechanistically, we identified that type I IFNs directly induce T-bet and Blimp-1 expression in CD4 T cells responding to Plasmodium blood-stage infection, which amplifies their production of IL-10 and IFN-γ. Furthermore, we show that these two Tr1-associated cytokines collaborate to suppress humoral immunity and parasite control.
Multiple reports show that type I IFNs are highly induced during malaria, yet the extent to which they regulate anti-Plasmodium immunity or disease is often conflicting. Clinical studies show that specific SNPs in the gene encoding ifnar1 are associated with resistance to cerebral malaria [50], and case-control studies in Angolian children and neuro-malaria patients in Thailand support that type I IFN responses are either associated with the development of cerebral malaria [51] or precede the induction of IFN-γ expression and severe disease [20]. These latter studies are consistent with our experiments showing that blocking type I IFN signaling during non-lethal experimental malaria abrogates the development of IFN-γ+T-bet+ Th1 responses. Similarly, multiple reports show that ifnar1 -/- mice have altered Th1 activity and are largely resistant to the development of acutely lethal P. berghei-induced experimental cerebral malaria (ECM) [17, 21, 25, 52]. In contrast, following infection with non-lethal P. yoelii or P. chabaudi spp., ifnar1 -/- mice exhibit either exacerbated disease and higher parasite burdens [22, 53] or no phenotype at all [18]. Although most studies have focused on the role of type I IFN signaling in either regulating myeloid dendritic cell stimulatory potential or Plasmodium parasite control during the first week of acute infection, to our knowledge no studies have examined whether type I IFN responses regulate humoral immunity during Plasmodium infection. To address this question, and to avoid pitfalls associated with altered immune system development or dysregulated hematopoiesis in ifnar1 -/- mice [54, 55], we employed transient blockade of type I IFN responses and chimeric/genetic approaches to evaluate CD4 T cell intrinsic and extrinsic roles for type I IFN signaling during experimental malaria. We found that IFNAR signaling blockade during experimental malaria enhanced the quantity and quality of the Tfh cell response, bolstered the magnitude of the germinal center reaction and elevated titers of parasite-specific antibody against MSP119, all of which were associated with improved control over parasite replication. Our chimeric studies show that type I IFN-mediated suppression of Tfh accumulation and humoral immunity is indirect.
During chronic LCMV infection, blocking type I IFN signaling, primarily the activity of IFNβ [56], enhances CD4 T cell function and accelerates resolution of persistent infection [27, 28]. However, the two arms of the adaptive immune response primarily responsible for limiting virus persistence and promoting resolution of LCMV, cytotoxic CD8 T cells and Tfh-dependent antibody responses, were not quantitatively or qualitatively enhanced [27, 28]. By comparison, during acute LCMV infection, type I IFNs are purported to directly inhibit Tfh cell differentiation via up-regulation of CD25, which sensitizes CD4 T cells to IL-2 signaling and promotes STAT5 binding to the Bcl-6 promoter, thereby blocking the Tfh-promoting activity of STAT3 [23]. Of note, administration of recombinant IFNβ during in vitro activation of naïve CD4 T cells induced CXCR5 and Bcl-6 expression in WT but not ifnar1 -/- CD4 T cells (not depicted and [24], highlighting that type I IFNs may positively regulate molecules important for the Tfh cell program in vitro. Thus, we examined possible CD4 T cell-intrinsic impacts of type I IFN signaling during experimental malaria. To do this, we performed co-adoptive transfer studies of WT and ifnar1 -/- CD4 T cells into Plasmodium infected mice, because analyses of humoral immunity and parasite control in tcrα -/- mice seeded with either WT or ifnar1 -/- cells may be confounded by the markedly reduced proliferation of CD4 T cells lacking the type I IFN receptor (e.g. Fig 5C). We found ifnar1 -/- and WT CD4 T cells differentiated into Tfh cells in equal proportions, despite reduced CD25 expression on ifnar1 -/- CD4 T cells. These data suggest that neither CD4 T cell-intrinsic type I IFN signaling nor IFN-mediated upregulation of CD25 directly limits Tfh development during experimental malaria. Instead, our data show that Plasmodium infection-induced type I IFNs promote the differentiation and function of T-bet+Blimp-1+ effector CD4 T cells. Pathogen-specific effector CD4 T cells are the major source of IL-10 following prolonged viral and parasitic infection. We found that IL-10 and IFN-γ expression were largely restricted to Blimp-1+ Tr1 cells, compared to all other Plasmodium infection-induced effector CD4 T cell subsets. Moreover, we identified that the effector cytokines of Tr1 cells, IL-10 and IFN-γ, constrain Tfh accumulation and protective humoral immunity. Our data supporting IL-2-independent induction of Blimp-1 during Plasmodium infection is also consistent with a recent report showing that IL-2 blockade failed to modulate Blimp-1 expression in LCMV-specific CD4 T cells [42].
Excessive Th1 activity and secretion of IFN-γ can limit the development and function of Tfh cells during malaria [15, 16, 57]. As noted, type I IFNs can increase CD4 T cell responsiveness to IL-2 signaling, which can stimulate STAT5 activation and skew CD4 T cells towards Th1 lineage commitment. Alternatively, in vitro studies show that type I IFNs can activate STAT4-mediated induction of T-bet and Th1 activity [24]. Consistent with these observations, we found that blocking type I IFN signaling during experimental malaria reduced T-bet expression in infection-induced Th1 cells. Here we extend these data and provide in vivo evidence that type I IFNs may directly induce T-bet to reinforce the Th1 program. Notably, ectopic expression of T-bet in antigen-specific CD8 T cells promotes Blimp-1 expression [58]. Furthermore, several studies show that during prolonged, highly inflammatory parasitic and viral infections, Th1 cells co-express the transcription factor Blimp-1, a transcriptional repressor that is required for Th1/Tr-1 production of IL-10 [42–44]. Tr1 cell production of IL-10 is known to limit pathogen control during chronic parasitic and viral infections [42, 43]. Thus, we assessed whether type I IFNs also impact Blimp-1 expression in CD4 T cells during experimental malaria or chronic LCMV infection. Strikingly, IFNAR signaling blockade decreased Blimp-1 expression in both parasite infection induced- and virus-specific CD4 T cells. Thus, the type I IFN-Blimp-1 axis represents a generalizable feature of prolonged/chronic pro-inflammatory infections. Importantly, compared to WT CD4 T cells, ifnar1 -/- CD4 T cells responding to malaria also exhibited a 30% decrease in the co-expression of T-bet and Blimp-1, demonstrating that CD4 T cell-intrinsic IFNAR signaling directly induces transcription factors important for Tr1 function. Accordingly, both antibody-mediated blockade and genetic ablation of IFNAR signaling resulted in markedly reduced Tr1 expression of IFN-γ and IL-10. These effects were also accompanied by reduced levels of serum IFN-γ and IL-10 in α-IFNAR-treated mice.
The importance of Tr1 cells in limiting inflammatory-mediated immunopathology during chronic viral and parasitic infections has been established [39, 40, 43, 59, 60]. However, Tr1 cell production of IL-10 can also hamper pathogen control [42, 43]. Defining the cellular and molecular mechanisms that govern Tr1 cell differentiation and function is therefore of interest and has therapeutic implications for Th1-inflammatory and autoimmune diseases, including cancer. Previous reports have identified that the inflammatory cytokines IL-27 and IL-12 drive Blimp-1 and IL-10 expression in both antigen-specific Tr1 and CD8 T cells. [61–63]. Furthermore, ICOS is linked to Tr1 development and activity [64], and recent studies show that systemic IL-27 and ICOS cooperate to regulate Tr1 activity during experimental malaria [65]; however, in these reports neither IL-27R signaling nor ICOS was essential for Tr1 development during P. yoelii malaria [65]. By contrast, we show that type I IFNs act directly on pathogen-specific CD4 T cells to induce co-expression of T-bet and Blimp-1 and their downstream Tr1-assocatied effector molecules IFN-γ and IL-10. Our in vivo data are also consistent with studies demonstrating IFNβ can trigger IL-10 production by CD4 T cells in vitro [66]. While CD4 T cell-derived IL-2 has been linked to Blimp-1 and IL-10 expression in CD8 T cells [67], we found that IFNβ-mediated Blimp-1 induction in in vitro stimulated CD4 T cells did not involve IL-2 signaling. Thus, our data uncover a previously unrecognized circuit and an additional mechanism for Tr1 development in vivo during highly inflammatory, Th1-biased infections.
One of the most striking and consistent differences between Plasmodium infection-induced effector CD4 T cells recovered from control rIgG- and α-IFNAR-treated mice, or WT and ifnar1 -/- effector CD4 T cells following adoptive transfer, was their diminished capacity to co-produce IL-10 and IFN-γ when IFNAR signaling was abrogated. While excessive IFN-γ is known to limit anti-Plasmodium humoral immunity [15, 57], only a limited number of studies have examined the impact of IL-10 on the germinal center reaction and humoral immunity, with variable results reported [68–72]. Although IL-10 can constrain Tfh differentiation following immunization [69], whether IL-10 plays a role in promoting or inhibiting Tfh cell differentiation, maintenance or function during infection remains less clear. Notably, a recent study identified that frequencies of IFN-γ and IL-10 co-producing CD4 T cells were increased in Ugandan children who presented with >2 malaria episodes/year, compared to children who presented with <2 malaria episodes/year [73]. Although this study did not assess humoral immunity (nor could causality be determined), these observations are consistent with Tr1 responses limiting protective immunity.
The capacity of IL-10 to modulate humoral immunity is likely multifaceted. Our data show that blockade of IL-10R signaling abrogates humoral immunity during experimental malaria. However, simultaneously blocking IL-10R signaling and neutralizing IFN-γ enhances parasite control and parasite-specific antibody responses. IL-10 can also limit the activity and function of antigen presenting cells (APC) [74], with potential impacts on Tfh priming. Conversely, IL-10 can signal via STAT3, which is important for promoting Tfh development [23]. Of note, IL-10 can also function in a feedback loop to limit the activity of effector Th1 cells during chronic virus infection [42]. In that study, IL-10 expression was Blimp-1-dependent, and prolonged TCR engagement and IRF4 activity were mechanistically linked to Blimp-1 induction. Our data show that type I IFN signaling directly and additionally contributes to Blimp-1 induction in cells responding to either Plasmodium or chronic LCMV infection, further supporting that type IFN-mediated induction of Blimp-1 and subsequent expansion of immunosuppressive Tr1 cells represents a generalizable feature of inflammatory Th1-biased infections.
Collectively, our results identify a previously unrecognized inhibitory circuit wherein CD4 T cell intrinsic type I IFN-signaling directly induces a T-bet-Blimp-1 axis in responding Plasmodium infection-induced Tr1 cells, thereby promoting expression of IFN-γ and IL-10 that cooperate to inhibit Tfh accumulation and expression of ICOS. Our data also support that blockade of IFNAR signaling or neutralization of Tr1 effector molecules IFN-γ and IL-10 during malaria may bolster Plasmodium-specific Tfh responses, enhance parasite control, and promote the establishment of humoral immunity. Our report identifies additional mechanisms that limit Plasmodium-specific antibody responses and highlights pathways that warrant consideration as potential targets when designing an efficacious malaria vaccine or novel immunotherapeutics to combat malarial disease.
Materials and Methods
Ethic statement
All animal experiments were conducted in accordance with the Animal Welfare Act and the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. OUHSC animal facilities have full accreditation from the Association for Assessment and Accreditation of Laboratory Animal Care and are PHS-assured (Assurance Number: # A3165-01). All animal procedures were approved by the OUHSC Animal Care and Use Committee (IACUC) under protocol 15–088 and the Office of Animal Welfare Assurance (OAWA), which oversees the administration of the IACUC at OUHSC.
Mice, Plasmodium and LCMV infection, and biologics
C57BL/6 wild type, tcrα -/-, Blimp-1-eYFP reporter, ifnar1 -/-, and il10rb -/- mice (6–8 weeks, 16–21 g) were purchased from Jackson Laboratories. Plasmodium yoelii (clone 17XNL, obtained from MR4 (ATCC)) was routinely passaged through mosquitoes and mouse infections were initiated by serial transfer of 106 parasite-infected red blood cells via tail vein injection. LCMV clone 13 infection was administered intravenously at a dose of 106 PFU. Parasitemia was measured using flow cytometry as described [75]. One day prior to P. yoelii infection, mice were injected i.p. with either 1.5 mg of MOPC isotype control or MAR1-5A3 (α-IFNAR) monoclonal antibodies, followed by 0.75 mg doses on days 2 and 4 p.i. Anti-IL-10 receptor (clone 1B1.3a), anti-IFN-γ (clone XMG1.2) were injected i.p. in 200 and 500 μg doses on days 7 and 10 p.i., respectively. All biologics were acquired from BioXcell.
Bone marrow chimeras
For TWT and Tifnar-/- chimeras, WT recipient mice were irradiated with 6.5 and 5.5 Gy, separated by 12 hours. Bone marrow from tcrα -/- and ifnar -/- or WT mice was mixed 1:9 and 107 cells were injected i.v. Mice were maintained on oral sulfamethoxazole for 2 weeks. Chimerism was assessed at 6 weeks in peripheral blood using congenic markers. Chimerism in the T cell compartment was 65–70% among 20 mice in two independent experiments. Mice were infected with P. yoelii at 8 weeks.
Flow cytometry
Mouse splenocytes were subjected to red blood cell lysis, washed and subsequently stained using fluorescently labeled antibodies against mouse CD4 (clone GK1.5), CD11a (clone M17/4), CD19 (clone 6D5), CD25 (clone PC61), B220 (clone RA3-6B2), CD44 (clone IM7), CD49b (clone DX5), CD95 (clone Jo2), CD98 (clone RL388), CD138 (clone 281–2), ICOS (clone 7E.17G9), LAG-3 (clone eBioC9B7W), PD-1 (clone RMP1-30), T and B cell activation antigen (clone GL-7), IgD (clone 11-26c.2a), or IgM (clone RMM-1). Reagents were acquired from Biolegend, Tonbo, eBioscience or BD Bioscience. Mouse splenocytes from LCMV cl13-infected mice were also stained with GP61-80 tetramer reagents (NIH) for one hour at room temperature before performing additional surface staining. In some experiments, cells were permeabilized with cytofix/cytoperm (BD Bioscience) followed by intracellular staining using anti-mouse IL-10 (JES5-16E3) and anti-mouse IFN-γ, (XMG1.2; Biolegend) or anti-IL-2 (JES6-5H4; eBioscience). For analysis of T follicular helper cells, splenocytes were incubated for 60 min at 4°C with rat anti-mouse CXCR5 (2G8; BD) and subsequently stained for 30 min at 4°C in the presence of biotinylated goat anti-rat IgG (Jackson), followed by staining with fluorochrome-conjugated anti-CD4, anti-CD44, anti-PD-1, and streptavidin-APC. T-bet (4B10), Bcl-6 (K112-91), and Blimp-1 (5E7), were stained after fixation and permeabilization using the FoxP3 staining buffer set (eBioscience). Samples were acquired using a Stratedigm S1200Ex flow cytometer and data was analyzed using FlowJo software (Tree Star, Inc., Ashland OR).
MSP119 and Cytokine ELISA
Plates (Nunc) were coated with recombinant MSP119 (MR4) blocked with 2.5% BSA/5% normal goat serum and MSP119-specific IgG was detected in pre-diluted serum samples using HRP-conjugated goat anti-mouse-IgG, -IgG2b, or -IgM (Jackson ImmunoResearch). The SureBlue Reserve TMB Kit (KPL) was used as substrate and absorbance was analyzed with a Spectra Max 340 (Molecular Devices). For serum cytokine analyses, plates were coated with 2 μg/mL of IL-10 or IFN-γ capture antibodies (eBioscience), blocked with 2.5% BSA/5% fetal calf serum. Serum samples were subsequently applied at a 1:4 dilution. Wells were washed, incubated with biotinylated detection antibodies (eBioscience) and developed with streptavidin-HRP at room temperature for 30 minutes before applying SureBlue Reserve TMB substrate as described above.
In vitro T cell culture
Naïve CD4 T cells were enriched from spleens of Blimp-1-eYFP reporter mice and stimulated with plate-bound α-CD3/α-CD28 for 3 days while treating with or without 1000 U/ml or 5000 U/ml of rIFNβ +/- blocking antibodies against IL-2 (JES6-1A12; used at a concentration of 10 μg /mL).
Statistical analyses
Statistical analyses were performed using GraphPad Prism 6 software (GrapPad). Specific tests of statistical significance are detailed in figure legends.
Supporting Information
(A) Mice were infected with 10 6 P. yoelii infected red blood cells (pRBCs) and then administered either MOPC (isotype) or α-IFNAR antibodies at the indicated time points (arrows). Parasitemia (% of RBCs infected) kinetics from two independent experiments are shown. (B-C) Representative dot plots illustrating that GC B cell (B) and Tfh (C) responses are Plasmodium infection-induced.
(EPS)
(A) Representative flow plots depicting the proportion of CD44 hi Blimp-1-eYFP + splenic CD4 T cells in naïve and Plasmodium infected mice. (B) Representative flow plots depicting the proportion of splenic GP61-80 specific CD4 T cells (left) and their subsequent expression of Blimp-1-eYFP (right) 14 days following LCMV infection. (C) Summary data (n = 5 mice/group) showing Blimp-1-eYFP expression (gMFI) in GP61-80 specific and effector (CD44hiCD11ahi) CD4 T cells on day 14 post LCMV cl13 infection.
(EPS)
(A-B). Analyses of LAG-3 and CD49b expression on Plasmodium infection-induced CD44 hi Blimp-1-eYFP + splenic CD4 T cells on day 10 p.i. (C) Positive control staining to verify CD49b reagent, which is also a pan-NK cell marker. NK cells were identified via NK1.1 and CD3ε staining.
(EPS)
(A) Representative flow plots showing the frequency of splenic GC B cells in rIgG-, α-IL-10R-, α-IFN-γ, or α-IFN-γ+α-IL-10R-treated mice on d14 p.i. (B) Summary data (n = 5 mice/group) depicting the frequency of GC B cells on day 14 p.i.
(EPS)
(A) Representative dot plots and histograms depicting Tfh differentiation among WT and ifnar -/- CD4 T cells recovered from the same Plasmodium-infected tcrα -/- mice on day 14 p.i. (B) Summary graph displaying the total number of recovered WT and ifnar1 -/- CD44hi effector CD4+ T cells. (C-D) Summary data showing proportion of Tfh cells (C) among recovered WT and ifnar1 -/- CD44hi CD4+ T cells and their expression of Bcl-6 (gMFI) (D). (E-F) Representative dot plots depicting the proportion of PD-1hiCXCR5+ Tfh cells (E) and GC B cells (F) recovered on day 23 p.i. from Plasmodium infected mixed bone marrow chimeric mice with either WT (TWT) or ifnar -/- (Tifnar-/-) T cell compartments. (G) Summary data depicting the total number of GC B cells in Plasmodium infected TWT and Tifnar-/- mice.
(EPS)
(A) Representative flow plots depicting the proportion of GP 61-80 specific CD4 T cells and their subsequent expression of CD25 (right) on day 14 post LCMV clone 13 infection. (B) Cumulative data (Mean +/- SD) showing the relative expression of CD25 (gMFI) on GP61-80 specific and splenic effector (CD44hiCD11ahi) CD4+ T cells on day 14 post LCMV clone 13 infection from control and α-IFNAR-treated mice.
(EPS)
Acknowledgments
We thank Christopher Hunter and Bob Axtell for critical feedback, and the Flow Cytometry Laboratory at OUHSC for technical assistance.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported in part by grants from the National Institute of Allergy and Infectious Disease (T32AI007633 to RAZ; 1K22AI099070 and R01AI125446 to NSB; R01AI053108 to DJJC) and the American Heart Association (16PRE27660002 to JJG). NSB is also an OK-INBRE scholar supported by a grant from the National Institute of General Medical Sciences (8P20GM103447). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Organization WH. World Malaria Report 2015. (Geneva: World Health Organization; ); 2015. [Google Scholar]
- 2. Crompton PD, Moebius J, Portugal S, Waisberg M, Hart G, Garver LS, et al. Malaria immunity in man and mosquito: insights into unsolved mysteries of a deadly infectious disease. Annual review of immunology. 2014;32:157–87. Epub 2014/03/25. 10.1146/annurev-immunol-032713-120220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crompton PD, Kayala MA, Traore B, Kayentao K, Ongoiba A, Weiss GE, et al. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(15):6958–63. Epub 2010/03/31. 10.1073/pnas.1001323107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kinyanjui SM, Bejon P, Osier FH, Bull PC, Marsh K. What you see is not what you get: implications of the brevity of antibody responses to malaria antigens and transmission heterogeneity in longitudinal studies of malaria immunity. Malaria journal. 2009;8:242 Epub 2009/10/29. 10.1186/1475-2875-8-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kinyanjui SM, Conway DJ, Lanar DE, Marsh K. IgG antibody responses to Plasmodium falciparum merozoite antigens in Kenyan children have a short half-life. Malaria journal. 2007;6:82 Epub 2007/06/30. 10.1186/1475-2875-6-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nature immunology. 2008;9(7):725–32. Epub 2008/06/20. 10.1038/ni.f.205 . [DOI] [PubMed] [Google Scholar]
- 7. Portugal S, Pierce SK, Crompton PD. Young lives lost as B cells falter: what we are learning about antibody responses in malaria. Journal of immunology. 2013;190(7):3039–46. Epub 2013/03/26. 10.4049/jimmunol.1203067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kometani K, Kurosaki T. Differentiation and maintenance of long-lived plasma cells. Current opinion in immunology. 2015;33:64–9. Epub 2015/02/14. 10.1016/j.coi.2015.01.017 . [DOI] [PubMed] [Google Scholar]
- 9. Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM. The generation of antibody-secreting plasma cells. Nature reviews Immunology. 2015;15(3):160–71. Epub 2015/02/24. 10.1038/nri3795 . [DOI] [PubMed] [Google Scholar]
- 10. Crotty S. Follicular helper CD4 T cells (TFH). Annual review of immunology. 2011;29:621–63. Epub 2011/02/15. 10.1146/annurev-immunol-031210-101400 . [DOI] [PubMed] [Google Scholar]
- 11. Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nature medicine. 2013;19(4):494–9. Epub 2013/03/12. 10.1038/nm.3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamamoto T, Lynch RM, Gautam R, Matus-Nicodemos R, Schmidt SD, Boswell KL, et al. Quality and quantity of TFH cells are critical for broad antibody development in SHIVAD8 infection. Science translational medicine. 2015;7(298):298ra120 Epub 2015/08/01. 10.1126/scitranslmed.aab3964 . [DOI] [PubMed] [Google Scholar]
- 13. Butler NS, Kulu DI. The regulation of T follicular helper responses during infection. Current opinion in immunology. 2015;34:68–74. Epub 2015/03/03. 10.1016/j.coi.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Obeng-Adjei N, Portugal S, Tran TM, Yazew TB, Skinner J, Li S, et al. Circulating Th1-Cell-type Tfh Cells that Exhibit Impaired B Cell Help Are Preferentially Activated during Acute Malaria in Children. Cell reports. 2015;13(2):425–39. Epub 2015/10/07. 10.1016/j.celrep.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zander RA, Obeng-Adjei N, Guthmiller JJ, Kulu DI, Li J, Ongoiba A, et al. PD-1 Co-inhibitory and OX40 Co-stimulatory Crosstalk Regulates Helper T Cell Differentiation and Anti-Plasmodium Humoral Immunity. Cell host & microbe. 2015;17(5):628–41. Epub 2015/04/22. 10.1016/j.chom.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ryg-Cornejo V, Ioannidis LJ, Ly A, Chiu CY, Tellier J, Hill DL, et al. Severe Malaria Infections Impair Germinal Center Responses by Inhibiting T Follicular Helper Cell Differentiation. Cell reports. 2016;14(1):68–81. Epub 2016/01/05. 10.1016/j.celrep.2015.12.006 . [DOI] [PubMed] [Google Scholar]
- 17. Haque A, Best SE, Ammerdorffer A, Desbarrieres L, de Oca MM, Amante FH, et al. Type I interferons suppress CD4(+) T-cell-dependent parasite control during blood-stage Plasmodium infection. European journal of immunology. 2011;41(9):2688–98. Epub 2011/06/16. 10.1002/eji.201141539 . [DOI] [PubMed] [Google Scholar]
- 18. Kim CC, Nelson CS, Wilson EB, Hou B, DeFranco AL, DeRisi JL. Splenic red pulp macrophages produce type I interferons as early sentinels of malaria infection but are dispensable for control. PloS one. 2012;7(10):e48126 Epub 2012/11/13. 10.1371/journal.pone.0048126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luty AJ, Perkins DJ, Lell B, Schmidt-Ott R, Lehman LG, Luckner D, et al. Low interleukin-12 activity in severe Plasmodium falciparum malaria. Infection and immunity. 2000;68(7):3909–15. Epub 2000/06/17. 10.1128/IAI.68.7.3909-3915.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rhodes-Feuillette A, Bellosguardo M, Druilhe P, Ballet JJ, Chousterman S, Canivet M, et al. The interferon compartment of the immune response in human malaria: II. Presence of serum-interferon gamma following the acute attack. Journal of interferon research. 1985;5(1):169–78. Epub 1985/01/01. 10.1089/jir.1985.5.169 . [DOI] [PubMed] [Google Scholar]
- 21. Sharma S, DeOliveira RB, Kalantari P, Parroche P, Goutagny N, Jiang Z, et al. Innate immune recognition of an AT-rich stem-loop DNA motif in the Plasmodium falciparum genome. Immunity. 2011;35(2):194–207. Epub 2011/08/09. 10.1016/j.immuni.2011.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu J, Tian L, Yu X, Pattaradilokrat S, Li J, Wang M, et al. Strain-specific innate immune signaling pathways determine malaria parasitemia dynamics and host mortality. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(4):E511–20. Epub 2014/01/30. 10.1073/pnas.1316467111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ray JP, Marshall HD, Laidlaw BJ, Staron MM, Kaech SM, Craft J. Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 cells. Immunity. 2014;40(3):367–77. Epub 2014/03/19. 10.1016/j.immuni.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakayamada S, Poholek AC, Lu KT, Takahashi H, Kato M, Iwata S, et al. Type I IFN induces binding of STAT1 to Bcl6: divergent roles of STAT family transcription factors in the T follicular helper cell genetic program. Journal of immunology. 2014;192(5):2156–66. Epub 2014/02/04. 10.4049/jimmunol.1300675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haque A, Best SE, Montes de Oca M, James KR, Ammerdorffer A, Edwards CL, et al. Type I IFN signaling in CD8- DCs impairs Th1-dependent malaria immunity. The Journal of clinical investigation. 2014;124(6):2483–96. Epub 2014/05/03. 10.1172/JCI70698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sheehan KC, Lai KS, Dunn GP, Bruce AT, Diamond MS, Heutel JD, et al. Blocking monoclonal antibodies specific for mouse IFN-alpha/beta receptor subunit 1 (IFNAR-1) from mice immunized by in vivo hydrodynamic transfection. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2006;26(11):804–19. Epub 2006/11/23. 10.1089/jir.2006.26.804 . [DOI] [PubMed] [Google Scholar]
- 27. Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013;340(6129):207–11. Epub 2013/04/13. 10.1126/science.1235214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340(6129):202–7. Epub 2013/04/13. 10.1126/science.1235208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nagai T, Devergne O, Mueller TF, Perkins DL, van Seventer JM, van Seventer GA. Timing of IFN-beta exposure during human dendritic cell maturation and naive Th cell stimulation has contrasting effects on Th1 subset generation: a role for IFN-beta-mediated regulation of IL-12 family cytokines and IL-18 in naive Th cell differentiation. Journal of immunology. 2003;171(10):5233–43. Epub 2003/11/11. . [DOI] [PubMed] [Google Scholar]
- 30. Le Bon A, Schiavoni G, D'Agostino G, Gresser I, Belardelli F, Tough DF. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14(4):461–70. Epub 2001/05/05. 10.1016/S1074-7613(01)00126-1 . [DOI] [PubMed] [Google Scholar]
- 31. Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nature immunology. 2012;13(2):188–95. Epub 2011/12/14. 10.1038/ni.2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Butler NS, Schmidt NW, Vaughan AM, Aly AS, Kappe SH, Harty JT. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell host & microbe. 2011;9(6):451–62. Epub 2011/06/15. 10.1016/j.chom.2011.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guthmiller JJ, Zander RA, Butler NS. Measurement of the T Cell Response to Preerythrocytic Vaccination in Mice. Methods Mol Biol. 2015;1325:19–37. Epub 2015/10/10. 10.1007/978-1-4939-2815-6_2. 26450376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McDermott DS, Varga SM. Quantifying antigen-specific CD4 T cells during a viral infection: CD4 T cell responses are larger than we think. Journal of immunology. 2011;187(11):5568–76. Epub 2011/11/02. 10.4049/jimmunol.1102104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schmidt NW, Butler NS, Badovinac VP, Harty JT. Extreme CD8 T cell requirements for anti-malarial liver-stage immunity following immunization with radiation attenuated sporozoites. PLoS pathogens. 2010;6(7):e1000998 Epub 2010/07/27. 10.1371/journal.ppat.1000998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kimura D, Miyakoda M, Kimura K, Honma K, Hara H, Yoshida H, et al. Interleukin-27-Producing CD4(+) T Cells Regulate Protective Immunity during Malaria Parasite Infection. Immunity. 2016;44(3):672–82. Epub 2016/03/13. 10.1016/j.immuni.2016.02.011 . [DOI] [PubMed] [Google Scholar]
- 37. Stone EL, Pepper M, Katayama CD, Kerdiles YM, Lai CY, Emslie E, et al. ICOS coreceptor signaling inactivates the transcription factor FOXO1 to promote Tfh cell differentiation. Immunity. 2015;42(2):239–51. Epub 2015/02/19. 10.1016/j.immuni.2015.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leavenworth JW, Verbinnen B, Yin J, Huang H, Cantor H. A p85alpha-osteopontin axis couples the receptor ICOS to sustained Bcl-6 expression by follicular helper and regulatory T cells. Nature immunology. 2015;16(1):96–106. Epub 2014/12/02. 10.1038/ni.3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Couper KN, Blount DG, Wilson MS, Hafalla JC, Belkaid Y, Kamanaka M, et al. IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS pathogens. 2008;4(2):e1000004 Epub 2008/04/11. 10.1371/journal.ppat.1000004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Freitas do Rosario AP, Lamb T, Spence P, Stephens R, Lang A, Roers A, et al. IL-27 promotes IL-10 production by effector Th1 CD4+ T cells: a critical mechanism for protection from severe immunopathology during malaria infection. Journal of immunology. 2012;188(3):1178–90. Epub 2011/12/30. 10.4049/jimmunol.1102755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, et al. Conventional T-bet(+)Foxp3(-) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. The Journal of experimental medicine. 2007;204(2):273–83. Epub 2007/02/07. 10.1084/jem.20062175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Parish IA, Marshall HD, Staron MM, Lang PA, Brustle A, Chen JH, et al. Chronic viral infection promotes sustained Th1-derived immunoregulatory IL-10 via BLIMP-1. The Journal of clinical investigation. 2014;124(8):3455–68. Epub 2014/07/09. 10.1172/JCI66108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Montes de Oca M, Kumar R, de Labastida Rivera F, Amante FH, Sheel M, Faleiro RJ, et al. Blimp-1-Dependent IL-10 Production by Tr1 Cells Regulates TNF-Mediated Tissue Pathology. PLoS pathogens. 2016;12(1):e1005398 Epub 2016/01/15. 10.1371/journal.ppat.1005398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Neumann C, Heinrich F, Neumann K, Junghans V, Mashreghi MF, Ahlers J, et al. Role of Blimp-1 in programing Th effector cells into IL-10 producers. The Journal of experimental medicine. 2014;211(9):1807–19. Epub 2014/07/31. 10.1084/jem.20131548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nature medicine. 2013;19(6):739–46. 10.1038/nm.3179 . [DOI] [PubMed] [Google Scholar]
- 46. Choi YS, Yang JA, Yusuf I, Johnston RJ, Greenbaum J, Peters B, et al. Bcl6 expressing follicular helper CD4 T cells are fate committed early and have the capacity to form memory. Journal of immunology. 2013;190(8):4014–26. Epub 2013/03/15. 10.4049/jimmunol.1202963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Starbeck-Miller GR, Xue HH, Harty JT. IL-12 and type I interferon prolong the division of activated CD8 T cells by maintaining high-affinity IL-2 signaling in vivo. The Journal of experimental medicine. 2014;211(1):105–20. Epub 2013/12/25. 10.1084/jem.20130901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ray JP, Staron MM, Shyer JA, Ho PC, Marshall HD, Gray SM, et al. The Interleukin-2-mTORc1 Kinase Axis Defines the Signaling, Differentiation, and Metabolism of T Helper 1 and Follicular B Helper T Cells. Immunity. 2015;43(4):690–702. Epub 2015/09/28. 10.1016/j.immuni.2015.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4(+)CD25(-)Foxp3(-) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. The Journal of experimental medicine. 2007;204(2):285–97. Epub 2007/02/07. 10.1084/jem.20061886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aucan C, Walley AJ, Hennig BJ, Fitness J, Frodsham A, Zhang L, et al. Interferon-alpha receptor-1 (IFNAR1) variants are associated with protection against cerebral malaria in the Gambia. Genes and immunity. 2003;4(4):275–82. Epub 2003/05/23. 10.1038/sj.gene.6363962 . [DOI] [PubMed] [Google Scholar]
- 51. Ball EA, Sambo MR, Martins M, Trovoada MJ, Benchimol C, Costa J, et al. IFNAR1 controls progression to cerebral malaria in children and CD8+ T cell brain pathology in Plasmodium berghei-infected mice. Journal of immunology. 2013;190(10):5118–27. Epub 2013/04/16. 10.4049/jimmunol.1300114 . [DOI] [PubMed] [Google Scholar]
- 52. Palomo J, Fauconnier M, Coquard L, Gilles M, Meme S, Szeremeta F, et al. Type I interferons contribute to experimental cerebral malaria development in response to sporozoite or blood-stage Plasmodium berghei ANKA. European journal of immunology. 2013;43(10):2683–95. Epub 2013/06/20. 10.1002/eji.201343327 . [DOI] [PubMed] [Google Scholar]
- 53. Voisine C, Mastelic B, Sponaas AM, Langhorne J. Classical CD11c+ dendritic cells, not plasmacytoid dendritic cells, induce T cell responses to Plasmodium chabaudi malaria. International journal for parasitology. 2010;40(6):711–9. Epub 2009/12/09. 10.1016/j.ijpara.2009.11.005 . [DOI] [PubMed] [Google Scholar]
- 54. Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458(7240):904–8. Epub 2009/02/13. 10.1038/nature07815 . [DOI] [PubMed] [Google Scholar]
- 55. Sato T, Onai N, Yoshihara H, Arai F, Suda T, Ohteki T. Interferon regulatory factor-2 protects quiescent hematopoietic stem cells from type I interferon-dependent exhaustion. Nature medicine. 2009;15(6):696–700. Epub 2009/06/02. 10.1038/nm.1973 . [DOI] [PubMed] [Google Scholar]
- 56. Ng CT, Sullivan BM, Teijaro JR, Lee AM, Welch M, Rice S, et al. Blockade of interferon Beta, but not interferon alpha, signaling controls persistent viral infection. Cell host & microbe. 2015;17(5):653–61. Epub 2015/05/15. 10.1016/j.chom.2015.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ryg-Cornejo V, Ly A, Hansen DS. Immunological processes underlying the slow acquisition of humoral immunity to malaria. Parasitology. 2016;143(2):199–207. Epub 2016/01/09. 10.1017/S0031182015001705 . [DOI] [PubMed] [Google Scholar]
- 58. Yeo CJ, Fearon DT. T-bet-mediated differentiation of the activated CD8+ T cell. European journal of immunology. 2011;41(1):60–6. Epub 2010/12/25. 10.1002/eji.201040873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Freitas do Rosario AP, Langhorne J. T cell-derived IL-10 and its impact on the regulation of host responses during malaria. International journal for parasitology. 2012;42(6):549–55. Epub 2012/05/03. 10.1016/j.ijpara.2012.03.010 . [DOI] [PubMed] [Google Scholar]
- 60. Riley EM, Wahl S, Perkins DJ, Schofield L. Regulating immunity to malaria. Parasite Immunol. 2006;28(1–2):35–49. 10.1111/J.1365-3024.2006.00775.X. WOS:000234982300006. [DOI] [PubMed] [Google Scholar]
- 61. Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nature immunology. 2007;8(12):1380–9. Epub 2007/11/13. 10.1038/ni1541 . [DOI] [PubMed] [Google Scholar]
- 62. Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nature immunology. 2007;8(12):1372–9. Epub 2007/11/13. 10.1038/ni1540 . [DOI] [PubMed] [Google Scholar]
- 63. Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nature immunology. 2007;8(12):1363–71. Epub 2007/11/13. 10.1038/ni1537 . [DOI] [PubMed] [Google Scholar]
- 64. Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. Journal of immunology. 2009;183(2):797–801. Epub 2009/07/03. 10.4049/jimmunol.0901233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Villegas-Mendez A, Shaw TN, Inkson CA, Strangward P, de Souza JB, Couper KN. Parasite-Specific CD4+ IFN-gamma+ IL-10+ T Cells Distribute within Both Lymphoid and Nonlymphoid Compartments and Are Controlled Systemically by Interleukin-27 and ICOS during Blood-Stage Malaria Infection. Infection and immunity. 2015;84(1):34–46. Epub 2015/10/16. 10.1128/IAI.01100-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P, et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nature medicine. 2010;16(4):406–12. Epub 2010/03/30. 10.1038/nm.2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sun J, Dodd H, Moser EK, Sharma R, Braciale TJ. CD4+ T cell help and innate-derived IL-27 induce Blimp-1-dependent IL-10 production by antiviral CTLs. Nature immunology. 2011;12(4):327–34. Epub 2011/02/08. 10.1038/ni.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. Journal of immunology. 2007;179(12):8180–90. Epub 2007/12/07. 10.4049/jimmunol.179.12.8180 . [DOI] [PubMed] [Google Scholar]
- 69. Cai G, Nie X, Zhang W, Wu B, Lin J, Wang H, et al. A regulatory role for IL-10 receptor signaling in development and B cell help of T follicular helper cells in mice. Journal of immunology. 2012;189(3):1294–302. Epub 2012/07/04. 10.4049/jimmunol.1102948 . [DOI] [PubMed] [Google Scholar]
- 70. Chacon-Salinas R, Limon-Flores AY, Chavez-Blanco AD, Gonzalez-Estrada A, Ullrich SE. Mast cell-derived IL-10 suppresses germinal center formation by affecting T follicular helper cell function. Journal of immunology. 2011;186(1):25–31. Epub 2010/11/26. 10.4049/jimmunol.1001657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rousset F, Garcia E, Defrance T, Peronne C, Vezzio N, Hsu DH, et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(5):1890–3. Epub 1992/03/01. 10.1073/pnas.89.5.1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sun K, Torres L, Metzger DW. A detrimental effect of interleukin-10 on protective pulmonary humoral immunity during primary influenza A virus infection. Journal of virology. 2010;84(10):5007–14. Epub 2010/03/05. 10.1128/JVI.02408-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jagannathan P, Eccles-James I, Bowen K, Nankya F, Auma A, Wamala S, et al. IFNgamma/IL-10 co-producing cells dominate the CD4 response to malaria in highly exposed children. PLoS pathogens. 2014;10(1):e1003864 Epub 2014/01/15. 10.1371/journal.ppat.1003864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nature reviews Immunology. 2010;10(3):170–81. Epub 2010/02/16. 10.1038/nri2711 . [DOI] [PubMed] [Google Scholar]
- 75. Malleret B, Claser C, Ong AS, Suwanarusk R, Sriprawat K, Howland SW, et al. A rapid and robust tri-color flow cytometry assay for monitoring malaria parasite development. Scientific reports. 2011;1:118 Epub 2012/02/23. 10.1038/srep00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Mice were infected with 10 6 P. yoelii infected red blood cells (pRBCs) and then administered either MOPC (isotype) or α-IFNAR antibodies at the indicated time points (arrows). Parasitemia (% of RBCs infected) kinetics from two independent experiments are shown. (B-C) Representative dot plots illustrating that GC B cell (B) and Tfh (C) responses are Plasmodium infection-induced.
(EPS)
(A) Representative flow plots depicting the proportion of CD44 hi Blimp-1-eYFP + splenic CD4 T cells in naïve and Plasmodium infected mice. (B) Representative flow plots depicting the proportion of splenic GP61-80 specific CD4 T cells (left) and their subsequent expression of Blimp-1-eYFP (right) 14 days following LCMV infection. (C) Summary data (n = 5 mice/group) showing Blimp-1-eYFP expression (gMFI) in GP61-80 specific and effector (CD44hiCD11ahi) CD4 T cells on day 14 post LCMV cl13 infection.
(EPS)
(A-B). Analyses of LAG-3 and CD49b expression on Plasmodium infection-induced CD44 hi Blimp-1-eYFP + splenic CD4 T cells on day 10 p.i. (C) Positive control staining to verify CD49b reagent, which is also a pan-NK cell marker. NK cells were identified via NK1.1 and CD3ε staining.
(EPS)
(A) Representative flow plots showing the frequency of splenic GC B cells in rIgG-, α-IL-10R-, α-IFN-γ, or α-IFN-γ+α-IL-10R-treated mice on d14 p.i. (B) Summary data (n = 5 mice/group) depicting the frequency of GC B cells on day 14 p.i.
(EPS)
(A) Representative dot plots and histograms depicting Tfh differentiation among WT and ifnar -/- CD4 T cells recovered from the same Plasmodium-infected tcrα -/- mice on day 14 p.i. (B) Summary graph displaying the total number of recovered WT and ifnar1 -/- CD44hi effector CD4+ T cells. (C-D) Summary data showing proportion of Tfh cells (C) among recovered WT and ifnar1 -/- CD44hi CD4+ T cells and their expression of Bcl-6 (gMFI) (D). (E-F) Representative dot plots depicting the proportion of PD-1hiCXCR5+ Tfh cells (E) and GC B cells (F) recovered on day 23 p.i. from Plasmodium infected mixed bone marrow chimeric mice with either WT (TWT) or ifnar -/- (Tifnar-/-) T cell compartments. (G) Summary data depicting the total number of GC B cells in Plasmodium infected TWT and Tifnar-/- mice.
(EPS)
(A) Representative flow plots depicting the proportion of GP 61-80 specific CD4 T cells and their subsequent expression of CD25 (right) on day 14 post LCMV clone 13 infection. (B) Cumulative data (Mean +/- SD) showing the relative expression of CD25 (gMFI) on GP61-80 specific and splenic effector (CD44hiCD11ahi) CD4+ T cells on day 14 post LCMV clone 13 infection from control and α-IFNAR-treated mice.
(EPS)
Data Availability Statement
All relevant data are within the paper.