Abstract
Recent evidence suggests that hydrogen sulfide (H2S) has cytoprotective and anti-aging effects. However, the mechanisms for such properties are not fully understood. Here, we show that the expression of the main H2S producing enzyme, CBS, and production of H2S are coordinately diminished in replicative senescent adult human dermal fibroblasts. The reduced production of H2S falls within the same time-frame that the hallmarks of replicative senescence appear including accumulation of SA–β-Gal, enhanced expression of p16, p21, and RRM2B while the expression of RRM2, hTERT, SIRT1, NAMPT, and NAD/NADH ratio all fall. Exogenous H2S increases the expression of hTERT, NAMPT, SIRT1 and NAD/NADH ratio in treated cells. Moreover, H2S safeguards the expression of hTERT in a NAMPT and SIRT1 dependent manner and delays the onset of replicative senescence as evidenced by reduced accumulation of age associated SA–β-Gal and cessation of proliferation. Postponement of loss of cell proliferative capacity without risk of mutagenesis shows implications for use of H2S in delaying the adverse effects of senescence in organisms.
Introduction
There are several lines of evidence that the gasotransmitter, H2S has cytoprotective and life extension properties. Recently, it was shown that the generation of reactive oxygen species is increased in knockouts of mpst-1, a major enzyme that drives the production of hydrogen sulfide in C. elegans and this deficit is overcome by the administration of GY4137 that exposes the short-lived mutants to hydrogen sulfide [1]. This treatment also extends the lifespan of normal animals and delays the onset of detrimental impact of senescence as assessed by pharyngeal contraction and defecation [1]. The extension of lifespan by hydrogen sulfide, which requires SIR-2.1 activity, affords the animals other health-promoting effects including stress resistance and improved thermotolerance [2].
It is known that calorie restriction promotes longevity by increasing SIRT1 expression [3]. In yeast and Drosophila, calorie restriction extends life-span by increasing Sir2 activity and by activating Sir2 deacetylase. Senescence is thought to be due to a progressive loss of cell function and/or cell loss over time. SIRT1 reduces stress induced apoptotic cell loss by deacetylation of the DNA repair factor, Ku70. Deacetylated Ku70, in turn, reduces apoptosis by sequestering the proapoptotic factor, Bax, away from the mitochondria. Thus, by inducing SIRT1 expression, calorie restriction promotes long-term survival of cells which are irreplaceable [3]. It was recently shown that the effect of calorie restriction on life extension is associated with an increase in production of hydrogen sulfide with a cysteine and methionine deficient diet being required for such an enhanced production [4].
In light of such evidence, here, we tested the hypothesis that replicative senescence is associated with a progressive loss in ability of cells to produce hydrogen sulfide and that supporting fibroblasts with an exogenous source of hydrogen sulfide delays replicative senescence that ultimately leads to cessation of proliferation. Data shown here support the view that the life extension properties of hydrogen sulfide, at least in part, is due to its impact in safeguarding against senescence in a NAMPT and SIRT1 dependent manner.
Materials and Methods
Reagents and cell culture
Cell viability was confirmed by Trypan Blue staining (Sigma-Aldrich, St Louis, MO). Chemicals were from Sigma-Aldrich, TRIZOL® and reverse transcriptase (RevertAid® Reverse Transcriptase) were from Thermo Scientific (Carlsbad, CA). NAMPT siRNA was purchased from Santa Cruz Biotechnology (Dallas, TX). Oligonucleotides were generated by IDT (Coralville, IA). Transfection reagent was purchased from Santa Cruz Biotechnology. Adult human dermal fibroblasts (aHDF) cells were obtained from ATCC (Manassas, VA) or Lonza (Walkersville, MD). Cells were maintained in Fibroblast Growth Medium (FGM, Lonza) with 2% fetal bovine serum and growth factors in a 37°C incubator with 5% CO2.
Determination of Population Doublings (PD)
Culture dishes were seeded in triplicates with 3 x 105 aHDF cells. We calculated PD based on the following formula: log ((number of cells harvested)/(number of cells seeded))/log2 + previous PD. Since the PD of cells received from manufacturer was not known, we defined the first PD after initial culture as 0 [5].
Staining for Senescence-Associated β-Galactosidase (SA-β-Gal)
SA–β-Gal staining was performed as described previously [6].
Measurement of H2S production
H2S production was measured by WPI instrument as described previously (Sarasota, FL) [7,8].
Real-time PCR
Real-time PCR was performed using iTaq® Universal SYBR® Green Supermix (Bio-Rad; Hercules, CA) and LightCycler® 96 (Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s instruction. Primers were purchased from IDT (S1 Table).
Immunoblotting
Immunoblotting is described previously [8]. 10 μg of total protein lysates for Nampt and β Actin and 30 μg of total protein lysates for Sirt1 were used. For hTERT blotting, nuclear extraction was performed [9] and 100 μg of nuclear extracted lysates were used. Used antibodies are; anti-hTERT mouse monoclonal (clone 2C4; EMD Millipore), anti-Nampt mouse monoclonal (Sigma), anti-Sirt1 rabbit polyclonal (Sigma), and anti-β Actin-HRP (sc1616-HRP; Santa Cruz). The substrate used in this study was ECL® Prime Western Blotting Detection Reagent (GE Healthcare). The membranes were scanned using C-Digit® (LI-COR) and analyzed by Image Studio® (LI-COR).
Telomerase activity assay
Cells were harvested and lysed in CHAPS buffer (0.5% CHAPS, 10 mM Tris-HCl, pH = 7.5., with 1.5. mM MgCl2, 1 mM EGTA, 10% glycerol). The telomeric repeat amplification protocol (TRAP) assay was performed as described by Kim et al. [10,11]. Briefly, PCR was performed using primers listed in S1 Table as follows: first incubation at 30°C for 30 min, second incubation at 95°C for 3 min, followed by a 30 cycle amplification (95°C for 30 s, 59°C for 30 s, and 72°C for 1 min). The products were run on a 15% polyacrylamide gel (Bio-Rad) in 0.5x TBS and the bands were stained with SYBR Gold Nucleic Acid Gel Stain (Life Technologies). Relative activity of telomerase was calculated by dividing the density of the all ladders to the density of the bands in internal control (The TRAP internal control “TSNT” was synthesized as reported previously (S1 Table) [11]. Densitometric analysis was performed using ImageJ (NIH, Bethesda, MD).
NAD assay
NAD assay was performed using EnzyChrom® NAD+/NADH+ Assay kit (BioAssay Systems, Hayward, CA), according to the manufacturer’s instruction.
siRNA transfection
NAMPT siRNA and Scrambled siRNAs (control siRNAs) were purchased from Santa Cruz Biotechnology. SIRT1 siRNA and oligonucleotides were generated by IDT. The sequence of SIRT1 siRNA was described previously [12]. Each oligonucleotide was dissolved in 100 μM Duplex Buffer (100 mM Potassium Acetate, 30 mM HEPES, pH 7.5) and mixed in equal molar amounts, with a final concentration of 10 μM per oligonucleotide. Oligonucleotides were annealed at 94°C for 2 minutes and then cooled to room temperature for 2 hours. Transfection of siRNA into aHDF cells was carried out at 37°C and with 5% CO2 for 2 days using transfection reagent (Santa Cruz Biotechnology) in DMEM medium without serum and antibiotics. The culture medium was replaced with fibroblast medium (FGM2, Lonza, Walkersville, MD) without or with 1 μM NaHS and cells were cultured for an additional 3 days.
SIRT1 activity assay
SIRT1 activity assay was performed using Universal SIRT Activity Assay Kit (Abcam, Cambridge, MA) according to the manufacturer’s instruction. Briefly, 5 x 105 of aHDF cells were transfected with NAMPT siRNA or Scrambled-siRNA. After 2 days, fresh medium was added without or with 1 μM NaHS and cells were cultured for an additional 3 days. Sample cells were collected and nuclear fractions were prepared, as described previously [13]. SIRT1 activity was normalized to total protein of each sample.
Statistics
All assays were done in 3–6 replicate cultures, in at least three independent experiments. Data are shown as means ± SEM. p values were determined by comparing the data from treated cells against control cells. Data were subjected to the two tailed t-test for determination of means and p values. p values less than 0.05 were considered significant. p values are shown as <0.05 (*), <0.005 (**) or <0.0005 (***).
Results
Replicative senescence leads to reduced production of hydrogen sulfide
We assessed the production of H2S in young (population doubling; PD: 5.9) and replicative senescent (PD: 18.8) aHDF cells. Consistent with previous reports, senescent cells show accumulation of senescence-associated β-galactosidase (SA–β-Gal) (68%, Fig 1A), increase in expression of p16, p21, RRM2B and decreased expression of RRM2 [14] (S1 Fig). The expression hTERT was also diminished by about 16 fold in senescent aHDF cells (Fig 1B). As compared to young cells, the NAD/NADH ratio which is a measure of metabolic activity was also decreased in senescent cells (Fig 1C).
Fig 1. Production of H2S is downregulated in replicatively senescent cells.
(A) Representative images of SA-β-Gal staining in young (PD: 5.9) and senescent (PD: 18.8) aHDF cells. Scale bars, 100 μm. (B) Real-time PCR analysis of expression of hTERT in young (PD: 5.9) and senescent (PD: 18.8) aHDF cells. The expression of hTERT was normalized to the expression level of β-ACTIN. (C) NAD/NADH ratio in young (PD: 5.9) and senescent (PD: 18.8) aHDF cells. Real-time PCR analysis of expression of CBS (D), MST (E), and CSE (F) in young (PD: 5.9) and senescent (PD: 18.8) aHDF cells. The expression of CBS, MST, and CSE was normalized to the expression level of β-ACTIN. (G) 1 x 106 cells of young (PD: 5.9) and senescent (PD: 18.8) aHDF cells were incubated in PBS at 37°C for 1 hour and then H2S was measured in culture supernatants. Mean values are shown along with error bars. *; p<0.05, **; p<0.005, ***; p<0.0005, n.s.; not significant.
Then, we assessed whether the expression of three H2S-producing enzymes, CBS, MST and CSE [15–17] is altered by senescence. The expression of CBS was decreased in senescent cells while the expression of MST and CSE remained the same in young and replicatively senescent cells (Fig 1D–1F). The production level of H2S in replicative senescent cells was diminished by 63% in senescent cells (Fig 1G).
Exogenous H2S upregulates the expression of hTERT and increases PD in aHDF cells
We tested whether the lower expression of hTERT in senescent aHDF cells is due to down-regulation of H2S production in senescent cells. Treatment of young aHDF cells with NaHS within the reported physiological level of H2S [18] (0.01 to 100 μM) significantly upregulated the expression of hTERT with 1 μM being the optimal concentration for maximum hTERT expression (Fig 2A). This finding was confirmed by using other cell types (S2A–S2C Fig), and as evidenced by immunoblotting (NaHS enhanced hTERT in treated cells; Fig 2B). However, treatment with NaHS failed to reduce SA-β-Gal or to upregulate the expression of hTERT in senescent aHDF cells (~65% SA-β-Gal+) (S3A and S3B Fig), suggesting that H2S induced hTERT expression is suppressed in senescent cells.
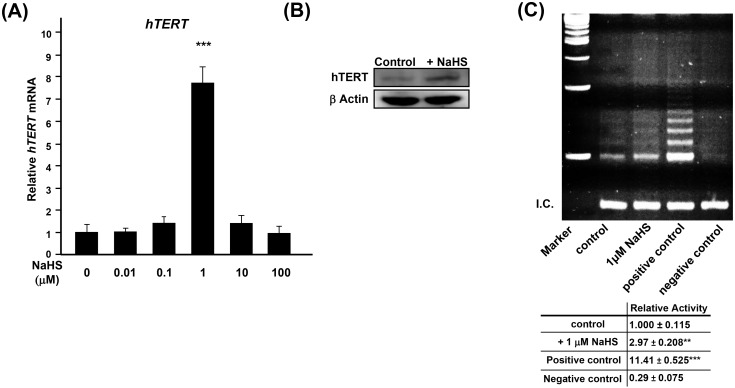
Fig 2. Exogenous H2S increases the expression of hTERT as well as the activity of telomerase.
(A) Real-time PCR analysis of the expression of hTERT in young (PD: 5.9) aHDF cells, treated with NaHS for 3 days. The expression of hTERT was normalized to the level of expression of β-ACTIN. Expression of untreated control was regarded as 1.0. (B) Immunoblotting of hTERT in aHDF cells without or with 1 μM NaHS for 7 days. 100 μg of the indicated nuclear extracts were subjected for immunoblotting. β-Actin was used as a loading control. (C) Telomerase activity in young (PD: 3.2) aHDF cells without or with treated with 1 μM NaHS for 7 days. Positive control was MDA-MB-231 cell lysate, and negative control was buffer alone. Bottom panel shows quantified means ± error bars from three independent assays. Relative activity of telomerase was calculated by dividing the density of all ladders to the density of the bands in internal control, indicated as internal control (I.C.).
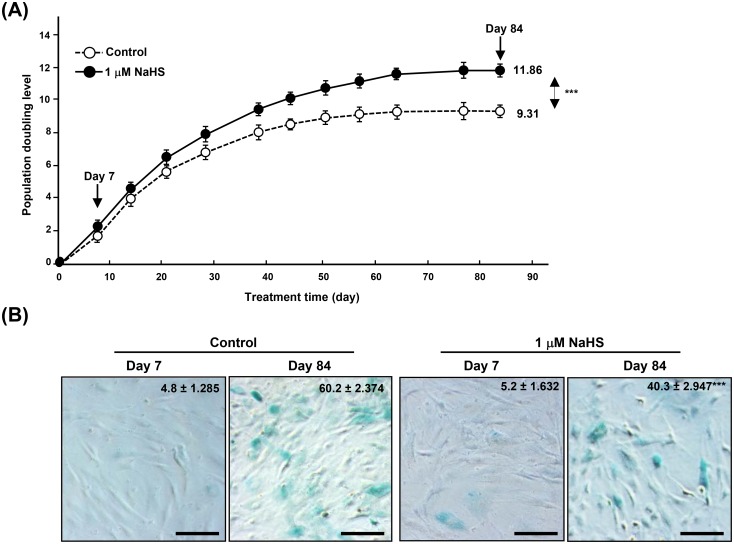
Up-regulation of expression of hTERT in young aHDF cells treated with NaHS was associated with an increase in the activity of telomerase (Fig 2C). We, then, investigated whether the increased expression of hTERT and telomerase activity increases PD, young aHDF cells were treated weekly with 0, 1, and 100 μM of NaHS for 84 days, and PD was calculated. As shown in Fig 3A, as compared to PD of the untreated control groups, treatment of aHDF cells with 1 μM NaHS caused a significant increase in PD. This increase was lost in cells that were treated with greater (100 μM) concentration of NaHS (S4 Fig). Consistent with these data, the percentage of SA-β-Gal positive cells was reduced in aHDF cells that were treated with 1 μM of NaHS (Fig 3B).
Fig 3. Exogenous H2S increases PD and suppresses SA-β-Gal expression.
(A) PD of cells treated without or with 1 μM NaHS. The population doubling of the first confluent cultures was designated as 0. (B) Representative images of SA–β-Gal staining in cells shown in Fig 3A. Mean values ± error bars of number of SA-β-Gal positive cells are shown on the right-upper corner of each image. *; p<0.05, ***; p<0.0005, n.s.; not significant. Scale bars, 100 μm.
H2S mediated hTERT expression is NAMPT and SIRT1 dependent
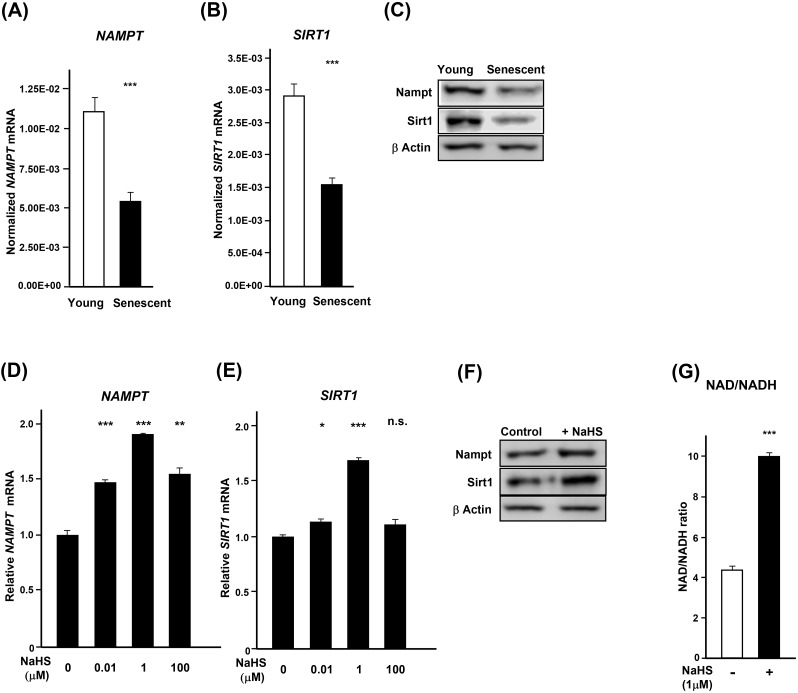
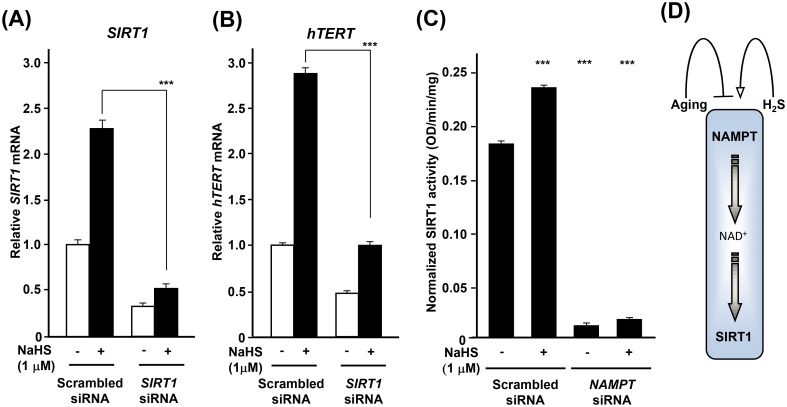
The life extension afforded by hydrogen sulfide [2,19,20] might be mediated, at least in part, by increasing the expression of NAMPT (nicotinamide phosphoribosyl transferase) that regulates metabolism together with SIRT1, a factor involved in the maintenance of integrity of telomeres [21]. Among the seven Sir2 homologues in mammalian cells (SIRT1 to -7), SIRT1 is most closely related to Sir2 which is known to be a major life-span regulator in C. elegans [2]. Nicotinamide adenine dinucleotide (NAD+) is a coenzyme that mediates many redox reactions and regulates NAD+-consuming enzymes such as Sirtuin family of NAD—dependent protein deacetylases. The biosynthesis of NAD+ is mediated by NAMPT. For these reasons, we examined whether the effect of H2S on hTERT is NAMPT and SIRT1 dependent. In senescent aHDF cells, the expression of NAMPT and SIRT1 diminished with age (Fig 4A–4C). Treatment of young aHDF cells (PD: 5.9) with NaHS increased the expression of NAMPT and SIRT1, in a dose dependent manner with 1 μM inducing maximum expression (Fig 4D and 4E). These data were further verified by immunoblotting (Fig 4F). The treatment also caused a coordinate increase in the ratio of NAD to NADH (Fig 4G). Treatment of aHDF cells with SIRT1 siRNA suppressed the NaHS induced expression of SIRT1 (Fig 5A and S5 Fig) and concomitantly prevented the expression of hTERT (Fig 5B). Whereas the siRNA to NAMPT reduced expression of NAMPT, it did not reduce the expression of SIRT1 (S5 Fig). However, suppression of NAMPT decreased the activity of SIRT1 (Fig 5C) and led to a decrease in the expression of hTERT (S5 Fig).
Fig 4. NaHS-treatment increases expression of NAMPT and SIRT1.
(A and B) The expression of NAMPT and SIRT1 in young (PD: 5.9) and senescent (PD: 18.8) was assessed by real-time PCR and normalized to the expression level of β-ACTIN. (C) Immunoblotting of Nampt and Sirt1 in young (PD: 5.9) and senescent (PD: 18.8) aHDF cells. β-Actin was used as a loading control. (D and E) Young (PD: 5.9) aHDF cells were treated without and with NaHS for 3 days, and RNA samples were then subjected to real-time PCR for assessment of NAMPT and SIRT1. The expression levels of NAMPT and SIRT1 were normalized to the levels of expression of β-ACTIN. (F) Immunoblotting of Nampt and Sirt1 in NaHS-treated young (PD: 5.9) aHDF cells. β-Actin was used as a loading control. (G) NAD/NADH ratio in young (PD: 5.9) aHDF cells treated without and with NaHS for 7 days. Data were normalized to the total amount of protein.
Fig 5. H2S induces hTERT expression in a NAMPT/SIRT1-dependent manner.
(A and B) Downregulation of SIRT1 suppresses the expression of hTERT. Young (PD: 5.9) aHDF cells (3 x 105 cells) were transfected with SIRT1 siRNA for 2 days, and these were treated without or with NaHS for 3 days. Total RNAs from these cells were subjected to real-time PCR analysis for SIRT1 (A) and hTERT (B). Data were normalized to the level of expression of β-ACTIN. The expression level of SIRT1 and hTERT in cells treated with Scrambled siRNA without NaHS treatment was regarded as 1.0. (C) Downregulation of NAMPT suppresses the activity of SIRT1. Young (PD: 5.9) aHDF cells (3 x 105 cells) were transfected with NAMPT siRNA for 2 days, and then the cells were treated without or with 1 μM NaHS for 3 days. Nuclear proteins were extracted and used for measurement of SIRT1 activity. Mean values ± error bars were normalized to the amount of total cell protein. (D) Mode of action of H2S in opposing senescence. *; p<0.05, **; p<0.005, ***; p<0.0005, n.s.; not significant.
Discussion
We demonstrated that the production of H2S as well as the expression of CBS both decrease upon aging. We further show that the downregulation of hTERT, SIRT1, NAMPT, and NAD/NADH ratio can be delayed by H2S and that long-term effect of H2S is to maintain telomerase expression, and to postpone replicative senescence as evidenced by increasing population doublings in aHDF cells treated with exogenous H2S. Thus, H2S maintains a threshold level of telomerase activity which contributes to its life-span extension properties.
H2S plays a bioenergetics role in Krebs cycle in mitochondria [22]. Modis et al showed that low concentrations of H2S elicited an increase of mitochondrial function, including an increase cellular pool of ATP and improved cell viability, whereas higher concentrations of H2S were inhibitory [22,23]. Our data show 1 μM of NaHS is optimal for promoting hTERT expression (Fig 2A) and in increasing PD (Fig 3A and S4 Fig). Regardless of site of action, H2S leads to an increase in cellular pool of ATP energy yield which results in suppressing cellular senescence in aHDF cells.
Previously, we have shown that H2S upregulates NAMPT and increases mitochondrial bioenergetics [7,8]. Although it is still not clear how H2S controls NAMPT, Huang et al reported that H2S suppresses the expression of microRNA34a by activating Nrf2 after hepatic ischemia/reperfusion injury [24]. Choi et al demonstrated that microRNA34a reduced NAMPT/NAD+ level [25]. Based on such finding H2S might negatively regulate NAMPT by suppression of microRNA34a. However, further studies are required to address the molecular mechanisms of NAMPT by H2S.
Others have shown the relation of NAMPT, NAD and SIRT1 [26,27]. It has been shown that SIRT1 by deacetylation of c-MYC [28] transcriptionally increases the activation of c-MYC and correspondingly increases the amount of acetylated H4 histone at the hTERT promoter [21]. In addition, FOXO3a, a downstream target of SIRT1, potentiated hTERT gene transcription by binding to c-MYC promoter. This upregulated c-MYC which was recruited to the hTERT promoter, leads to the of hTERT gene activation [29]. Intriguingly, NaHS-treatment increases the expression of FOXO3a in aHDF cells (data not shown); thus, it seems that SIRT1 upregulates hTERT through FOXO3a/c-MYC and increases the lifespan of human fibroblasts.
Mammalian senescence is dependent on the mammalian NAD-dependent deacetylase, Sirt1, and Nampt-mediated systemic NAD biosynthesis [30]. Based on our findings, H2S regulates the expression of NAMPT and SIRT1 in a dose dependent manner and coordinately sets the NAD/NADH ratio. H2S also regulates hTERT expression, and this function is dependent on both NAMPT mRNA expression and SIRT1 activity. Sir2 and its orthologues play an important role in controlling longevity in model organisms as diverse as yeast to worms and flies [31]. Among sirtuins, it has been shown that SIRT1 delays senescence and extends life-span in both male and female mice [32]. In light of such findings, the life extension afforded by H2S might be mediated, at least in part, through activation of SIRT1 (Fig 5D). Consistent with these results, the treatment of human umbilical vascular endothelial cells with H2S, delayed the H2O2 and nicotinamide induced pre-mature senescence by SIRT1 activation [33,34]. The impact of H2S on NAMPT/SIRT1, likely, has global effects since it has been shown that RNA-mediated knockdown of NAMPT or NMNAT-1 in MCF-7 breast cancer cells reduced total cellular NAD+ levels and globally altered pattern of gene expression [35]. Together, the postponement of loss of cell proliferative capacity by H2S without the risk of mutagenesis suggests that H2S can be used in delaying the adverse effects of senescence in organisms.
Supporting Information
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
Acknowledgments
Authors thank Drs Junji Magae and Elena A. Ostrakhovitch for critical reading of the manuscript.
Abbreviations
- H2S
hydrogen sulfide
- aHDF
adult human dermal fibroblasts
- SA-β-Gal
Senescence-Associated β-Galactosidase
- RRM2
ribonucleotide reductase M2
- RRM2b
ribonucleotide reductase M2B
- CSE
cystathionine-β-lyase
- CBS
cystathionine-β-synthase
- MST
3-mercaptopyruvate sulftransferase
- hTERT
catalytic subunit of human telomerase
- NaHS
sodium hydrosulfide
- HRP
horseradish peroxidase
- Ct
cycle threshold
- NAMPT
Nicotinamide phosphoribosyltransferase
- s
second
- h
hour
- PD
population doubling
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1.Qabazard B, Li L, Gruber J, Peh MT, Ng LF, Kumar SD, et al. (2014) Hydrogen sulfide is an endogenous regulator of aging in Caenorhabditis elegans. Antioxid Redox Signal 20: 2621–2630. 10.1089/ars.2013.5448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller DL, Roth MB (2007) Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. Proc Natl Acad Sci U S A 104: 20618–20622. 10.1073/pnas.0710191104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, et al. (2004) Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305: 390–392. 10.1126/science.1099196 [DOI] [PubMed] [Google Scholar]
- 4.Hine C, Harputlugil E, Zhang Y, Ruckenstuhl C, Lee BC, Brace L, et al. (2015) Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 160: 132–144. 10.1016/j.cell.2014.11.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenwood SK, Hill RB, Sun JT, Armstrong MJ, Johnson TE, Gara JP, et al. (2004) Population doubling: a simple and more accurate estimation of cell growth suppression in the in vitro assay for chromosomal aberrations that reduces irrelevant positive results. Environ Mol Mutagen 43: 36–44. 10.1002/em.10207 [DOI] [PubMed] [Google Scholar]
- 6.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 92: 9363–9367 10.1073/pnas.92.20.9363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostrakhovitch EA, Akakura S, Sanokawa-Akakura R, Goodwin S, Tabibzadeh S (2015) Dedifferentiation of cancer cells following recovery from a potentially lethal damage is mediated by H2S-Nampt. Exp Cell Res 330: 135–150. 10.1016/j.yexcr.2014.09.027 [DOI] [PubMed] [Google Scholar]
- 8.Sanokawa-Akakura R, Ostrakhovitch EA, Akakura S, Goodwin S, Tabibzadeh S (2014) A H2S-Nampt dependent energetic circuit is critical to survival and cytoprotection from damage in cancer cells. PLoS One 9: e108537 10.1371/journal.pone.0108537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schreiber E, Harshman K, Kemler I, Malipiero U, Schaffner W, Fontana A (1990) Astrocytes and glioblastoma cells express novel octamer-DNA binding proteins distinct from the ubiquitous Oct-1 and B cell type Oct-2 proteins. Nucleic Acids Res 18: 5495–5503 10.1093/nar/18.18.5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, et al. (1994) Specific association of human telomerase activity with immortal cells and cancer. Science 266: 2011–2015 10.1126/science.7605428 [DOI] [PubMed] [Google Scholar]
- 11.Kim NW, Wu F (1997) Advances in quantification and characterization of telomerase activity by the telomeric repeat amplification protocol (TRAP). Nucleic Acids Res 25: 2595–2597 10.1093/nar/25.13.2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takayama K, Ishida K, Matsushita T, Fujita N, Hayashi S, Sasaki K, et al. (2009) SIRT1 regulation of apoptosis of human chondrocytes. Arthritis Rheum 60: 2731–2740. 10.1002/art.24864 [DOI] [PubMed] [Google Scholar]
- 13.Abmayr SM, Yao T, Parmely T, Workman JL (2006) Preparation of nuclear and cytoplasmic extracts from mammalian cells. Curr Protoc Mol Biol Chapter 12: Unit 12 11 10.1002/0471142727.mb1201s75 [DOI] [PubMed] [Google Scholar]
- 14.Kuo ML, Sy AJ, Xue L, Chi M, Lee MT, Yen T, et al. (2012) RRM2B suppresses activation of the oxidative stress pathway and is up-regulated by p53 during senescence. Sci Rep 2: 822 10.1038/srep00822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kery V, Bukovska G, Kraus JP (1994) Transsulfuration depends on heme in addition to pyridoxal 5'-phosphate. Cystathionine beta-synthase is a heme protein. J Biol Chem 269: 25283–25288 [PubMed] [Google Scholar]
- 16.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, et al. (2008) H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 322: 587–590. 10.1126/science.1162667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, et al. (2009) 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal 11: 703–714. 10.1089/ARS.2008.2253 [DOI] [PubMed] [Google Scholar]
- 18.Calvert JW, Coetzee WA, Lefer DJ (2010) Novel insights into hydrogen sulfide—mediated cytoprotection. Antioxid Redox Signal 12: 1203–1217. 10.1089/ars.2009.2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei Y, Kenyon C (2016) Roles for ROS and hydrogen sulfide in the longevity response to germline loss in Caenorhabditis elegans. Proc Natl Acad Sci U S A 113: E2832–2841. 10.1073/pnas.1524727113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qabazard B, Ahmed S, Li L, Arlt VM, Moore PK, Sturzenbaum SR (2013) C. elegans aging is modulated by hydrogen sulfide and the sulfhydrylase/cysteine synthase cysl-2. PLoS One 8: e80135 10.1371/journal.pone.0080135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamashita S, Ogawa K, Ikei T, Udono M, Fujiki T, Katakura Y (2012) SIRT1 prevents replicative senescence of normal human umbilical cord fibroblast through potentiating the transcription of human telomerase reverse transcriptase gene. Biochem Biophys Res Commun 417: 630–634. 10.1016/j.bbrc.2011.12.021 [DOI] [PubMed] [Google Scholar]
- 22.Szabo C, Ransy C, Modis K, Andriamihaja M, Murghes B, Coletta C, et al. (2014) Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br J Pharmacol 171: 2099–2122. 10.1111/bph.12369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Modis K, Coletta C, Erdelyi K, Papapetropoulos A, Szabo C (2013) Intramitochondrial hydrogen sulfide production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J 27: 601–611. 10.1096/fj.12-216507 [DOI] [PubMed] [Google Scholar]
- 24.Huang X, Gao Y, Qin J, Lu S (2014) The role of miR-34a in the hepatoprotective effect of hydrogen sulfide on ischemia/reperfusion injury in young and old rats. PLoS One 9: e113305 10.1371/journal.pone.0113305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi SE, Fu T, Seok S, Kim DH, Yu E, Lee KW, et al. (2013) Elevated microRNA-34a in obesity reduces NAD+ levels and SIRT1 activity by directly targeting NAMPT. Aging Cell 12: 1062–1072. 10.1111/acel.12135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imai S, Kiess W (2009) Therapeutic potential of SIRT1 and NAMPT-mediated NAD biosynthesis in type 2 diabetes. Front Biosci (Landmark Ed) 14: 2983–2995 10.2741/3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rehan L, Laszki-Szczachor K, Sobieszczanska M, Polak-Jonkisz D (2014) SIRT1 and NAD as regulators of ageing. Life Sci 105: 1–6. 10.1016/j.lfs.2014.03.015 [DOI] [PubMed] [Google Scholar]
- 28.Mao B, Zhao G, Lv X, Chen HZ, Xue Z, Yang B, et al. (2011) Sirt1 deacetylates c-Myc and promotes c-Myc/Max association. Int J Biochem Cell Biol 43: 1573–1581. 10.1016/j.biocel.2011.07.006 [DOI] [PubMed] [Google Scholar]
- 29.Yamashita S, Ogawa K, Ikei T, Fujiki T, Katakura Y (2014) FOXO3a potentiates hTERT gene expression by activating c-MYC and extends the replicative life-span of human fibroblast. PloS One 9: e101864 10.1371/journal.pone.0101864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imai S (2009) From heterochromatin islands to the NAD World: a hierarchical view of aging through the functions of mammalian Sirt1 and systemic NAD biosynthesis. Biochim Biophys Acta 1790: 997–1004. 10.1016/j.bbagen.2009.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imai S, Guarente L (2014) NAD+ and sirtuins in aging and disease. Trends Cell Biol 24: 464–471. 10.1016/j.tcb.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, et al. (2013) Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab 18: 416–430. 10.1016/j.cmet.2013.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng M, Qiao W, Cui J, Liu L, Liu H, Wang Z, et al. (2014) Hydrogen sulfide delays nicotinamide-induced premature senescence via upregulation of SIRT1 in human umbilical vein endothelial cells. Mol Cell Biochem 393: 59–67. 10.1007/s11010-014-2046-y [DOI] [PubMed] [Google Scholar]
- 34.Suo R, Zhao ZZ, Tang ZH, Ren Z, Liu X, Liu LS, et al. (2013) Hydrogen sulfide prevents H(2)O(2)-induced senescence in human umbilical vein endothelial cells through SIRT1 activation. Mol Med Rep 7: 1865–1870. 10.3892/mmr.2013.1417 [DOI] [PubMed] [Google Scholar]
- 35.Zhang T, Berrocal JG, Frizzell KM, Gamble MJ, DuMond ME, Krishnakumar R, et al. (2009) Enzymes in the NAD+ salvage pathway regulate SIRT1 activity at target gene promoters. J Biol Chem 284: 20408–20417. 10.1074/jbc.M109.016469 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.