Abstract
Introduction
Currently, there is no consensus on dementia diagnostics in adults with intellectual disabilities (ID). There are three types of assessments available: direct cognitive tests, test batteries, and informant reports.
Methods
A systematic literature search was conducted in four databases yielding 9840 records. Relevant studies were identified and selected using predefined inclusion and exclusion criteria and then coded and classified according to assessment type. This was completed by two independent researchers, with a third consulted when discrepancies arose. The review collates diagnostic instruments and presents strengths and weaknesses.
Results
Overall 47 studies met the search criteria, and 43 instruments were extracted from the selected studies. Of which, 10 instruments were classified as test batteries, 23 were classified as direct cognitive tests, and the remaining 10 were informant reports.
Discussion
This review can recommend that cognitive test batteries can offer the most practical and efficient method for dementia diagnosis in individuals with ID.
Keywords: Dementia, Diagnosis, Intellectual disability, Cognitive testing, Test battery, Informant reports, Assessment tools
1. Introduction
An intellectual disability (ID), similar to the UK specific term learning disability, onsets during the developmental period and is characterized by impairments of general mental abilities that impact adaptive functioning in three main domains: conceptual, social, and practical (American Psychological Association, 2013). [1] Various studies discussed throughout this review refer specifically to Down syndrome (DS). This is the most common genetic ID disorder seen in clinical practice. DS is caused 94% of the time by nondisjunction of chromosome 21 and 3%–5% of the time by translocation. The IQ of people with DS falls within the mild to moderately severe ID spectrum [2].
The life expectancy of individuals with ID is increasing due to improvements in medicine and living circumstances [3], [4]. Individuals with mild ID are even experiencing life spans equal to those of the general population [5]. Adults with ID are subsequently in a position where age-related illnesses are becoming a greater concern. The most notable of these illnesses is dementia, for which an individual's age is the strongest risk factor (e.g., Daviglus et al [6]). Dementia of the Alzheimer's type is a cognitive impairment that gradually onsets, is progressive, and leads to interference with social and occupational functioning [7]. Dementia can be caused by a variety of underlying pathology. For example, Alzheimer's disease (AD) [8] consists of amyloid plaques and neurofibrillary tangles in the cerebral cortex, temporal lobe cortex, and hippocampus, among other brain areas [9].
Furthermore, individuals with ID often experience onset of aging characteristics earlier than in the general population [10], and this is reflected in age of dementia diagnosis. Onset of dementia usually occurs among older adults over the age of 65 years; however, in individuals with DS, onset is usually around the early 50s [11].
The literature has shown substantial conflict in prevalence estimates of dementia in ID populations with and without DS when compared to the general population. Dementia has been shown to be common in older adults with ID but prevalence rates reported differ according to the diagnostic criteria applied [12]. It was found that diagnoses of dementia are substantially higher than in the general population, for people who have ID but do not have DS [13]. 21.6% of participants were diagnosed with dementia, compared to 5.7% that was expected in a non-ID group with this age structure. This was further supported by Strydom et al. [14] who highlighted the incidence rate of dementia in ID to be five times higher than that of older adults in the general population. Other studies have shown prevalence rates to only be comparable or higher than in the general population (e.g., Strydom et al. [15]). Opposing studies have shown risk of dementia to be equivalent to or lower than in the general population (e.g., Zigman et al. [16]). The variety in prevalence estimates further highlights the divergence in the understanding and application of dementia diagnostics for individuals with ID.
Stronger evidence has been established regarding dementia rates in individuals with ID and DS. Incidence of early-onset dementia of the Alzheimer's type has been shown to be higher than in the general population (e.g., Bush et al. [17]). Genetic findings have suggested that owing to the complex etiology of DS and the triplication of the amyloid precursor protein gene on chromosome 21, DS could be considered a model of early-onset dementia [18]. Almost all adults with DS over the age of 35–40 years show neuropathologic changes characteristic of AD [19], including senile plaques and neurofibrillary tangles. Although this does not necessarily reflect a clinical diagnosis, genetic evidence has merely begun to highlight similarities between the neuropathology of the two conditions. Unsurprisingly however, individuals with DS in many cases have been shown to be at higher risk of developing Alzheimer's disease than the general population (e.g., Nieuwenhuis-Mark [20]).
There is a need for further clarification of the difference in prevalence rates between the three populations, individuals from the general population with no pre-existing impairment, individuals with ID but without DS and individuals with ID and DS. Regardless of comparisons to the general population, evidence does show that the prevalence rates of dementia in ID increase dramatically between the ages of 40 and 60 years [21]. Therefore, dementia diagnostic assessments should be targeted at this age group or before.
Diagnosing dementia can be an incredibly difficult and complicated process. This is remarkably more complex in individuals with ID, as dementia and related pathology is manifested in areas of functioning that are, more than likely, already impaired by the intellectual disability [22]; thus leading to inherent difficulties in assessing cognitive functioning to aid with dementia diagnostics in people with ID [21]. There is no agreement in the literature or in practice on how dementia diagnosis should be informed in ID populations (e.g., Moran et al. [23]).
Assessments within the general population that build up a picture to aid the clinician when diagnosing dementia involves direct cognitive tests that indicate progressive cognitive decline in areas such as short-term and long-term memory, orientation, communication and mood, among others. But these tests are frequently not appropriate for individuals with ID as they often require abilities that individuals with ID may find more difficult due to their pre-existing impairment. The tests are not often developed for use in ID populations, and therefore, they do not reliably screen for dementia in this group [24]. Moreover, there are no normed data for this population, and thus, results cannot be interpreted meaningfully [23]. Consequently, floor effects are often observed on the chosen test and problems of accuracy in diagnosis ensue. There are three potential assessment methods that practitioners can apply to help inform diagnosis. These include a single test that directly assesses the individual's cognitive functioning, a test battery which comprises of multiple tests that assess a range of cognitive functions and lastly, informant reports which are completed by a carer or close relative who can report on the individual's functioning.
This review aims to collate existing instruments used in the diagnosis of dementia in individuals with ID. The instruments will then be coded according to whether they are (1) a direct cognitive test, (2) informant report, or (3) a test battery. The review will then discuss the benefits of each type of test. This review shall build on previous reviews by presenting an up to date overview of the instruments available, as well as discussing instruments that have been proposed for diagnostics in adults with ID but have yet to be established as such. This could include instruments that are designed for use in the general population, in the intellectually disabled populations or in people who have already been diagnosed with dementia. This review will therefore help clinicians to extend their knowledge of the potential cognitive assessments available as well as discuss non-cognitive assessments being used and give recommendations based on previous literature.
2. Methods
2.1. Literature search
A systematic literature search was conducted in four databases: PubMed, Science Direct, Google Scholar and PsycInfo. These databases were selected due to the depth and breadth that they offer in literature searching and their relevance to the reviewed topic. The search string included various terms for (1) the measure of interest (e.g., Alzheimer's disease, Dementia, Dementia of Alzheimer's type) and (2) the output of interest (e.g., diagnosis, assessment, instrument, screening tool). The search was performed once for the (3) specified population (e.g., intellectual disability, learning disability, mental retardation) and again for (4) Down syndrome, due to the well documented increased risk of dementia of Alzheimer's type in this subgroup of individuals with ID. Table 1 shows the logic of the search strategy. References of included studies were also hand-searched, to include further relevant studies. Both English and non-English publications were sought after. However due to searches being conducted in English only, publications that had been originally written in English or translated into English were able to be included.
Table 1.
Search string logic
| Search Terms | Output | Measure | Population |
|---|---|---|---|
| Synonyms | Informant report, direct test, test battery, diagnosis, diagnostic, screening, assessment, tool, questionnaire, Scale | Dementia, Alzheimer's disease, Dementia of Alzheimer's type | Intellectual Disability, Learning Disability, Mental Retardation, Developmental Disability, Down Syndrome, Downs Syndrome. |
| Combined and Truncated | Inform* OR Informant Report* OR diagnos* OR screen OR screening* OR instrument* OR tool* OR Assess* OR questionnaire OR Scale* | Dement* OR Alzheimer* | ((Intellectual* OR mental* OR learning OR developmental*) AND (disab* OR retard*)) OR (Down* AND syndrom*) |
Relevant studies were identified and selected using the following inclusion criteria. Identified studies should be suitable dementia assessments for individuals with ID; this included informant reports, independent direct cognitive tests, or test batteries. Test batteries were included with both cognitive assessment and noncognitive assessment reported by an informant. Direct cognitive tests that are not yet used for dementia assessment but test a specific aspect of cognitive functioning like memory, intelligence, or orientation in an intellectually disabled population were included. Participants in selected studies included participants with ID that were classified as mild, moderate, severe, with or without the presence of DS. Included studies compared individuals with ID to individuals with ID who had already been diagnosed with dementia. Reviews, guidance documents, and dissertation projects were included when they pertained to the topic to consider and build on previous findings. These publications included discussion of dementia diagnostics in ID, instruments used for cognitive assessment, informant reporting for the purpose of dementia assessment, and guidance documents regarding dementia diagnostics or assessment in individuals with ID.
Studies were excluded if the instruments presented were not suitable for use in ID or DS populations. The instrument did not need to have been used for the purpose of diagnosis as of yet, but if it had been shown to be tolerated well by participants with ID and had been suggested for use in dementia assessment, then it was considered in this review. Diagnostic checklists and criteria were excluded as this review aimed to assess instruments that assess an individual with ID's functioning, either via an informant or directly, to aid the practitioner to complete checklists and criteria for dementia diagnosis. Checklists, although helpful when making the final decision regarding diagnosis, require heavy input from trained clinicians. This review sought to identify assessment methods that can be completed before input from the clinician as this will give the opportunity for diagnosis to be made more efficiently. Likewise, medical tests or studies focusing on biological or genetic markers were also excluded, due to their differential emphasis in the diagnostic process. Studies looking at interventions and treatments were also excluded due to lack of relevance to the diagnostic process.
2.2. Extraction of information and coding of instruments
Instruments were extracted from included studies and coded according to whether they were (1) an independent direct cognitive test completed by the individual, (2) an informant report completed by a carer or consultee on behalf of the individual or (3) a test battery consisting of multiple tests. Instruments were then put into the table that corresponds with the given code. Therefore if an instrument was coded as a direct cognitive test then it was placed into Table 2. Informant reports were added to Table 3, and finally Test batteries were placed into Table 4. Test batteries contain many different independent direct cognitive tests and informant reports, if the instrument was included in a battery it was described in Table 4, although it may be applicable to Table 2 or 3, this is to avoid repetition, so when considering which instrument to use do bear in mind that individual tests contained within test batteries are also available (see Table 4 for references). Instruments were further coded to highlight the level of ID and whether DS was present during the specified study. Tables therefore are displayed with non-DS participants denoted first, starting with mild ID, then moderate, and finally severe. After this, studies that compared ID participants without DS to participants with equivalent level of ID and DS as well. Finally, the tables displayed studies conducted with participants who have ID and DS. Selection and coding of studies were completed by two independent researchers (JEK, SS), with a third independent researcher consulted when discrepancies arose (RD).
Table 2.
Direct cognitive tests
| Author (Year) | Country and setting (clinical or applied) | Test name | Ability tested | Ppts | Type of ID | Groups | Outcome (what was sig?) >< | Comments |
|---|---|---|---|---|---|---|---|---|
| McDaniel (2000) [25] | United States—Applied setting (quiet room in their unit) | Dementia Rating Scale (DRS)[26] | General cognitive ability | 84 ppts Aged: 14–60 years |
Mild ID (n = 32) Moderate ID (n = 42) Severe ID (n = 10) |
1 = Mild 2 = Moderate 3 = Severe |
1 > 2 (sig) on Total Score and all subtests except Construction 2 > 3 (sig) on all measures. |
DRS can provide info about the cognitive strengths and weaknesses of individuals with ID. DRS can be administered to a wide range of individuals with ID. |
| Pyo et al. (2010) [27] | United States—Applied Setting (separate room with a family or staff member present to make ppts feel more comfortable) | The revised Picture Recognition Memory Test (r-PRMT) [28] | Visual recognition memory | 59 ppts (26 cases, 33 controls) Age: 40+ |
Moderate to severe | 1 = DAT cases with DS (n = 15) 2 = DAT cases without DS (n = 11) 3 = Controls with DS (n = 9) 4 = Controls without DS (n = 24) |
Controls > Cases on r-PRMT Controls with non-DS etiologies scored much lower with a wider score spread, resulting in significant overlap with the score distribution of DAT cases. Effect sizes indicated that ppts with DS were 5.35 for r-PRMT immediate and 4.44 for r-PRMT delayed which were significantly larger compared to non-DS ppts who showed effect sizes of 0.73 and 1.02, respectively. |
r-PRMT may be effective at identifying DAT among moderate to severe from DS, however high false positive rate. |
| The Modified Objective Memory Test (OMT) | Recall memory | Cases = controls on OMT (no sig difference) | ||||||
| Test for severe impairment (TSI) [29] | Mental status as a whole, including immediate memory recall and delayed recall. | Cases = controls on TSI (no sig difference) | ||||||
| The Neuropsychology (NEPSY) Comprehension of Instructions [31] | Language comprehension | Cases = controls on The NEPSY (no sig difference) | ||||||
| Shultz et al. (2004) [30] | United States—Applied setting (designated rooms at ppts' group homes or workshops) | The Shultz Mental Status Exam | Overall mental Status | 38 ppts aged: 45–74 years |
ID without DS (32%) and ID with DS (68%) | Cases = Dementia Controls = Non dementia |
Both performance tasks discriminated between groups. The performance tasks were related to dementia and IQ, but not age or sex. | Both the Shultz Mental Status Exam and the paired associate learning task were able to detect cases versus controls and therefore could be informative when diagnosing dementia in ID. |
| Paired Associate Learning Task (modified from [32]) | Visual Spatial Explicit Memory | |||||||
| Krinsky-McHale et al. (2002) [33] | United States—Potentially a clinical setting but this is not specified. | Selective Reminding Test (SRT) [34] Modified for use in this population [35] |
Explicit Memory | 155 ppts | Down Syndrome vs individuals with ID but no DS. Equivalent level of ID between groups. | Cases 1 = DS with DAT Controls 1 = S without DAT Cases 2 = ID without DS with DAT Controls 2 = ID without DS without DAT |
Cases 1 < controls 1 and cases 2 < controls 2 on long-term storage and retrieval processing abilities | These declines preceded other DAT symptoms, in most cases by more than 1 year & sometimes up to 3 years. Results confirm SRT can detect affected memory processes during early dementia in adults with DS. |
| Das et al. (1995) [36] | Canada and United States—Applied setting (quiet rooms located in a workshop, group or independent living setting) | Dementia Rating Scale (DRS)[26] | General cognitive ability | 63 ppts Age: 40–49 years or 50–62 years |
Down syndrome vs individuals with ID but no DS. Equivalent level of ID between groups. | Younger cases = DS aged 40–49 years Younger controls = non-DS aged 40–49 years Older cases = DS aged 50–62 years Older controls = non-DS aged 50–62 years |
Older cases < younger cases, younger controls, older controls | Older DS individuals performed most poorly on the tasks involving planning and attention. DRS indicates good clinical utility. PPVT-r also discriminated effectively. Matrix was found to be too difficult for individuals with moderate to severe ID to complete. |
| Peabody Picture Vocab Test—revised [37] | Receptive vocabulary | |||||||
| Matrix—Analysis Test—expanded form [38] | Nonverbal measure of intelligence | |||||||
| Nelson et al. (2007) [39] | United States—Clinical Setting | Simple visual discrimination | Visual discrimination learning | 19 ppts Age: 24–55 years Mean = 40 |
Down syndrome | Results demonstrated good reliability and validity of select tests. | ||
| Reversal learning | Executive function | Reversal and landmark 0: Sensitivity 71.43 and specificity 72.73 |
Sensitivity and specificity not given for tests individually. | |||||
| Delayed non-match to sample | Object recognition | Delayed non-match to sample and landmark 4: sensitivity, 72.73; specificity 27.27 |
||||||
| Landmark stimulus—response task | Spatial learning and memory | Landmark 4: sensitivity 75; specificity 60 | ||||||
| McCarron et al (2014) [40] | Ireland & United States—Clinical Setting (Memory clinic) | Downs Syndrome Mental Status Exam (DMSE) [41] | Overall mental status | 77 ppts Aged: 35+ years |
Down syndrome | Cases = dementia Controls = nondementia |
Average age of diagnosis = 55.41 (SD = 7.14) Median survival = 7 years after diagnosis Cases sig older than controls |
DMSE was effective at picking up changes in functioning 1 year before diagnosis. |
| Kay et al. (2003) [42] | UK—Clinical Setting | Prudhoe cognitive functioning test (PCFT) | Overall mental status, including: orientation, recall, language, praxis, and calculation. | 87 ppts Aged: 20+ years |
Down syndrome | No dementia cases participated, the sample was made up of individuals with DS only. | PCRT sig. correlated with Adaptive Behaviour Scale (ABS—[43]) given to carers. PCRT sig. correlated with degree of ID More subjects with high levels (i.e., profound to untestable) of ID obtained very low or zero scores on PCFT. |
PCFT = reliable quantitative measure of cognitive function in DS. Floor effects suggest that PCRT is limited to detecting cognitive decline to those who are less disabled. |
| Devenny et al. (2002) [44] | United States—Applied Setting (Quiet rooms in ppts' day program or at their residence) | Cued Recall Test (CRT) [45], [46] | Cued memory recall | 160 ppts | Down syndrome | Cases = with DS and early stage DAT Controls = DS with no DAT Controls 2 = ID no DS and no DAT |
Cut-off value ≤23 on the TS = sensitivity: 94.7%, specificity: 93.9%, positive predictive value: 81.9% when cases compared to controls 2. | Usefulness of CRT needs to be confirmed with longitudinal data. Memory declines can occur several years before DAT identification. |
| Tyrrell et al. (2001) [47] | Ireland—Potentially a clinical setting but not clearly stated. | Downs Syndrome Mental Status Exam (DMSE)[41] | Overall mental status. | 285 ppts Aged: 35–74 mean age ±SD = 46.5 ± 8.2 years |
Down Syndrome | Cases = DS with dementia Controls = DS without dementia |
Sig different Median scores in Cases vs Controls for DMSE. | |
| Test for Severe Impairment (TSI)[29] | Mental Status as a whole, including immediate memory recall and delayed recall. | Sig different Median scores in Cases vs Controls for TSI. | No floor or ceiling effects in individuals with moderate and severe ID. | |||||
| Deb et al. (1999) [48] | UK—Setting not clearly stated. | The Mini Mental Status Exam (MMSE)[49] | Overall mental status | 62 ppts Aged: 35+ years |
Down syndrome | Cases = DS with Dementia (n = 26) Controls = DS without dementia (n = 36) |
MMSE could only be completed by 34 (55%) ppts with DS. 30 ppts got MMSE score less than 24 (the usual cut-off for the diagnosis of possible dementia), 23 ppts (77%; of the 30) did not have a diagnosis of dementia. |
MMSE not able to be administered to all ppts with DS. And did not accurately identify cases or controls. |
| Hon et al. (1999) [50] | UK—Applied Setting (Ppts' home or day center) | Cambridge Cognitive Examination (CAMCOG) | Overall cognitive functioning | 74 ppts Aged: 30+ years |
Down syndrome | 1 = Younger DS 2 = Older DS |
CAMCOG scores = well distributed. 8 ppts (11%) scored 0. 1 > 2 (sig) on total CAMCOG score 1 > 2 (sig) on 6 out of 7 subscales. |
CAMCOG useful unless ID is severe. May need some modifications to make it more accessible. Better than MMSE as well. |
| Pennington et al. (2003) [51] | United States—Applied Setting | Cambridge Neuropsychological Test Automated Battery, Paired Associates Learning (CANTAB-PAL—Robbins [52]) | Visual-spatial explicit memory | 56 ppts | Down Syndrome | 1 = Children without DS 2 = Children with DS |
Study was not assessing dementia but does show that the test is well tolerated in DS populations. | CANTAB-PAL was designed for use for assessing dementia in general population. But this study indicates that CANTAB-PAL may be able to be used in assessment of dementia in ID. |
| Boanda et al. (2008) [53] | Spain—Clinical Setting | The Mini Mental Status Exam (MMSE) [49] | Overall mental status | 45 ppts Age: 40+ years |
Down syndrome | Cases = Alzheimer's disease (AD) Cases 2 = Potential AD Control = Absence of AD |
MMSE performance sig. correlated with total and cognitive DMR scores as well as SIB scores. | MMSE = useful for assessing cognition. |
Abbreviations: ID, intellectual disabilities; DS, Down syndrome; DAT, Dementia Alzheimer's type; ppts, participants.
NOTE. Tests highlighted in bold indicate repeated use within studies. Age is denoted in years.
Table 3.
Instruments based on informant reports
| Author (Year) | Country and setting (clinical or applied) | Test name | Ability tested | Ppts and age | Type of ID | Groups | Outcome (what was sig?) >< | Comments |
|---|---|---|---|---|---|---|---|---|
| Zeilinger et al. (2015) [58] | United States—applied (in large residential care homes) | The National Task Group—early detection screen for dementia [59] | Dementia Status | 221 carers | ID. All participants are cared for. | Groups | Four feasibility dimensions of use of the NTG-EDSD were reported on by carers. However, data from the NTG-EDSD were not assessed directly. All feasibility dimensions were rated good to very good and 80% of the carers found the NTG-EDSD useful or very useful in the early detection of dementia. |
Reliability and validity of the instrument for clinical use in aiding dementia diagnostic assessment was not assessed. Therefore, further research is needed before use of this instrument. |
| Lin et al. (2014) [55] | Taiwan—Setting is not clearly stated but potentially an applied setting. | Dementia Screening Questionnaire for Individuals with Intellectual Disabilities (DSQIID[60]) | Dementia status | 459 ppts Aged: 45+ years |
ID of varying degree | Cases = Dementia Controls = Nondementia |
Was used to identify cases and controls in this study. 16.3% of ppts in this study were identified as being demented based on the DSQIID. |
Although originally designed for use in DS is an effective tool for diagnosing dementia in ID. |
| Activities of Daily living Questionnaire (ADL [54]) | Daily functioning | Disability level and comorbidity can explain 10% of the ADL score variation. Dementia conditions can only explain 3% of the ADL score variation in the study. |
ADL would not be an effective tool for diagnosing dementia in ID | |||||
| de Vreese et al. (2011) [61] | Italy—Applied setting | Assessment for adults with Developmental Disabilities Scale (AADS-I [61]) | Behavior | 63 ppts | All ID included | Good reliability and validity found. | Useful for detecting dementia if used longitudinally. | |
| Kirk et al. (2006) [62] | UK—Setting is not clearly stated. | Dementia questionnaire for mentally retarded people (DMR)[56] | Dementia status Behavior |
88 ppts Aged: 40+ |
Varying ID (n = 76) And DS (n = 12) |
All ppts completed both tests | DMR significantly related to ABS | Would need to use both to assess an individual for dementia diagnosis as neither covers the full range of factors effected by dementia. |
| The Adaptive Behaviour Scale (ABS-RC2) [43] | ABS significantly related to DMR | 2 questionnaires showed significant relationships. | ||||||
| Shultz et al. (2004) [30] | United States—Applied setting (rooms at group homes or workshops) | The Dementia Scale for Down Syndrome (DSDS) | Dementia status | 38 ppts Aged: 45–74 |
ID without DS (32%) and ID with DS (68%) | Cases = Dementia Controls = Non dementia |
Both dementia scales discriminated between groups. | All informant reports used were able to detect cases vs controls and therefore could be informative to clinicians looking to make a decision regarding dementia diagnostics for people with ID. |
| Dementia questionnaire for mentally retarded people (DMR)[56] | Dementia status | The dementia scales were not related to premorbid IQ, age, or sex. | ||||||
| Reiss screen for maladaptive behavior[63] | Adaptive behaviour | Various Reiss screen subscales also discriminated between groups. | ||||||
| Prasher et al. (2004) [64] | UK—Setting is not clearly stated. | Adaptive Behaviour Dementia Questionnaire (ABDQ [64]) | Behavior | 150 ppts (83 male 67 females) Mean age: 44 years |
Down syndrome | Cases = Diagnosed DAT during 5 year study Controls = remained non dementia throughout. |
The scale has good reliability and validity. Overall accuracy = 92%. |
First tool designed specifically for detecting DAT in DS. |
| Lin et al. (2014) [65] | Taiwan—Setting is not clearly stated but potentially an applied setting. | Dementia Screening Questionnaire for Individuals with Intellectual Disabilities (DSQIID—[60]) | Dementia Status | 196 ppts Aged: 15–48 years |
Down syndrome | Younger = adolescent ppt Older = adult ppts |
Older > younger on DSQIID scores. Older age (P = .001) and comorbid conditions (P = .003) were significantly associated with DSQIID scores. Age (P < .01), severe disability level (P < .05), and comorbid condition (P < .01) significantly explained 13% of variation in DSQIID scores after adjusting for sex, education level, and multiple disabilities. |
DSQIID used well to diagnose dementia here in DS but need to consider other demographic factors that play a large influence on dementia status. |
| Ball et al. (2004) [66] | UK—Setting is not clearly stated. | Modified version of Cambridge examination for mental disorders of the elderly (CAMDEX) | General cognitive functioning | 74 ppts at first visit and 56 ppts at repeat 6 years later Aged: 30+ years |
Down syndrome | CAMDEX-based diagnosis of AD shown to be consistent with objectively observed cognitive decline (good concurrent validity) and to be a good predictor of future diagnosis. Inter-rater reliability was good with Kappa >0.8 for 91% of items and >0.6 for all items. |
Modified CAMDEX informant interview useful when diagnosing dementia in ID and DS. | |
| McCarron et al. (2014) [40] | Ireland & United States—Clinical Setting (Memory clinic) | Daily Living Skills Questionnaire (DLSQ) [67] | Daily Functioning | 77 ppts Aged: 35+ years |
Down syndrome | Cases = dementia Controls = non dementia |
Over 14 year follow-up average age of diagnosis = 55.41 years (SD = 7.14). Median survival of 7 years after diagnosis. Cases older than controls (sig) Decline in DLSQ score was shown 3-4 years prior to diagnosis. Presence of dementia also associated with epilepsy and sensory impairments. |
Changes in DLSQ indicated diagnosis 3–4 years apriori. More effective than direct tests used (DMSE and TSI) Also informative about variables that are associated with dementia diagnosis. |
| Dementia Questionnaire for Mentally retarded people (DMR)[56] | Among instruments used DMR most sensitive to tracking change in symptoms over time before diagnosis, reporting changes 5 years prior to diagnosis. Direct tests used only reported changes 1 year prior to diagnosis. | DMR most effective at reporting changes in functioning. | ||||||
| Deb (2007) [60] | UK—Setting is not clearly stated. | Dementia Screening Questionnaire for Individuals with Intellectual Disabilities (DSQIID—[60]) | Dementia Status | 193 ppts Aged: 23–77 years Mean age = 55 years |
Down syndrome | Sensitivity = 0.92 and specificity = 0.97 On DSQIID score of 20. Internal consistency (a1/4 0.91) for all its 53 items, and good test–retest and inter-rater reliability. Good construct validity was established by dividing the items into 4 factors. |
Valid and reliable screening method for dementia in DS. | |
| Kay (2003) [42] | UK—Clinical setting | Adaptive Behaviour Scale (ABS) | Behavior | 87 ppts Aged: 20+ years |
Down syndrome | No dementia cases participated, the sample was made up of individuals with DS only. | Significantly correlated with direct test Prudhoe cognitive functioning test (PCFT—see Table 1) ABS correlated significantly with the degree of ID. |
Was able to obtain scores for all levels of ID including profound, whereas the direct test was not able to. |
| Deb et al. (1999) [48] | UK—Setting is not clearly stated. | Dementia Questionnaire for persons with Mentally Retardation (DMR)[56] | Dementia Status | 62 ppts Aged: 35+ with DS. |
Down syndrome | Cases = Dementia (n = 26) Controls = non dementia (n = 36) |
DMR and DSDS showed good positive correlation. A similar positive correlation was found between the overall DSDS score and the scores in the main subcategories of the DMR. Direct test used (MMSE) could not be completed by all ppts. |
Informant scales, rather than the direct tests, were more useful for the diagnosis of dementia in people with an intellectual disability. |
| Dementia Scale for Down Syndrome (DSDS[68]) | Dementia Status |
Abbreviations: ID, intellectual disabilities; DS, Down syndrome; DAT, dementia Alzheimer's type; ppts, participants.
NOTE. Tests highlighted in bold indicate repeated use within studies. Age is denoted in years.
Table 4.
Test batteries
| Author | Battery name—designed for… | Informant reports contained in battery | Ability tested | Direct tests contained in battery | Ability tested | Ppts, age, and group | Type of ID | Outcome and comments |
|---|---|---|---|---|---|---|---|---|
| Burt et al. (2000) [69]—United States | Working Groups Battery—designed for dementia diagnosis in ID. |
|
|
|
|
1–1.5 hours to administer. Longitudinal administration is crucial to observing clinical change. |
||
| Palmer (2006) [82] - USA | Not given—designed for dementia assessment in individuals with Mental Retardation. |
|
|
|
22 ppts Aged: 33–66 years Groups: Cases = Dementia Controls = matched for IQ, age, presence of DS and sex but no dementia present. |
Mild or Moderate ID. |
2–2.5 hours to administer. Cases < Controls in all areas assessed. |
|
| Van der Wardt et al. (2011) [87]—UK, applied setting | Cognitive computerized test battery for individual's with intellectual disabilities (CCIID)—designed to assess IQ in individuals with ID. | N/A | N/A |
|
|
Reliability and validity studies were conducted in various ID populations and showed the CCIID to be a valid and reliable instrument for testing IQ. | ID all levels |
30 minutes to administer. Originally designed as an IQ test for verifying eligibility for Paralympic sporting events, but has been suggested for use in dementia assessment – not yet tested however for this purpose. |
| Silverman et al. (2004) [89]—United States, applied setting (ppts' residence or day program facility) |
|
|
|
|
273 ppts Aged: 45+ years After testing grouped into:
|
All levels of ID. | 2 hours to administer. 18 month longitudinal analysis presented. Findings suggest that by conducting a full assessment of cognitive abilities like presented here, diagnosis of dementia can be made a lot more rapid and accurate. |
|
| Das et al. (1995) [36]—United States and Canada, applied setting (quiet rooms in workshops, group or independent living setting) | Das Naglieri Cognitive Assesment System—designed to assess cognitive decline due to aging among individuals with Downs Syndrome. | N/A | N/A |
|
63 ppts Aged: 50–62 Groups
|
ID with DS or ID without DS with equivalent level of ID. |
1.5–4 hours to administer – a lot of variation in time taken. 2 < than all other groups on all tasks. Seen most on tasks requiring planning and attention. |
|
| Crayton et al. (1997) [97]—UK | Neuropsychological Assesment of dementia in adults with intellectual disability—designed for dementia assessment in Downs syndrome. | Cognitive test battery was compared to…
|
|
|
|
70 ppts Aged: 28+ Mean Age: 42.8 Groups
|
DS |
1.5 hours to administer. VABS and all neuropsychological tests negatively correlated (sig) – preexisting global cog impairment shown on these tests No difference between age groups (1, 2, and 3) on neuropsychological deficits. – because of screening method used before study. 2 & 3 < 1 performance on memory tests (sig) Results suggest sensitive tests that were used could be useful in dementia diagnostic process. |
| Oliver et al. (1998) [101] – UK | Different test batteries were collated, including the CANTAB and CAMCOG, plus extra tests added for the purpose of this study. (Please see across) – designed to detect age-related cognitive change in DS. |
|
|
|
|
57 ppts Aged: 30+ Groups
|
DS |
Does not state how long the battery took to administer. 28.3% of ppts showed severe cognitive deterioration, like apraxia or agnosia. A higher prevalence of these impairments was associated with older age. Rate of cognitive deterioration also ↑ w/age & degree of pre-existing cognitive impairment. Deterioration in memory, learning and orientation preceded the acquisition of aphasia, agnosia and apraxia. Pattern of cognitive deterioration seen with individuals who have DS in this study is comparable to the pattern reported in individuals who have Alzheimer's disease but do not have DS. |
| Jozsvai et al. (2002) [104]—UK, Clinical Setting | Not given—designed to assess cognitive decline in DS. |
|
|
|
|
35 ppts Aged: 28+ years Groups: Cases = diagnosed DAT using DSDS (n = 12) Controls = without DAT (n = 23) |
DS
|
Doesn't state how long the battery took to administer. FLUD and IO shown to be most useful tests in battery—must be wary of practice effects though. BNT and BD, most effected by aging & had least diagnostic ability. |
| Johansson et al. (2002) [106]—Sweden | Not given—designed to assess dementia in DS. | Informants were interviewed with questions regarding the ppts abilities in the following aspects and any changes observed in these abilities:
|
|
9 ppts Aged: 26–56 Groups:
|
DS |
Ppt section took 1.5–2 hours to administer. Advocates a combination of testing and interviewing in order to gain a full clinical picture. |
||
| Witts (1998) [107]—UK, applied setting (adult training centers. | Severe Impairment Battery (SIB [108])—designed to assess cognitive functioning of the severely demented client. |
|
-Adaptive behavior | Battery tests focus on:
|
33 ppts Mean age = 36 years |
DS |
20 minutes to administer. Good reliability and validity found. No floor effects encountered. Should be used longitudinally. |
|
Abbreviations: ID, intellectual disabilities; DS, Down syndrome; DAT, dementia Alzheimer's type; ↑, increases; ppts, participants.
NOTE. Tests highlighted in bold indicate repeated use within studies. Age is denoted in years.
3. Results
3.1. Results of the literature search
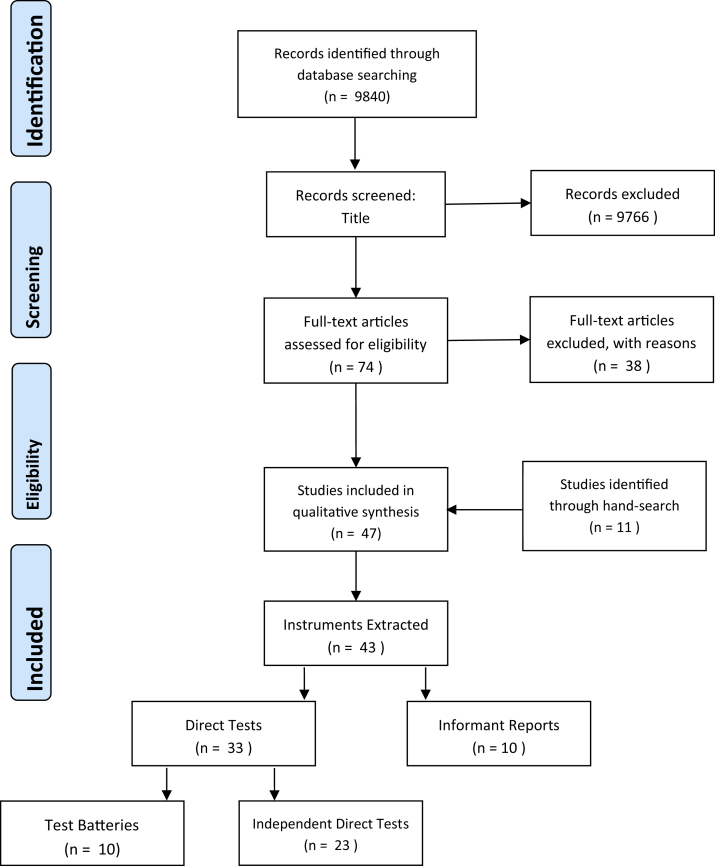
The literature searches conducted in all four databases yielded a total of 9840 studies. After excluding duplicates, screening titles and abstracts, 74 studies remained. These were assessed in full text, a further 34 studies were excluded at this point for not meeting the inclusion criteria. Thirty-six studies remained, and their references were hand searched manually, identifying 12 additional relevant studies. An overview of the whole search and the results is shown in Fig. 1. In total, 48 studies met the search criteria.
Fig. 1.
A PRISMA flow diagram detailing the search strategy and results.
A total of 44 instruments were found in the 47 included studies. There were 33 instruments to be completed by the individual and 11 to be completed by the carer or consultee. Of the 33 tests completed by the individual, 10 test batteries were identified, and 23 independent direct tests were identified. In the following sections, the instruments extracted are described in further detail.
3.2. Direct cognitive tests
During the literature search, 23 instruments coded as direct cognitive test batteries were identified; these are listed in Table 2. They each assess an aspect of cognitive functioning hypothesized to be associated with dementia, and therefore useful to assess when considering making a diagnosis. Various aspects of memory were the most frequently tested cognitive function. Memory domains included visual recognition, visual spatial, explicit, recall, and cued recall. Numerous tests sought to take a snapshot of overall cognitive functioning and mental status. Alternatively individual cognitive domains assessed included learning, various aspects of language, object recognition, executive function, and intelligence, among others. Many tests still incurred floor effects when participants were classed as having severe ID, reducing the tests potential for practical usage (e.g., PCFT [42]; MMSE [48]; CAMCOG [50]). The comments column in Table 2 denotes when a test has encountered this problem.
3.3. Informant reports
The informant reports highlighted by the studies in this review are detailed in Table 3. A total of 11 informant reports were reviewed. The informant reports nearly all assessed either behavior, dementia status, or daily functioning. These are noncognitive symptoms of dementia that indirectly indicate changes in cognitive functioning. When the participant is classed as having severe ID, these methods are more favored, as they do not require the individual to complete any tests that they may potentially find distressing. All informant reports in Table 3 were shown to be effective during the dementia diagnostic procedure, except for the Activities of Daily Living Questionnaire (ADL [54]), which was shown to not be effective in this population [55]. The Dementia Questionnaire for Mentally Retarded people (DMR [56]), which has been renamed at the Dementia Questionnaire for people with Learning Disabilities (DLD [57]) and Adaptive Behaviour Scale (ABS [43]) was highlighted to be most effective when used together, as they can cover a wide range of factors affected by dementia. This suggests that both adaptive behavior and general cognitive functioning that are assessed with these two scales are implicated in dementia diagnosis.
3.4. Test batteries
There were 10 test batteries identified in the literature search; which are listed in Table 4. Of these 10 batteries, four were designed for individuals with ID and five for individuals with Down syndrome. The remaining battery was designed for individuals in the general population who are already severely demented, rather than for use as an assessment battery. Eight of the batteries contained sections for informant reports as well, whereas two of the batteries chose to focus on just the participant's cognitive abilities. The test batteries varied in length from 20 minutes (severe impairment battery), up to 4 hours (Das Naglieri Cognitive Assessment System).
3.5. Other studies assessed
Table 5 shows the other studies reviewed. Although these studies did not present specific instruments, they discussed instruments available and presented their recommendations for diagnosis of dementia for people with ID and Down syndrome. Overall seven other studies were assessed, of which five were classed as literature reviews and two were classed as recommendation or advise documents, issued with the intention of informing practitioners of which route to take when diagnosing dementia in people with ID. All the documents agreed that a consensus should be reached to further research and benefit clinical practice. Many of the studies also highlighted how crucial longitudinal use of the chosen screening instrument is to accurately inform diagnosis [113] further argues for individually tailored assessment techniques to be employed, as many of the studies noted that the variability in cognition of individuals with ID makes it almost impossible to recommend one set instrument. Therefore selecting appropriate tests for the individual is key. Some studies also note the use of a multidisciplinary approach to most successfully inform diagnosis. This requires obtaining knowledge of the patient's history and making observations of not only their cognitive functioning, but also emotional, motivational and daily functioning.
Table 5.
Other studies reviewed
| Author (year) | Country | Document | Summary of key points |
|---|---|---|---|
| Moran et al. (2013) [23] | United States | Advise document |
|
| Zelinger et al. (2013) [24] | Austria | Literature review |
|
| Nieuwenhuis-Mark (2009) [20] | The Netherlands | Literature review |
|
| Krinksky-McHale et al. (2013) [109] | United States | Literature review |
|
| Nagdee (2011) [110] | South Africa | Literature review |
|
| Strydom et al. (2003) [111] | UK | Literature review |
|
| Suh (2013) [112] | Hong Kong | Advise document |
|
4. Discussion
4.1. Summary
In this review, instruments that are used in the assessment of dementia in individuals with intellectual disabilities (IDs) were systematically collected and described. This review also presents information regarding the available instruments in a more accessible and condensed form for clinicians to use to inform decisions regarding dementia diagnostics for individuals with ID. Furthermore, strengths and weaknesses of each type of instrument were discussed.
The three categories of diagnostic instruments presented are direct cognitive tests, informant reports, and test batteries. Previous reviews agree that consensus needs to be reached to advance assessment of dementia in ID (e.g., Zeilinger et al. [24]). Clinicians currently lean toward using instruments that they are previously familiar or comfortable with; however, this has resulted in disparity in the instruments being used across clinical settings. By reaching a consensus, benefits will not only be seen in assessment efficiency and communication between health professionals but also in treatment. Earlier treatment has been suggested to maintain the highest possible level of cognitive functioning, whereas dementia is mild [114].
Many studies agreed that memory impairment is crucial to dementia diagnosis and therefore included assessments of various aspects of memory in their recommendations of instruments. Some studies chose to assess other cognitive domains either in conjunction with memory assessments or instead of, for example, tests of orientation, language, intelligence, executive functioning, to name a few. Although the study by Crayton et al. [97] observed a similar clinical progression in the participants with ID and dementia that is often seen in individuals with dementia but no pre-existing ID, the numerous different cognitive domains tested in the included studies highlight how onset, course, and progression of dementia can notably differ from person to person.
With this in mind, it is vital to consider the level of intellectual disability that the instrument will be most suitable for assessing. It is important to also note that instruments often differ in their applicability to clinical or applied settings. All instruments discussed can be administered in both settings; however, some instruments are better suited to one setting or the other. In any case, level of distraction, how comfortable the participant is, length of the test, and accuracy of information gathered always need to be considered when deciding where to administer various instruments.
4.2. Direct cognitive tests
Evaluation of the direct cognitive tests showed many instruments that are appropriate for application with people who have ID. Studies assessed a variety of levels of ID, DS only, as well as comparing participants with DS to individuals with equivalent level of ID but no DS. Therefore, the instruments assessed present a range of levels of ID and could be applicable across the population, if administered correctly.
Multiple studies indicated good clinical utility for the Dementia Rating Scale (DRS [26]) and the Downs Syndrome Mental Status Exam (DMSE [41]). Furthermore, the study by McCarron et al. [40] commented that the DMSE was particularly useful in detecting cognitive changes 1 year before dementia diagnosis and therefore could prove useful in early detection. Studies looking at the DRS only included a total of 147 participants [25], [36], whereas studies looking at the DMSE included 362 participants [40], [47]. Therefore, to further support the findings of these studies, more research will need to be completed using the DRS, particularly in a larger sample size. No studies evaluated showed the DRS or the DMSE to be unsuitable for dementia diagnostics in ID. Both instruments were used in applied and clinical settings in the reported studies, indicating their flexibility in application and therefore good potential for use in informing dementia diagnostics.
Additionally, the modified version of the Selective Reminding Test (SRT [35]) was shown to have good utility in early detection, picking up cognitive changes between 1 and 3 years before dementia diagnosis [33]. Although there were no studies opposing this conclusion, this was only shown in one study of 155 participants; therefore, further research is required to support the clinical utility of the SRT.
Discrepancies in the effectiveness of the mini mental status examination (MMSE [49]) and the test for severe impairment (TSI [29]) arose. For example, the study by Boada et al. [53] was able to show the MMSE discriminated effectively between people with ID and people with ID and dementia. Similarly, Tyrrell et al. [46] replicated findings using the TSI. However, studies are inconsistent as Deb et al. [48] found the MMSE to show no significant difference between people with ID with and without dementia. Pyo et al. [27] also found no significant difference with the TSI.
The direct cognitive assessments shown to be most effective in this literature review included the DRS, DMSE, and SRT; however, each requires further assessment in larger sample sizes. Several studies noted the importance of tests being administered longitudinally, as there are no normative data for individuals with an ID as of yet. If used longitudinally clinicians can observe any cognitive decline, which could be very informative and necessary for making a decision regarding dementia diagnosis. Having said that, Margallo-Lana [115] highlighted findings that longitudinal follow-up is not useful in people with severe ID. So, test selection needs to be carefully tailored to the level of functioning of the individual and the setting in which the testing can take place.
4.3. Informant reports
Instruments classed as informant reports evaluated noncognitive concepts, such as activities of daily living and functioning, as individuals with dementia find many activities of daily living difficult due to decline in episodic memory [116]. Informants are often in a good position to observe these changes. Furthermore, informants reporting on everyday functioning, prospectively or retrospectively, are much more effective than reporting on changes in memory [117]. These noncognitive concepts have also been shown to hold greater significance to individuals with ID and their carers than evaluation of cognitive changes [118]. Although the effectiveness of informant reports often varies from study to study (e.g., Jozsvai et al. [119]), in the studies reviewed here, informant reports were shown overall to be an effective way of aiding in dementia diagnostics. As informant reports are not completed by the participant, they are exceedingly suitable for individuals who have severe ID. A variety of both clinical and applied settings were used in the reviewed studies and no studies commented on the setting being inappropriate for the assessment, but again level of distraction and accuracy of data do always need to be considered when deciding where to administer instruments.
In all the studies that compared informant reports to direct cognitive tests, informant reports were shown to be more effective than cognitive assessments [40], [42], [48]. The Daily Living Skills Questionnaire (DLSQ [67]) was noted to be effective in early detection, showing changes indicative of dementia 3–4 years before diagnosis. The Dementia Screening Questionnaire for Individuals with Intellectual Disabilities (DSQIID [60]) was administered to 848 participants across numerous reviewed studies and each found the questionnaire to be informative. However, the study by Lin et al. [55], [65] did note that other demographic factors that influence dementia status do need to be considered alongside DSQIID administration.
Results on the Activities of Daily Living Questionnaire (ADL [54]) were better explained by disability level and comorbidity than dementia status, and therefore, this was the only informant report reviewed to be shown to be unsuitable for use in dementia diagnostics for people with ID.
4.4. Test batteries
Test batteries reviewed contained a variety of instruments including both direct cognitive tests and informant reports. All batteries reviewed were effective in discriminating between ID dementia cases and ID controls, and none described floor effects, indicating promise for clinical utility. However, Jozsvai et al. [104] found the Boston Naming Task (BNT [78]) and the Block Design Test (BD from WISC-R [93]) contained in their test battery to be most affected by aging. Thus, these two tests were shown to have least diagnostic utility out of the battery. So, if a practitioner was to select this test battery, it is advised that these tests be removed.
The Cognitive Computerized Test Battery for Individual's with Intellectual Disabilities (CCIID [87]) is yet to be studied for the purpose of dementia diagnostics in individuals with ID. However, the CCIID has been validated in adults with ID. Moving forward, this battery should be assessed in a demented ID sample before clinical utility. Similarly, the Das Naglieri Cognitive Assessment System is yet to be assessed comparing ID dementia cases to ID controls. Das et al. [36] assessed cognitive decline as a result of aging that occurs among adults with DS with this test battery and showed the battery to be effective at detecting age-related cognitive decline. However, research has not yet assessed its utility in discriminating between dementia cases and controls in an ID or DS sample. Therefore, further research would need to be carried out to determine this battery's usefulness in aiding with dementia diagnosis.
Test batteries assess a range of cognitive abilities without relying on informants. Consequently, to best inform dementia diagnostics administering a test battery longitudinally can highlight any decline and track cognitive functioning in the years before dementia onset to best aid a clinician in making a diagnostic decision. Test batteries, however, do have numerous practical implications that need to be considered. Many require touch screen laptops, which are costly if the technology is not already available to the clinician. The laptops would also need to be near an available plug socket to administer tests without interruption, which might not be practical in an applied setting, which limits their potential utility. Paper and pen forms of certain cognitive tests are available, so if it is not feasible to have technology then the same concept of assessing a range of cognitive functions can be applied.
The direct tests and informant reports recommended above and described in the tables can help in deciding which tests to administer. However, comparing dementia cases to controls before clinical utility is advised where limited evidence is available on use for people with ID, which is often the case for numerous instruments presented throughout this review. Likewise, it must be noted that all test batteries presented require further testing to validate their clinical utility in an appropriate sample, particularly those with concerns noted above.
4.5. Combining methods
Previous reviews argue that a combination of methods can best inform dementia diagnosis in individuals with ID (e.g., Burt et al. [120]). Johansson et al. [106] describe how cognitive testing and informant interviewing could be the most effective way to combine methods and gain a full clinical picture. Combining methods for diagnosis, although effective, may be time consuming, and therefore the combination of methods chosen need to be carefully considered. This further supports the recommendation of the use of a test battery to aid diagnosis, as a number of batteries presented contain informant reports as well as cognitive assessments.
4.6. Limitations of this review
This review has some limitations. Most notably, instruments that compiled the test batteries were not evaluated individually as direct cognitive tests. To improve this research, instruments used within the batteries could be assessed individually as well as part of the battery. However, owing to the benefits highlighted in this review of test batteries, it was felt that test batteries would be of more benefit to clinicians to be presented as a whole.
4.7. Conclusion
In summary, it can be recommended that when diagnosing dementia in individuals with ID test, batteries can offer the most informative assessment of cognition. This could be alongside informant reports or a battery that contains informant reports to provide valuable information on the daily functioning of the individual as well as an overall assessment of cognitive functioning. Tables provided highlight previous validation of test batteries, and before selecting a battery clinicians should review literature presented. Particularly considering the length of the test battery, the level of ID of the individual being assessed and the setting in which the instrument will be administered. It may be advised to complete a shorter instrument when the ID is more severe. In this case, the CCIID or the SIB each takes 30 minutes or less to administer. Nonetheless, breaks should be offered to participants throughout testing, and it is always possible to split testing sessions into multiple shorter sessions.
Completing a test battery that covers both informant reports of daily functioning and assesses a full range of cognitive abilities is advised. This can enable clinicians to gain a more in-depth account of a participant's functioning and symptoms, hence can best inform a decision regarding dementia diagnosis.
Research in context.
-
1.
Systematic review: A systematic literature search was conducted in four databases (PubMed, Science Direct, Google Scholar, and PsycInfo). Studies were identified and selected using predefined inclusion and exclusion criteria; coded and classified according to assessment type by two independent researchers, with a third consulted when discrepancies arose.
-
2.
Interpretation: The review collates diagnostic instruments and presents strengths and weaknesses of each type of assessment and each individual test. Findings indicated that test batteries to offer the most practical and efficient method of assessment. The variation in areas of functioning affected by dementia in individuals' with ID can be assessed with a battery to best inform a clinician's decision.
-
3.
Future directions: The review proposes specific test batteries that could be most beneficial; however, small sample sizes in cited studies suggest that further studies need to investigate the use of these batteries in larger samples of individuals with ID to better highlight diagnostic potential for each test.
References
- 1.American Psychiatric Association . American Psychiatric Pub; Washington DC: 2013. Diagnostic and statistical manual of mental disorders (DSM-5®) [Google Scholar]
- 2.Stanton L.R., Coetzee R.H. Down's syndrome and dementia. Adv Psychiatr Treat. 2004;10:50–58. [Google Scholar]
- 3.Eyman R.K., Call T.L., White J.F. Life expectancy of persons with Down syndrome. Am J Ment Retard. 1991;95:603–612. [PubMed] [Google Scholar]
- 4.Janicki M.P., Ackerman L., Jacobson W. State developmental disabilities/ageing plans and planning for an older developmentally disabled population. Ment Retard. 1985;23:297–301. [PubMed] [Google Scholar]
- 5.Patja K., Iivanainen M., Vesala H., Oksanen H., Ruoppila I. Life expectancy of people with intellectual disability: A 35-year follow-up study. J Intellect Disabil Res. 2000;44:591–599. doi: 10.1046/j.1365-2788.2000.00280.x. [DOI] [PubMed] [Google Scholar]
- 6.Daviglus M.L., Plassman B.L., Pirzada A., Bell C.C., Bowen P.E., Burke J.R. Risk factors and preventive interventions for Alzheimer disease. Arch Neurol. 2011;68:1185–1190. doi: 10.1001/archneurol.2011.100. [DOI] [PubMed] [Google Scholar]
- 7.Diagnostic and statistical manual-text revision (DSM-IV-TRim) American Psychiatric Association; Washington DC: 2000. [Google Scholar]
- 8.Ford A.H. Neuropsychiatric aspects of dementia. Maturitas. 2014;79:209–215. doi: 10.1016/j.maturitas.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Wilcock G.K., Esiri M.M. Plaques, tangles and dementia: A quantitative study. J Neurol Sci. 1982;56:343–356. doi: 10.1016/0022-510x(82)90155-1. [DOI] [PubMed] [Google Scholar]
- 10.Lin J., Wu C., Lin P., Lin L., Chu C.M. Early onset ageing and service preparation in people with intellectual disabilities: Institutional managers' perspective. Res Dev Disabil. 2011;32:188–193. doi: 10.1016/j.ridd.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Janicki M.P., Dalton A.J. Prevalence of dementia and impact on intellectual disability services. Ment Retard. 2000;38:276–288. doi: 10.1352/0047-6765(2000)038<0276:PODAIO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Strydom A., Livingston G., King M., Hassiotis A. Prevalence of dementia in intellectual disability using different diagnostic criteria. Br J Psychiatry. 2007;191:150–157. doi: 10.1192/bjp.bp.106.028845. [DOI] [PubMed] [Google Scholar]
- 13.Cooper S. High prevalence of dementia among people with learning disabilities not attributable to Down's syndrome. Psychol Med. 1997;27:609–616. doi: 10.1017/s0033291796004655. [DOI] [PubMed] [Google Scholar]
- 14.Strydom A., Chan T., King M., Hassiotis A., Livingston G. Incidence of dementia in older adults with intellectual disabilities. Res Dev Disabil. 2013;34:1881–1885. doi: 10.1016/j.ridd.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 15.Strydom A., Shooshtari S., Lee L., Raykar V., Torr J., Tsiouris J. Dementia in older adults with intellectual disabilities—epidemiology, presentation, and diagnosis. J Policy Pract Intellect Disabilities. 2010;7:96–110. [Google Scholar]
- 16.Zigman W.B., Schupf N., Devenny D.A., Miezejeski C., Ryan R., Urv T.K. Incidence and prevalence of dementia in elderly adults with mental retardation without down syndrome. Am J Ment Retard. 2004;109:126–141. doi: 10.1352/0895-8017(2004)109<126:IAPODI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Bush A., Beail N. Risk factors for dementia in people with Down syndrome: issues in assessment and diagnosis. Am J Ment Retard. 2004;109:83–97. doi: 10.1352/0895-8017(2004)109<83:RFFDIP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Rohn T.T., McCarty K.L., Love J.E., Head E. Is Apolipoprotein E4 an Important Risk Factor for Dementia in Persons with Down Syndrome? J Parkinsons Dis Alzheimers Dis. 2014;1 doi: 10.13188/2376-922x.1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deb S., McHugh R. Academic Press; San Diego: 2010. Chapter eight - dementia among persons with Down syndrome. International review of research in mental retardation; pp. 221–255. [Google Scholar]
- 20.Nieuwenhuis-Mark R.E. Diagnosing Alzheimer's dementia in Down syndrome: Problems and possible solutions. Res Dev Disabil. 2009;30:827–838. doi: 10.1016/j.ridd.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Wilson L.R., Annus T., Zaman S.H., Holland A.J. Understanding the process; links between Down Syndrome and dementia. In: Watchman K., editor. Intellectual Disability and Dementia; Research into practice. Jessica Kingsley Publishers; London: 2014. [Google Scholar]
- 22.Holland A.J. Ageing and learning disability. Br J Psychiatry. 2000;176:26–31. doi: 10.1192/bjp.176.1.26. [DOI] [PubMed] [Google Scholar]
- 23.Moran J.A., Rafii M.S., Keller S.M., Singh B.K., Janicki M.P. The national task group on intellectual disabilities and dementia practices consensus recommendations for the evaluation and management of dementia in adults with intellectual disabilities. Mayo Clin Proc. 2013;88:831–840. doi: 10.1016/j.mayocp.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 24.Zeilinger E.L., Stiehl K.A., Weber G. A systematic review on assessment instruments for dementia in persons with intellectual disabilities. Res Dev Disabil. 2013;34:3962–3977. doi: 10.1016/j.ridd.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 25.McDaniel W.F., McLaughlin T. Further support for using the dementia rating scale in the assessment of neuro-cognitive functions of individuals with mental retardation. Clin Neuropsychol. 2000;14:72–75. doi: 10.1076/1385-4046(200002)14:1;1-8;FT072. [DOI] [PubMed] [Google Scholar]
- 26.Mattis S. Psychological Assessment Resources; Odessa, FL: 1988. Dementia rating scale (DRS) [Google Scholar]
- 27.Pyo G., Ala T., Kyrouac G.A., Verhulst S.J. A pilot study of a test for visual recognition memory in adults with moderate to severe intellectual disability. Res Dev Disabil. 2010;31:1475–1480. doi: 10.1016/j.ridd.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Pyo G., Kripakaran K., Curtis K., Curtis R., Markwell S. A preliminary study of the validity of memory tests recommended by the working group for individuals with moderate to severe intellectual disability. J Intellect Disabil Res. 2007;51:377–386. doi: 10.1111/j.1365-2788.2006.00886.x. [DOI] [PubMed] [Google Scholar]
- 29.Albert M., Cohen C. The test for severe impairment: An instrument for the assessment of patients with severe cognitive dysfunction. J Am Geriatr Soc. 1992;40:449–453. doi: 10.1111/j.1532-5415.1992.tb02009.x. [DOI] [PubMed] [Google Scholar]
- 30.Shultz J., Aman M., Kelbley T., LeClear Wallace C., Burt D.B., Primeaux-Hart S. Evaluation of screening tools for dementia in older adults with mental retardation. Am J Ment Retard. 2004;109:98–110. doi: 10.1352/0895-8017(2004)109<98:EOSTFD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 31.Korkman M., Kirk U., Kemp S. Psychological Corporation; Chicago: 1998. NEPSY: A developmental neuropsychological assessment. [Google Scholar]
- 32.Taylor D.V., Sandman C.A., Touchette P., Hetrick W.P., Barron J.L. Naltrexone improves learning and attention in self-injurious individuals with developmental disabilities. J Dev Phys Disabil. 1993;5:29–42. [Google Scholar]
- 33.Krinsky-McHale S., Devenny D., Silverman W. Changes in explicit memory associated with early dementia in adults with Down's syndrome. J Intellect Disabil Res. 2002;46:198–208. doi: 10.1046/j.1365-2788.2002.00365.x. [DOI] [PubMed] [Google Scholar]
- 34.Buschke H. Selective reminding for analysis of memory and learning. J Verbal Learning Verbal Behav. 1973;12:543–550. [Google Scholar]
- 35.Hill AL, Wisniewski KE, Devenny-Phatate D, Silverman W. Cognitive functioning of older people with Down syndrome. In Eighth International Congress of the International Association for Scientific Study of Mental Deficiency, Dublin, Ireland; 1988.
- 36.Das J., Divis B., Alexander J., Parrila R.K., Naglieri J.A. Cognitive decline due to aging among persons with Down syndrome. Res Dev Disabil. 1995;16:461–478. doi: 10.1016/0891-4222(95)00030-5. [DOI] [PubMed] [Google Scholar]
- 37.Dunn L.M., Dunn L.M. American Guidance Service; Circle Pines: 1981. Peabody picture vocabulary test: Forms L and M. [Google Scholar]
- 38.Naglieri J.A. Merrill; Columbus, OH: 1985. Matrix analogies test: Expanded form. [Google Scholar]
- 39.Nelson L.D., Scheibel K.E., Ringman J.M., Sayre J.W. An experimental approach to detecting dementia in Down syndrome: a paradigm for Alzheimer's disease. Brain Cogn. 2007;64:92–103. doi: 10.1016/j.bandc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 40.McCarron M., McCallion P., Reilly E., Mulryan N. A prospective 14-year longitudinal follow-up of dementia in persons with down syndrome. J Intellect Disabil Res. 2014;58:61–70. doi: 10.1111/jir.12074. [DOI] [PubMed] [Google Scholar]
- 41.Haxby J.V. Neuropsychological evaluation of adults with down's syndrome: Patterns of selective impairment in non-demented old adults. J Intellect Disabil Res. 1989;33:193–210. doi: 10.1111/j.1365-2788.1989.tb01467.x. [DOI] [PubMed] [Google Scholar]
- 42.Kay D., Tyrer S., Margallo-Lana M., Moore P., Fletcher R., Berney T. Preliminary evaluation of a scale to assess cognitive function in adults with down's syndrome: The prudhoe cognitive function test. J Intellect Disabil Res. 2003;47:155–168. doi: 10.1046/j.1365-2788.2003.00451.x. [DOI] [PubMed] [Google Scholar]
- 43.Nihira K., Lambert N.M., Leland H. Pro-ed; Washington DC: 1993. Adaptive behavior scale: Residential and community. Examiner's manual. [Google Scholar]
- 44.Devenny D.A., Zimmerli E.J., Kittler P., Krinsky-McHale S.J. Cued recall in early-stage dementia in adults with Down's syndrome. J Intellect Disabil Res. 2002;46:472–483. doi: 10.1046/j.1365-2788.2002.00417.x. [DOI] [PubMed] [Google Scholar]
- 45.Buschke H. Cued recall in amnesia. J Clin Exp Neuropsychol. 1984;6:433–440. doi: 10.1080/01688638408401233. [DOI] [PubMed] [Google Scholar]
- 46.Grober E., Buschke H. Genuine memory deficits in dementia. Dev Neuropsychol. 1987;3:13–36. [Google Scholar]
- 47.Tyrrell J., Cosgrave M., McCarron M., McPherson J., Calvert J., Kelly A. Dementia in people with Down's syndrome. Int J Geriatr Psychiatry. 2001;16:1168–1174. doi: 10.1002/gps.502. [DOI] [PubMed] [Google Scholar]
- 48.Deb S., Braganza J. Comparison of rating scales for the diagnosis of dementia in adults with Down's syndrome. J Intellect Disabil Res. 1999;43:400–407. doi: 10.1046/j.1365-2788.1999.043005400.x. [DOI] [PubMed] [Google Scholar]
- 49.Folstein M.F., Folstein S.E., McHugh P.R. MIni-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 50.Hon J., Huppert F.A., Holland A.J., Watson P. Neuropsychological assessment of older adults with down's syndrome: An epidemiological study using the cambridge cognitive examination (CAMCOG) Br J Clin Psychol. 1999;38:155–165. doi: 10.1348/014466599162719. [DOI] [PubMed] [Google Scholar]
- 51.Pennington B.F., Moon J., Edgin J., Stedron J., Nadel L. The neuropsychology of down syndrome: Evidence for hippocampal dysfunction. Child Dev. 2003;74:75–93. doi: 10.1111/1467-8624.00522. [DOI] [PubMed] [Google Scholar]
- 52.Robbins T.W., James M., Owen A.M., Sahakian B.J., McInnes L., Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5:266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- 53.Boada M., Alegret M., Buendia M., Hernández I., Viñas G., Espinosa A. The usefulness of standard neuropsychological testing for adults with Down syndrome and dementia. Int Med Rev Down Syndr. 2008;12:2–7. [Google Scholar]
- 54.Mahoney F.I., Barthel D.W. Functional evaluation: The barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 55.Lin J., Lin L., Hsia Y., Hsu S., Wu C., Chu C.M. A national survey of caregivers' perspective of early symptoms of dementia among adults with an intellectual disability based on the DSQIID scale. Res Autism Spectr Disord. 2014;8:275–280. [Google Scholar]
- 56.Evenhuis H. Evaluation of a screening instrument for dementia in ageing mentally retarded persons. J Intellect Disabil Res. 1992;36:337–347. doi: 10.1111/j.1365-2788.1992.tb00532.x. [DOI] [PubMed] [Google Scholar]
- 57.Evenhuis H., Kengen M.M., Eurlings H.A. Pearson; San Antonio: 2007. Dementia questionnaire for people with learning disabilities (DLD) [Google Scholar]
- 58.Zeilinger E.L., Gärtner C., Janicki M.P., Esralew L., Weber G. Practical applications of the NTG-EDSD for screening adults with intellectual disability for dementia: A German-language version feasibility study. J Intellect Dev Disabil. 2015;41:1–8. [Google Scholar]
- 59.Esralew L., Janicki M.P., DiSipio M., Jokinen J., Keller S.M., Members of the National Task Group Section on Early Detection and Screening . Vol. 16. NADD Bulletin; New York: 2013. pp. 47–54. (National Task Group Early Detection Screen for Dementia (NTG-EDSD) manual). [Google Scholar]
- 60.Deb S., Hare M., Prior L., Bhaumik S. Dementia screening questionnaire for individuals with intellectual disabilities. Br J Psychiatry. 2007;190:440–444. doi: 10.1192/bjp.bp.106.024984. [DOI] [PubMed] [Google Scholar]
- 61.De Vreese L.P., Mantesso U., De Bastiani E., Marangoni A., Gomiero T. Psychometric evaluation of the Italian version of the AADS questionnaire: A caregiver-rated tool for the assessment of behavioral deficits and excesses in persons with intellectual disabilities and dementia. Int Psychogeriatr. 2011;23:1124–1132. doi: 10.1017/S1041610211000342. [DOI] [PubMed] [Google Scholar]
- 62.Kirk L.J., Hick R., Laraway A. Assessing dementia in people with learning disabilities: The relationship between two screening measures. J Intellect Disabil. 2006;10:357–364. doi: 10.1177/1744629506070053. [DOI] [PubMed] [Google Scholar]
- 63.Reiss S.P. International Diagnostic Systems; Worthington: 1987. Reiss screen for maladaptive behavior: Version 1.1. [Google Scholar]
- 64.Prasher V., Farooq A., Holder R. The adaptive behaviour dementia questionnaire (ABDQ): Screening questionnaire for dementia in Alzheimer's disease in adults with down syndrome. Res Dev Disabil. 2004;25:385–397. doi: 10.1016/j.ridd.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 65.Lin J.D., Chen W.X., Hsu S.W., Lin L.P., Lin F.G., Tang C.C. Primary caregivers' awareness and perception of early-onset dementia conditions in adolescents and young and middle-aged adults with Down syndrome. Res Dev Disabil. 2014;35:1934–1940. doi: 10.1016/j.ridd.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 66.Ball S., Holland A., Huppert F., Treppner P., Watson P., Hon J. The modified CAMDEX informant interview is a valid and reliable tool for use in the diagnosis of dementia in adults with downs syndrome. J Intellect Disabil Res. 2004;48:611–620. doi: 10.1111/j.1365-2788.2004.00630.x. [DOI] [PubMed] [Google Scholar]
- 67.National Institute of Aging . National Institute of Aging, Laboratory of Neurosciences; 1989. The daily living skills questionnaire. [Google Scholar]
- 68.Gedye A. Gedye Research and Consulting; Vancouver: 1995. Dementia scale for Down syndrome. Manual. [Google Scholar]
- 69.Burt D., Aylward E.H. Test battery for the diagnosis of dementia in individuals with intellectual disability. J Intellect Disabil Res. 2000;44:175–180. doi: 10.1046/j.1365-2788.2000.00264.x. [DOI] [PubMed] [Google Scholar]
- 70.Cosgrave M.P., McCarron M., Anderson M., Tyrrell J., Gill M., Lawlor B.A. Cognitive decline in down syndrome: A validity/reliability study of the test for severe impairment. Am J Ment Retard. 1998;103:193–197. doi: 10.1352/0895-8017(1998)103<0193:CDIDSA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 71.Thorndike R.L., Hagen E.P., Sattler J.M. Riverside Publishing Company; Chicago: 1986. Stanford-binet intelligence scale. [Google Scholar]
- 72.Seltzer G.B. Waisman Centre, University of Wisconsin; Madison, WI: 1997. Modified FULD object memory evaluation. [Google Scholar]
- 73.Pyo G., Curtis K., Curtis R., Markwell S. A validity study of the working group's orientation test for individuals with moderate to severe intellectual disability. J Intellect Disabil Res. 2009;53:780–786. doi: 10.1111/j.1365-2788.2009.01191.x. [DOI] [PubMed] [Google Scholar]
- 74.Bruininks R.H., Woodcock R., Weatherman R., Hill B. Riverside Publishing; Itasca, IL: 1996. SIB-R: Scales of independent behavior-revised. [Google Scholar]
- 75.Moss M.B., Albert M.S., Butters N., Payne M. Differential patterns of memory loss among patients with Alzheimer's disease, huntington's disease, and alcoholic korsakoff's syndrome. Arch Neurol. 1986;43:239–246. doi: 10.1001/archneur.1986.00520030031008. [DOI] [PubMed] [Google Scholar]
- 76.Pyo G., Ala T., Kyrouac G.A., Verhulst S.J. A validity study of the Working Group's Autobiographical Memory Test for individuals with moderate to severe intellectual disability. Res Dev Disabil. 2011;32:70–74. doi: 10.1016/j.ridd.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 77.Aylward E., Burt D. American Association on Mental Retardation; Washington, DC: 1998. Test battery for the diagnosis of dementia in individuals with intellectual disability. Report of the working group for the establishment of criteria for the diagnosis of dementia in individuals with intellectual disability. [DOI] [PubMed] [Google Scholar]
- 78.Kaplan E., Goodglass H., Weintraub S. Lea & Febiger; Philadelphia: 1983. The boston naming test. [Google Scholar]
- 79.McCarthy D. Psychological Corporation; New York: 1972. McCarthy scales of children's abilities. [Google Scholar]
- 80.Tiffin J., Asher E.J. The purdue pegboard: Norms and studies of reliability and validity. J Appl Psychol. 1948;32:234. doi: 10.1037/h0061266. [DOI] [PubMed] [Google Scholar]
- 81.Beery K.E. Modern Curriculum Press; NJ: 1997. The beery-buktenica VMI: Developmental test of visual-motor integration with supplemental developmental tests of visual perception and motor coordination: Administration, scoring, and teaching manual. [Google Scholar]
- 82.Palmer G.A. Neuropsychological profiles of persons with mental retardation and dementia. Res Dev Disabil. 2006;27:299–308. doi: 10.1016/j.ridd.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 83.Visser F., Aldenkamp A., Van Huffelen A., Kuilman M. Prospective study of the prevalence of Alzheimer-type dementia in institutionalized individuals with Down syndrome. Am J Ment Retard. 1997;101:400–412. [PubMed] [Google Scholar]
- 84.D'Elia L., Satz P., Uchiyama C., White T. Psychological assessment resources; Odessa FL: 1996. Color trails test. [Google Scholar]
- 85.Spreen O., Strauss E. Oxford University Press; New York: 1998. A compendium of neurological tests. Administration, Norms and Commentary. [Google Scholar]
- 86.Fuld P.A. Guaranteed stimulus-processing in the evaluation of memory and learning. Cortex. 1980;16:255–271. doi: 10.1016/s0010-9452(80)80061-x. [DOI] [PubMed] [Google Scholar]
- 87.Van Der Wardt V., Bandelow S., Hogervorst E. Nova Science Publishers; New York: 2011. Development of the cognitive computerized test battery for individuals with intellectual disabilities for the classification of athletes with intellectual disabilities. [Google Scholar]
- 88.Corsi PM. Human memory and the medical temporal region of the brain. Dissertation Abstracts International. 1972;34:891B.
- 89.Silverman W., Schupf N., Zigman W., Devenny D., Miezejeski C., Schubert R. Dementia in adults with mental retardation: Assessment at a single point in time. Am J Ment Retard. 2004;109:111–125. doi: 10.1352/0895-8017(2004)109<111:DIAWMR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 90.Wisniewski K., Hill A. Clinical aspects of dementia in mental retardation and developmental disabilities. In: Wisniewski H., Janicki M., editors. Aging and Developmental Disabilities. Brookes; Baltimore: 1985. pp. 195–210. [Google Scholar]
- 91.Nihira K., Foster R., Shellhaas M., Leland H. The Association; Washington, DC: 1974. American association on mental deficiency adaptive behavior scale. [Google Scholar]
- 92.Beery K., Buktenica N. Modern Curriculum Press; Cleveland: 1989. The VMI. Developmental Test of Visual-Motor Integration. [Google Scholar]
- 93.Wechsler D. Psychological Corporation; New York: 1974. Manual for the wechsler intelligence scale for children, revised. [Google Scholar]
- 94.Teuber H.L., Battersby W.S., Bender M.B. Changes in visual searching performance following cerebral lesions. Am J Physiol. 1949;159:592. [Google Scholar]
- 95.Naglieri J.A., Das J. Construct and criterion-related validity of planning, simultaneous, and successive cognitive processing tasks. J Psychoeduc Assess. 1987;5:353–363. [Google Scholar]
- 96.Das J., Mishra R.K. Assessment of cognitive decline associated with aging: A comparison of individuals with down syndrome and other etiologies. Res Dev Disabil. 1995;16:11–25. doi: 10.1016/0891-4222(94)00032-5. [DOI] [PubMed] [Google Scholar]
- 97.Crayton L., Oliver C., Holland A., Bradbury J., Hall S. The neuropsychological assessment of age related cognitive deficits in adults with Down's syndrome. J Appl Res Intellect Disabil. 1997;11:255–272. [Google Scholar]
- 98.Sparrow S.S., Balla D.A., Cicchetti D.V. American Guidance Service; Circle Pines, MN: 1984. Vineland adaptive behavior scales: Interview edition, survey form manual. [Google Scholar]
- 99.Dunn L., Dunn L., Whetton C., Pintilie D. NFER-nelson; Windsor: 1982. The British Picture Vocabulary Scale (short form) [Google Scholar]
- 100.Roth M., Tym E., Mountjoy C.Q., Huppert F.A., Hendrie H., Verma S. CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry. 1986;149:698–709. doi: 10.1192/bjp.149.6.698. [DOI] [PubMed] [Google Scholar]
- 101.Oliver C., Crayton L., Holland A., Hall S., Bradbury J. A four year prospective study of age-related cognitive change in adults with Down's syndrome. Psychol Med. 1998;28:1365–1377. doi: 10.1017/s0033291798007417. [DOI] [PubMed] [Google Scholar]
- 102.Terman L.M., Merrill M.R. Houghton-Mifflin; Boston: 1960. Stanford Binet Intelligence Scale. [Google Scholar]
- 103.Sahakian B.J., Morris R.G., Evenden J.L., Heald A., Levy R., Philpot M. A comparative study of visuospatial memory and learning in Alzheimer-type dementia and parkinson's disease. Brain. 1988;111:695–718. doi: 10.1093/brain/111.3.695. [DOI] [PubMed] [Google Scholar]
- 104.Jozsvai E., Kartakis P., Collings A. Neuropsychological test battery to detect dementia in down syndrome. J Dev Disabil. 2002;9:27–34. [Google Scholar]
- 105.Fuld P.A. Psychological testing in the differential diagnosis of the dementias. Alzheimers Dis Senile Demen Relat Disord. 1978;7:185–193. [Google Scholar]
- 106.Johansson E., Terenius O. Development of an instrument for early detection of dementia in people with down syndrome. J Intellect Dev Disabil. 2002;27:325–345. [Google Scholar]
- 107.Witts P., Elders S. The ‘Severe impairment battery’: Assessing cognitive ability in adults with Down syndrome. Br J Clin Psychol. 1998;37:213–216. doi: 10.1111/j.2044-8260.1998.tb01295.x. [DOI] [PubMed] [Google Scholar]
- 108.Saxton J., McGonigle K., Swihart A., Boller F. Thames Valley Test Company; London: 1993. The severe impairment battery. [Google Scholar]
- 109.Krinsky-McHale S.J., Silverman W. Dementia and mild cognitive impairment in adults with intellectual disability: issues of diagnosis. Dev Disabil Res Rev. 2013;18:31–42. doi: 10.1002/ddrr.1126. [DOI] [PubMed] [Google Scholar]
- 110.Nagdee M. Dementia in intellectual disability: a review of diagnostic challenges. Afr J Psychiatry. 2011;14:194–199. doi: 10.4314/ajpsy.v14i3.1. [DOI] [PubMed] [Google Scholar]
- 111.Strydom A., Hassiotis A. Diagnostic instruments for dementia in older people with intellectual disability in clinical practice. Aging Ment Health. 2003;7:431–437. doi: 10.1080/13607860310001594682. [DOI] [PubMed] [Google Scholar]
- 112.Suh G.H. Adoption of two standard deviation notions of mental retardation for the diagnosis of dementia. East Asian Arch Psychiatry. 2013;23:78. [PubMed] [Google Scholar]
- 113.Nagdee M., O'Brien G. MacKeith Press; London: 2009. Dementia in developmental disability; pp. 10–30. [Google Scholar]
- 114.Seltzer B., Zolnouni P., Nunez M., Goldman R., Kumar D., Ieni J. Efficacy of donepezil in early-stage Alzheimer disease: A randomized placebo-controlled trial. Arch Neurol. 2004;61:1852–1856. doi: 10.1001/archneur.61.12.1852. [DOI] [PubMed] [Google Scholar]
- 115.Margallo-Lana M.L., Moore P.B., Kay D.W.K., Perry R.H., Reid B.E., Berney T.P. Fifteen-year follow-up of 92 hospitalized adults with Down's syndrome: incidence of cognitive decline, its relationship to age and neuropathology. J Intellect Disabil Res. 2007;51:463–477. doi: 10.1111/j.1365-2788.2006.00902.x. [DOI] [PubMed] [Google Scholar]
- 116.Mokhtari M., Aloulou H., Tiberghien T., Biswas J., Racoceanu D., Yap P. New trends to support independence in persons with mild dementia: A mini-review. Gerontology. 2012;58:554–563. doi: 10.1159/000337827. [DOI] [PubMed] [Google Scholar]
- 117.Jamieson-Craig R., Scior K., Chan T., Fenton C., Strydom A. Reliance on carer reports of early symptoms of dementia among adults with intellectual disabilities. J Policy Pract Intellect Disabilities. 2010;7:34–41. [Google Scholar]
- 118.Cooper S., Prasher V.P. Maladaptive behaviours and symptoms of dementia in adults with down's syndrome compared with adults with intellectual disability of other aetiologies. J Intellect Disabil Res. 1998;42:293–300. doi: 10.1046/j.1365-2788.1998.00135.x. [DOI] [PubMed] [Google Scholar]
- 119.Jorm A. Methods of screening for dementia: A meta-analysis of studies comparing an informant questionnaire with a brief cognitive test. Alzheimer Dis Assoc Disord. 1997;11:158–162. [PubMed] [Google Scholar]
- 120.Burt D.B., Primeaux-Hart S., Loveland K.A., Cleveland L.A., Lewis K.R., Lesser J. Aging in adults with intellectual disabilities. Am J Ment Retard. 2005;110:268–284. doi: 10.1352/0895-8017(2005)110[268:AIAWID]2.0.CO;2. [DOI] [PubMed] [Google Scholar]