Abstract
Objective
The objective of this study was to investigate whether cancer specific survival in rectal cancer patients is affected by patient-related factors, conditional on radiation treatment.
Methods
359 invasive rectal cancer patients who consented and provided questionnaire data for a population-based case-control study of colorectal cancer in Metropolitan Detroit were included in this study. Their vital status was ascertained through to the population-based cancer registry. Hazard ratios (HR) for cancer specific and other deaths and 95% confidence intervals (CIs) were calculated according to selected patients' characteristics, stratified by radiation status, using joint Cox proportional hazards models.
Results
A total of 159 patients were found to be deceased after the median follow-up of 9.2 years, and 70% of them were considered to be cancer specific. Smoking and a history of diabetes were associated with an increased probability of deaths from other causes (HR 3.20, 95% CI 1.72–5.97 and HR 2.02, 95% CI 0.98–4.16, respectively), while regular use of non-steroidal anti-inflammatory drugs (NSAIDs) was inversely correlated with cancer-specific mortality (HR 0.50, 95% CI 0.30–0.81). Furthermore, the associations of smoking and NSAIDs with the two different types of deaths (cancer vs others) significantly varied with radiation status (P-values for the interactions= 0.014 for both). In addition, we observed a marginally significantly reduced risk of cancer specific deaths in the patients who had the relative ketogenic diet overall (HR=0.49, 95% 0.23–1.02).
Conclusion
Further research is warranted to confirm these results in order to develop new interventions to improve outcome from radiation treatment.
Keywords: Non-steroidal anti-inflammatory drugs, smoking, diet, rectal cancer, survival
Introduction
The treatment of rectal cancer has evolved significantly during the past few decades and radiation is now part of the standard protocol for rectal cancer management [1–3]. It is primarily given for stage 2 and 3 cases as neo-adjuvant therapy before surgery, with or without concomitant administration of chemotherapeutic agents. Introduction of these preoperative treatments along with better surgical techniques have been ascribed to recent improvements in overall survival from rectal cancer at the population level [4–6] as well as to improved quality of life by allowing more sphincter preserving surgeries [7,8]. Yet, local and distant recurrences occur in a fraction of the patients. Several tumor related factors, including tumor size, tumor location, and local extension, and other treatment-related factors, such as negative surgical margin, radiation dose, and pathological response to radiation, concomitant chemotherapy use and type of agents used, have been associated with local recurrence and survival [1,2,9]. In addition, systemic markers, CEA and pro-inflammatory reactants, such as CRP and neutrophil count, have been shown to predict outcome of neoadjuvant radiation [10,11]
Biophysiologically, hypoxic lesions and tumors with increased anaerobic glycolysis are known to be radioresistant [12,13]. There are a number of pharmacological modifiers which alleviate hypoxia and correct aberrant tumor metabolism by reducing glycolysis and enhancing mitochondrial oxidation [12,13]. Many of these compounds are already in clinical use or testing for cancer and other conditions [14,15]. In addition, human lifestyle and behavioral factors possibly modulate these pathways. Short term starvation and ketogenic diets, which lead to lower availability of glycolysis substrate, have been demonstrated to enhance the effect of radiation treatment in mouse glioma models [16,17]. Smoking may cause a low-grade chronic hypoxic condition due to elevated carbon monoxide levels and pharmacological actions of nicotine [18,19]. Some over the counter medications such as Aspirin and Ibuprofen are known to act on these pathways [20,14].
To date, there has been minimal study of whether these patient-related factors indeed modify outcome of radiation treatment for rectal cancer patients. Leveraging the data collected for a population-based case-control study of colorectal cancer in Metropolitan Detroit, and survival linkage to its population-based cancer registry, we sought to address whether cancer specific survival in rectal cancer patients is affected by their medical history and lifestyle factors, conditional on radiation treatment. We focus on patients' factors that potentially intervene in the atmospheric and nutritional environments of a tumor, namely smoking, medications and body composition and macronutrient intake, based on the literature review cited above and others.
Methods
Study subjects and data acquisition
The subjects for this study were derived from rectal cancer cases who were enrolled in a population-based case-control study of colorectal cancer in Metropolitan Detroit, details of which have been reported elsewhere [21,22]. The study was approved by the Wayne State University Human Investigation Committee. Eligible cases were diagnosed between January 1, 2003 and September 30, 2005, were histologically confirmed, were and between 45 and 80 years of age, and were identified through the Metropolitan Detroit Cancer Surveillance System (MDCSS), which is a founding member of National Cancer Institute supported Surveillance Epidemiology and End Results (SEER) Program. There were 359 invasive rectal cancer cases among the 1163 final eligible colorectal cancer patients that consented and provided valid questionnaire data to the parent study. At enrolment, the study participants were interviewed over the telephone using structured questionnaires regarding their usual diet and other risk factors for colorectal cancer for the time-period preceding cancer diagnosis (approximately 2 years prior to the interview) to rule out influences of the disease, and provided a biospecimen for genotyping assays. A validated semi-quantitative food frequency questionnaire, Block 98.2 (Block Dietary Data Systems, Berkeley, CA), was used to estimate daily nutrient (including individual fatty acid groups) intake. Energy-adjusted nutrient intake was calculated by means of the residual method [23]. Participants were also queried about regular use of selected common medications, which was defined as at least 3–4 times per week for 6 months or longer. Body mass index (kg/m2) and physical activity (weighted sum of time the subject spent per 24 hours) were calculated as described previously [21,22]. Smoking and alcohol use status was dichotomized, current smoker vs not and daily alcohol drinker vs not, respectively. Information concerning clinical and histological characteristics of tumor, vital status (as of July 1, 2014) and cause of death was obtained through linkage with MDCSS. Causes of deaths (ICD10) were divided into two groups, cancer specific (cancers of the intestinal tract, liver and unknown primary sites) or other deaths according to one of the site-specific schemes for cause-specific deaths used in SEER program. The classification was made without knowledge of patients' radiation therapy status.
Statistical analysis
We adopted the joint Cox proportional hazards models proposed by Xue et al [24] for evaluating multiple outcomes in prospective cohort studies to analyze simultaneously cancer specific deaths and other deaths. This model was originally proposed for the analysis of competing risk events [25]. Hazard ratios (HR) for deaths and 95% confidence intervals (CIs) were calculated according to selected patients' characteristics, and were adjusted for age, sex and stage of the disease, for all patients, patients treated by radiation and those not treated by radiation. These basic covariates were screened from those associated with overall survival at least at p<0.10 level. Continuous covariates were grouped into quintiles based on distributions of the cases and controls combined to calculate HRs compared with the lowest quintile (the reference category). We performed tests for linear trend in HRs for these variables using ordinal scores for each category. Furthermore, we tested the interactions between radiation treatment and these ordinal or binary covariates by including their multiplicative interaction terms. A difference in the HRs between two groups of the events was tested based on their regression coefficients and standard errors using a Z test. All statistical analyses were performed using SAS version 9.2.
Results
38% (137 out of 359) of the patients received radiation treatment as part of the initial course of cancer targeted therapy (Table 1). Among those who had also surgical resection (N=121), 49% had radiation preoperatively and 54% had postoperatively (3% for both) (data not shown). Most (92%) of the patients treated with radiation also received chemotherapy and the patients treated with radiation were diagnosed at more advanced stage than those without radiation (P<0.01). A total of 159 patients were found to be deceased after the median follow-up of 9.2 years, and 70% of them were considered to be cancer specific.
Table 1.
Characteristics of 359 rectal cancer patients enrolled in an epidemiologic study by radiation therapy status
| Characteristics | No radiation therapy (N=222) |
Radiation therapy (N=137) |
|||
|---|---|---|---|---|---|
| No. | (%)* | No. | (%)* | ||
| Age | 45–49 | 17 | 8% | 11 | 8% |
| 50–54 | 49 | 22% | 27 | 20% | |
| 55–59 | 38 | 17% | 28 | 20% | |
| 60–64 | 25 | 11% | 22 | 16% | |
| 65–69 | 31 | 14% | 16 | 12% | |
| 70–74 | 28 | 13% | 20 | 15% | |
| 75–80 | 34 | 15% | 13 | 9% | |
| sex | Female | 109 | 49% | 63 | 46% |
| Male | 113 | 51% | 74 | 54% | |
| Race | African | 63 | 28% | 21 | 15% |
| Other | 159 | 72% | 116 | 85% | |
| Cancer stage | Local/regional | 154 | 69% | 68 | 50% |
| Node or distant metastasis | 68 | 31% | 69 | 50% | |
| Surgery | No | 25 | 11% | 16 | 12% |
| Local excision | 59 | 27% | 15 | 11% | |
| Segmental resection | 114 | 51% | 65 | 47% | |
| Proctectomy | 24 | 11% | 41 | 30% | |
| Chemotherapy | No | 185 | 83% | 11 | 8% |
| Received | 37 | 17% | 126 | 92% | |
| Vital status | Alive | 132 | 59% | 68 | 50% |
| Died of cancer | 59 | 27% | 53 | 39% | |
| Died of other causes | 31 | 14% | 16 | 12% | |
column percentages within groups (no radiation or radiation)
Smoking and a history of diabetes were associated with an increased probability of deaths from other causes (HR 3.20, 95% CI 1.72–5.97 and HR 2.02, 95% CI 0.98–4.16, respectively), while there were no associations between these two factors and cancer specific death. On the other hand regular use of non-steroidal anti-inflammatory drugs (NSAIDs) was inversely correlated with cancer-specific mortality (HR 0.50, 95% CI 0.30–0.81). As a result, the HRs associated with these three factors were significantly different from cancer specific to other deaths (P=0.019, 0.015 and 0.041, respectively). There were no overall associations of any of the nutritional factors or physical activity with either type of outcomes (Table 2).
Table 2.
Hazard ratio (HR) and 95% confidence interval (CI) for cancer specific and other deaths associated with selected patient characteristics
| Patient characteristics | Patients | Cancer specific death | Other death | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|
| Events | HR | 95% CI | Events | HR | ||||
| Smoking status | No | 281 | 84 | 1.00 | 31 | 1.00 | ||
| Yes | 77 | 28 | 1.25* | 0.79–1.98 | 16 | 3.20* | 1.72–5.97 | |
| Alcohol | Not daily | 280 | 90 | 1.00 | 33 | 1.00 | ||
| Daily | 79 | 22 | 0.76 | 0.45–1.27 | 14 | 1.61 | 0.85–3.03 | |
| Non-steroidal anti-inflammatory drugs | No | 245 | 89 | 1.00 | 28 | 1.00 | ||
| Used | 114 | 23 | 0.50* | 0.30–0.81 | 19 | 1.11* | 0.61–2.02 | |
| Diabetes | No | 313 | 100 | 1.00 | 36 | 1.00 | ||
| Ever | 46 | 12 | 0.66* | 0.37–1.18 | 11 | 2.02* | 0.98–4.16 | |
| Physical activity index | <27.14 | 71 | 33 | 1.00 | 9 | 1.00 | ||
| 27.14–28.6 | 73 | 23 | 0.80 | 0.45–1.40 | 11 | 1.22 | 0.50–2.99 | |
| 28.6–30.5 | 71 | 16 | 0.63 | 0.35–1.15 | 13 | 1.57 | 0.68–3.63 | |
| 30.6–34.5 | 72 | 16 | 0.52 | 0.27–0.99 | 8 | 0.94 | 0.37–2.44 | |
| >34.5 | 72 | 24 | 0.94 | 0.52–1.69 | 6 | 0.74 | 0.26–2.10 | |
| Trend | P=0.397 | P=0.490 | ||||||
| BMI (kg/m2) | <23.76 | 71 | 24 | 1.00 | 8 | 1.00 | ||
| 23.77–25.85 | 72 | 23 | 1.06 | 0.60–1.88 | 11 | 1.93 | 0.78–4.78 | |
| 25.86–28.4 | 72 | 19 | 0.67 | 0.36–1.27 | 9 | 1.09 | 0.42–2.86 | |
| 28.5–32.55 | 72 | 22 | 0.69 | 0.38–1.28 | 10 | 1.09 | 0.42–2.85 | |
| >32.56 | 72 | 23 | 0.99 | 0.57–1.73 | 9 | 1.36 | 0.53–3.53 | |
| Trend | P=0.526 | P=0.992 | ||||||
| Calories (kcal/day) | <1490 | 71 | 22 | 1.00 | 13 | 1.00 | ||
| 1490–1940 | 72 | 21 | 1.18 | 0.63–2.22 | 7 | 0.65 | 0.25–1.72 | |
| 1941–2450 | 72 | 22 | 1.19 | 0.64–2.18 | 9 | 0.88 | 0.38–2.05 | |
| 2451–3080 | 72 | 20 | 0.85 | 0.44–1.65 | 9 | 0.91 | 0.38–2.18 | |
| >3081 | 72 | 27 | 1.44 | 0.74–2.79 | 9 | 1.10 | 0.43–2.79 | |
| Trend | P=0.592 | P=0.746 | ||||||
| % fat | <32.0 | 71 | 23 | 1.00 | 11 | 1.00 | ||
| 32.0–36.1 | 72 | 28 | 1.36 | 0.76–2.46 | 11 | 1.18 | 0.50–2.75 | |
| 36.2–39.6 | 72 | 28 | 1.71 | 0.96–3.05 | 9 | 1.09 | 0.44–2.68 | |
| 39.7–42.7 | 72 | 15 | 0.78 | 0.41–1.50 | 8 | 0.80 | 0.32–2.02 | |
| >42.8 | 72 | 18 | 0.85 | 0.45–1.60 | 8 | 0.82 | 0.32–2.13 | |
| Trend | P=0.246 | P=0.475 | ||||||
| % carbohydrate | <40.3 | 71 | 16 | 1.00 | 10 | 1.00 | ||
| 40.3–44.8 | 72 | 21 | 1.48 | 0.75–2.92 | 8 | 0.86 | 0.33–2.23 | |
| 44.9–49.1 | 72 | 23 | 1.69 | 0.88–3.25 | 11 | 1.10 | 0.47–2.57 | |
| 49.2–53.9 | 72 | 26 | 1.69 | 0.86–3.32 | 6 | 0.51 | 0.18–1.47 | |
| >=54.0 | 72 | 26 | 1.55 | 0.78–3.08 | 12 | 1.02 | 0.41–2.54 | |
| Trend | P=0.202 | P=0.772 | ||||||
| Glycemic load (g/day) | <84.5 | 71 | 19 | 1.00 | 8 | 1.00 | ||
| 84.6–109.6 | 72 | 23 | 1.18 | 0.61–2.28 | 12 | 1.61 | 0.66–3.92 | |
| 109.7–145 | 72 | 22 | 0.96 | 0.51–1.82 | 10 | 1.37 | 0.55–3.40 | |
| 145.5–183 | 72 | 17 | 0.86 | 0.43–1.70 | 8 | 1.09 | 0.42–2.85 | |
| >=183 | 72 | 31 | 1.61 | 0.86–3.03 | 8 | 1.52 | 0.57–4.08 | |
| Trend | P=0.303 | P=0.726 | ||||||
HR: adjusted for age, sex and stage, based on joint Cox proportional hazards models
HRs significantly differed between cancer specific and other deaths (p<0.05)
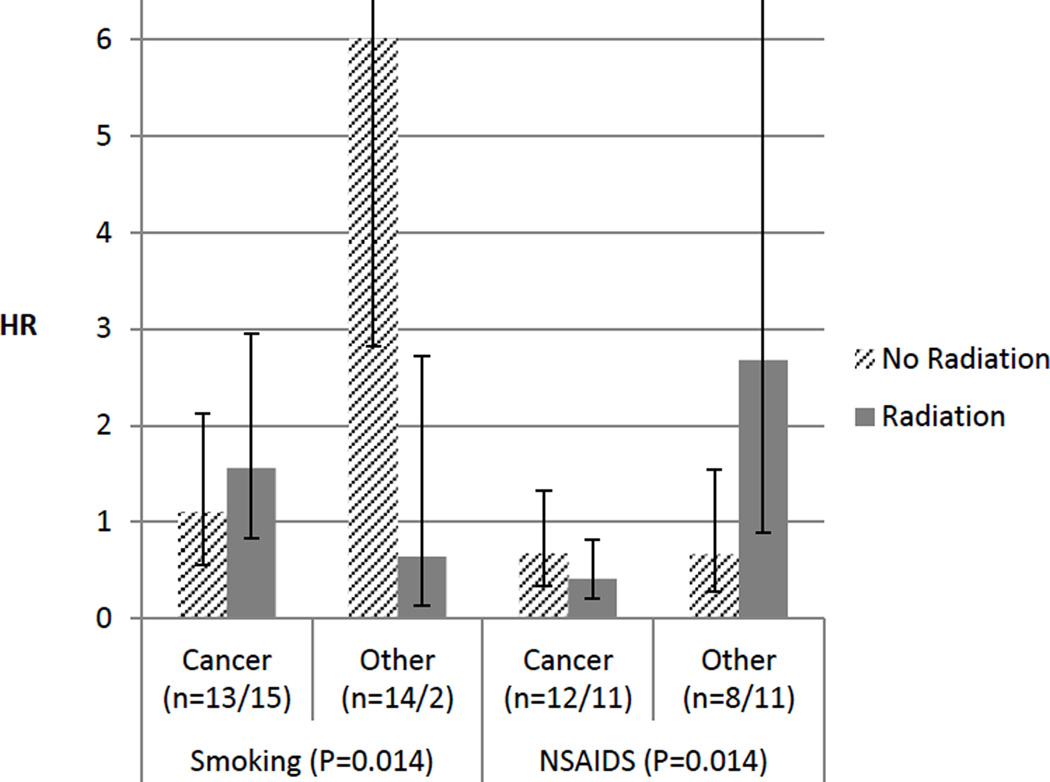
We further analyzed the data, stratified by radiation therapy status. As illustrated in Fig 1, among the patients who were irradiated, smoking tended to increase cancer specific deaths but to reduce other deaths, while the increased risk of deaths from other causes associated with smoking was apparent among those not irradiated (HR=6.01 95% CI 2.82–12.78). The HR for cancer specific deaths was significantly reduced in only the regular NSAID users who had radiation (HR=0.41 95% CI 0.21–0.81), compared with non-users with radiation, which was also significantly different (P=0.005) from the corresponding HR for other deaths in the patients who were irradiated (2.68, 95% CI 0.89–8.08). The association of these two factors (smoking and NSAIDs) with the two different types of deaths (cancer vs others) significantly varied with radiation status (P-values for the interactions= 0.014 for both). Further adjustment of these two factors each other did not change the significance of the associations (data not shown). There were no monotonic increasing or decreasing trends in risk of either type of deaths with any nutritional factor by radiation status. However, among the patients with radiation therapy, cancer specific HR was decreased with higher calories from fat (top vs bottom HR 0.63 95% CI 0.26–1.53), while that for other deaths was reduced with high glycemic load (top vs bottom HR 0.19, 95% CI 0.02–1.66).
Fig. 1.
Hazard ratios (HRs) for cancer specific deaths and for other deaths associated with smoking and non-steroidal anti-inflammatory drugs (NSAIDS) use status, stratified by history radiation therapy (striped bars: no radiation, solid bars: radiation). The extended lines from the top of the bars indicate 95% confidence intervals. The "n" in the parentheses indicates the numbers of each event for no radiation and radiation groups and the p-values in the parentheses those for the interactions between smoking/NSAIDS with radiation therapy status and causes of deaths.
We also analyzed whether a relative ketogenic diet, defined as >=40% calorie from fat and <100g/day glycemic load based on overall distributions, influences the outcome of radiation therapy (Table 3). We observed a marginally significantly reduced risk of cancer specific death in the patients who had the relative ketogenic diet overall (HR=0.49, 95% 0.23–1.02). Although the cancer specific HR associated with the ketogenic diet differed minimally by radiation treatment status, it was significantly lower than the corresponding HR for other deaths in the irradiated group only (P= 0.025).
Table 3.
Hazard ratio (HR) and 95% confidence interval (CI) for cancer specific and other deaths associated with ketogenic diet by radiation therapy status
| Patient groups |
Diet type | Patients | Cancer specific death | Other death | 95% CI | |||
|---|---|---|---|---|---|---|---|---|
| Events | HR | 95% CI | Events | HR | ||||
| All patients | Non-ketogenic diet | 311 | 104 | 1.00 | 41 | 1.00 | ||
| Ketogenic-diet | 48 | 8 | 0.49 | 0.23–1.02 | 6 | 0.80 | 0.34–1.87 | |
| No radiation | Non-ketogenic diet | 192 | 55 | 1.00 | 29 | 1.00 | ||
| Ketogenic-diet | 30 | 4 | 0.49 | 0.16–1.49 | 2 | 0.35 | 0.08–1.52 | |
| Radiation | Non-ketogenic diet | 119 | 49 | 1.00 | 12 | 1.00 | ||
| Ketogenic-diet | 18 | 4 | 0.47* | 0.18–1.27 | 4 | 2.52* | 0.82–7.69 | |
| Interaction of ketogenic diet with radiation and cause of deaths | P=0.111 | |||||||
Ketogenic diet was defined as >=40% calorie from fat and <100g/ day glycemic load
HR: adjusted for age, sex and stage, based on joint Cox proportional hazards models
HRs significantly differed between cancer specific and other deaths (p<0.05)
Discussion
The results of the present study demonstrate that some patient-related factors may exert disparate effects on cancer specific deaths and other deaths. Furthermore, their effects varied appreciably by radiation treatment status, suggesting a possibility that such patient-related factors may alter radiation sensitivity or radiation toxicity. These observations may have clinical translational value as such patient-related factors are modifiable, as opposed to tumor characteristics.
In our study population, smoking was associated with an increased risk of non-cancer specific deaths. Although cigarette smoking has been convincingly associated with an increased risk of colorectal neoplasms, the association with colorectal cancer itself has been rather modest, compared with more pronounced association with adenoma [26–28], suggesting a role in earlier stage of carcinogenesis. In contrast, smoking has been more firmly linked to other causes of deaths, such as cardiovascular diseases and respiratory diseases including lung cancer [29]. The fact that these causes of deaths comprised a substantial proportion (~50%) of non-cancer specific deaths is likely to account for the observed association.
Data from both observational studies and clinical trials suggest that regular NSAID and aspirin use reduces not only cancer incidence but also cancer mortality [30,31]. The evidence has been particularly strong and consistent for colorectal cancer, as these drugs have been demonstrated to reduce the risk of polyp recurrence as well as the incidence and mortality of colorectal cancers [30]. Although the preventive effects on cancer incidence and mortality are generally seen after a latent period of about 10 years [32], recent studies have highlighted their potential short term benefits on cancer incidence, death, survival and risk of metastasis [31,33–35]. Thus, reduced risk of cancer specific mortality observed in our study is consistent with these observations.
Interestingly, these two personal exposures had differential effects on cancer-specific and other deaths depending on radiation therapy status. Hypoxic cells are not only resistant to radiation because of low oxygen level per se, but also induce angiogenesis and fibrosis, as well as are important selective force in the progression of cancer, i.e., local invasion and distant metastasis [36–39]. These pathophysiological changes are mediated by a nuclear transcriptional regulator, hypoxia-inducible factor (HIF) [37,38]. Accordingly, over-expression of HIF-1α and other hypoxia-responsive genes has been associated with increased risk of treatment failure and recurrence and reduced overall and disease-free survival [40–44]. Not only does smoking cause a low-grade hypoxia due to elevated carbon monoxide levels [18], but also nicotine induces HIF-1α expression leading to radioresistance in a lung cancer xenograft model [19].
HIF-1α activation leads to the change in tumor energy metabolism from mitochondrial respiration to anaerobic glycolysis which is considered a hallmark of cancer in order to meet increased energy need for rapid cell proliferation (so called Warburg Effect) [45,46] and, metabolic intermediates of the glycolytic pathway, such as lactate and pyruvate, also confer radioresistance and promote tumor progression [47,12]. Aspirin and Ibuprofen, commonly used NSAIDs, interfere with these pathways, through inhibition of key enzymes, such as mTOR and COX2 [20,14]. These are potential underlying mechanisms may account for the suggestive association of smoking and NSAIDs use with cancer specific mortality that appeared to be more pronounced in the patients who underwent radiation.
A growing body of evidence also supports the suggestion that ketogenic conditions (via short term starvation or high fat/low carbohydrate diet) are useful to target the Warburg Effect by limiting a substrate for anaerobic glycolysis [48], and thus deter tumor progression/proliferation [15,12] and potentiate the effects of radiation or chemoradiation therapies in animal models [16,17]. Therapeutic ketogenic diets typically prescribed for epilepsy use a very fat/carbohydrate plus protein ratio, i.e., 4:1 [17], beyond regular weight loss diets, and thus was not translatable to our study population. Instead, we defined a relative ketogenic diet based on overall distribution of percent fat and glycemic loads. Even so, our data were supportive for the potential effects of ketogenic diet in reducing cancer specific mortality.
The strengths of the study include the population-based study design, high quality follow-up [49], sufficient follow-up time to identify a substantial number of cancer specific mortalities, use of a competing risk model and a variety of personal exposure data which are usually not available for clinical samples. On the other hand we realize several limitations. First, survival bias may be a concern as patients who succumbed quickly were not able to be included in the study as the case ascertainment through MDCSS followed by physician notification processes took 3–5 months from diagnosis and because patients who are gravely ill or on continuous treatment are generally unwilling to participate. This may have lowered the proportion of the patients with aggressive tumor or advanced stage, for which radiation treatment may be more often prescribed and thus may have resulted in biased estimates if such patients differed from those who participated in the study in the personal characteristics associated with cancer specific mortality. Second, we acknowledge that the classification of causes of death has influences on the results of the analyses and that there was some room for misclassification due to a lack of access to medical records. Deaths from complication from radiation toxicity could have been misclassified as non-cancer specific death. However, even if patients who were radiosensitive due to a certain characteristic suffered from such a complication, this should have led to a weakening of the interaction, rather than strengthening. Also, as is the nature of observational studies, we acknowledge that uncontrolled differences exist between patients who were treated by radiation and those who were not concerning a number of unmeasured variables, which may be confounded in the observed associations, and that the findings may not be considered to be as clear-cut or definitive as those from clinical trials. Furthermore, the parent study was designed to explore etiological risk factors of colorectal cancer, and consequently exposure data we collected reflect those before cancer diagnosis which could change after diagnosis and during treatment.
The present study was based on a posteriori analysis and involves multiple comparisons. Thus, we realize that some of the results may be simply a chance finding and a caution should be exercised in the interpretation of the results. In addition, when patients were grouped according to cause of deaths, radiation status and personal characteristics, the numbers of the patients and events in each stratum became considerably small. This may result in inaccurate risk estimates with wide confidence intervals and also restricted the number of covariates to be included in the statistical models. Finally, we are aware that other statistical models for competing risks in survival analysis exist [50,51], but the model used here has been considered to provide directly comparable risk estimates across all types of outcomes included.
Conclusions
Despite these limitations, this study provides novel observations that corroborate laboratory data and are potentially clinically useful to improve outcomes from radiation treatment if they are validated. Thus, the insights gained from this study may possibly lead to higher efficacy of radiotherapy in future patients by means of life-style modifications, which has been proven to be a cost effective intervention. Confirmatory prospective studies are necessary, including a larger sample size, better exposure assessment and more clearly defined patient populations, in order to develop new intervention protocol to be tested in clinical trials.
Acknowledgments
We thank Ms. Barbara Rusin, Dr. Maria Samerson and Ms. Ann Bankowski from Wayne State University for technical support and/or collection/selection of blood samples from cases and controls.
Funding Source(s)
This work was supported by Research Grants R01-CA93817 from National Cancer Institute and R21 DE023181fom National Institute of Dental and Craniofacial Research and Bridge Funding from Wayne State University.
Footnotes
Conflict of Interest statement:
Ikuko Kato declares no conflicts of interest.
Gregory Dyson declares no conflicts of interest.
Michael Snyder declares no conflicts of interest.
Hyeong-Reh Kim declares no conflicts of interest.
Richard K. Severson declares no conflicts of interest.
Statement of Ethical standards: The study has been approved by the appropriate ethics committee and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All persons gave their informed consent prior to their inclusion in the study.
References
- 1.Phang PT, Wang X. Current controversies in neoadjuvant chemoradiation of rectal cancer. Surgical oncology clinics of North America. 2014;23(1):79–92. doi: 10.1016/j.soc.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Glynne-Jones R, Harrison M, Hughes R. Challenges in the neoadjuvant treatment of rectal cancer: balancing the risk of recurrence and quality of life. Cancer radiotherapie : journal de la Societe francaise de radiotherapie oncologique. 2013;17(7):675–685. doi: 10.1016/j.canrad.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 3.Glimelius B. Neo-adjuvant radiotherapy in rectal cancer. World journal of gastroenterology : WJG. 2013;19(46):8489–8501. doi: 10.3748/wjg.v19.i46.8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birgisson H, Talback M, Gunnarsson U, Pahlman L, Glimelius B. Improved survival in cancer of the colon and rectum in Sweden. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2005;31(8):845–853. doi: 10.1016/j.ejso.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Martijn H, Voogd AC, van de Poll-Franse LV, Repelaer van Driel OJ, Rutten HJ, Coebergh JW. Improved survival of patients with rectal cancer since 1980: a population-based study. European journal of cancer (Oxford, England: 1990) 2003;39(14):2073–2079. doi: 10.1016/s0959-8049(03)00493-3. [DOI] [PubMed] [Google Scholar]
- 6.Spitale A, Franzetti-Pellanda A, Mazzola P, Richetti A, Mazzuchelli L, Bordoni A. Impact of preoperative radiotherapy on survival in locally advanced rectal cancer: an observational population-based study from the South of Switzerland. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP) 2012;21(2):139–146. doi: 10.1097/CEJ.0b013e32834c9c56. [DOI] [PubMed] [Google Scholar]
- 7.Ceelen W, Pattyn P, Boterberg T, Peeters M. Pre-operative combined modality therapy in the management of locally advanced rectal cancer. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2006;32(3):259–268. doi: 10.1016/j.ejso.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R. Preoperative versus postoperative chemoradiotherapy for rectal cancer. The New England journal of medicine. 2004;351(17):1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 9.Kong M, Hong SE, Choi WS, Kim SY, Choi J. Preoperative concurrent chemoradiotherapy for locally advanced rectal cancer: treatment outcomes and analysis of prognostic factors. Cancer Res Treat. 2012;44(2):104–112. doi: 10.4143/crt.2012.44.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carruthers R, Tho LM, Brown J, Kakumanu S, McCartney E, McDonald AC. Systemic inflammatory response is a predictor of outcome in patients undergoing preoperative chemoradiation for locally advanced rectal cancer. Colorectal Disease. 2012;14(10):e701–e707. doi: 10.1111/j.1463-1318.2012.03147.x. [DOI] [PubMed] [Google Scholar]
- 11.Toiyama Y, Inoue Y, Saigusa S, Kawamura M, Kawamoto A, Okugawa Y, Hiro J, Tanaka K, Mohri Y, Kusunoki M. C-Reactive Protein as Predictor of Recurrence in Patients with Rectal Cancer Undergoing Chemoradiotherapy Followed by Surgery. Anticancer Research. 2013;33(11):5065–5074. [PubMed] [Google Scholar]
- 12.Meijer TWH, Kaanders JHAM, Span PN, Bussink J. Targeting Hypoxia, HIF-1, and Tumor Glucose Metabolism to Improve Radiotherapy Efficacy. Clinical Cancer Research. 2012;18(20):5585–5594. doi: 10.1158/1078-0432.CCR-12-0858. [DOI] [PubMed] [Google Scholar]
- 13.Toustrup K, Sørensen BS, Nordsmark M, Busk M, Wiuf C, Alsner J, Overgaard J. Development of a Hypoxia Gene Expression Classifier with Predictive Impact for Hypoxic Modification of Radiotherapy in Head and Neck Cancer. Cancer Research. 2011;71(17):5923–5931. doi: 10.1158/0008-5472.CAN-11-1182. [DOI] [PubMed] [Google Scholar]
- 14.Semenza Gregg L. Hypoxia-Inducible Factors in Physiology and Medicine. Cell. 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. doi: http://dx.doi.org/10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov. 2011;10(9):671–684. doi: 10.1038/nrd3504. [DOI] [PubMed] [Google Scholar]
- 16.Safdie F, Brandhorst S, Wei M, Wang W, Lee C, Hwang S, Conti PS, Chen TC, Longo VD. Fasting Enhances the Response of Glioma to Chemo- and Radiotherapy. PLoS ONE. 2012;7(9):e44603. doi: 10.1371/journal.pone.0044603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdelwahab MG, Fenton KE, Preul MC, Rho JM, Lynch A, Stafford P, Scheck AC. The Ketogenic Diet Is an Effective Adjuvant to Radiation Therapy for the Treatment of Malignant Glioma. PLoS ONE. 2012;7(5):e36197. doi: 10.1371/journal.pone.0036197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hedblad B, Engström G, Janzon E, Berglund G, Janzon L. COHb% as a marker of cardiovascular risk in never smokers: Results from a population-based cohort study. Scandinavian Journal of Public Health. 2006;34(6):609–615. doi: 10.1080/14034940600590523. [DOI] [PubMed] [Google Scholar]
- 19.Warren GW, Romano MA, Kudrimoti MR, Randall ME, McGarry RC, Singh AK, Rangnekar VM. Nicotinic modulation of therapeutic response in vitro and in vivo. International Journal of Cancer. 2012;131(11):2519–2527. doi: 10.1002/ijc.27556. [DOI] [PubMed] [Google Scholar]
- 20.Din FVN, Valanciute A, Houde VP, Zibrova D, Green KA, Sakamoto K, Alessi DR, Dunlop MG. Aspirin Inhibits mTOR Signaling, Activates AMP-Activated Protein Kinase, and Induces Autophagy in Colorectal Cancer Cells. Gastroenterology. 2012;142(7):1504–1515. e1503. doi: 10.1053/j.gastro.2012.02.050. doi: http://dx.doi.org/10.1053/j.gastro.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato I, Land S, Majumdar AP, Barnholtz-Sloan J, Severson RK. Functional polymorphisms to modulate luminal lipid exposure and risk of colorectal cancer. Cancer epidemiology. 2010;34(3):291–297. doi: 10.1016/j.canep.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato I, Majumdar AP, Land SJ, Barnholtz-Sloan JS, Severson RK. Dietary fatty acids, luminal modifiers, and risk of colorectal cancer. International journal of cancer Journal international du cancer. 2010;127(4):942–951. doi: 10.1002/ijc.25103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. American Journal of Epidemiology. 1986;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 24.Xue X, Kim MY, Gaudet MM, Park Y, Heo M, Hollenbeck AR, Strickler HD, Gunter MJ. A Comparison of the Polytomous Logistic Regression and Joint Cox Proportional Hazards Models for Evaluating Multiple Disease Subtypes in Prospective Cohort Studies. Cancer Epidemiology Biomarkers & Prevention. 2013;22(2):275–285. doi: 10.1158/1055-9965.EPI-12-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lunn M, Don M. Applying Cox Regression to Competing Risks. Biometrics. 1995;51(2):524–532. [PubMed] [Google Scholar]
- 26.Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. Jama. 2008;300(23):2765–2778. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- 27.Botteri E, Iodice S, Raimondi S, Maisonneuve P, Lowenfels AB. Cigarette smoking and adenomatous polyps: a meta-analysis. Gastroenterology. 2008;134(2):388–395. doi: 10.1053/j.gastro.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Secretan B, Straif K, Baan R, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V. A review of human carcinogens—Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. The Lancet Oncology. 2009;10(11):1033–1034. doi: 10.1016/s1470-2045(09)70326-2. doi: http://dx.doi.org/10.1016/S1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 29.National Center for Chronic Disease P, Health Promotion Office on S, Health. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014. Reports of the Surgeon General. [Google Scholar]
- 30.IARC. Non-Steroidal Anti-Inflammatory Drugs. Lyon, France: IARC; 1997. IARC Handbooks of Cancer Prevention, vol 1. [Google Scholar]
- 31.Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. The Lancet Oncology. 2012;13(5):518–527. doi: 10.1016/S1470-2045(12)70112-2. doi: http://dx.doi.org/10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- 32.Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. The Lancet. 2007;369(9573):1603–1613. doi: 10.1016/S0140-6736(07)60747-8. doi: http://dx.doi.org/10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 33.Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, Lee R, Belch JF, Wilson M, Mehta Z, Meade TW. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379(9826):1602–1612. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 34.Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379(9826):1591–1601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 35.Bastiaannet E, Sampieri K, Dekkers OM, de Craen AJM, van Herk-Sukel MPP, Lemmens V, van den Broek CBM, Coebergh JW, Herings RMC, van de Velde CJH, Fodde R, Liefers GJ. Use of Aspirin postdiagnosis improves survival for colon cancer patients. Br J Cancer. 2012;106(9):1564–1570. doi: 10.1038/bjc.2012.101. doi: http://www.nature.com/bjc/journal/v106/n9/suppinfo/bjc2012101s1.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corvo R, Antognoni P, Sanguineti G. Biological predictors of response to radiotherapy in head and neck cancer: recent advances and emerging perspectives. Tumori. 2001;87(6):355–363. doi: 10.1177/030089160108700601. [DOI] [PubMed] [Google Scholar]
- 37.Semenza GL. Targeting HIF-1 for cancer therapy. Nature reviews Cancer. 2003;3(10):721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 38.Semenza GL. Intratumoral hypoxia, radiation resistance, and HIF-1. Cancer cell. 2004;5(5):405–406. doi: 10.1016/s1535-6108(04)00118-7. [DOI] [PubMed] [Google Scholar]
- 39.Yoo Y-G, Christensen J, Huang LE. HIF-1α Confers Aggressive Malignant Traits on Human Tumor Cells Independent of Its Canonical Transcriptional Function. Cancer Research. 2011;71(4):1244–1252. doi: 10.1158/0008-5472.CAN-10-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu XG, Xing CG, Feng YZ, Chen J, Deng C. Clinical significance of immunohistochemical expression of hypoxia-inducible factor-1alpha as a prognostic marker in rectal adenocarcinoma. Clinical colorectal cancer. 2006;5(5):350–353. doi: 10.3816/ccc.2006.n.005. [DOI] [PubMed] [Google Scholar]
- 41.Rasheed S, Harris AL, Tekkis PP, Turley H, Silver A, McDonald PJ, Talbot IC, Glynne-Jones R, Northover JM, Guenther T. Hypoxia-inducible factor-1alpha and-2alpha are expressed in most rectal cancers but only hypoxia-inducible factor-1alpha is associated with prognosis. Br J Cancer. 2009;100(10):1666–1673. doi: 10.1038/sj.bjc.6605026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Z, He X, Xia W, Huang Q, Zhang Z, Ye J, Ni C, Wu P, Wu D, Xu J, Qiu F, Huang J. Prognostic value and clinicopathological differences of HIFs in colorectal cancer: evidence from meta-analysis. PLoS One. 2013;8(12):e80337. doi: 10.1371/journal.pone.0080337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silva P, Slevin NJ, Sloan P, Valentine H, Cresswell J, Ryder D, Price P, Homer JJ, West CML. Prognostic Significance of Tumor Hypoxia Inducible Factor–1α Expression for Outcome After Radiotherapy in Oropharyngeal Cancer. International Journal of Radiation Oncology Biology Physics. 2008;72(5):1551–1559. doi: 10.1016/j.ijrobp.2008.07.051. doi: http://dx.doi.org/10.1016/j.ijrobp.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 44.Liang X, Zheng M, Jiang J, Zhu G, Yang J, Tang Y. Hypoxia-inducible factor-1 alpha, in association with TWIST2 and SNIP1, is a critical prognostic factor in patients with tongue squamous cell carcinoma. Oral Oncology. 2011;47(2):92–97. doi: 10.1016/j.oraloncology.2010.11.014. doi: http://dx.doi.org/10.1016/j.oraloncology.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 45.Yeung SJ, Pan J, Lee MH. Roles of p53, Myc and HIF-1 in Regulating Glycolysis — the Seventh Hallmark of Cancer. Cell Mol Life Sci. 2008;65(24):3981–3999. doi: 10.1007/s00018-008-8224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garber K. Energy Deregulation: Licensing Tumors to Grow. Science. 2006;312(5777):1158–1159. doi: 10.1126/science.312.5777.1158. [DOI] [PubMed] [Google Scholar]
- 47.Quennet V, Yaromina A, Zips D, Rosner A, Walenta S, Baumann M, Mueller-Klieser W. Tumor lactate content predicts for response to fractionated irradiation of human squamous cell carcinomas in nude mice. Radiotherapy and Oncology. 2006;81(2):130–135. doi: 10.1016/j.radonc.2006.08.012. doi: http://dx.doi.org/10.1016/j.radonc.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 48.Woolf EC, Scheck AC. The Ketogenic Diet for the Treatment of Malignant Glioma. Journal of Lipid Research. 2014 doi: 10.1194/jlr.R046797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kato I, Yee CL, Dombi GWK, J F, Severson RK. Race and other factors associated with differential follow-up in a population-based cancer registry. J Registr Manag. 2007;34:129–134. [Google Scholar]
- 50.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. 1988:1141–1154. [Google Scholar]
- 51.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]