Abstract
Introduction
Emerging evidence indicates associations between extra-central nervous system (CNS) bacterial infections and an increased risk for dementia; however, epidemiological evidence is still very limited.
Methods
This study involved a retrospective cohort of a national sample of US veterans (N = 417,172) aged ≥56 years. Extended Cox proportional hazard models adjusted for demographic characteristics and medical and psychiatric comorbidities determined the associations between systemic and localized extra-CNS bacterial infections occurring >2 years before the initial dementia diagnosis and the risk for dementia.
Results
Exposure to any extra-CNS bacterial infection was associated with a significantly increased risk for dementia (hazard ratio [HR] = 1.20 [95% confidence interval = 1.16–1.24]). Independently, septicemia (HR = 1.39 [1.16–1.66]), bacteremia (HR = 1.22 [1.00–1.49]), osteomyelitis (HR = 1.20 [1.06–1.37]), pneumonia (HR = 1.10 [1.02–1.19]), urinary tract infections (HR = 1.13 [1.08–1.18]), and cellulitis (HR = 1.14 [1.09–1.20]) were associated with a significantly increased risk for dementia.
Discussion
Both systemic and localized extra-CNS bacterial infections are associated with an increased risk for developing dementia.
Keywords: Dementia, Bacterial infections, Septicemia, Bacteremia, Pneumonia, Osteomyelitis, Urinary tract infections, Cellulitis, Veterans
1. Introduction
Dementia is a chronic degenerative disorder that is characterized by progressive global cognitive dysfunction. Emerging evidence from animal models indicates associations between extra-central nervous system (CNS) inflammatory events such as extra-CNS bacterial infections and the neuropathogenesis of dementia syndromes. For example, systemic inflammation—induced by extra-CNS injection of bacterial lipopolysaccharide—has been found to induce, potentiate, and exacerbate the development and propagation of dementia neuropathology in the brain of transgenic mice [1], [2], [3], [4]. Moreover, experiments also indicate that extra-CNS inflammatory events can induce various cognitive decrements including impaired memory [4], [5], [6], attention [7], [8], and executive functioning [8]. These findings suggest that extra-CNS bacterial infections could trigger, exacerbate, and potentiate the development and spread of dementia neuropathology, cause cognitive impairments, and possibly increase the risk for dementia among humans.
Indeed, emerging epidemiological research has linked extra-CNS bacterial infections to an increased risk for subsequent dementia among humans. For example, history of two or more extra-CNS infections was associated with a significantly increased risk for dementia among individuals aged 84 years or older [9], whereas collectively, previous hospitalization for cellulitis, urinary tract infections (UTIs), septicemia, and bacteremia was associated with a significantly increased risk for dementia in a community-based cohort [10].
Epidemiological evidence also shows significant associations between severe or systemic extra-CNS bacterial infections and increased risk for both cognitive impairments and dementia in humans. For example, sepsis significantly increased the risk for long-term cognitive moderate–severe cognitive impairments up to 8 years after the infection [11]. In addition, sepsis significantly increased risk for dementia among intensive care recipients after discharge (hazard ratio [HR] = 1.40 [95% confidence interval {CI} = 1.28–1.53]) [12].
Hospitalization for pneumonia has also been associated with a significantly increased risk for subsequent dementia [10], [13], as has chronic osteomyelitis that was demonstrated in a retrospective cohort study involving 17,238 newly diagnosed patients and 68,944 age- and gender-matched controls (relative risk = 1.6 [95% CI = 1.4–1.83]) [14].
Nevertheless, the epidemiological evidence for the association between extra-CNS bacterial infections during adulthood and the risk of dementia is still very limited; thus, further investigation is required. Moreover, the independent associations between less severe localized (e.g., cellulitis and UTIs) and other outpatient extra-CNS bacterial infections on the risk for dementia remain undetermined. Prior research also lacked sufficient adjustment for psychiatric comorbidities such as posttraumatic stress disorder, depression, and psychotic disorders (e.g., bipolar disorder) that have been associated with the risk for infections [15], [16], [17] and dementia [18], [19], [20], [21]. Thus, the purpose of this study was to conduct a comprehensive assessment of the associations between several systemic and localized extra-CNS bacterial infections including septicemia, bacteremia, pneumonia, osteomyelitis, septic arthritis, cellulitis, and UTIs and the risk for developing dementia in a national sample of United States (US) veterans with adjustment for demographic characteristics and medical and psychiatric comorbidities.
2. Methods
2.1. Study design and population
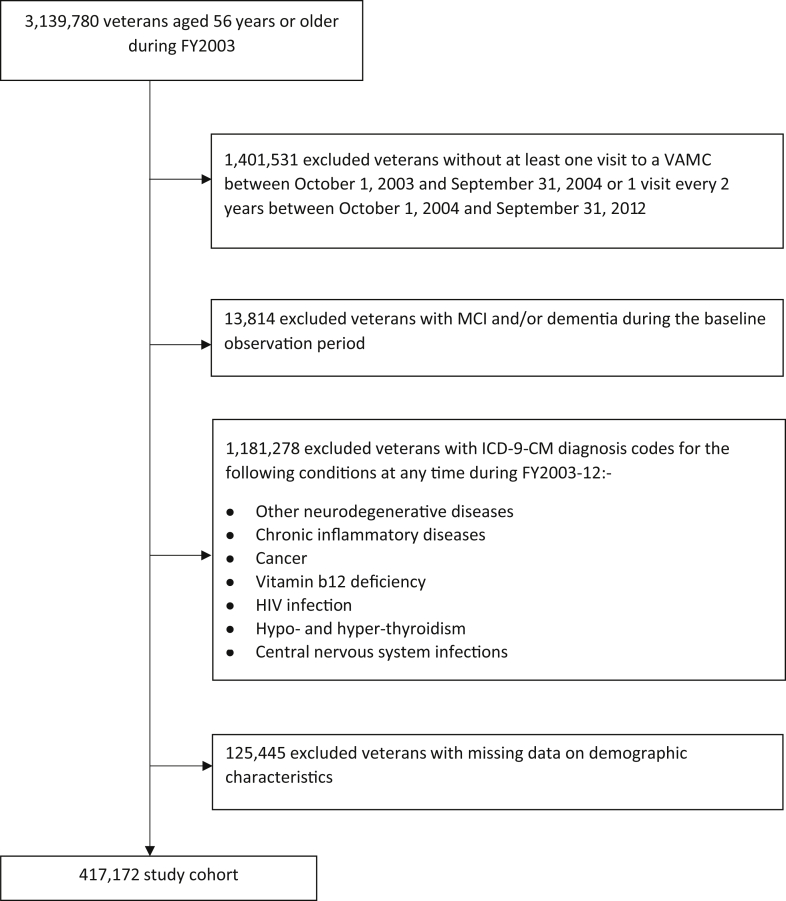
The study used a retrospective cohort study design. The sample population that comprised 3,139,780 veterans aged 56 years and older during fiscal year (FY) 2003 enrolled and receiving health care at any Veterans Health Administration (VHA) care facility in the US veterans were excluded (Fig. 1) if 1) they died or had clinical encounters containing International Classification of Diseases—9th edition (ICD-9) diagnosis codes for dementia or mild cognitive impairment during the baseline observation period (i.e., from October 01, 2002 through September 31, 2004); 2) had less than one VHA encounter every 2 years during the follow-up period (FY 2004–2012); and 3) had an ICD-9 code (Appendix A, Table A.1) for other neurodegenerative disorders, cancer, chronic inflammatory diseases, or conditions associated with potentially reversible or non-neurodegenerative cognitive impairments at any time during the study period. Thus, the final sample consisted of 417,172 veterans with complete information on all variables of interest. The Institutional Review Board of the University of Iowa and the Research and Development Committee of the Iowa City Veterans Affairs Health Care System reviewed and approved this study.
Fig. 1.
Subject selection process.
2.2. Data sources
Data were derived from the VHA national repository of databases including 1) the Patient Treatment File (PTF) Bed Section Files and 2) the Outpatient Care Files (OCF). The PTF Bed Section Files identify all VHA inpatient admissions and include data elements such as demographic characteristics and principal and secondary ICD-9 diagnoses. The OCF database contains data including demographic characteristics and principal and secondary ICD-9 diagnoses for each outpatient encounter at all VHA facilities. Unique identifiers allowed the linkage of the individual databases to create a comprehensive longitudinal medical record for each subject's health and health-care utilization.
2.3. Diagnosis of extra-CNS bacterial infections
Systemic and localized extra-CNS bacterial infections of interest included septicemia, bacteremia, pneumonia, osteomyelitis, septic arthritis, UTI, and cellulitis and were diagnosed using ICD-9 diagnosis codes [22], [23] (Appendix A, Table A.2) in the PTF Bed Section Files and OCF. All participants with ICD-9 diagnosis codes for the extra-CNS bacterial infections of interest either as primary or as secondary diagnosis during the follow-up period were classified as having an extra-CNS bacterial infection. To account for the association between preclinical (or the delayed diagnostic recording of) dementia and the increased risk of certain extra-CNS bacterial infections, only infections occurring >2 years before the date of the first dementia diagnosis were considered among participants with a diagnosis of dementia. Controls were participants without a diagnosis of an extra-CNS bacterial infection during the study period.
2.4. Follow-up and censoring
We collected data from the veterans' medical records until a code for dementia was identified in the medical record or until they were censored. Veterans were considered censored if they 1) did not develop dementia by the end of the 9-year follow-up period, 2) ceased to have any contact with a VHA facility during the follow-up period, or 3) died during the follow-up period.
2.5. Dementia diagnosis
The diagnosis of dementia was determined from the PTF Bed Section Files and OCF using ICD-9 diagnosis codes for dementias based on the previous studies [18], [19] and included 290.0x, 290.10, 290.11, 290.12, 290.13, 290.20, 290.21, 290.3x, and 331.2 (senile dementias); 290.40, 290.41, 290.42, and 290.43 (vascular dementia); 294.10, 294.11, and 294.8x (dementia not otherwise specified); 331.0 (Alzheimer's disease); 331.11 and 331.19 (frontotemporal dementia), and 331.82 (Lewy Body dementia).
2.6. Measurement of other covariates and potential confounders
Data on potential confounders were extracted from the PTF and OCF and included demographic characteristics (i.e., age, gender, race/ethnicity, and annual income), medical comorbidity (i.e., traumatic brain injury, hypertension, ischemic heart disease, cerebrovascular disease, atherosclerosis, diabetes mellitus, hyperlipidemia, chronic obstructive pulmonary disease, chronic kidney disease, chronic liver disease, and peptic ulcer disease/gastritis), and psychiatric comorbidity (i.e., posttraumatic stress disorder [PTSD], major depression, schizophrenia, bipolar disorder, anxiety disorder, and alcohol, tobacco, and illicit substance abuse). Medical and psychiatric comorbidities were diagnosed using ICD-9 codes (Appendix A, Table A.3).
2.7. Statistical analysis
Data were analyzed quantitatively using SAS® software, version 9.3, and alpha was set at 0.05. Mann–Whitney U (after assessment for normality) or chi-square test determined whether veterans with a diagnosis of an extra-CNS bacterial infection and the controls differed in terms of the continuous and categorical baseline covariates, respectively.
Extended Cox proportional hazards models with age as the time scale and extra-CNS bacterial infections as time-dependent covariates were used to determine whether the extra-CNS bacterial infections of interest were associated with the risk of developing dementia. The analysis was conducted in several steps. First, we assessed for potential collinearity among the medical and psychiatric covariates using phi coefficients and excluded one covariate from each pair of moderate strongly positively correlated comorbidities (ɸ ≥ 0.2) based on relevance in extant literature and/or clinical experience. Thus, medical and psychiatric covariates included in our adjustments were traumatic brain injury, hypertension, ischemic heart disease, cerebrovascular disease, atherosclerosis, diabetes mellitus, chronic obstructive pulmonary disease, chronic kidney disease, chronic liver disease, peptic ulcer disease/gastritis, bipolar disorder, PTSD, schizophrenia, and alcohol abuse.
Second, we determined the overall association between any extra-CNS bacterial infection and the risk for dementia in unadjusted and models adjusted for demographic characteristics and medical and psychiatric comorbidities.
Third, we considered each extra-CNS bacterial infection of interest as an independent risk factor for dementia and assessed the association between each particular extra-CNS bacterial infection of interest with the risk for dementia in unadjusted and models including adjustments for demographic characteristics and medical and psychiatric comorbidities.
3. Results
The average age of the participants at baseline was 67.7 (±8.1) years, and mean follow-up duration for all participants was 9.03 (±1.1) years. Most participants (97.9%) were male and 82% were white.
Of the 417,172 veterans in the final sample, 87,400 (21%) veterans had a diagnosis of at least one extra-CNS bacterial infection during the study period. Table 1 provides a summary of baseline characteristics of the veterans with and without a diagnosis of an extra-CNS bacterial infection during the study period. The most commonly diagnosed infection was UTI, whereas septic arthritis was least frequent (Table 2).
Table 1.
Comparison of the baseline characteristics of participants with and without a diagnosis of an extra-CNS bacterial infection during the study period
| Variables | No infections (n = 329,772) Mean (SD) or n (%) |
Infection (n = 87,400) Mean (SD) or n (%) |
P value |
|---|---|---|---|
| Demographic characteristics | |||
| Age | 68.12 (8.00) | 66.12 (8.27) | <.001 |
| Gender | <.001 | ||
| Male | 323,851 (98.20) | 84,685 (96.89) | |
| Race | <.001 | ||
| White | 277,803 (84.24) | 64,610 (73.92) | |
| Black | 30,312 (9.19) | 14,663 (16.78) | |
| Other | 21,675 (6.75) | 8127 (9.30) | |
| Income (percentile) | <.001 | ||
| 25th | 91,005 (27.60) | 13,304 (15.22) | |
| 50th | 74,213 (22.50) | 30,065 (34.40) | |
| 75th | 77,001 (23.35) | 27,293 (31.23) | |
| >75th | 78,553 (26.55) | 16,738 (19.15) | |
| Medical comorbidities | |||
| Traumatic brain injury | 659 (0.20) | 447 (0.51) | <.001 |
| Hypertension | 228,220 (69.21) | 65,476 (74.92) | <.001 |
| Ischemic heart disease | 99,549 (30.19) | 26,377 (30.18) | .965 |
| Cerebrovascular disease | 22,010 (6.67) | 8370 (9.58) | <.001 |
| Atherosclerosis | 3778 (1.15) | 2470 (2.83) | <.001 |
| Hyperlipidemia | 166,347 (50.44) | 41,896 (47.94) | <.001 |
| Diabetes | 85,024 (25.78) | 32,687 (37.40) | <.001 |
| Chronic obstructive pulmonary disease | 6282 (1.90) | 3691 (4.22) | <.001 |
| Kidney disease | 5960 (1.81) | 3101 (3.55) | <.001 |
| Liver disease | 111 (0.03) | 37 (0.04) | .226 |
| Gastric/peptic ulcer disease | 9441 (2.86) | 3566 (4.08) | <.001 |
| Psychiatric comorbidities | |||
| Posttraumatic stress disorder | 15,724 (4.7) | 6950 (7.95) | <.001 |
| Depression | 32,417 (9.83) | 13,056 (14.94) | <.001 |
| Bipolar disorder | 2920 (0.89) | 1571 (1.80) | <.001 |
| Substance abuse | |||
| Tobacco use | 30,132 (9.14) | 13,579 (15.54) | <.001 |
| Alcohol abuse | 36,605 (11.10) | 16,893 (19.33) | <.001 |
| Drug abuse | 2736 (0.83) | 2207 (2.53) | <.001 |
Abbreviations: CNS, central nervous system; SD, standard deviation.
Table 2.
Prevalence of extra-CNS bacterial infections
| Extra-CNS bacterial infection | N | % |
|---|---|---|
| Septicemia | 3484 | 0.84 |
| Bacteremia | 2368 | 0.57 |
| Pneumonia | 15,613 | 3.74 |
| Osteomyelitis | 5005 | 1.20 |
| Septic arthritis | 1000 | 0.24 |
| UTI | 40,186 | 9.63 |
| Cellulitis | 45,346 | 10.87 |
Abbreviations: CNS, central nervous system; UTI, urinary tract infection.
Compared with the controls, veterans with a diagnosis of at least one extra-CNS bacterial infection during the study period were significantly younger (mean age 66 vs 68 years, P < .001) and were significantly (P < .001) more likely to have diagnoses of most of the medical and psychiatric comorbidities known to be associated with the risk for dementia including hypertension, diabetes mellitus, cerebral vascular disease, atherosclerosis, chronic obstructive pulmonary disease, peptic ulcer disease, depression, bipolar disorder, alcohol abuse, tobacco use, and illicit drug abuse at baseline.
By our surveillance methods, 25,639 (6.15%) veterans developed dementia during the follow-up period. In unadjusted cox proportional hazard model, a diagnosis of at least one extra-CNS bacterial infection was associated with a significantly increased risk for dementia (HR = 1.50 [95% CI = 1.45–1.55]), which remained significant after adjustment for demographic characteristics and medical and psychiatric comorbidities (HR = 1.20 [95% CI = 1.16–1.24]) (Table 3).
Table 3.
Unadjusted and adjusted Cox proportional HRs for extra-CNS bacterial infections versus no infection and the risk for dementia
| Infection | Unadjusted |
Adjusted∗ |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Any infection | 1.50 (1.45–1.55) | <.001 | 1.18 (1.14–1.22) | <.001 |
| Septicemia | 2.09 (1.75–2.49) | <.001 | 1.39 (1.16–1.66) | <.001 |
| Bacteremia | 1.88 (1.54–2.29) | <.001 | 1.22 (1.00–1.49) | .046 |
| Pneumonia | 1.54 (1.43–1.67) | <.001 | 1.10 (1.02–1.19) | .013 |
| Osteomyelitis | 1.73 (1.52–1.97) | <.001 | 1.20 (1.06–1.37) | .006 |
| Septic arthritis | 1.43 (1.07–1.90) | .014 | 1.04 (0.78–1.39) | .772 |
| UTI | 1.44 (1.38–1.51) | <.001 | 1.13 (1.08–1.18) | <.001 |
| Cellulitis | 1.49 (1.42–1.56) | <.001 | 1.14 (1.09–1.20) | <.001 |
Abbreviations: CI, confidence interval; CNS, central nervous system; HR, hazard ratio; UTI, urinary tract infection.
Adjusted for demographics characteristics and medical and psychiatric comorbidity.
In unadjusted analyses, all extra-CNS bacterial infections of interest were each individually associated with an increased risk for dementia (Table 3). However, after adjustment for demographic characteristics and medical and psychiatric comorbidities, septicemia (HR = 1.39 [95% CI = 1.16–1.66]), bacteremia (HR = 1.22 [95% CI = 1.0–1.49]), pneumonia (HR = 1.10 [95% CI = 1.02–1.19]), osteomyelitis (HR = 1.20 [95% CI = 1.06–1.37]), UTI (HR = 1.13 [95% CI = 1.08–1.18]), and cellulitis (HR = 1.14 [95% CI = 1.09–1.20]) were each found to be separately associated with a statistically significant increased risk for dementia (Table 3). Although septic arthritis was associated with an increased risk for dementia, this result was not statistically significant (HR = 1.04 [95% CI = 0.78–1.39]).
We used subcohorts of individuals whose first infection was or included UTI or cellulitis and the controls to conduct several sensitivity analyses. First, we determined the impact of other concurrent or subsequent extra-CNS bacterial infections on the UTI and cellulitis associated risk for dementia, separately. Results showed both UTI and cellulitis significantly increased the risk for dementia even after adjustment for potential confounders and other concurrent or subsequent extra-CNS bacterial infections, HR = 1.51 (95% CI = 1.44–1.60) and HR = 1.28 (95% CI = 1.21–1.34), respectively.
Second, we determined the associations between recurrent UTIs and cellulitis with the risk for dementia. Infections were considered recurrent if they occurred >60 days after the previous infection. After adjustment for potential confounders, results showed that the risk for dementia increased significantly with both increasing number of UTI and cellulitis episodes, HR = 1.18 (95% CI = 1.16–1.20) and HR = 1.03 (95% CI = 1.01–1.06), respectively.
4. Discussion and limitations
The purpose of this study was to determine the associations between systemic and localized extra-CNS bacterial infections, that is, septicemia, bacteremia, pneumonia, osteomyelitis, septic arthritis, cellulitis, and UTI with risk of developing dementia in a large nationally representative sample of US veterans aged 56 years and older. Results showed statistically significant joint and separate associations between extra-CNS bacterial infections including septicemia, bacteremia, pneumonia, osteomyelitis, cellulitis, and UTI, with the risk for developing dementia after adjusting for demographic characteristics and medical and psychiatric comorbidities. These results were consistent with prior studies. For example, Shah et al. [13] and Tate et al. [10] found a significant association between hospitalization for pneumonia and an increased risk for subsequent dementia, whereas Tseng et al. [14] found a significant association between a diagnosis of chronic osteomyelitis and an increased risk for dementia. To our knowledge, our study is the first study to assess the independent impacts of UTI, cellulitis, and septic arthritis and on the risk for dementia. Results showed significant associations between UTI and cellulitis with the risk for dementia even after adjustment for other concurrent or subsequent more severe extra-CNS bacterial infections. Although septic arthritis was associated with an increased risk for dementia, this result was not statistically significant after adjustment for potential confounders possibly because of the low prevalence of septic arthritis in our sample.
4.1. Mechanisms of the association between infections and the risk for dementia
The association between severe extra-CNS bacterial infections or sepsis and acute cognitive dysfunction (i.e., sepsis associated encephalopathy or delirium) is well established. Sepsis has been associated with various detrimental central nervous system effects including decreased metabolism [24], oxidative stress [25], and impaired microcirculation [26], all of which are capable of causing permanent neuronal dysfunction and degeneration that might manifest as chronic cognitive deficits or dementia. Indeed, sepsis has been associated with an increased risk of long-term cognitive dysfunction [11] and dementia [12], and our results examining the associations between sepsis and the risk for dementia were consistent with prior research.
Alternatively, sepsis and other extra-CNS bacterial infections might be associated with dementia neuropathogenesis because of the effects of extra-CNS inflammatory events on the CNS. Extra-CNS bacterial infections have been associated with significant inflammation [27] and significantly increased levels of circulating pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α [27], [28]. Evidence indicates that circulating extra-CNS generated cytokines can activate CNS astrocytes and microglia [29], [30], which in turn can cause neuronal death and injury through various mechanisms including release of nitric oxide and reactive oxygen species, activation of the complement cascade, and neglect of essential neuroprotective roles [30], [31], [32], [33], [34]. In addition, circulating extra-CNS generated pro-inflammatory cytokines have been linked to blood–brain barrier disruption [35], [36], which can also cause neuronal injury, apoptosis [37], and dementia [38]. Furthermore, both activated CNS microglia and astrocytes and damaged or necrotic neurons release various chemokines and cytokines directly into the CNS, which cause further neuronal injury, and the recruitment and activation of additional astrocytes and microglia [39], [40], thus exacerbating the extra-CNS inflammation–induced neurotoxicity. These findings suggest that extra-CNS bacterial infections could result in a self-sustaining cycle of progressive neurodegeneration and, possibly, dementia [41].
Evidence also indicates that extra-CNS bacterial infections can directly induce neuronal destruction and apoptosis in the absence of inflammatory cytokine-mediated effects. For example, Murray et al. [42] found that peripherally injected bacterial liposaccharide directly stimulated CNS endothelium and primed microglia despite effective blockage of systemic cytokine synthesis. Thus, extra-CNS bacterial infections—either directly or indirectly through effects of systemic inflammation on the brain and blood brain barrier—pose risks for permanent and progressive neuronal dysfunction and degeneration that might manifest as chronic cognitive dysfunction or dementia.
However, because dementia neuropathology is likely to develop over several decades [43], [44], an alternative mechanism for the associations between extra-CNS bacterial infections and the risk for dementia exits. For example, it could be that extra-CNS infections during late adulthood exacerbate the development and spread of underlying CNS neuropathology because of infections earlier in life or other causes. Evidence from animal models indicates minimal activation of CNS astrocytes and microglia after initial stimulation by extra-CNS inflammatory events [45]; however, subsequent stimulation of previously exposed or primed astrocytes and microglia results in a markedly exaggerated inflammatory response [45], [46]. Moreover, Njie et al. [47] found microglia derived from aged animals secrete significantly greater amounts of pro-inflammatory cytokines IL-6 and TNF-α in response to stimulation compared with those from young animals. Henry et al. [48] also reported exaggerated cytokine production in aged microglia, whereas Damani et al. [49] found aged microglia to be associated with a more sustained inflammatory responses. Indeed, systemic inflammation has been found to exacerbate and potentiate the propagation of underlying dementia neuropathology in the brains of transgenic mice [10], [11], [12], [13].
Our results showed significant combined and separate associations between both systemic and localized extra-CNS bacterial infections and an increased risk for dementia. Moreover, the association between extra-CNS bacterial infections and the risk for dementia was stronger for severe or systemic compared with the less severe or localized extra-CNS bacterial infections. Also, the risk for dementia increased significantly with increasing number of UTIs and cellulitis episodes. Taken together, these findings could indicate a dose–response type of association. However, further studies are required to elucidate the exact mechanisms for the associations between extra-CNS bacterial infections and dementia pathogenesis.
4.2. Strengths and limitations
Our study has several limitations. First, we did not adjust for nonbacterial extra-CNS infections such as viral and fungal infections, which might also result in systemic inflammation. Nevertheless, bacterial infections have been found to induce a more robust systemic inflammatory response as evidenced by significantly higher serum concentrations of cytokines including IL-1Ra, IL-2, IL-6, and TNF-α compared with viral infections [37], and we adjusted for many potential confounders and effect modifiers including psychiatric comorbidity not accounted for in the previous analyses.
Second, we used ICD-9 codes for diagnosis of the primary exposures, outcomes, and medical and psychiatric comorbidities, and this could result in possible misclassification bias in our analyses. However, the agreement between inpatient diagnoses abstracted from the VHA administrative files using ICD-9 codes and written medical records for various medical conditions (including most of our variables of interest) has been estimated at 98.3% [50]. Positive predictive values associated with ICD-9 codes in detecting clinical bacterial infections in the VHA database range from 70% for pneumonia and cellulitis to 95% for osteomyelitis [22].
Third, our sample was mainly white and male and because veterans can elect to use services other than the VHA for primary health care and the fact that our sample was restricted largely to regular users of the VHA, our findings might not be generalizable to females, non-whites, all military veterans, or nonmilitary populations. In addition, it is possible that certain infections were diagnosed outside the VHA and not included in our analysis. Nevertheless, our sample was largely restricted to regular VHA system users and included both in and outpatient data to capture most infections in the sample.
Strengths of this study include the retrospective cohort study design, very large sample size, adjustment for multiple potential confounders, and time-dependent analysis for the effect of infections. In addition, because the VHA maintains electronic records on veterans across the country, we believe our study is largely representative of the overall veteran population.
4.3. Conclusions
In conclusion, systemic and localized extra-CNS bacterial infections are collectively and independently associated with an increased risk of developing dementia among older US veterans. Thus, prevention of extra-CNS bacterial infections could positively influence the risk for developing dementia among older adults. Further basic and epidemiological research in different populations is recommended to elucidate the mechanisms and associations between systemic bacterial infections and the risk for dementia.
Research in context.
-
1.
Systematic review: We searched PubMed, Web of Science, and EBSCO databases. Emerging experimental and epidemiological evidence suggests an association between extra-central nervous system (CNS) bacterial infections and the risk for dementia. However, the epidemiological evidence is still very limited and requires further investigation. Moreover, the independent associations between urinary tract infections, cellulitis, and septic arthritis with the risk for dementia remain undetermined.
-
2.
Interpretation: Systemic and localized extra-CNS bacterial infections are jointly and independently associated with an increased risk for developing dementia. The association was stronger for severe or systemic compared with the less severe or localized extra-CNS bacterial infections, which could indicate a dose–response type of association.
-
3.
Future directions: Prevention of extra-CNS bacterial infections could influence the risk for developing dementia among older adults. Further basic and epidemiological research in different populations is recommended to elucidate the mechanisms and associations between extra-CNS bacterial infections and the risk for dementia.
Acknowledgments
Support for this project was provided by the Department of Veterans Affairs Health Services Research and Development Service (Abrams: CDA 10-016). None of these sponsors had any role in the study design, methods, analyses, and interpretation or in preparation of the manuscript and the decision to submit it for publication. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2016.08.004.
Supplementary data
References
- 1.Guo J.T., Yu J., Grass D., de Beer F.C., Kindy M.S. Inflammation dependent cerebral deposition of serum amyloid a protein in a mouse model of amyloidosis. J Neurosci. 2002;22:5900–5909. doi: 10.1523/JNEUROSCI.22-14-05900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kitazawa M., Oddo S., Yamasaki T.R., Green K.N., LaFerla F.M. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dpendent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. J Neurosci. 2005;25:8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheng J.G., Bora S.H., Xu G., Borchelt D.R., Price D.L., Koliatsos V.E. Lipopolysaccharide-induced-neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid peptide in APPswe transgenic mice. Neurobiol Dis. 2003;14:133–145. doi: 10.1016/s0969-9961(03)00069-x. [DOI] [PubMed] [Google Scholar]
- 4.Sy M., Kitazawa M., Medeiros R., Whitman L, Cheng D, Lane TE. Inflammation induced by infection potentiates tau pathological features in transgenic mice. Am J Pathol. 2011;178:2811–2822. doi: 10.1016/j.ajpath.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J.W., Lee Y.K., Yuk D.Y., Choi D.Y., Ban S.B., Oh K.W. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation. 2008;5:37. doi: 10.1186/1742-2094-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valero J., Mastrella G., Neiva I., Sánchez S., Malva J.O. Long-term effects of an acute and systemic administration of LPS on adult neurogenesis and spatial memory. Front Neurosci. 2014;8:83. doi: 10.3389/fnins.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holden J.M., Meyers-Manor J.E., Overmier J.B., Gahtan E., Sweeney W., Miller H. Lipopolysaccharide-induced immune activation impairs attention but has little effect on short-term working memory. Behav Brain Res. 2008;194:138–145. doi: 10.1016/j.bbr.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 8.Culley DJ, Snayd M., Baxter M.G., Xie Z., Lee I.H., Rudolph J. Systemic inflammation impairs attention and cognitive flexibility but not associative learning in aged rats: possible implications for delirium. Front Aging Neurosci. 2014;6:107. doi: 10.3389/fnagi.2014.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn N., Mullee N., Perry H., Holmes C. Association between dementia and infectious disease evidence from a case-control study. Alzheimer Dis Assoc Disord. 2005;19:91–94. doi: 10.1097/01.wad.0000165511.52746.1f. [DOI] [PubMed] [Google Scholar]
- 10.Tate J.A., Snitz B.E., Alvarez K.A., Nahin R.L., Weissfeld L.A., Lopez O. Infection hospitalization increases risk of dementia in the elderly. Crit Care Med. 2014;42:1037–1046. doi: 10.1097/CCM.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwashyna T.J., Ely E.W., Smith D.M., Langa K.M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerra C., Linde-Zwirble W.T., Wunsch H. Risk factors for dementia after critical illness in elderly Medicare beneficiaries. Crit Care. 2012;16:R233. doi: 10.1186/cc11901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah F.A., Pike F., Alvarez K., Angus D., Newman A.B., Lopez O. Bidirectional relationship between cognitive function and pneumonia. Am J Respir Crit Care Med. 2013;188:586–592. doi: 10.1164/rccm.201212-2154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tseng C.H., Huang W.S., Muo C.H., Kao C.H. Increased risk of dementia among chronic osteomyelitis patients. Eur J Clin Microbiol Infect Dis. 2015;34:153–159. doi: 10.1007/s10096-014-2200-1. [DOI] [PubMed] [Google Scholar]
- 15.Benros M.E., Waltoft B.L., Nordentoft M., Ostergaard S.D., Eaton W.W., Krogh J. Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMA Psychiatry. 2013;70:812–820. doi: 10.1001/jamapsychiatry.2013.1111. [DOI] [PubMed] [Google Scholar]
- 16.Boscarino J.A. Diseases among men 20 years after exposure to severe stress: implications for clinical research and medical care. Psychosom Med. 1997;59:605–614. doi: 10.1097/00006842-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Seminog O.O., Goldacre M.J. Risk of pneumonia and pneumococcal disease in people with severe mental illness: English record linkage studies. Thorax. 2013;68:171–176. doi: 10.1136/thoraxjnl-2012-202480. [DOI] [PubMed] [Google Scholar]
- 18.Qureshi S.U., Kimbrell T., Pyne J.M., Magruder K.M., Hudson T.J., Petersen N.J. Greater prevalence and incidence of dementia in older veterans with posttraumatic stress disorder. J Am Geriatr Soc. 2010;58:1627–1633. doi: 10.1111/j.1532-5415.2010.02977.x. [DOI] [PubMed] [Google Scholar]
- 19.Yaffe K., Vittinghoff E., Lindquist K., Barnes D., Covinsky K.E., Neylan T. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67:608–613. doi: 10.1001/archgenpsychiatry.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao Y., Huang C., Zhao K., Ma L., Qiu X., Zhang L. Depression as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Int J Geriatr Psychiatry. 2013;28:441–449. doi: 10.1002/gps.3845. [DOI] [PubMed] [Google Scholar]
- 21.Wu K.Y., Chang C.M., Liang H.Y., Wu C.S., Chia-Hsuan Wu E., Chen C.H. Increased risk of developing dementia in patients with bipolar disorder: a nested matched case-control study. Bipolar Disord. 2013;15:787–794. doi: 10.1111/bdi.12116. [DOI] [PubMed] [Google Scholar]
- 22.Schneeweiss S., Robicsek A., Scranton R., Zuckerman D., Solomon D.H. Veteran's affairs hospital discharge databases coded serious bacterial infections accurately. J Clin Epidemiol. 2007;60:397–409. doi: 10.1016/j.jclinepi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Angus D.C., Linde-Zwirble W.T., Lidicker J., Clermont G., Carcillo J., Pinsky M.R. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Semmler A., Hermann S., Mormann F., Weberpals M., Paxian S.A., Okulla T. Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism. J Neuroinflammation. 2008;15:38. doi: 10.1186/1742-2094-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barichello T., Fortunato J.J., Vitali A.M., Feier G., Reinke A., Moreira J.C. Oxidative variables in the rat brain after sepsis induced by cecal ligation and perforation. Crit Care Med. 2006;34:886–889. doi: 10.1097/01.CCM.0000201880.50116.12. [DOI] [PubMed] [Google Scholar]
- 26.Taccone FS, Su F., Pierrakos C., He X., James S., Dewitte O. Cerebral microcirculation is impaired during sepsis: an experimental study. Crit Care. 2010;14:R140. doi: 10.1186/cc9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holub M., Lawrence D.A., Andersen N., Davidová A., Beran O., Marešová V. Cytokines and chemokines as biomarkers of community-acquired bacterial infection. Mediators Inflamm. 2013;2013:190145. doi: 10.1155/2013/190145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cannon J.G., Tompkins R.G., Gelfand J.A., Michie H.R., Stanford G.G., van der Meer J.W. Circulating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin fever. J Infect Dis. 1990;161:79–84. doi: 10.1093/infdis/161.1.79. [DOI] [PubMed] [Google Scholar]
- 29.Hannestad J., Gallezot J., Schafbauer T., Lim K., Kloczynski T., Morris E.D. Endotoxin-induced systemic inflammation activates microglia: [11c]pbr28 positron emission tomography in nonhuman primates. Neuroimage. 2012;63:232–239. doi: 10.1016/j.neuroimage.2012.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreno B., Jukes J.P., Vergara-Irigaray N., Errea O., Villoslada P., Perry V.H. Systemic inflammation induces axon injury during brain inflammation. Ann Neurol. 2011;70:932–942. doi: 10.1002/ana.22550. [DOI] [PubMed] [Google Scholar]
- 31.Dheen S.T., Kaur C., Ling E. Microglial activation and its implications in the brain diseases. Curr Med Chem. 2007;14:1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- 32.Fuller S., Steele M., Münch G. Activated astroglia during chronic inflammation in Alzheimer's disease—do they neglect their neurosupportive roles? Mutat Res. 2010;690:40–49. doi: 10.1016/j.mrfmmm.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Kaindl A.M., Degos V., Peineau S., Gouadon E., Chhor V., Loron G. Activation of microglial N-methyl-D aspartate receptors triggers inflammation and neuronal cell death in the developing and mature brain. Ann Neurol. 2012;72:536–549. doi: 10.1002/ana.23626. [DOI] [PubMed] [Google Scholar]
- 34.Ma D., Jin S., Li E., Doi Y., Parajuli B., Noda M. The neurotoxic effect of astrocytes activated with toll-like receptor ligands. J Neuroimmunol. 2013;254:10–18. doi: 10.1016/j.jneuroim.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Boveri M., Kinsner A., Berezowski V., Lenfant A.M., Draing C., Cecchelli R. Highly purified lipoteichoic acid from gram-positive bacteria induces in vitro blood-brain barrier disruption through glia activation: role of pro-inflammatory cytokines and nitric oxide. Neuroscience. 2006;137:1193–1209. doi: 10.1016/j.neuroscience.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Farkas G., Márton J., Nagy Z., Mándi Y., Takács T., Deli M.A. Experimental acute pancreatitis results in increased blood-brain barrier permeability in the rat: a potential role for tumor necrosis factor and interleukin 6. Neurosci Lett. 1998;242:147–150. doi: 10.1016/s0304-3940(98)00060-3. [DOI] [PubMed] [Google Scholar]
- 37.de Vries H.E., Blom-Roosemalen M.C., van Oosten M., de Boer A.G., van Berkel T.J., Breimer D.D. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol. 1996;64:37–43. doi: 10.1016/0165-5728(95)00148-4. [DOI] [PubMed] [Google Scholar]
- 38.Erickson M.A., Banks W.A. Blood–brain barrier dysfunction as a cause and consequence of Alzheimer's disease. J Cereb Blood Flow Metab. 2013;33:1500–1513. doi: 10.1038/jcbfm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Semmler A., Frisch C., Debeir T., Ramanathan M., Okulla T., Klockgether T. Long-term cognitive impairment, neuronal loss and reduced cortical cholinergic innervation after recovery from sepsis in a rodent model. Exp Neurol. 2007;204:733–740. doi: 10.1016/j.expneurol.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Pais T.F., Figueiredo C., Peixoto R., Braz M.H., Chatterjee S. Necrotic neurons enhance microglial neurotoxicity through induction of glutaminase by a MyD88-dependent pathway. J Neuroinflammation. 2008;5:43. doi: 10.1186/1742-2094-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griffin W.S. Inflammation and neurodegenerative diseases. Am J Clin Nutr. 2006;83:470S–474S. doi: 10.1093/ajcn/83.2.470S. [DOI] [PubMed] [Google Scholar]
- 42.Murray C.L., Skelly D.T., Cunningham C. Exacerbation of CNS inflammation and neurodegeneration by systemic LPS treatment is independent of circulating IL-1β and IL-6. J Neuroinflammation. 2011;8:50. doi: 10.1186/1742-2094-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braak H., Thal D.R., Ghebremedhin E. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 44.Braak H., Tredici K.D. The pathological process underlying Alzheimer's disease in individuals under thirty. Acta Neuropathol. 2011;121:171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- 45.Püntener U., Booth S.G., Perry V.H., Teeling J.L. Long-term impact of systemic bacterial infection on the cerebral vasculature and microglia. J Neuroinflammation. 2012;9:146. doi: 10.1186/1742-2094-9-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cunningham C., Wilcockson D.C., Campion S., Lunnon K., Perry V.H. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Njie E.G., Boelen E., Stassen F.R., Steinbusch H.W., Borchelt D.R., Streit W.J. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol Aging. 2012;33:195.e1–195.e12. doi: 10.1016/j.neurobiolaging.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henry C.J., Huang Y., Wynne A.M., Godbout J.P. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Damani M.R., Zhao L., Fontainhas A.M., Amaral J., Fariss R.N., Wong W.T. Age-related alterations in the dynamic behavior of microglia. Aging Cell. 2011;10:263–276. doi: 10.1111/j.1474-9726.2010.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kashner M.T. Agreement between administrative files and written medical records: a case of the Department of Veterans Affairs. Med Care. 1998;36:1324–1336. doi: 10.1097/00005650-199809000-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.