Abstract
Introduction
On the basis of the proxy measures of cognitive reserve, we created a middle age self-report risk score for early prediction of dementia.
Methods
We used a longitudinal population-based study of 2602 individuals with a replication sample (N = 1011). Risk score at a mean age of 47 years was based on questions on educational and occupational attainments. Cognitive status at a mean age of 74 was determined via two validated telephone instruments.
Results
The prevalence of dementia was 10% after a mean follow-up of 28 years. Risk score was a good predictor of dementia: area under the curve = 0.77 (95% confidence interval, 0.74–0.80). The risk of dementia decreased as a function of risk score from 36% to 0%. The risk score was significantly associated with cognition after a mean follow-up of 39 years in the replication sample.
Discussion
Self-report risk score predicted cognitive functioning and dementia risk 20–40 years later.
Keywords: Education, Early identification, Cognitive reserve, Dementia, Middle age, Occupation, Risk score, Twins
Highlights
-
•
We created a middle age self-report risk score that predicts dementia in 20–40 years.
-
•
Risk score consisted of questions on educational and occupational attainments.
-
•
These measures are considered as proxies of cognitive reserve.
-
•
Risk score is easily acquired low-cost tool for early identification of dementia risk.
-
•
Educational and occupational attainments build cognitive reserve against dementia.
1. Introduction
Alzheimer's disease (AD) that accounts for about 60%–70% of aging-related dementias is typically diagnosed after the age of 65 years but the disease process may start even decades before the diagnosis [1]. Because there is no medication to stop or reverse the AD progress, there is a need for early identification of individuals at highest dementia risk. Here, risk scores consisting of multiple risk factors can be of great utility, as no individual factor predicts the disease.
Numerous studies have provided models for predicting dementia [2], [3], but only eight longitudinal studies have created a risk score for prediction of dementia [4], [5], [6], [7], [8], [9], [10], [11]. Two of the risk scores have been created for those with type 2 diabetes [10], [11], two risk scores were aimed to predict dementia in primary care patients [7], [9], and four risk scores were created using population- or community-based samples [4], [5], [6], [8]. Seven of the eight risk scores were created on samples aged ≥60 years. Furthermore, many of the risk scores used measures that require in-person attendance with a health care provider such as lipid levels, neuropsychological testing, or brain imaging.
Use of middle age risk scores are common in the context of cardiovascular disease [12], but considering dementia prediction, only one risk score, namely, cardiovascular risk factors, aging, and dementia (CAIDE), was based on middle age measures, but again in-laboratory measures were included [5]. One risk score was based solely on self-report measures [8], but the baseline information was acquired in old age, so it may not be optimal for early identification as risk factor levels may change during the preclinical phases of the disease.
In addition to risk scores of dementia, cardiovascular risk scores have also been used to predict cognitive status in old age [13]. Cardiovascular risk scores in middle age are significant predictors of old age cognitive functioning and change, AD, and all-cause dementia [2], [14]. In line with this, dementia risk scores have demonstrated the predictive utility of cardiovascular factors in detecting individuals at highest risk for dementia [5], [13], [15].
All previous risk scores have included the educational level as a risk factor, and consistent observations suggest education as a strong contributor to the total risk score with age being the most robust component. However, studies have not always rigorously tested the contribution of other risk factors along with the effects of age and education. Importantly, no predictive score has been based on only protective factors of educational and occupational attainment: factors that are considered as components of cognitive reserve (CR).
CR hypothesis states that those with higher levels of reserve can tolerate aging-related pathology and risk factors better and develop dementias later than those with lower levels of reserve [16]. The level of CR is commonly operationalized by measures that are related to individuals' premorbid general cognitive ability [17]. Proxy measures of CR, such as educational and occupational attainment, are associated with decreased risk of dementia [18], [19]. To date, no middle age risk scores in longitudinal design have been based solely on proxy measures of CR. We aimed to develop a middle age self-report risk score that predicts the risk of dementia for more than a period of 20–40 years by using indicators of CR.
2. Methods
2.1. Study design and participants
The participants of our study were all Finnish twins from same-sex pairs born before 1958 who were enrolled in the longitudinal population-based Finnish Twin Cohort (FTC) study with a participation rate of about 90% at the baseline data collection in 1975. FTC study design and data collection are described in detail elsewhere [20].
The creation of the risk score was based on a discovery set of FTC participants. In 1999–2007, all twins who were aged ≥65 years (born before 1938) were invited to participate in a telephone interview for a screening of dementia (participation rate of 70%). Two validated instruments were used: a telephone assessment for dementia (TELE) [21] and the telephone interview for cognitive status (TICS) [22]. Telephone interview protocol and a validation of the Finnish versions of the instruments are described in detail elsewhere [23], [24]. For the current analysis, the risk score was validated in a sample of 1086 individuals with TELE and TICS data from the ongoing data collection (started in 2014) from cohorts 1938–1944 (participation rate of 64%).
Answering and returning completed questionnaire was considered as consent in postal questionnaire data collection in 1975; the study was approved by the National Board of Health. During the course of the cohort study, participants were repeatedly informed about the study, and that they may withdraw from it whenever they wish. Written informed consent was obtained from all participants of the telephone interview protocol. Ethical committee of Hospital District of Southwest Finland had approved the protocol for telephone interview.
2.2. Procedures
The questionnaire in 1975 included questions on educational and occupational attainment. Education was categorized into five groups: (1) ≤3 years, (2) 4–6 years, (3) 7–9 years, (4) 10–11 years, and (5) ≥12 years. Four work-related questions included self-reported occupational status and information on the working environment of current or previous (if not working at the moment) position. Working status was categorized in three groups: (1) not working (because of old age/disability pension or unemployment), (2) homemaker, or (3) working or studying. Working environment was defined with three questions. One question asked was if the work was (1) very monotonous, (2) somewhat monotonous/somewhat variable, or (3) very variable. Physical working environment was categorized as (1) working mainly outdoors/both indoors and outdoors or (2) working indoors. Work-related nonphysical versus physical activity was categorized into four categories: (1) heavy manual labor, (2) manual labor (requiring lifting in addition to standing and walking), (3) light manual labor (requiring standing and walking but no other manual labor), and (4) nonmanual labor (mainly sitting). Age at the time of 1975 postal questionnaire was categorized into ≤39, 40–46, 47–53, 54–59, ≥60 years. The APOE status (ε4 carriers vs. ε4 non-carriers) was determined by genotyping of two single-nucleotide polymorphisms from DNA samples collected at follow-up (rs429358 and rs7412) [25]. Cardiovascular disease status was based on International Classification of Diseases 9 rubric codes 390–399 and 410–449 acquired from hospital discharge registry records and use of fully reimbursable medications for these causes until year 2004.
At follow-up, TELE and TICS yielded a continuous normally distributed total score and also classification into dementia, mild impairment in cognitive functioning (MICF), and cognitively normal. We note that the term MICF does not refer to established clinical criteria of mild cognitive impairment [26], [27] but simply refers to those whose TELE/TICS scores fall between cutoff scores of dementia and cognitively intact. We asked four questions about the independence in daily activities: (1) Are you able to take care of your household? (2) Are you able to get around outside? (3) Are you able to do shopping? (4) Are you able to dress and undress yourself? Participants reported if they were able to perform the activity independently, with the help from others or were not able to perform the activity. In addition to individual questions, we also created a variable that indicates whether a person was completely independent or if person had any difficulties in daily activities (i.e., needed help to perform any activity).
On calculating beta coefficients for our risk score, our primary definition of dementia was based on cases who were classified as having dementia by both TELE and TICS, and individuals with intact cognition were cognitively intact according to both the TELE and TICS. The cutoff scores of dementia were <16 for TELE and <22.5 for TICS [24]. The cutoff scores for intact cognition were >17.5 for TELE and >26.5 for TICS. Dementia versus normal cognition classification excluded individuals with intermediate scores on TELE (16–17.5) and TICS (22.5–26.5) and those whose cognitive status was in disagreement between TELE and TICS.
After creating the risk score, we used classification into demented versus nondemented individuals in all subsequent analyses; here the aforementioned criteria for dementia was applied and all others were considered as nondemented. We also used the classification of demented, MICF, and cognitively normal separately for TELE and TICS.
2.3. Statistical analysis
We followed the procedure of Kivipelto et al. [5] in calculating the risk score. First, we ran logistic regression models with one variable at a time predicting the dementia status (i.e., dementia vs. cognitively intact). Covariates included sex, age, and follow-up time between 1975 questionnaire and TICS. All significant predictors of dementia status were included in a multivariate logistic regression model. On the basis of β coefficients from the multivariate logistic regression model, we assigned a score for age, education, and work-related variables. Sex and follow-up time were used as covariates. Integer scores were obtained by multiplying the smallest β coefficient of −0.521 by −2 (smallest coefficient was −0.467 in the model without age). Subsequently, all β coefficients were multiplied by −2 and the values were rounded to nearest integer. Finally, scores of all variables were summed to create a risk score that represents the level of CR in middle age. This score was called CR score as higher scores indicated higher levels of educational and occupational attainments.
We used receiver operating characteristic curve analysis to calculate the area under the curve (AUC) and reported sensitivity, specificity, and positive predictive and negative predictive values of the CR score as a predictor of dementia status.
In addition to CR score including age, we also calculated a CR score without age in the model. This score allows to look at the CR score in different age groups but is also useful in subsequent analyses that are described subsequently.
We also analyzed the association between the CR score in middle age and the total TELE/TICS score as a continuous variable in a linear regression model. We also included a history of cardiovascular disease and APOE genotype, to test if the CR score predicts dementia along with the cardiovascular disease and APOE status (ε4 carriers vs. ε4 non-carriers). Family income at the time of 1975 questionnaire was used as an additional covariate. Analyses were done by using Stata statistical package. Clustered family data (twins within families) was taken into account in analyses [28].
3. Results
3.1. Main analyses
The discovery sample of 2602 individuals born before 1938 with TELE/TICS included 1355 men (52.1%) and 1247 women (47.9%). The mean age at the time of the TICS was 74.4 years (standard deviation [SD] = 5.3) and time of follow-up ranged from 23 to 31 years (M = 27.8; SD = 2.2). The mean age at the time of 1975 questionnaire data collection was 46.7 years (SD = 6.2) with a range from 38.4 to 69.5 years.
The number of demented individuals was 265 (10.2%), 1252 (48.1%) were defined as cognitively healthy, 823 (31.6%) had MICF according to either or both TELE and TICS, and 262 (10.1%) were those whose cognitive status was not in agreement with TELE and TICS instruments (Table 1). The majority (51.9%) of those with dementia, but only 8.3% of cognitively healthy individuals reported difficulties at least in one daily activity (Supplementary Table 1). According to the TELE, 449 (17.3%) individuals were demented, 642 (24.7%) individuals had MICF, and 1511 (58.1%) had intact cognition. According to the TICS, 343 (13.2%) were demented, 638 (24.5%) had MICF, and 1621 (62.3%) had intact cognition.
Table 1.
Cognitive status as measured with TELE and TICS
| TELE score (0–20) | TICS score (0–38) |
||
|---|---|---|---|
| Normal >26.5 | MICF 22.5–26.5 | Dementia <22.5 | |
| Normal >17.5 | 1252 | 244 | 15 |
| MICF 16–17.5 | 328 | 251 | 63 |
| Dementia <16 | 41 | 143 | 265 |
Abbreviations: MICF, mild impairment in cognitive functioning; TELE, telephone assessment for dementia; TICS, telephone interview for cognitive status.
A total of 2284 individuals had data for all educational and occupational variables from the 1975 questionnaire. The modal characteristics were 6 years of education (N = 1214) and currently at work (N = 1887) in 1975.
We derived beta coefficients from the multivariate logistic model to create the middle age CR score (Table 2). The CR score with age ranged from 3 to 25 and was normally distributed. We categorized continuous score into 10 groups each representing more than 100 individuals; this variable was included in further analyses.
Table 2.
Multivariate logistic model results. Beta coefficients (P values in parentheses) and scores assigned to each variable of the cognitive reserve score
| Variable | Model with age |
Model without age |
||
|---|---|---|---|---|
| β coefficient | Score | β coefficient | Score | |
| Education (in years) | ||||
| 0–3 | Reference | 0 | Reference | 0 |
| 4–6 | −1.927 (<.001) | 4 | −1.865 (<.001) | 4 |
| 7–8 | −3.155 (<.001) | 6 | −2.967 (<.001) | 6 |
| 9 | −3.139 (<.001) | 6 | −3.123 (<.001) | 6 |
| 10–11 | −3.487 (<.001) | 7 | −3.412 (<.001) | 7 |
| 12 or more | −5.679 (<.001) | 11 | −5.344 (<.001) | 11 |
| Work status | ||||
| Not working | Reference | 0 | Reference | 0 |
| Homemaker | −0.548 (.215) | 1 | −1.462 (<.001) | 3 |
| Working | −0.688 (.107) | 1 | −1.787 (<.001) | 4 |
| Nature of work | ||||
| Very monotonous | Reference | 0 | Reference | 0 |
| Somewhat monotonous/somewhat variable | −0.852 (.081) | 2 | −0.737 (.077) | 1 |
| Very variable | −0.917 (.073) | 2 | −0.708 (.109) | 1 |
| Work environment | ||||
| Outdoors/outdoors and indoors | Reference | 0 | Reference | 0 |
| Indoors | −0.552 (.009) | 1 | −0.467 (.016) | 1 |
| Physicality of work | ||||
| Heavy manual labor | Reference | 0 | Reference | 0 |
| Manual labor: standing and walking + lifting and carrying | −0.521 (.041) | 1 | −0.628 (.006) | 1 |
| Manual labor: standing and walking | −0.730 (.025) | 1 | −0.939 (.003) | 2 |
| Mainly sitting, very little physical activity required | −0.722 (.026) | 1 | −0.915 (.002) | 2 |
| Age (in years) | - | |||
| ≥60 | Reference | 0 | - | - |
| 54–59 | −1.890 (<.001) | 4 | - | - |
| 47–53 | −3.110 (<.001) | 6 | - | - |
| 40–46 | −3.789 (<.001) | 8 | - | - |
| ≤39 | −4.648 (<.001) | 9 | - | - |
| Cognitive reserve score range | 0–25 | 0–19 | ||
Abbreviations: TELE, telephone assessment for dementia; TICS, telephone interview for cognitive status.
NOTE. On the basis of β coefficients from the multivariate logistic regression model, we assigned a score for age, education, and work-related variables. Outcome variable was dichotomous classification of dementia versus intact cognition, but the results were similar when using multinomial logistic regression model including also those who had intermediate TELE/TICS scores and those whose cognitive status was in disagreement between TELE and TICS. Results were also similar when using linear regression model with a continuous TELE/TICS score as outcome variable.
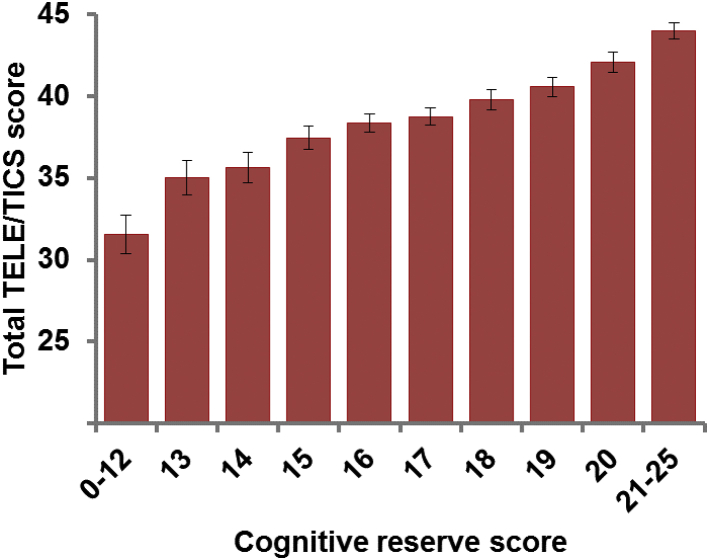
Contrasting those with dementia (9.4%, N = 214) and those without dementia (N = 2070), the CR score had the AUC of 0.77 (95% confidence interval [CI], 0.74–0.80). The prevalence of dementia decreased from 36.5% in the lowest scoring group to 0% in the highest scoring group (Table 3). Using a cutoff score of <16, sensitivity was 69% and specificity was 70% with positive predictive value of 19% and negative predictive value of 96%. With this cutoff, 70% were correctly classified. The results were similar when using the cutoff scores of dementia for TELE and TICS separately; further the prevalence of MICF was also decreased from the lowest scoring group to the highest scoring group (Supplementary Table 2). Fig. 1 shows the mean TELE/TICS score by CR score.
Table 3.
The prevalence (N in parentheses) of dementia in the old age (>65 years) by middle age cognitive reserve (CR) score category
| Cognitive status | CR score |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0–12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21–25 | |
| Dementia | 36.5% (74) | 17.7% (23) | 13.9% (22) | 10.0% (28) | 7.6% (21) | 7.1% (30) | 3.8% (8) | 1.9% (5) | 1.9% (3) | 0% (0) |
| No dementia | 63.5% (129) | 82.3% (107) | 86.1% (136) | 90.0% (253) | 92.4% (254) | 92.9% (391) | 96.2% (201) | 98.1% (263) | 98.1% (158) | 100% (178) |
| Total | 203 | 130 | 158 | 281 | 275 | 421 | 209 | 268 | 161 | 178 |
| OR | 1.00 (REF) | 0.376 | 0.292 | 0.194 | 0.152 | 0.137 | 0.071 | 0.034 | 0.034 | - |
| 95% CI | 0.220–0.644 | 0.171–0.497 | 0.120–0.315 | 0.090–0.258 | 0.086–0.218 | 0.033–0.153 | 0.010–0.115 | 0.010–0.108 | ||
Abbreviations: OR, odds ratio; CI, confidence interval; TELE, telephone assessment for dementia; TICS, telephone interview for cognitive status; REF, reference category for logistic model.
NOTE. Cases with dementia were categorized as demented according to both TELE and TICS instruments; all others are considered as nondemented.
Fig. 1.
Mean total TELE/TICS score by cognitive reserve score category (error bars represent 95% confidence intervals). Linear regression analysis controlling for sex, age, and the follow-up time indicated significant (F[4, 1597] = 167.12; P < .0001) association between the cognitive reserve score without age and the total TELE/TICS score (coefficient = 1.06 [95% CI, 0.96–1.15]). TELE, telephone assessment for dementia; TICS, telephone interview for cognitive status.
As education contributes strongly to the risk of dementia, we conducted an additional analysis restricted to those individuals who had 6 years of education. Again, the dementia risk decreased linearly as a function of better middle age CR score: 36.2% in the lowest scoring group and 1.3% in the highest scoring group (AUC = 0.70 [95% CI, 0.65–0.74]; Supplementary Table 3).
CR score without age ranged from 1 to 19 and was categorized into eight groups (Table 2). Using the cutoff score of <12, dementia was more prevalent in those with lower CR score in all age groups (Supplementary Tables 4 and 5).
3.2. Models with cardiovascular disease and APOE genotype
We included cardiovascular disease and APOE genotype (ε4 carriers vs. ε4 non-carriers) as additional variables in the models. CR score was a significant predictor of the dementia status and cognitive performance in all models, also when including family income as an additional covariate (Table 4, Supplementary Table 6).
Table 4.
Logistic models with middle age cognitive reserve (CR) score (without age), cardiovascular disease status, APOE genotype, and family income
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|
| N | 2284 | 2284 | 1671 | 1671 | 2158 |
| CR score | −0.378; P < .001 | −0.375; P < .001 | −0.357; P < .001 | −0.351; P < .001 | −0.355; P < .001 |
| Cardiovascular disease | - | 0.255; P = .130 | - | 0.389; P = .060 | - |
| APOE (ε4 carriers vs. ε4 non-carriers) | - | - | 0.373; P = .080 | 0.366; P = .087 | - |
| Family income | - | - | - | −0.035; P = .556 |
Abbreviations: TELE, telephone assessment for dementia; TICS, telephone interview for cognitive status.
NOTE. Dependent variable is dementia status (dementia vs. no dementia). All models include sex, age, and follow-up time as covariates. See Supplementary Table 6 for corresponding analyses with a continuous TELE/TICS score as a dependent variable.
3.3. Replication: Extending the predictive power of the CR score among those in their 30s
Replication sample included twins who were in their 30s (32–38 years; M = 34.7; SD = 1.7) at the time of 1975 questionnaire. A total of 1086 participants (as of April 2016) had TELE/TICS data at a mean age of 73.9 (71–76 years; SD = 1.1) and of these 1011 had complete data for calculating the CR score. At a mean follow-up of 39.1 years (SD = 0.75; 37–40 years), 1.7% (N = 17) were demented (4.5%; N = 46 and 3.4%; N = 34 according to the TELE and TICS separately).
AUCs were 0.66 (95% CI, 0.58–0.73) for TELE and 0.72 (95% CI, 0.65–0.79) for TICS. Using a CR score cutoff point of <12, there was a significant difference in the prevalence of dementia: TELE 7.4% versus 3.2% (F[1, 629] = 9.745, P = .0019) and TICS 6.8% versus 1.8% (F[1, 629] = 16.220, P = .0001) in the low and high CR score groups, respectively. Controlling for sex, age, and follow-up time, linear regression analysis indicated significant (F[4, 629] = 43.63, P < .001) positive association between the CR score and total TELE/TICS score (coefficient = 0.67 [95% CI, 0.55–0.78]).
4. Discussion
We created the first solely education and occupation-based middle age self-report risk score that predicts the risk of cognitive impairment in old age. Our risk score consisted of self-report measures of educational and occupational attainments and is considered to reflect the level of CR in middle age. Higher CR score was related to better cognitive functioning and lower risk of dementia in old age. Looking at different age groups separately, the pattern of our CR score–cognitive status association was very similar.
An easily acquired and low-cost risk score was able to predict dementia status 20–40 years later, which supports its utility in detecting individuals at highest risk for dementia. Previously, middle age CAIDE score has shown to be a useful instrument for early identification of dementia risk [5], [15]. The accuracy of our risk score is comparable with the CAIDE risk score, which has been the only predictive score based on middle age measures. Our score is based solely on self-report measures, which make it a very low-cost and easily accessible approach. Other composite scores of CR have shown that also leisure time activities predict cognitive status [29], [30], [31].
We created two models: one with a score assigned for age and the other without age. The score with age is aimed for clinical use. The other score can be used for research purposes. The fact that our risk score consists only of protective factors make it possible to study known risk factors (e.g., cardiovascular) and our score simultaneously to see if the protective and risk factors have additive and interactive effects on the risk of dementia.
Protective effect of higher educational attainment on dementia risk has been well replicated [19]. Similar to many previous risk scores, education was an influential component of our risk score. We conducted additional analysis by focusing on individuals with the same level of formal education and found that the results were not only driven by educational attainment. Our finding of middle age occupational attainment as a predictor of old age cognition is in line with the earlier literature [18]. Although the associations between individual components of our risk score and dementia have been shown in earlier studies, our study is the first to create a risk score based on educational and occupational information and to show that such a risk score is a strong predictor of dementia.
Our risk score did not include any direct health-related measures such as cardiovascular risk factors. Strength of this approach is that we can use our risk score and other risk factors as separate predictors of old age cognition. In fact, the results of the present study indicated that our risk score was a significant predictor of old age cognition and dementia status even when controlling for cardiovascular disease history or APOE genotype. We note that previous risk scores do not differentiate between education and health-related risk factors. Therefore, two individuals with similar risk scores can have divergent profiles: one with a lower educational level and no health-related risks and other with a higher educational level and health-related problems.
Our approach will also allow to test if the protective and risk factors have interactive effects on old age cognition, for example, do the level of protective factors moderate the association between cardiovascular risk factors and old age cognition. Such an analysis can inform more about the CR hypothesis [16]. If both proxy measures of CR and health-related risk factors are included in the same risk score, it is not possible to separate the effects of CR and risk factors on old age cognition. For example, we can test if our risk score moderates the cardiovascular risk factor–cognitive functioning or leisure time activity–cognitive functioning associations.
Although our results support the idea that higher educational and occupational attainments are causally related to lower dementia risk, these results may also reflect the initial cognitive ability level; that is, those with higher cognitive ability may obtain a higher educational level and occupational status.
A limitation is our dementia definition that was based on telephone interviews. TELE and TICS do not provide exact diagnostic decision of dementia, but a number of studies have indicated that these are useful screening instruments for dementia [23], [32], [33], [34]. Moreover, our definition of dementia required dementia status based on both TELE and TICS instruments. Furthermore, we found that most of those with dementia had at least some difficulties in daily activities. Limitation in our younger replication sample was a small number of participants who met the criteria for dementia according to both the TELE and TICS. Therefore, we used TELE and TICS dementia definitions separately in the replication sample. Further research is needed to investigate our approach in different populations and with different dementia classifications.
In addition to risk reduction, policies supporting the increase in CR have been recognized as an important component of primary prevention of global burden of dementia [35]. Our results stress the importance of educational and occupational factors as important cornerstones of building of CR against dementia. Globally, many people have access to education, but there are still many countries where people have limited access to education or an average educational level is low. Still, life expectancy is increasing in many low- and middle-income countries resulting in more cases of dementias. Policies investing in education and improving working environments may help to increase CR against dementia especially in countries with low levels of education. Considering secondary prevention, our easily acquired and low-cost risk score based on self-reported educational and occupational attainments can be used for early identification of individuals with high dementia risk. Early identification is important both in public health perspective and in promoting healthy cognitive aging for all individuals. Tools for early identification can also benefit drug and life style intervention trials.
4.1. Conclusions
For clinical utility, our risk score is a low-cost easily acquired tool for early identification of individuals with high risk for cognitive impairment and dementia. For research purposes, including only protective factors allows to investigate additive and interactive effects of our risk score and other risk factors (e.g., cardiovascular) on cognitive impairment and dementia. Our results support the role of educational attainment and working environment as important factors on building CR against dementia.
Research in context.
-
1.
Systematic review: We performed search on PubMed using terms “risk score” AND “dementia,” and “risk score” AND “Alzheimer's” in June 2016 and identified eight longitudinal studies that have created a risk score for prediction of Alzheimer's Disease or all-cause dementia.
-
2.
Interpretation: We created the first self-report middle age summary score that predicts the risk of dementia in old age and consists only of educational and occupational factors that are related to cognitive reserve (CR). The accuracy of our risk score is comparable with the CAIDE risk score, which has been the only predictive score based on middle age measures.
-
3.
Future directions: Our risk score can be used for early identification of individuals at increased risk for dementia. This tool can also benefit drug and life style intervention trials. Our study highlights the importance of educational attainment and work-related environment as important cornerstones in building of CR against dementia.
Acknowledgments
The authors acknowledge Ulla Kulmala-Gråhn, Kristiina Saanakorpi, Maarit Mantere, Riitta Sipilä, and Maarit Hanhiala for conducting telephone interviews and Pia Ruokolinna for data entry. The authors thank all the twins who participated in the Finnish Twin Cohort study.
Footnotes
Funding: This study was supported by the Academy of Finland Center of Excellence in Complex Disease Genetics (213506, 129680), the Academy of Finland (265240, 263278 [J.K.], 257075 [E.V.]), Juho Vainio Foundation (E.V.), and Sigrid Juselius Foundation (J.O.R.). The funders had no role in study design, data collection, data analysis, data interpretation, or in writing of the report.
Author Contributions: J.O.R., M.K., and J.K. contributed to the conception and design of the study protocol. E.V. designed the research questions, performed the literature research, analyzed the data, and drafted the manuscript. J.O.R., N.L., K.H., M.K., and J.K. contributed to data collection and revising the manuscript.
Conflicts of interest: The authors have no conflicts of interest.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2016.08.003.
Supplementary data
References
- 1.Jack C.R., Jr., Knopman D.S., Jagust W.J., Shaw L.M., Aisen P.S., Weiner M.W. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang E.Y., Harrison S.L., Errington L., Gordon M.F., Visser P.J., Novak G. Current developments in dementia risk prediction modelling: an updated systematic review. PLoS One. 2015;10:e0136181. doi: 10.1371/journal.pone.0136181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephan B.C., Tang E., Muniz-Terrera G. Composite risk scores for predicting dementia. Curr Opin Psychiatry. 2016;29:174–180. doi: 10.1097/YCO.0000000000000235. [DOI] [PubMed] [Google Scholar]
- 4.Barnes D.E., Covinsky K.E., Whitmer R.A., Kuller L.H., Lopez O.L., Yaffe K. Predicting risk of dementia in older adults: the late-life dementia risk index. Neurology. 2009;73:173–179. doi: 10.1212/WNL.0b013e3181a81636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kivipelto M., Ngandu T., Laatikainen T., Winblad B., Soininen H., Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5:735–741. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 6.Reitz C., Tang M.X., Schupf N., Manly J.J., Mayeux R., Luchsinger J.A. A summary risk score for the prediction of Alzheimer disease in elderly persons. Arch Neurol. 2010;67:835–841. doi: 10.1001/archneurol.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jessen F., Wiese B., Bickel H., Eifflander-Gorfer S., Fuchs A., Kaduszkiewicz H., AgeCoDe Study Group Prediction of dementia in primary care patients. PLoS One. 2011;6:e16852. doi: 10.1371/journal.pone.0016852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anstey K.J., Cherbuin N., Herath P.M., Qiu C., Kuller L.H., Lopez O.L. A self-report risk index to predict occurrence of dementia in three independent cohorts of older adults: the ANU-ADRI. PLoS One. 2014;9:e86141. doi: 10.1371/journal.pone.0086141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walters K., Hardoon S., Petersen I., Iliffe S., Omar R.Z., Nazareth I. Predicting dementia risk in primary care: development and validation of the Dementia Risk Score using routinely collected data. BMC Med. 2016;14:6. doi: 10.1186/s12916-016-0549-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Exalto L.G., Biessels G.J., Karter A.J., Huang E.S., Katon W.J., Minkoff J.R. Risk score for prediction of 10 year dementia risk in individuals with type 2 diabetes: a cohort study. Lancet Diabetes Endocrinol. 2013;1:183–190. doi: 10.1016/S2213-8587(13)70048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta H.B., Mehta V., Tsai C.L., Chen H., Aparasu R.R., Johnson M.L. Development and validation of the RxDx-Dementia Risk Index to predict dementia in patients with type 2 diabetes and hypertension. J Alzheimers Dis. 2015;49:423–432. doi: 10.3233/JAD-150466. [DOI] [PubMed] [Google Scholar]
- 12.D'Agostino R.B., Sr., Vasan R.S., Pencina M.J., Wolf P.A., Cobain M., Massaro J.M. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 13.Kaffashian S., Dugravot A., Elbaz A., Shipley M.J., Sabia S., Kivimaki M. Predicting cognitive decline: a dementia risk score vs. the Framingham vascular risk scores. Neurology. 2013;80:1300–1306. doi: 10.1212/WNL.0b013e31828ab370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison S.L., Ding J., Tang E.Y., Siervo M., Robinson L., Jagger C. Cardiovascular disease risk models and longitudinal changes in cognition: a systematic review. PLoS One. 2014;9:e114431. doi: 10.1371/journal.pone.0114431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Exalto L.G., Quesenberry C.P., Barnes D., Kivipelto M., Biessels G.J., Whitmer R.A. Midlife risk score for the prediction of dementia four decades later. Alzheimers Dement. 2014;10:562–570. doi: 10.1016/j.jalz.2013.05.1772. [DOI] [PubMed] [Google Scholar]
- 16.Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vuoksimaa E., Panizzon M.S., Chen C.H., Eyler L.T., Fennema-Notestine C., Fiecas M.J. Cognitive reserve moderates the association between hippocampal volume and episodic memory in middle age. Neuropsychologia. 2013;51:1124–1131. doi: 10.1016/j.neuropsychologia.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valenzuela M.J., Sachdev P. Brain reserve and dementia: a systematic review. Psychol Med. 2006;36:441–454. doi: 10.1017/S0033291705006264. [DOI] [PubMed] [Google Scholar]
- 19.Meng X., D'Arcy C. Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS One. 2012;7:e38268. doi: 10.1371/journal.pone.0038268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaprio J., Koskenvuo M. Genetic and environmental factors in complex diseases: the older Finnish Twin Cohort. Twin Res. 2002;5:358–365. doi: 10.1375/136905202320906093. [DOI] [PubMed] [Google Scholar]
- 21.Gatz M., Reynolds C.A., John R., Johansson B., Mortimer J.A., Pedersen N.L. Telephone screening to identify potential dementia cases in a population-based sample of older adults. Int Psychogeriatr. 2002;14:273–289. doi: 10.1017/s1041610202008475. [DOI] [PubMed] [Google Scholar]
- 22.Brandt J., Spencer M., Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- 23.Jarvenpaa T., Rinne J.O., Raiha I., Koskenvuo M., Lopponen M., Hinkka S. Characteristics of two telephone screens for cognitive impairment. Dement Geriatr Cogn Disord. 2002;13:149–155. doi: 10.1159/000048646. [DOI] [PubMed] [Google Scholar]
- 24.Laitala V.S., Kaprio J., Koskenvuo M., Raiha I., Rinne J.O., Silventoinen K. Coffee drinking in middle age is not associated with cognitive performance in old age. Am J Clin Nutr. 2009;90:640–646. doi: 10.3945/ajcn.2009.27660. [DOI] [PubMed] [Google Scholar]
- 25.Virta J.J., Heikkila K., Perola M., Koskenvuo M., Raiha I., Rinne J.O. Midlife cardiovascular risk factors and late cognitive impairment. Eur J Epidemiol. 2013;28:405–416. doi: 10.1007/s10654-013-9794-y. [DOI] [PubMed] [Google Scholar]
- 26.Petersen R.C., Smith G.E., Waring S.C., Ivnik R.J., Tangalos E.G., Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 27.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams R.L. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 29.Leon I., Garcia-Garcia J., Roldan-Tapia L. Estimating cognitive reserve in healthy adults using the Cognitive Reserve Scale. PLoS One. 2014;9:e102632. doi: 10.1371/journal.pone.0102632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lojo-Seoane C., Facal D., Guardia-Olmos J., Juncos-Rabadan O. Structural model for estimating the influence of cognitive reserve on cognitive performance in adults with subjective memory complaints. Arch Clin Neuropsychol. 2014;29:245–255. doi: 10.1093/arclin/acu007. [DOI] [PubMed] [Google Scholar]
- 31.Valenzuela M.J., Sachdev P. Assessment of complex mental activity across the lifespan: development of the Lifetime of Experiences Questionnaire (LEQ) Psychol Med. 2007;37:1015–1025. doi: 10.1017/S003329170600938X. [DOI] [PubMed] [Google Scholar]
- 32.Castanho T.C., Amorim L., Zihl J., Palha J.A., Sousa N., Santos N.C. Telephone-based screening tools for mild cognitive impairment and dementia in aging studies: a review of validated instruments. Front Aging Neurosci. 2014;6:16. doi: 10.3389/fnagi.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manly J.J., Schupf N., Stern Y., Brickman A.M., Tang M.X., Mayeux R. Telephone-based identification of mild cognitive impairment and dementia in a multicultural cohort. Arch Neurol. 2011;68:607–614. doi: 10.1001/archneurol.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beeri M.S., Werner P., Davidson M., Schmidler J., Silverman J. Validation of the modified telephone interview for cognitive status (TICS-m) in Hebrew. Int J Geriatr Psychiatry. 2003;18:381–386. doi: 10.1002/gps.840. [DOI] [PubMed] [Google Scholar]
- 35.Wu Y.T., Fratiglioni L., Matthews F.E., Lobo A., Breteler M.M., Skoog I. Dementia in western Europe: epidemiological evidence and implications for policy making. Lancet Neurol. 2016;15:116–124. doi: 10.1016/S1474-4422(15)00092-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.