Trend analysis of the massive international hide trade in Amazonia reveals differential resilience to hunting for aquatic and terrestrial wildlife.
Keywords: Amazonian historical ecology, empty forest, commercial and subsistence hunting, hide and skin trade, refuges, hunting sustainability, wildlife conservation, neotropical wildlife, wildlife resilience, source-sink dynamics
Abstract
The Amazon basin is the largest and most species-rich tropical forest and river system in the world, playing a pivotal role in global climate regulation and harboring hundreds of traditional and indigenous cultures. It is a matter of intense debate whether the ecosystem is threatened by hunting practices, whereby an “empty forest” loses critical ecological functions. Strikingly, no previous study has examined Amazonian ecosystem resilience through the perspective of the massive 20th century international trade in furs and skins. We present the first historical account of the scale and impacts of this trade and show that whereas aquatic species suffered basin-wide population collapse, terrestrial species did not. We link this differential resilience to the persistence of adequate spatial refuges for terrestrial species, enabling populations to be sustained through source-sink dynamics, contrasting with unremitting hunting pressure on more accessible aquatic habitats. Our findings attest the high vulnerability of aquatic fauna to unregulated hunting, particularly during years of severe drought. We propose that the relative resilience of terrestrial species suggests a marked opportunity for managing, rather than criminalizing, contemporary traditional subsistence hunting in Amazonia, through both the engagement of local people in community-based comanagement programs and science-led conservation governance.
INTRODUCTION
The Amazon basin is one of the world’s richest and most critical natural environments, both in regulating climate (1) and sustaining biodiversity at a global scale (2). Threats posed by habitat loss, fire, and climate change are well documented (1, 3–5). A more insidious threat is overhunting (6–9), which results in a defaunation process that can cascade onto ecosystem functioning (9–12). Although it has been proposed that defaunation due to the massive 20th century international trade in Amazonian furs and skins led to an “empty forest” scenario (13–15), it is remarkable that the magnitude and impacts of this trade have never been quantified, despite the insight that such a study would provide into ecosystem resilience. Existing knowledge of Amazonian resilience to hunting is based on studies that are temporally or spatially restricted and focused only on terrestrial species (6, 8, 10, 16–22). Here, we present the first systematic account of the history, scale, and outcomes of the globally significant Amazonian hide trade throughout the 20th century and discuss the consequences of our findings for present-day management and conservation.
In the late 19th century, the rubber boom brought about a complete social and economic restructuring of the Amazon frontier. About half a million colonists entered the region to extract rubber across all major river basins; an immense fleet of steamships arose for transportation and trade; and a network of traveling river merchants purchased forest products from extractivists in a debt peonage regime known as aviamento, extending from hinterland rubber groves to commercial export warehouses in Manaus and Belém (23–26). After rubber prices collapsed in 1912 due to competition from Malaysian plantations, enterprises that did not go bankrupt were obliged to find substitute products (27). The international trade in Amazonian animal hides, which was previously minimal, grew considerably and persisted for about 80 years, supplying markets in the United States, Europe, and south-southeastern Brazil (27).
Our analysis is based on previously obscure data from port registries, commercial records, and cargo manifests of animal hide shipments in the central-western Brazilian Amazon in the 20th century. These are collated and systematized here for the first time, following an exhaustive search of surviving primary archive sources. Many of the documents containing these records no longer exist, so a major contribution of this work has been to trace surviving documents and their whereabouts so that the history of the hide trade can be reconstructed (see Materials and Methods and text S1).
The available shipment data typically consisted of total hide weight for all species combined; however, for a subset of records chiefly relating to exports and occasionally to landings, the composition of the shipment by species was available. We developed a novel trend model to enable us to combine these two sources of information to estimate an individual harvest trend for each species over time. This approach avoids the bias that would result from modeling the trends only in the subset of species-specific data, if not adjusted for by knowledge of the total harvest over time (28), which would underestimate harvests in the 1930s relative to those in the more data-rich 1960s and thus overestimate population resilience (see Data and approach).
Amazonian hunters in the 20th century were largely opportunistic forest dwellers, who engaged in hunting primarily for meat and traded in animal hides to supplement their subsistence living and income from other forest products (see text S2). Among the 89,000 extractivists in the central-western Brazilian Amazon recorded in the 1950 census, only 528 declared themselves to be professional hunters (29–32). Because of the unregulated and opportunistic nature of hunting practices, there is no information about the level of hunting effort over time, but conversely, there is strong justification for assuming an intensification of effort as the human population increased. Hunting effort is unlikely to have decreased in response to declines in exploited populations because the wide range of commercially attractive species ensured that hunters could trade whatever they could catch, and since animal skins constituted just one of many extractive products shipped by the fluvial transport network, the opportunity to sell hides persisted even if the volume of trade diminished. It is therefore reasonable to assume that harvest trends reflected animal population status to some degree, especially in the case where low harvests were returned despite strong market incentives and a high human population.
To use our modeled harvest trend curves to draw inference on population resilience during the hide trade, we focused on a comparison between two periods of peak exploitation: the 1930s–1940s and the 1960s. Each period saw a sharp increase in the total harvest of all species combined (Fig. 1), and hunting incentives were strong due to high market pelt prices (Fig. 2). However, the rural human population in the central-western Brazilian Amazon was 68% larger in the 1960s than in the 1940s (fig. S1), so it is reasonable to assume that hunting effort was higher in the later period. Thus, species that disappeared from the harvest in the 1960s had presumably experienced widespread population collapse. Although it cannot be proved that this was due to overhunting, the circumstantial evidence is strong, especially when considered alongside anecdotal evidence from hunters of the day (see text S2). Conversely, a greater resilience to exploitation can be deduced for species whose harvests remained buoyant in the 1960s.
Fig. 1. Overview of the international trade in Amazonian animal hides through the 20th century.
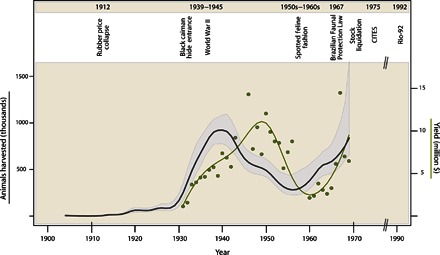
Modeled annual harvests for 20 species from the whole central-western Brazilian Amazon region (dark gray line), which include landings at Manaus and additional exports from other hinterland ports. 95% CIs obtained by bootstrap (gray area). Annual yields, converted to U.S. dollars indexed to 2015 prices, from extant hide export records from the central-western Brazilian Amazon (green dots and green trend line); these extant records represent a subset of the total modeled yield.
Fig. 2. Annual harvests and average prices for the main terrestrial and aquatic/semiaquatic species that were hunted commercially for hides and pelts in the central-western Brazilian Amazon, 1904–1969.
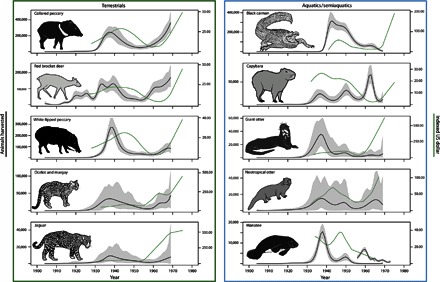
Modeled total commercial harvests including those exported internationally [black lines ± 95% confidence interval (CI) regions in gray] and hide prices converted to U.S. dollars indexed to 2015 prices (green lines).
RESULTS
Overview of the international trade in Amazonian animal hides and pelts
Based on our models of harvest trends, we estimate that from 1904 to 1969, 23.3 million (21.6 million to 26.8 million) wild mammals and reptiles representing at least 20 species were commercially hunted for their hides, comprising 13.9 million (12.7 million to 15.1 million) terrestrial mammals, 1.9 million (1.7 million to 2.3 million) aquatic and semiaquatic mammals, and 7.5 million (6.2 million to 10.6 million) reptiles (Table 1 and table S1).
Table 1. Estimated numbers of animals hunted for their hides in the central-western Brazilian Amazon (1904–1969).
Historical peak shows the year and estimated number of animals corresponding to maximum harvest for each species. Harvest change indicates the percentage change in modeled harvest for each species between a 5-year period centered on the overall pre-1965 peak harvest year for that species and the final 5-year period of exploitation from 1965 to 1969. The first peak occurred between 1937 and 1943 for every species except the capybara (1963). The final harvest for the manatee comprises meat production instead of hides and is taken from 1969 to 1973; see text for details. 95% bootstrapped CIs are shown in parentheses.
| Species | Total (1904–1969) | Historical peak | Year | Harvest change |
| Terrestrial | ||||
| Collared peccary (Pecari tajacu) | 5,443,795 (4,740,807–6,177,067) | 363,425 (238,190–500,988) | 1969 | 15 (−22, 68) |
| Red brocket deer (Mazama americana) | 4,152,218 (3,685,451–4,570,403) | 169,885 (109,431–249,834) | 1969 | 16 (−16, 71) |
| White-lipped peccary (Tayassu pecari) | 3,110,753 (2,598,553–3,626,290) | 273,408 (212,667–356,238) | 1939 | −67 (−78, −51)* |
| Ocelot/margay (Leopardus pardalis/Leopardus wiedii) | 804,080 (529,517–1,223,279) | 44,448 (6,690–115,648) | 1969 | −13 (−66, 145) |
| Jaguar (Panthera onca) | 182,564 (112,533–313,385) | 9,344 (2,807–20,318) | 1938 | −30 (−88, 249) |
| Aquatic/semiaquatic | ||||
| Black caiman (Melanosuchus niger) | 4,415,469 (3,978,153–4,846,254) | 313,907 (249,474–390,660) | 1943 | −92 (−95, −87)* |
| Capybara (Hydrochoerus hydrochaeris) | 1,040,533 (896,826–1,223,881) | 86,687 (61,431–115,778) | 1963 | −75 (−84, −61)* |
| Giant otter (Pteronura brasiliensis) | 386,491 (265,399–581,032) | 35,589 (18,175–58,149) | 1937 | −88 (−96, −64)* |
| Neotropical otter (Lontra longicaudis) | 362,335 (203,411–636,137) | 14,919 (3,655–32,961) | 1937 | −20 (−82, 359) |
| Manatee (Trichechus inunguis) | 113,033 (92,658–138,583) | 15,872 (12,558–19,820) | 1938 | −91 (−94, −88)* |
*Percentage harvest change is significantly different from zero at the 5% level.
The overall harvest trend for all species combined (shown in Fig. 1) closely tracks events in 20th-century world history. After the 1912 rubber collapse, the Amazonian hide trade, previously minimal and mostly focused on red brocket deer (27), began to increase. The trade increased gradually through the 1910s and 1920s and then experienced a marked upturn in the 1930s, coinciding with the consolidation of the United States as the primary export market for Amazonian hides. With the Japanese capture of Malaysian rubber plantations at the outset of World War II, the United States made heavy investments in Brazilian rubber, prompting some 35,000 to 80,000 rubber tappers (so-called “soldiers of rubber”) to move to the western Brazilian Amazon (23–26), vastly increasing forest hunting effort. The accompanying peak in the hide trade during World War II saw at least 1 million hides harvested annually (Fig. 1). With an active ground war ensuing in Europe, nearly all hide production was exported to the United States during this time. Throughout World War II, prices of animal hides rose steadily, and wild animal hides and pelts came to top the list of extractive Amazonian export products after rubber (fig. S2 and text S3). After World War II, indexed hide prices as well as total harvest declined somewhat (Fig. 2). However, harvest trends at some localities continued to climb (Fig. 3).
Fig. 3. Time series of animal harvests at nine localities in the central-western Brazilian Amazon.
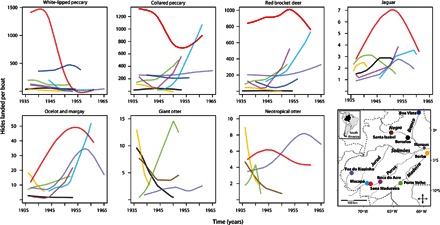
The curves show estimated number of hides transported per boat and are color-coded according to trade locality. Data are gleaned from cargo manifests of the J. G. Araujo Company.
The international fashion zeal for spotted felid furs in the 1950s and 1960s (33–36) prompted sharp increases in pelt prices (Fig. 2), motivating commercial harvesting of Amazonian animal hides to rise again and generating a second production peak of 860,000 hides in 1969 (Fig. 1). During the four decades from the 1930s to the 1960s, the 10 main commercially harvested species of the western Brazilian Amazon (Table 1) generated about $500 million in adjusted 2015 base-year U.S. currency according to our model. Although Brazil officially banned hunting with the 1967 Faunal Protection Law, loopholes allowing for the trade of stockpiled hides facilitated ongoing illegal hunting and exports until the ratification of the Convention on International Trade in Endangered Species (CITES) in 1975 (Fig. 1) (33, 36–39). The giant otter, neotropical otter, jaguar, ocelot, margay, manatee, and black caiman were all listed in CITES Appendix I, granting them maximum protection from exploitation. Illegal wildlife trade still persisted through the 1980s, when demand in Europe and the United States began to wane due to improved CITES enforcement and the declining popularity of furs in the fashion industry (34). Finally, the 1992 Rio Convention on Biological Diversity consolidated international awareness and put an end to the vogue in Amazonian animal hides as fashion accessories (40).
Differential resilience of aquatic and terrestrial species to 20th century commercial hunting
Modeled harvest trend curves for individual species imply that aquatic species mostly exhibited population collapse at a basin-wide scale (Figs. 2 and 4). The peak harvest of the giant otter in the 1960s was only 12% of that in the 1930s. The neotropical otter (a smaller, solitary species with a similar pelt) was more resilient, apparently replacing the giant otter in trade when the latter was driven to commercial extinction. Likewise, black caiman harvest in the 1960s attained only 8% of peak production in 1943. In 1964, the previously ignored spectacled caiman rapidly entered the market as a replacement (35). Demand for manatee hides dwindled in the 1950s when synthetic rubber became available, but demand for manatee meat persisted in Manaus until the early 1970s (41). Although nearly 16,000 manatees were commercially harvested in 1938, offtake in the 1970s declined to only 9% of this amount. The capybara (a semiaquatic species and the world’s largest rodent) exhibited a stepwise harvest pattern from 1930 to 1960, with a sudden decline during the 1960s, despite sharp rises in hide prices (Fig. 2).
Fig. 4. Harvest resilience displayed against habitat and demographic characteristics.
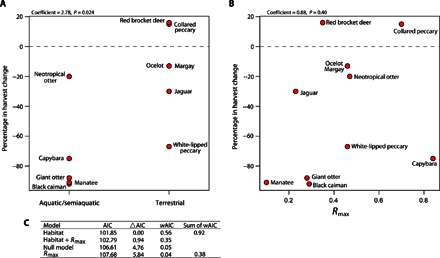
Resilience of game populations to historical commercial hunting (represented by the percentage change in harvests across time) and correlation with habitat type (aquatic/terrestrial) (A) and intrinsic rate of natural population growth (Rmax) (B). Fitting a general linear model provides some evidence of higher population resilience in species with higher reproductive rates, but resilience is better predicted by habitat type (C). Akaike information criterion (AIC) weights (wAIC) for each model are given by exp(−ΔAIC/2) divided by the sum of this quantity over the four specified models.
By contrast, terrestrial species such as red brocket deer and collared peccary showed higher harvests in the 1960s than in the 1930s, providing evidence of greater resilience to 20th century hunting activity. Of all terrestrial mammals, only the white-lipped peccary showed signs of population decline at both basin-wide and local scales (Figs. 2 and 3). Although its reproductive rate is intermediate between that of red brocket deer and collared peccary (table S2) (18), the white-lipped peccary lives in large herds, ranges over large territories, and can be slaughtered by the dozen, especially when the herd crosses a river (text S2) (42, 43).
About 180,000 jaguars were harvested in the central-western Brazilian Amazon during the fur trade. Although jaguar harvests peaked in 1938 with more than 9000 individuals, nearly 8000 individuals were still harvested in 1969 (Fig. 2). The sharp increase in international prices for felid pelts also led to innovations in trapping technology (33) so that jaguar and other smaller spotted cats (margay and ocelot) showed upward harvest trends beginning in the late 1950s. We expect our estimates of felid harvest in the 1960s to be conservative, because undeclared activity may have risen in the 1960s due to increased taxes on luxury pelts (33, 44).
Accessibility of aquatic and terrestrial habitats for harvest
To characterize the differential hunting pressure applied to terrestrial and aquatic species, we mapped all human settlements in the central-western Brazilian Amazon in the mid-20th century (see Materials and Methods). Under the assumption that hunting behavior was largely opportunistic and radiated out from settlements (8, 17, 20–22, 44), we considered hypothetical catchments of 5- and 10-km radius around the 3298 settlements. These catchment sizes are representative of those previously reported for the foraging behavior of Amazonian subsistence hunters (9, 17, 20–22, 45–47). We quantified the amount of aquatic and terrestrial habitat accessible to hunters under each hypothetical catchment size using preclassified imagery of floodplain areas (48). We estimated the proportions of harvestable areas and nonharvestable refuge areas for terrestrial habitat during the high-water season and for aquatic habitat during the low-water season.
Terrestrial habitats varied from 88 to 95% of the central-western Brazilian Amazon between the high- and low-water seasons, encompassing areas of 1,909,768 and 2,064,818 km2, respectively (table S3). During the high-water season, when the lowest amount of terrestrial habitat was available, the harvestable area near settlements ranged from 131,619 to 370,207 km2 under the 5- and 10-km catchment scenarios, comprising 7 to 19% of all terrestrial habitat. Meanwhile, flooded areas comprised between 110,927 and 265,976 km2 (5 to 12% of the focal area) during the low- and high-water seasons, respectively. Traditionally, Amazonian people live along rivers (Fig. 5), so 29 to 55% (32,167 to 60,899 km2) of the total aquatic habitat during the low-water season could have been commercially exploited in the 1950s and 1960s under the 5- and 10-km catchment scenarios (table S3).
Fig. 5. Two hypothetical hunting area scenarios displayed against terrestrial and aquatic habitats in the central-western Brazilian Amazon during the mid-20th century.
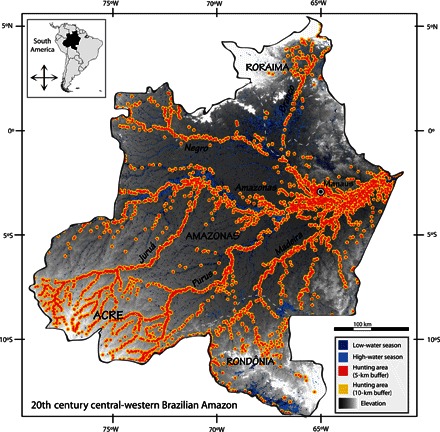
Hunting catchment areas were obtained from 5- and 10-km buffer radii (red and yellow, respectively) around 3298 historical settlements in the 1950s and 1960s. These are predominantly nonindigenous settlements; locations of indigenous settlements in this period are mostly unknown. Low- and high-water seasons (dark and light blue, respectively) were reclassified from available raster imagery (48) for the focal area. Dashed lines delimit Brazilian state frontiers (state name in upper case). River names are in italic bold. See Materials and Methods for further details of the spatial analyses performed.
Hunting sustainability based on maximum production and refuge-harvestable area models
The sustainability of hunting practices is often assessed by sustainability indices such as the Robinson-Redford production index (49, 50), which assesses whether animals are removed by hunting at a greater rate than they can be replaced naturally at a maximum production level for the species. Maximum production is defined as the maximum number of animals that can be added to the population annually through reproduction under ideal conditions (49, 50). The Robinson-Redford index does not take account of the availability of unharvested refuge areas that may replenish the exploited population.
We applied the Robinson-Redford production index to our harvest estimates for terrestrial species (see Materials and Methods). Our conservative estimates for commercial offtake, disregarding both smuggled and deteriorated skins and animals killed but not retrieved, were higher than the estimated maximum production for all species under the 5-km terrestrial catchment scenario (table S4). This would imply unsustainable harvests for terrestrial species according to this index. Under the 10-km terrestrial catchment scenario, which corresponds to a lower hunting intensity per unit area, only the collared peccary harvest would be considered sustainable in both the 1930s and the 1960s according to this index (table S4).
An alternative method of assessing hunting sustainability is the refuge-harvestable area model of Joshi and Gadgil (51), which focuses on the relative sizes of harvested and unharvested areas relative to the reproductive capability of the species in question (see Materials and Methods). Results are shown in table S5. Despite the simplicity of the Joshi and Gadgil model (51), its predictions on hunting sustainability are broadly consistent with the conclusions from our trend analysis (Table 1 and Figs. 2 and 4) and support the overall conclusion that aquatic habitats were more vulnerable to open-access, unregulated hunting than terrestrial habitats (table S5). For most aquatic species, refuge size (Arefuge) was lower than the area required to achieve maximum sustainable yield (AMSY), especially for the 10-km catchment scenario which may be more realistic in aquatic territory, and consistent with the conclusion from our trend analysis that harvests were unsustainable for these species. By contrast, terrestrial species were calculated to have refuge sizes higher than AMSY (table S5), also consistent with our observed conclusion of largely sustained yields for these species.
DISCUSSION
Accessibility as the primary driver of population resilience to harvest in Amazonia
We have shown that the commercial exploitation of animal hides in the 20th century apparently led to population collapse for the affected aquatic wildlife species (52), signaling the possibility of an “empty river” phenomenon. Population collapses in aquatic species attest to their high vulnerability to unregulated hunting, particularly during years of severe drought (4, 53–55) when aquatic wildlife is confined to larger waterways that are generally accessible to hunters. Although our analysis focuses on only five species harvested for their hides, declines of large aquatic vertebrates such as manatee, Amazonian turtle (Podocnemis expansa), and the arapaima fish (Arapaima gigas) have been reported since the late 19th century (56–60). More recently, in the last few decades, several species of large, commercially important Amazonian fish have shown strong signs of population decline due to overfishing, including tambaqui (Colossoma macropomum) and Brachyplatystoma catfish (Pimelodidade) as well as arapaima (61–65). The cascading effects of these population declines at a basin-wide scale remain unknown.
By examining overall harvest trends at broad spatial scales, we found that aquatic or terrestrial habitat type was the most important predictor of harvest resilience across multiple species (Fig. 4). This does not diminish the importance of other biological and behavioral considerations in determining outcomes for individual species. Such considerations include reproductive rate, home-range size, and the heightened vulnerability of social and diurnal species. Epidemic diseases might also have suppressed the size or resilience of certain host populations (66). For instance, brucellosis and leptospirosis, increasingly introduced from domesticated animals, might have contributed to population declines for white-lipped peccary (67, 68). Although population trends for individual species are driven by a complex interplay of factors, the emergence of habitat type as the dominant predictor across multiple species permits strong conclusions about the pivotal role of habitat accessibility to the overall outcomes of 20th century commercial hunting.
Covering about 12 and 5% of the overall forest territory during the high- and low-water seasons, respectively, rivers and floodplains were relatively densely populated and easily accessible to hunters (Fig. 5). On the other hand, for most terrestrial species, forest interior areas provided refuges with reduced hunting pressure (Fig. 5, text S2, and table S3). When such refuges are sufficiently large, animal populations persist at large spatial scales regardless of the level of localized harvesting effort (51). Assuming that commercial hunting was limited to catchment areas of 5- and 10-km radius around the 3298 historical settlements in the central-western Brazilian Amazon during the mid-20th century, more than 80% of terrestrial habitat would have remained free of hunting, whereas more than 50% of aquatic habitat would have been accessible to hunters (see Materials and Methods, table S3, and text S2). We suggest that this was the main reason why large-bodied vertebrate populations generally persisted in the dense upland forests of terra firme, whereas they were nearly wiped out in the rivers and floodplains of várzeas and igapós.
Applying standard indices to assess hunting sustainability (49, 50) in the catchment areas generated by the 5- and 10-km radial scenarios suggested that annual harvests of terrestrial animals at the peaks of commercial exploitation were generally higher than annual population replenishment (table S4), which might suggest that the 20th-century hide trade should have driven terrestrial species to extinction. By contrast, applying refuge-harvestable area models (51) predicted sustainable harvests for terrestrial species (table S5). This contradiction, also observed in studies of the impacts of Amazonian subsistence hunting (6, 8, 9, 13, 16–24), suggests that more complex modeling is required to understand the dynamics and impacts of hunting through space and time (47), ideally incorporating both animal reproduction and dispersal, and the enduring effects of historical harvests.
Recent Amazonian historical ecology: A new picture
Whereas previous studies of historical ecology in Amazonia have focused on ancient human impacts (69–72), our work casts light on the impacts of more recent human activities on Amazonian biodiversity and resource exploitation while also providing a historical background for contemporary wildlife management and conservation (73).
Neglecting the recent historical context of harvests can lead to misdiagnosis of hunting sustainability. For instance, the marked depletion of white-lipped peccary populations along the Iaco River (municipality of Sena Madureira, state of Acre) in the 1990s has previously been attributed to the impact of local subsistence hunting in recent years (74). However, our data show that these peccary populations had already collapsed in the mid-1940s, at least in commercial catchment areas (Fig. 3), so the hypothesis of overexploitation by subsistence hunters needs to be weighed against that of slow population recovery. Neglecting historical context can also cause hunting impacts to be underestimated, for example when assessing the harvest of an already-depleted population using the Robinson-Redford production index (50, 75). Low harvests may be interpreted as sustainable, when in fact harvests are low due to previous overexploitation (50, 75). This is evident for white-lipped peccary harvests in the late 1960s, when our trend model suggests that overexploitation resulted in a 67% population reduction in the central-western Brazilian Amazon. Because harvests became lower than maximum production, a naïve application of the Robinson-Redford index might conclude that hunting was sustainable (table S4). Thus, the historical ecology of wildlife exploitation must be taken into account when assessing contemporary hunting sustainability.
Consideration of spatial ecology is also important. The enduring impacts of 20th century commercial hunting have widely varied across the study area (Fig. 3). In some regions, vulnerable animal populations have never recovered from the age of commercial hunting, perhaps due to unremitting pressure from subsistence hunting, conflict with humans, or disease. Examples include the giant otter (76, 77), black caiman (78), and manatee (79), which even now are still in the process of recolonizing several areas of the Amazon basin. Understanding these spatial and temporal historical forces is fundamental to comprehending the current status of animal populations and developing adequate concepts and strategies for managing subsistence hunting.
New perspectives on contemporary wildlife management in Amazonia
The fact that intense and sustained commercial harvest for over 50 years failed to extirpate ungulates and felid populations at a basin-wide scale suggests that Amazonian wildlife can be quite resilient to hunting as long as adequate source populations persist, so that the forest is at least not empty of these terrestrial species. Peccaries, deer, and tapirs, which in the central-western Brazilian Amazon supplied some 650,000 animals annually in the late 1930s to the international trade in animal hides, and 615,000 in 1969, continue to provide about 650,000 individuals annually in the same region today for consumption by subsistence hunters (8), notwithstanding differences in the spatial distribution of hunting effort over time (text S2).
For most of the terrestrial species we examined, our findings do not support the assumption that 20th-century commercial hunting resulted in a severe degree of defaunation at a basin-wide scale, which would have been to the large-scale detriment of ecosystem roles played by ungulates, such as seed predation and dispersal for hundreds of plant species (80–85), and top-down population regulation performed by jaguar, ocelot, and margay (86, 87). Instead, we suggest that some loss in ecosystem function probably occurred locally across harvested areas. This would have been particularly critical in regions where white-lipped peccary populations were overharvested (Fig. 3), affecting seed predation and seedling recruitment (10). Elevated hunting activity during the 20th century hide trade might also have affected nonhide species taken by hunters for food, such as large primates, tapirs, guans, and curassows, which are crucial for seed dispersal for many large-seeded tree species (10, 13, 80, 13, 80), and given their low fecundity rates, can be quite affected by subsistence hunting (18). Nevertheless, about 70% of all populations of the lowest-fecundity and most prized game species, such as ateline primates (Ateles and Lagothrix), currently occur at carrying capacity at both landscape and basin-wide scales (9, 17).
In addition to their ecological functions, large vertebrates also provide an essential food source for millions of forest dwellers (6–8). Subsistence hunting is a central pillar of Amazonian culture (47, 88–90), but its legal status is unclear due to conflicting Brazilian laws in animal conservation and human rights (91). Although Brazil’s historic Faunal Protection Law of 1967, and the subsequent ratification of CITES in 1975, certainly prevented further depletion of animal populations by commercial hunters, this regulation essentially criminalized all hunting and remains in force today, creating serious legal barriers to the development of subsistence game management strategies for traditional peoples in the Brazilian Amazon.
Legal regulation and management of subsistence hunting represents a tremendous conservation opportunity in Brazil and other Amazonian countries. The most successful natural resource management programs in Amazonia have engaged local communities directly in community-based comanagement (91–95). In an enormous and typically low-governance region such as Amazonia, the presence of forest dwellers and their traditional livelihoods can inhibit the large-scale clearing of forest and the extraction of natural resources for commercial purposes and can also serve as a political force opposing infrastructure projects and environmentally detrimental legislation (47, 96, 97). Involving traditional people is critical in wildlife conservation programs, given their inherent knowledge of natural systems and rapid management decision-making (98, 99). In extractive reserves and indigenous territories, human livelihoods are protected by law; thus, subsistence hunting is largely tolerated (91), and the use of participatory zoning (take and no-take zones) has been encouraged by recent Brazilian policies (100, 101). The resulting preservation of large unhunted refuge areas between dispersed human settlements creates a model for promising cost-effective strategies in hunting management across the Amazon, supported by refuge-harvestable (51) and source-sink spatial and temporal modeling (17, 47).
Ideally, community-based comanagement hunting programs should include support for monitoring the intensity and spatial spread of harvests, as well as animal reproduction and dispersal rates, which can be used to parameterize spatially explicit refuge-harvest dynamic models across natural landscapes. Regional variation in forest productivity, hunting intensity, carrying capacity (8), sociocultural practices (6, 47, 90, 102), and the background history of local animal exploitation (Fig. 3) should all be taken into account when designing management programs. Once our understanding of Amazonian wildlife ecology is improved, additional strategies may be adopted such as male-only harvests, age-specific harvests, and quotas (85). Protected areas or no-take zones alone might not be sufficient to secure all critical environments required by wildlife, especially for aquatic migratory species and those with large home ranges, such as jaguar and white-lipped peccary. Hence, conservation management in Amazonia requires a basin-wide approach to maintain the interconnectivity, integrity, and dynamics of the entire ecosystem, especially for aquatic wildlife (9, 103).
During the peak of the international trade in Amazonian hides, deforestation was almost nonexistent. Our suggestion that traditional subsistence hunting may represent more a management opportunity than a threat is restricted to roadless regions that remain largely forested, ideally officially protected, and where people still maintain traditional practices. Yet as Brazil and other Amazonian countries expand their road networks, as well as agribusiness and ranching frontiers and other infrastructure projects (1, 3, 5, 104), the accessibility of the once remote refuge areas has increased. The challenge is to understand whether in regions such as the Amazonian “arc of deforestation,” the dynamics of source-sink systems have been so irreparably disrupted that the landscape-scale sustainability of hunting can no longer operate. If large refuges with limited road and river accessibility cannot be maintained, the combined effects of deforestation, habitat fragmentation, human colonization, wildfire, disease outbreaks, and hunting will likely result in the decimation of wildlife (1, 3, 6, 12, 68, 105). Collapse of the basin-wide system of spatial refuges, which ensured the resilience of terrestrial species even during the heyday of 20th-century commercial hunting, would indeed result in an empty forest.
MATERIALS AND METHODS
Study area
The study area comprises the states of Amazonas, Acre, Rondônia, and Roraima in North Brazil, covering 2,185,172 km2 and mostly consisting of Amazonian forest (see Fig. 5).
Game species
Focal species included 10 large-bodied mammals: Sirenia, Trichechidae: manatee (Trichechus inunguis); Rodentia, Caviidae: capybara (Hydrochoerus hydrochaeris); Carnivora, Felidae: ocelot (Leopardus pardalis) and margay (Leopardus wiedii), which are combined for analysis, and jaguar (Panthera onca); Carnivora, Mustelidae: neotropical otter (Lontra longicaudis) and giant otter (Pteronura brasiliensis); Cetartiodactyla, Tayassuidae: collared peccary (Pecari tajacu) and white-lipped peccary (Tayassu pecari); Cetartiodactyla, Cervidae: red brocket deer (Mazama americana); and one reptile: Crocodylia, Alligatoridae: black caiman (Melanosuchus niger), the largest Amazonian vertebrate. Other species (not temporally analyzed) included common agouti (Dasyprocta spp.), Amazonian brocket deer (Mazama nemorivaga), tapir (Tapirus terrestris), iguana (Iguana iguana), tegu lizard (Tupinambis teguixin), caiman lizard (Dracaena guianensis), boa (Boa constrictor), anaconda (Eunectes murinus), and spectacled caiman (Caiman crocodilus).
Commercial offtake records from the central-western Brazilian Amazon
Throughout the 20th century, commercial and port records in Amazonia were monitored by the Finance Secretariat of Amazonas; the Departments of Statistics of the states of Amazonas and Acre; the Manaus Harbour Ltd., which ran the Port of Manaus concession for Amazonas state until the mid-1960s; the Commercial Association of Amazonas; and the Development Commission for the State of Amazonas (CODEAMA). Together, these various data sources, which were systematized and analyzed here for the first time, yield a wealth of information about extractive industries in western Amazonia for about 120 years. Unfortunately, successive government administrations and managers discarded nearly all of the original information in hardcopy documents. A major challenge in systematizing the records was to determine which data sources were still extant and where to find them. Some surviving documents were found in libraries and museums in Manaus, Amazonas, including the Cosme Ferreira Filho Library (Commercial Association of Amazonas), the Geography and History Institute of Amazonas (IGHA), the Amazonian Museum of the Federal University of Amazonas (UFAM), the Amazonas State Public Library, the Museum of the Port of Manaus, the library of the National Institute for Amazonian Research, and the Mario Ypiranga Monteiro Library. Some documents were also found in the libraries of the Brazilian Institute of Geography and Statistics (IBGE) at both Rio de Janeiro and Rio Branco (state of Acre). For the list of primary historical documents, see text S1. Descriptions of the primary historical documents systematized are below.
(1) Amazonas state official commercial records. Annual reports have been produced by the Amazonas state government from 1852 to the present. Some reports contain tables summarizing extractive products exported, with amounts and prices for the states of Amazonas and, occasionally, Acre. Hide exports per species were available for 1852, 1857, 1858, 1860, 1864, 1867, 1873, 1875–1886, 1888, 1895, 1896, 1898, 1899, 1903–1919, 1921–1933, 1935–1940, and 1943.
(2) Manáos Harbour Ltd. port records. The concession owner of the Port of Manaus from 1902 to the 1960s—the Manáos Harbour Ltd.—published annual reports (the Trafego do Porto de Manaus) of total cargo in transit through the port, either as landings or as exports. For hides, data are presented in kilograms of hides of all species combined for the 1904–1952 period.
(3) ACA commercial records. The Commercial Association of Amazonas (ACA) published periodic journals containing rich qualitative and quantitative information about the hide trade, among other extractive products. The two monthly journals were Revista da Associação Comercial (1908–1941) and Boletim da Associação Comercial (1941–1973), amounting to a total of 515 issues. Data on exports per species for the state of Amazonas were available for 1908–1918, 1925–1933, 1943, 1946, 1948–1950, and 1959. Data on overall exports and landings for all species combined were available for 1934–1941 and 1945–1956.
(4) Corel cargo manifests. The daily commercial newspaper Informativo Corel published cargo manifests for all boats and ships that landed at the port of Manaus, and their regions of origin, whether Amazonian hinterland or overseas. Data quality is variable, ranging from detailed information about the number of hides per species per boat to less informative summaries of total kilograms in general categories such as “wildlife skins,” “caiman,” and “luxury skins” (fantasia, as carnivore pelts were named locally). We found newspaper issues detailing these records for 1957, 1959, 1962, 1965, 1968, 1969, and 1971.
(5) IBGE commercial records. Commercial records of numbers of hides exported were published annually by the IBGE, available for 1960–1969 by state and for some species. We converted numbers of hides to kilograms by multiplying offtake by the average weight of hide per species, to reconcile the IBGE records with the other data sources. Unfortunately, there is no specific mention of exports of jaguar or neotropical otter hides, unlike other species. Another problem concerns the lack of distinction between collared and white-lipped peccaries and the two species of caimans. Export data from Acre state were also available for 1943. The Brazilian Annual Statistics publication organized by the IBGE is available at http://biblioteca.ibge.gov.br.
(6) Aury Medeiro’s Acre records. Total hide exports aggregated across species from the state of Acre were available for 1961–1970 in Medeiros (106).
(7) Carvalho’s Amazonas records. This seminal paper on the Amazonian hide trade provides data on the kilograms of hides exported by the state of Amazonas between 1950 and 1965 (36), collected by the Departamento Estadual de Estatística do Amazonas (DEE) (see below). The units of kilograms, rather than individual hides, were not adequately specified in the original paper (36) but were clarified in a personal communication with J. C. M. Carvalho by D. Domning (41). We used records mainly from 1950 to 1954, with the exception of 1965 for the production of jaguar pelts, because after this period, data were mostly underestimated. Additionally, Carvalho (36) published a record of 4.9 million kilograms for black caiman hides produced in 1950, which we did not include in our analysis because it is inconsistent with other records for the same year.
(8) DEE commercial records. The now defunct department of public statistics of the state of Amazonas (DEE) collected commercial statistics from the 1930s to the 1960s. In addition to the data presented by Carvalho (36), we used this source to supplement information on hide exports from Amazonas by species from 1963 to 1965.
(9) CODEAMA commercial records. The now defunct CODEAMA was created in the 1960s. We used its records for hide exports per species from Amazonas state for 1966–1969.
(10) J. G. Araujo Company’s cargo manifests. We were able to analyze about 2000 original, privately held, and previously inaccessible shipping invoices and cargo manifests of the J. G. Araujo Company, a family merchant empire based in Manaus that lasted from the 1870s to the 1990s, when it finally went bankrupt. The company, a driving force in the rubber boom economy of Amazonas state at the turn of the 20th century (107, 108), later became a major exporter of animal hides (27). The company received hides from at least 130 localities, former seringais, distributed throughout all the main river basins of the Amazon (27). This unique, previously unavailable data set was essential for studying regional variations in the hide trade.
Data and approach
The status of exploited wildlife populations is best indicated by the numbers of landed hides for each species shipped from the hinterlands. However, data records distinguishing market landings by species are scarce, although they are often available for exports; moreover, data are frequently presented in terms of kilograms of hides rather than numbers of individual hides. Although exports do give some indication of population status, quantities exported were generally less than quantities extracted in each year, due to substantial stockpiling of hides. For 45 years in which both export and extraction data were available, hide exports across species summed to 13.8 million kilograms, whereas extractions summed to 21.6 million kilograms. These issues demanded a new approach to data modeling.
We adopted a two-pronged approach, such that data on total extractions, aggregated across species and measured in kilograms, were modeled simultaneously with data on the composition of traded hides by species insofar as these were available, and the composition of the available data in each year was assumed to be indicative of the composition of total extractions in that year. This dual approach enabled us to estimate trends in the totals harvested for each species over time.
We reconstructed two independent data sets:
(1) Total kilograms extracted annually, aggregated across species. Because data records distinguishing market landings by species are scarce, we constructed a time series of kilograms of hides extracted for all species combined, annually from 1904 to 1969. We attempted to capture as much as possible of the total hide extraction in the central-western Brazilian Amazon by summing records from four time series: (i) hides landed at the Port of Manaus arising from the states of Amazonas, Acre, Rondônia, and Roraima (any landings in Manaus corresponding to extractions from Peru, Bolivia, and Colombia were excluded); and, additionally, harvests that were not landed (and thus went unrecorded) in the Port of Manaus but were exported directly from the ports of (ii) Acre state, (iii) Rondônia state, and (iv) the fluvial ports of the middle-Amazon River in Amazonas state. These data were generally available for 54 of the 66 years from 1904 to 1969, although for the 1950s, not all of the four time series were available. We tended to select the largest amount per year across the historical documents. We represent this time series of total kilograms extracted by X1904 , …, X1969. The total extraction data set was obtained from primary documents 1 to 6 and 9 (see text S1).
(2) Annual composition of available data by species. Most of the data that distinguished shipments by species arose from the export records, or occasionally landing records, of Amazonas and Acre states. The annual breakdown by species of available records, corresponding to the proportion of hide weight in kilograms attributed to each species, constitutes the species composition time series. Our approach is to use the estimated species composition in each year together with the estimated total extracted each year [described in (1)], to give an estimate of the kilograms of hide extracted for each species in each year.
To construct the species composition time series, we used species-specific landing data rather than export data where possible, especially for 1968 and 1969, due to the large volume of stockpiled hides exported after the Brazilian Faunal Protection Law was passed in 1967. Amazonas state exports included hides extracted from the states of Amazonas, Acre, Rondônia, and Roraima, which were normally landed at the Port of Manaus and then exported from there. A substantial amount was exported directly from the states of Acre, Rondônia, and Roraima. We preferably summed exports for all states to create the species composition records; however, for 26 of the 66 years, export data were available only for the state of Amazonas. We tended to select the largest amount for each species in each year across the historical documents, with the exception of the black caiman in 1950, which had an inconsistent outlier record of almost 5 million kilograms (see above).
Species-specific data were available for 55 of the 66 years from 1904 to 1969, but the number of records for individual species varied from 55 years for the red brocket deer to 22 years for the manatee. In each year t, we divided the amount in kilograms recorded for species i by the total amount recorded for all species in year t to obtain proportion Pit to be attributed to species i in that year. Thus, (P1t , P2t , …, PSt) is the proportional composition of species 1, 2, …, S in year t, where 0 ≤ Pit ≤ 1 for each species i, and P1t + … + PSt = 1 for each time t = 1904, ..., 1969. The species composition data were obtained from primary documents 1, 3, 4, 6, 7, 8, and 9 (see text S1).
Model for harvest trends
Let γi(t) represent the unknown number of kilograms extracted for species i in year t, for t = 1904, …, 1969 and for i = 1, …, S. Our approach is to estimate γi(t) as a smooth curve over time for each species i, such that the curves γi(t) simultaneously fit the data on annual total extraction aggregated across species and on annual composition by species. The total extraction aggregated across species in year t is and is fitted to the corresponding data X1904 , …, X1969. We used a gamma model for this component, specifically, Xt ~ Gamma(Γ(t), σ) for t = 1904 , …, 1969, where Gamma(Γ(t), σ) denotes the gamma distribution with mean Γ(t) and scale parameter σ. The proportional composition of extractions by species in year t is and is fitted to the corresponding species composition data (P1t , P2t , …, PSt) for each year t = 1904 , …, 1969. We used a multivariate Dirichlet model for this component, with parameter vector (γ1(t), …, γS(t)) × δ/ Γ(t). This model ensures that (P1t , P2t , …, PSt) is a vector of proportions summing to 1, such that Pit has mean γi(t) / Γ(t). The parameter δ controls the scatter of Pit about the mean, such that large values of δ describe a close fit. If there are missing species records in year t, the proportional composition of those species that do have records in year t follows a Dirichlet distribution that is easily derived from the full Dirichlet model.
We estimated the extraction trend curves γ1(t), …, γS(t) using cubic splines (109, 110), with the number and position of knots for the curve γi(t) determined by the number and position of data records for species i, up to a maximum of K knots. We estimated σ, δ, and the spline parameters for γ1(t), …, γS(t) by maximum likelihood, using custom code written in the statistical language R (111). We fitted the model for a range of values of K, and used AIC to select the final value of K. The log-likelihoods for the gamma and Dirichlet components were each summed over time and added together for the overall log-likelihood.
Bootstraps and CIs
For CIs, we used the parametric bootstrap (112). We generated replicate data and from the fitted model, preserving the pattern of missing data records found in the original data. We refitted the model for each of 500 replicates and constructed percentile CIs for quantities of interest using the 500 fitted results.
The intrinsic rate of natural increase (Rmax)
Estimates of Rmax were obtained from previous publications (113) or calculated using the Cole equation (114). Estimates for age of first reproduction, age of last reproduction, and annual birth rate of female offspring were obtained from published data (115–126) or from personal communications with V. M. da Silva for the manatee. All estimates are presented in table S2.
Population resilience to commercial hunting, and relationship with habitat type and Rmax
The resilience of each species to commercial hunting was assessed by the estimated percentage harvest change between two peak harvest periods. For species i, the estimated percentage change was given by , where denotes the sum of the estimated harvests over five consecutive years defined as peak period j, for j = 1, 2. The first peak period was centered on the overall pre-1965 peak year for species i, which fell between 1937 and 1943 for all species except the capybara (1963). The second peak period was defined as 1965–1969 for all species except the manatee, for which we used 1969–1973 due to meat production that continued into the 1970s. This analysis was performed in the R statistical language (111).
Tracking indexed hide prices
Prices of hides were obtained in the two historical Brazilian currencies over the study period (real and cruzeiro) or directly as U.S. dollars between 1926 and 1975. Historical notes on Brazilian currency are detailed in the study of Domning (41). Prices were converted to U.S. dollars and indexed (127) for the 2015 base year. Prices were generally obtained from primary historical documents 3 to 9 listed above, as well as from other references (128, 129). When species-specific hide prices were unavailable, we obtained prices by dividing the total revenue by the number of hides or weight traded per species per year.
Spatial analysis
Spatial analyses were performed in the R statistical language (111) using packages sp (130, 131) and raster (132). Historical localities in the central-western Brazilian Amazon, mostly nonindigenous, were obtained from maps and censuses of the IBGE in the 1950s (133, 134) and 1960s (135) and georeferenced in QGIS software (136). To represent a crude large-scale harvestable area in the central-western Brazilian Amazon in the 1950s and 1960s, we buffered (at 5 and 10 km) the centroids of all 3298 settlements following the typical radial spread of subsistence hunting effort, as previously reported (17, 20–22). This produced two hypothetical hunting catchment areas, which we rasterized using the raster package (132).
We extracted a mask encompassing the states of Acre, Amazonas, Roraima, and Rondônia from a preclassified mosaic (wetland extant, vegetation cover, and inundation state) at a 100-m resolution of the Japanese Earth Resource Satellite (JERS-1), which is free for use and is available on the ORNL DAAC website at http://daac.ornl.gov/index.shtml (48). We reclassified this mosaic to obtain flooded and nonflooded areas (aquatic and terrestrial habitat, respectively) in both low- and high-water seasons in the study area. Each of these four binary rasters was compared with the two binary rasters of harvestable areas with buffers of 5- and 10-km radius around the historical settlements, to gain the proportion of aquatic and terrestrial habitats that were accessible and inaccessible by the human population in the central-western Brazilian Amazon during the 1950s and 1960s, according to the two hypothetical scenarios.
Sustainability of commercial hunting in terrestrial species: Harvest versus production
We compared modeled harvests to the estimates of maximum production using the Robinson-Redford sustainability index (49, 50), one of the main analytical tools used to determine sustainable harvest rates in studies of subsistence hunting. Maximum production, measured in number of animals per square kilometer, is defined as the maximum number of animals that can be added to the population annually under ideal conditions, taking account only of reproduction and natural mortality. This maximal increase is generally assumed to occur when the population is at 60% of its carrying capacity K (49, 50). The index does not take account of animal dispersal into or out of the harvested area. We defined catchment areas for terrestrial habitats using a 5-km radius around all historical settlements, totaling 131,619 km2. The species-specific carrying capacities (K) were obtained from previous publications (49, 50, 137, 138).
Refuge size and finite rate of population increase (λ) for predicting the sustainability of hunting in the central-western Brazilian Amazon: The Joshi and Gadgil model
We compared the proportional area of refuges (inaccessible areas) under the 5- and 10-km buffer scenarios previously described with the proportional area of refuges required to achieve the MSY according to the formula of Joshi and Gadgil (51), using the expression
where α is the proportion of the total area that should be maintained as refuge to gain an MSY, and λ is the species-specific finite rate of population increase (51), calculated as . This relationship between reproductive rate and refuge size is based on an assumption of maximal hunting effort such that the whole population in the harvestable area (proportion 1 − α) is harvested in the time period, and repopulation takes place from the refuge (51).
To evaluate sustainability for the 10 main commercially hunted species during the Amazonian hide trade using the Joshi and Gadgil model, we calculated the size of refuges (Arefuge) by excluding the two hypothetical catchment area scenarios of 5 and 10 km around all settlements (Ahunt) from the total area of the central-western Brazilian Amazon (2,175,744 km2). We compared these to the estimated species-specific area required for maximum sustainable harvests: AMSY = αAhunt/(1 − α), where α = 1/λ. Sustainable harvests require Arefuge > AMSY. This simple model unrealistically assumes that each species entirely occupies its available habitat (terrestrial habitat for terrestrial species and aquatic habitat for aquatic and semiaquatic species) and that population density is homogeneous across the Amazon basin, so the index provides a crude but useful indicator. Results are summarized in table S5.
Supplementary Material
Acknowledgments
We thank the library managers of Manaus, Rio Branco, and Rio de Janeiro who helped locate historical documents; J. Fragoso, J. Valsecchi, J. Zuanon, J. Pezzuti, H. Bizri, and G. Ferraz made helpful comments on the manuscript; N. V. Joshi provided insightful explanation on the dynamics of refuge-harvestable systems; F. Benzecry (a former president of the now-defunct Canadense tannery) provided a valuable overview of the historical hide industry; dozens of former hunters from the Amazonian hinterlands provided precious feedback about hunting methods and population status during the trade in animal hides; and F. Figueiredo and M. Santos assisted with spatial analyses. We are grateful for the attentive editorial comments and suggestions of the referees. Funding: A.P.A. and E.M.V. thank CNPq (procs. 140222/2011-1 and 309458/2013-7), FAPEAM (proc. 062.00427/2013), and CAPES (proc. PDSE-14646/13-7) for funding support. This Ph.D. research was conducted at the National Institute of Amazonian Research (Manaus, Brazil) and at the University of Auckland (New Zealand). Author contributions: A.P.A., E.M.V., G.H.S., and F.R. designed the study. A.P.A. uncovered, collected, systematized, and analyzed the data. R.M.F. analyzed the data and performed modeling and statistical analyses. A.P.A., G.H.S., C.A.P., and R.M.F. wrote the paper. All authors discussed the results and commented on the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: Correspondence and requests for data sets and historical documents analyzed here should be addressed to A.P.A (aapardalis@gmail.com). Requests for models and statistical analysis should be addressed to R.M.F. (r.fewster@auckland.ac.nz) and A.P.A.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/10/e1600936/DC1
text S1. Historical documents list.
text S2. Comparing the impacts of contemporary subsistence hunting versus historical commercial hunting in Amazonia.
text S3. International demand for Amazonian hides through time.
fig. S1. Rural population in the central-western Brazilian Amazon.
fig. S2. Central-western Brazilian Amazon yields (U.S. dollars in 2015 currency equivalence) for foremost 20th century products.
table S1. Average hide weights of commercially hunted species.
table S2. Intrinsic rate of natural increase (Rmax) for game species and parameters required for its calculation by the Cole equation.
table S3. Area of terrestrial and aquatic habitats, and their accessibility by hunters, under two hunting catchment area scenarios (buffers of 5 and 10 km around all settlements) in the central-western Brazilian Amazon.
table S4. Comparison between the Robinson-Redford production index and commercial harvests at two historical peaks for terrestrial species.
table S5. Comparison of the minimum refuge area required for maximum sustainable harvests (AMSY) according to the Joshi and Gadgil model (α = 1/λ) to actual refuge area (Arefuge) in the central-western Brazilian Amazon.
REFERENCES AND NOTES
- 1.Malhi Y., Roberts J. T., Betts R. A., Killeen T. J., Li W., Nobre C. A., Climate change, deforestation, and the fate of the Amazon. Science 319, 169–172 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Pimm S. L., Jenkins C. N., Abell R., Brooks T. M., Gittleman J. L., Joppa L. N., Raven P. H., Roberts C. M., Sexton J. O., The biodiversity of species and their rates of extinction, distribution, and protection. Science 344, 1246752 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Nepstad D., Carvalho G., Barros A. C., Alencar A., Capobianco J. P., Bishop J., Moutinho P., Lefebvre P., Silva U. L. Jr, Prins E., Road paving, fire regime feedbacks, and the future of Amazon forests. Forest Ecol. Manag. 154, 395–407 (2001). [Google Scholar]
- 4.Phillips O. L., Aragão L. E. O. C., Lewis S. L., Fisher J. B., Lloyd J., López-González G., Malhi Y., Monteagudo A., Peacock J., Quesada C. A., van der Heijden G., Almeida S., Amaral I., Arroyo L., Aymard G., Baker T. R., Bánki O., Blanc L., Bonal D., Brando P., Chave J., de Oliveira Á. C. A., Cardozo N. D., Czimczik C. I., Feldpausch T. R., Freitas M. A., Gloor E., Higuchi N., Jiménez E., Lloyd G., Meir P., Mendoza C., Morel A., Neill D. A., Nepstad D., Patiño S., Peñuela M. C., Prieto A., Ramírez F., Schwarz M., Silva J., Silveira M., Thomas A. S., ter Steege H., Stropp J., Vásquez R., Zelazowski P., Dávila E. A., Andelman S., Andrade A., Chao K.-J., Erwin T., Di Fiore A., Honorio E. C., Keeling H., Killeen T. J., Laurance W. F., Cruz A. P., Pitman N. C. A., Vargas P. N., Ramírez-Angulo H., Rudas A., Salamão R., Silva N., Terborgh J., Torres-Lezama A., Drought sensitivity of the Amazon rainforest. Science 323, 1344–1347 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Fearnside P. M., Deforestation in Brazilian Amazonia: History, rates, and consequences. Conserv. Biol. 19, 680–688 (2005). [Google Scholar]
- 6.J. G. Robinson, E. L. Bennett, Hunting for Sustainability in Tropical Forests (Columbia Univ. Press, New York, 2000). [Google Scholar]
- 7.Milner-Gulland E. J., Bennett E. L., Wild meat: The bigger picture. Trends Ecol. Evol. 18, 351–357 (2003). [Google Scholar]
- 8.Peres C. A., Effects of subsistence hunting on vertebrate community structure in Amazonian forests. Conserv. Biol. 14, 240–253 (2000). [Google Scholar]
- 9.Peres C. A., Emilio T., Schietti J., Desmoulière S. J. M., Levi T., Dispersal limitation induces long-term biomass collapse in overhunted Amazonian forests. Proc. Natl. Acad. Sci. U.S.A. 113, 892–897 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terborgh J., Nuñez-Iturri G., Pitman N. C. A., Valverde F. H. C., Alvarez P., Swamy V., Pringle E. G., Paine C. E. T., Tree recruitment in an empty forest. Ecology 89, 1757–1768 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Bello C., Galetti M., Pizo M. A., Magnago L. F. S., Rocha M. F., Lima R. A. F., Peres C. A., Ovaskainen O., Jordano P., Defaunation affects carbon storage in tropical forests. Sci. Adv. 1, e1501105 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dirzo R., Young H. S., Galetti M., Ceballos G., Isaac N. J. B., Collen B., Defaunation in the Anthropocene. Science 345, 401–406 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Redford K. H., The empty forest. BioScience 42, 412–422 (1992). [Google Scholar]
- 14.Wilkie D. S., Bennett E. L., Peres C. A., Cunningham A. A., The empty forest revisited. Ann. N. Y. Acad. Sci. 1223, 120–128 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Stokstad E., The empty forest. Science 345, 396–399 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Novaro A. J., Redford K. H., Bodmer R. E., Effect of hunting in source-sink systems in the Neotropics. Conserv. Biol. 14, 713–721 (2000). [Google Scholar]
- 17.Levi T., Shepard G. H. Jr, Ohl-Schacherer J., Peres C. A., Yu D. W., Modelling the long-term sustainability of indigenous hunting in Manu National Park, Peru: Landscape-scale management implications for Amazonia. J. Appl. Ecol. 46, 804–814 (2009). [Google Scholar]
- 18.Bodmer R. E., Eisenberg J. F., Redford K. H., Hunting and the likelihood of extinction of Amazonian mammals. Conserv. Biol. 11, 460–466 (1997). [Google Scholar]
- 19.De Souza-Mazurek R. R., Pedrinho T., Feliciano X., Hilário W., Gerôncio S., Marcelo E., Subsistence hunting among the Waimiri Atroari indians in central Amazonia, Brazil. Biodivers. Conserv. 9, 579–596 (2000). [Google Scholar]
- 20.Sirén A., Hambäck P.. Machoa J., Including spatial heterogeneity and animal dispersal when evaluating hunting: A model analysis and an empirical assessment in an Amazonian community. Conserv. Biol. 18, 1315–1329 (2004). [Google Scholar]
- 21.Read J. M., Fragoso J. M. V., Silvius K. M., Luzar J., Overman H., Cummings A., Giery S. T., de Oliveira L. F., Space, place, and hunting patterns among indigenous peoples of the Guyanese Rupununi region. J. Lat. Am. Geogr. 9, 213–243 (2010). [Google Scholar]
- 22.Constantino P. A. L., Dynamics of hunting territories and prey distribution in Amazonian Indigenous Lands. Appl. Geogr. 56, 222–231 (2015). [Google Scholar]
- 23.S. Hecht, A. Cockburn, The Fate of the Forest: Developers, Destroyers, and Defenders of the Amazon (The University of Chicago Press, Chicago, 2010) [Google Scholar]
- 24.W. Dean, Brazil and the Struggle for Rubber: A Study in Environmental History (Cambridge Univ. Press, Cambridge, 1987). [Google Scholar]
- 25.A. C. F. Reis, O Seringal e o Seringueiro (Serviço de Informação Agrícola, Ministério da Agricultura, Rio de Janeiro, 1953). [Google Scholar]
- 26.R. Santos, História Econômica da Amazônia (1800-1920) (TA Queiroz, São Paulo, 1980). [Google Scholar]
- 27.Antunes A. P., Shepard G. H. Jr, Venticinque E. M., O comércio internacional de peles silvestres na Amazônia brasileira no século XX: The international trade in wild animals skins from the Brazilian Amazon in the 20th century. Bol. Mus. Para. Emílio Goeldi. Ciĕnc. Hum. 9, 487–518 (2014). [Google Scholar]
- 28.Pauly D., Zeller D., Catch reconstructions reveal that global marine fisheries catches are higher than reported and declining. Nat. Commun. 7, 10244 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Instituto Brasileiro de Geografia e Estatística (IBGE), Censos Demográficos e Econômicos – Território do Guaporé (IBGE, Rio de Janeiro, 1957), vol. 6. [Google Scholar]
- 30.Instituto Brasileiro de Geografia e Estatística (IBGE). Censos Demográficos e Econômicos – Território do Acre (IBGE, Rio de Janeiro, 1957), vol. 7. [Google Scholar]
- 31.Instituto Brasileiro de Geografia e Estatística (IBGE), Censos Demográficos e Econômicos – Estado do Amazonas (IBGE, Rio de Janeiro, 1957), vol. 8. [Google Scholar]
- 32.Instituto Brasileiro de Geografia e Estatística (IBGE), Censos Demográficos e Econômicos – Território do Rio Branco (IBGE, Rio de Janeiro, 1957), vol. 9. [Google Scholar]
- 33.Smith N. J. H., Spotted cats and the Amazon skin trade. Oryx 13, 362–371 (1976). [Google Scholar]
- 34.A. J. Loveridge, S. W. Wang, L. G. Frank, J. Seidensticker, People and wild felids: Conservation of cats and management of conflicts, in Biological Conservation of Wild Felids, D. W. Macdonald, A. J. Loveridge, Eds. (Oxford Univ. Press, Oxford, 2010), pp. 161–195. [Google Scholar]
- 35.Solari J., Ribeiro O., Chiodi A., Imagens de um massacre. Rev Real 67, 157–168 (1971). [Google Scholar]
- 36.J. C. M. Carvalho, A conservação da natureza e recursos naturais na Amazônia Brasileira, in Simpósio Sobre a Biota Amazônica, H. Lent, Ed. (CNPq, Rio de Janeiro, 1967), vol. 7., chap. 1, pp. 1–47. [Google Scholar]
- 37.J. N. Ceccatto, Lei de proteção à fauna, in Encontro Nacional sobre Conservação da Fauna e Recursos Faunísticos (Instituto Brasileiro de Desenvolvimento Florestal, Brasília, 1977), pp. 153–174. [Google Scholar]
- 38.Smith N. J., Utilization of game along Brazil’s transamazon highway. Acta Amazonica 6, 455–466 (1976). [Google Scholar]
- 39.Ayres J. M., Ayres C., Aspectos da caça no alto rio Aripuanã. Acta Amazonica 9, 287–298 (1979). [Google Scholar]
- 40.Mourão G. M., Utilização econômica da fauna silvestre no Brasil: O exemplo do jacaré-do-pantanal. Embrapa Pantanal 5, 1–4 (2000). [Google Scholar]
- 41.Domning D. P., Commercial exploitation of manatees Trichechus in Brazil c. 1785–1973. Biol. Conserv. 22, 101–126 (1982). [Google Scholar]
- 42.Peres C. A., Population status of white-lipped Tayassu pecari and collared peccaries T. tajacu in hunted and unhunted Amazonian forests. Biol. Conserv. 77, 115–123 (1996). [Google Scholar]
- 43.Fragoso J. M. V., Home range and movement patterns of white-lipped peccary (Tayassu pecari) herds in the Northern Brazilian Amazon. Biotropica 30, 458–469 (1998). [Google Scholar]
- 44.Jerozolimski A., Peres C. A., Bringing home the biggest bacon: A cross-site analysis of the structure of hunter-kill profiles in Neotropical forests. Biol. Conserv. 111, 415–425 (2003). [Google Scholar]
- 45.Albert B., Le Tourneau F.-M., Ethnogeography and resource use among the Yanomami: Toward a model of “reticular space”. Curr. Anthropol. 48, 584–592 (2007). [Google Scholar]
- 46.Renoux F., de Thoisy B., Hunting management: The need to adjust predictive models to field observations. Ethnobio. Conserv. 5, (2016). [Google Scholar]
- 47.Shepard G. H. Jr, Levi T., Neves E. G., Peres C. A., Yu D. W., Hunting in ancient and modern Amazonia: Rethinking sustainability. Am. Anthropol. 114, 652–667 (2012). [Google Scholar]
- 48.Hess L. L., Melack J. M., Affonso A. G., Barbosa C., Gastil-Buhl M., Novo E. M. L. M., Wetlands of the lowland Amazon basin: Extent, vegetative cover, and dual-season inundated area as mapped with JERS-1 synthetic aperture radar. Wetlands 35, 745–756 (2015). [Google Scholar]
- 49.J. G. Robinson, K. H. Redford, Sustainable harvest of neotropical forest animals, in Neotropical Wildlife Use and Conservation, J. G. Robinson, K. H. Redford, Eds. (The University of Chicago Press, Chicago, 1991), chap. 27, pp. 415–429. [Google Scholar]
- 50.J. G. Robinson, Calculating maximum sustainable harvests and percentage offtakes, in Hunting for Sustainability in Tropical Forests, J. G. Robinson, E. L. Bennett, Eds. (Columbia Univ. Press, New York, 2000), pp. 521–524. [Google Scholar]
- 51.Joshi N. V., Gadgil M., On the role of refugia in promoting prudent use of biological resources. Theor. Popul. Biol. 40, 211–229 (1991). [Google Scholar]
- 52.Smith N. J. H., Caimans, capybaras, otters, manatees, and man in Amazonia. Biol. Conserv. 19, 177–187 (1981). [Google Scholar]
- 53.Freitas C. E. C., Siqueira-Souza F. K., Humston R., Hurd L. E., An initial assessment of drought sensitivity in Amazonian fish communities. Hydrobiologia 705, 159–171 (2013). [Google Scholar]
- 54.Oberdorff T., Jézéquel C., Campero M., Carvajal-Vallejos F., Cornu J. F., Dias M. S., Duponchelle F., Maldonado-Ocampo J. A., Ortega H., Renno J. F., Tedesco P. A., Opinion paper: How vulnerable are Amazonian freshwater fishes to ongoing climate change?. J. Appl. Ichthyol. 31, 4–9 (2015). [Google Scholar]
- 55.Sorribas M. V., Paiva R. C. D., Melack J. M., Bravo J. M., Jones C., Carvalho L., Beighley E., Forsberg B., Costa M. H., Projections of climate change effects on discharge and inundation in the Amazon basin. Clim. Change 136, 555–570 (2016). [Google Scholar]
- 56.J. Veríssimo, A pesca na Amazônia (Livraria Classica de Alves, Rio de Janeiro, 1895). [Google Scholar]
- 57.Nunes-Pereira M., A pesca no rio Purus. A Voz do Mar: Bol. Com. Exec. Pesca 178–183 (1941). [Google Scholar]
- 58.Nunes-Pereira M. A., A pesca no rio Purus. A Voz do Mar: Bol Com Exec Pesca 186, 178–186 (1943). [Google Scholar]
- 59.Nunes-Pereira M. A., O peixe-boi da Amazônia. Bol. Minist. Agric. 3, 21–95 (1944). [Google Scholar]
- 60.Smith N. J. H., Aquatic turtles of Amazonia: An endangered resource. Biol. Conserv. 16, 165–176 (1979). [Google Scholar]
- 61.Bayley P. B., Petrere M. Jr, Amazon fisheries: Assessment methods, current status, and management options. Can. Spec. Publ. Fish. Aquat. Sci. 106, 385–398 (1989). [Google Scholar]
- 62.Campos C. P., Costa Sousa R. G., Catarino M. F., de Albuquerque Costa G., Freitas C. E. C., Population dynamics and stock assessment of Colossoma macropomum caught in the Manacapuru Lake system (Amazon Basin, Brazil). Fisheries Manag. Ecol. 22, 400–406 (2015). [Google Scholar]
- 63.Córdoba E. A., León Á. V. J., Bonilla-Castillo C. A., Petrere M. Jr, Peláez M., Duponchelle F., Breeding, growth and exploitation of Brachyplatystoma rousseauxii Castelnau, 1855 in the Caqueta River, Colombia. Neotrop. Ichthyol. 11, 637–647 (2013). [Google Scholar]
- 64.Petrere M. Jr, Barthem R. B., Córdoba E. A., Gómez B. C., Review of the large catfish fisheries in the upper Amazon and the stock depletion of piraíba (Brachyplatystoma filamentosum Lichtenstein). Rev. Fish Biol. Fish. 14, 403–414 (2004). [Google Scholar]
- 65.Castello L., Arantes C. C., Mcgrath D. G., Stewart D. J., De Sousa F. S., Understanding fishing-induced extinctions in the Amazon. Aquat. Conserv. 25, 587–598 (2015). [Google Scholar]
- 66.S. Cleaveland, G. R. Hess, A. Dobson, M. K. Laurenson, H. I. McCallum, M. Roberts, R. Woodroffe, The role of pathogens in biological conservation, in The Ecology of Wildlife Diseases, P. J. Hudson, A. Rizzoli, B. T. Grenfell, H. Heesterbeek, A. P. Dobson, Eds. (Oxford Univ. Press, Oxford, 2002), pp. 139–150. [Google Scholar]
- 67.M. Solorio, Avaliação sanitária da presença de doenças e caracterização dos padrões de caça de subsistência do queixada (Tayassu pecari) de vida livre na Amazônia Peruana, thesis, Universidade de São Paulo, Piracicaba (2010). [Google Scholar]
- 68.J. M. Fragoso, Desapariciones locales del bachiro labiado (Tayassu pecari) en la Amazonia: migraciones, sobre-cosecha, o epidemia, in Manejo de Fauna Silvestre en la Amazonia, T. G. Fang, R. E. Bodmer, R. Aquino, M. H. Valqui, Eds. (OFAVIM, La Paz, Bolivia, 1997), pp. 309–312. [Google Scholar]
- 69.Tollefson J., Amazon ecology: Footprints in the forest. Nature 502, 160–162 (2013). [DOI] [PubMed] [Google Scholar]
- 70.McMichael C. H., Piperno D. R., Bush M. B., Silman M. R., Zimmerman A. R., Raczka M. F., Lobato L. C., Sparse pre-Columbian human habitation in western Amazonia. Science 336, 1429–1431 (2012). [DOI] [PubMed] [Google Scholar]
- 71.Bush M. B., McMichael C. H., Piperno D. R., Silman M. R., Barlow J., Peres C. A., Power M., Palace M. W., Anthropogenic influence on Amazonian forests in pre-history: An ecological perspective. J. Biogeogr. 42, 2277–2288 (2015). [Google Scholar]
- 72.Clement C. R., Denevan W. M., Heckenberger M. J., Junqueira A. B., Neves E. G., Teixeira W. G., Woods W. I., The domestication of Amazonia before European conquest. Proc. Biol. Sci. 282, 20150813 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McClenachan L., Cooper A. B., Mckenzie M. G., Drew J. A., The importance of surprising results and best practices in historical ecology. BioScience 65, 932–939 (2015). [Google Scholar]
- 74.E. S. Martins, A caça de subsistência de extrativistas na Amazônia: Sustentabilidade, biodiversidade e extinção de espécies, thesis, Universidade de Brasília, Brasília (1992). [Google Scholar]
- 75.Milner-Gulland E. J., Akçakaya H. R., Sustainability indices for exploited populations. Trends Ecol. Evol. 16, 686–692 (2001). [Google Scholar]
- 76.Uscamaita M. R., Bodmer R., Recovery of the endangered giant otter Pteronura brasiliensis on the Yavarí-Mirín and Yavarí rivers: A success story for CITES. Oryx 44, 83–88 (2010). [Google Scholar]
- 77.Lima D. S., Marmontel M., Bernard E., Reoccupation of historical areas by the endangered giant river otter Pteronura brasiliensis (Carnivora: Mustelidae) in Central Amazonia, Brazil. Mammalia 78, 177–184 (2014). [Google Scholar]
- 78.Silveira R., Thorbjarnarson J. B., Conservation implications of commercial hunting of black and spectacled caiman in the Mamirauá Sustainable Development Reserve, Brazil. Biol. Conserv. 88, 103–109 (1999). [Google Scholar]
- 79.D. S. Souza, Peixe-boi da Amazônia (Trichechus inunguis natterer 1883): mortalidade e uso do habitat na reserva de desenvolvimento sustentável Piagaçu-Purus, Amazônia central, Brasil, thesis, Instituto Nacional de Pesquisas da Amazônia, Manaus, Amazonas (2015). [Google Scholar]
- 80.Bodmer R. E., Strategies of seed dispersal and seed predation in Amazonian ungulates. Biotropica 23, 255–261 (1991). [Google Scholar]
- 81.Beck H., A review of peccary-palm interactions and their ecological ramifications across the Neotropics. J. Mammal. 87, 519–530 (2006). [Google Scholar]
- 82.Silman M. R., Terborgh J. W., Kiltie R. A., Population regulation of a dominant rain forest tree by a major seed predator. Ecology 84, 431–438 (2003). [Google Scholar]
- 83.Hibert F., Sabatier D., Andrivot J., Scotti-Saintagne C., Gonzalez S., Prévost M.-F., Grenand P., Chave J., Caron H., Richard-Hansen C., Botany, genetics and ethnobotany: A crossed investigation on the elusive tapir’s diet in French Guiana. PLOS One 6, e25850 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wright S. J., The myriad consequences of hunting for vertebrates and plants in tropical forests. Perspect. Plant. Ecol. 6, 73–86 (2003). [Google Scholar]
- 85.Ripple W. J., Newsome T. M., Wolf C., Dirzo R., Everatt K. T., Galetti M., Hayward M. W., Kerley G. I. H., Levi T., Lindsey P. A., Macdonald D. W., Malhi Y., Painter L. E., Sandom C. J., Terborgh J., Van Valkenburgh B., Collapse of the world’s largest herbivores. Sci. Adv. 1, e1400103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Estes J. A., Terborgh J., Brashares J. S., Power M. E., Berger J., Bond W. J., Carpenter S. R., Essington T. E., Holt R. D., Jackson J. B. C., Marquis R. J., Oksanen L., Oksanen T., Paine R. T., Pikitch E. K., Ripple W. J., Sandin S. A., Scheffer M., Schoener T. W., Shurin J. B., Sinclair A. R. E., Soulé M. E., Virtanen R., Wardle D. A., Trophic downgrading of planet Earth. Science 333, 301–306 (2011). [DOI] [PubMed] [Google Scholar]
- 87.Ripple W. J., Estes J. A., Beschta R. L., Wilmers C. C., Ritchie E. G., Hebblewhite M., Berger J., Elmhagen B., Letnic M., Nelson M. P., Schmitz O. J., Smith D. W., Wallach A. D., Wirsing A. J., Status and ecological effects of the world’s largest carnivores. Science 343, 1241484 (2014). [DOI] [PubMed] [Google Scholar]
- 88.Descola P., Estrutura ou sentimento: A relação com o animal na Amazônia. MANA 4, 23–45 (1998). [Google Scholar]
- 89.Fausto C., Feasting on people: Eating animals and humans in Amazonia. Curr. Anthropol. 48, 497–530 (2007). [Google Scholar]
- 90.G. H. Shepard Jr., “Hunting in Amazonia” Encyclopaedia of the History of Science, Technology, and Medicine in Non-Western Cultures, http://link.springer.com/referenceworkentry/10.1007/978-94-007-3934-5_9909-1 (2014).
- 91.de Mattos Vieira M. A. R., von Muhlen E. M., Shepard G. H. Jr, Participatory monitoring and management of subsistence hunting in the Piagaçu-Purus reserve, Brazil. Conserv. Soc. 13, 254–264 (2015). [Google Scholar]
- 92.Castello L., Viana J. P., Watkins G., Pinedo-Vasquez M., Luzadis V. A., Lessons from integrating fishers of Arapaima in small-scale fisheries management at the Mamirauá Reserve, Amazon. Environ. Manag. 43, 197–209 (2009). [DOI] [PubMed] [Google Scholar]
- 93.R. E. Bodmer, P. E. Puertas, P. E. Community-based comanagement of wildlife in the Peruvian Amazon, in Hunting for Sustainability in Tropical Forests, J. G. Robinson, E. L. Bennett, Eds. (Columbia Univ. Press, New York, 2000), pp. 395–409. [Google Scholar]
- 94.Luzar J. B., Silvius K. M, Overman H., Giery S. T., Read J. M., Fragoso J. M. V., Large-scale environmental monitoring by indigenous peoples. BioScience 61, 771–781 (2011). [Google Scholar]
- 95.Fragoso J. M. V., Levi T., Oliveira L. F. B., Luzar J. B., Overman H., Read J. M., Silvius K. M., Line transect surveys underdetect terrestrial mammals: Implications for the sustainability of subsistence hunting. PLOS One 11, e0152659 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nepstad D., Schwartzman S., Bamberger B., Santilli M., Ray D., Schlesinger P., Lefebvre P., Alencar A., Prinz E., Fiske G., Rolla A., Inhibition of Amazon deforestation and fire by parks and indigenous lands. Conserv. Biol. 20, 65–73 (2006). [DOI] [PubMed] [Google Scholar]
- 97.Yu D. W., Levi T., Shepard G. H. Jr, Conservation in low-governance environments. Biotropica 42, 569–571 (2010). [Google Scholar]
- 98.Gadgil M., Berkes F., Folke C., Indigenous knowledge for biodiversity conservation. Ambio 22, 151–156 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Danielsen F., Burgess N. D., Balmford A., Monitoring matters: Examining the potential of locally-based approaches. Biodivers. Conserv. 14, 2507–2542 (2005). [Google Scholar]
- 100.Brazilian Federal Law, National System of Protected Areas. Federal Law 9985 (2000).
- 101.Brazilian Federal Law, National Policy for Environmental and Territorial Management of Indigenous Lands. Federal Law 7747 (2012).
- 102.N. van Vliet, R. Nasi, Embracing uncertainty for the sustainable management of hunting in tropical forests, paper presented at the XIV World Forestry Congress, Durban, South Africa, 7 to 11 September 2015. [Google Scholar]
- 103.R. Barthem, M. Goulding, Fishing the Future: A Scale for Amazon Freshwater Conservation (Amazon Conservation Association, Lima, 2006). [Google Scholar]
- 104.Benchimol M., Peres C. A., Widespread forest vertebrate extinctions induced by a mega hydroelectric dam in lowland Amazonia. PLOS One 10, e0129818 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peres C. A., Synergistic effects of subsistence hunting and habitat fragmentation on Amazonian forest vertebrates. Conserv. Biol. 15, 1490–1505 (2001). [Google Scholar]
- 106.A. Medeiros, Couros e Peles Silvestres: Produção, Comércio, Industrialização e Exportação (Rio de Janeiro, 1972). [Google Scholar]
- 107.Stevens A. W., Exploring the Valley of the Amazon in a Hydroplane. Natl. Geogr. Mag. 49, 353–420 (1926). [Google Scholar]
- 108.G. MacCreagh, White Waters and Black (Century Co, New York, 1926). [Google Scholar]
- 109.Fewster R. M., Buckland S. T., Siriwardena G. M., Baillie S. R., Wilson J. D., Analysis of population trends for farmland birds using generalized additive models. Ecology 81, 1970–1984 (2000). [Google Scholar]
- 110.T. J. Hastie, R. J. Tibshirani, Generalized Additive Models (Chapman and Hall, London, 1990). [Google Scholar]
- 111.R Development Core Team, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, 2010). [Google Scholar]
- 112.B. Efron, R. J. Tibshirani, An Introduction to the Bootstrap (Chapman and Hall, London, 1993). [Google Scholar]
- 113.Robinson J. G., Redford K. H., Intrinsic rate of natural increase in Neotropical forest mammals: Relationship to phylogeny and diet. Oecologia 68, 516–520 (1986). [DOI] [PubMed] [Google Scholar]
- 114.Cole L. C., The population consequences of life history phenomena. Q. Rev. Biol. 29, 103–137 (1954). [DOI] [PubMed] [Google Scholar]
- 115.Rodrigues F. R., Da Silva V. M. F., Barcellos J. F. M., Lazzarini S. M., Reproductive anatomy of the female Amazonian manatee Trichechus inunguis Natterer, 1883 (Mammalia: Sirenia). Anat. Rec. 291, 557–564 (2008). [DOI] [PubMed] [Google Scholar]
- 116.E. Staib, Eco-etología del Lobo de Río (Pteronura brasiliensis) en el sureste del Peru, thesis, Sociedad Zoológica de Frankfort (2005). [Google Scholar]
- 117.Da Silveira R., Campos Z., Thorbjarnarson J., Magnusson W. E., Growth rates of black caiman (Melanosuchus niger) and spectacled caiman (Caiman crocodilus) from two different Amazonian flooded habitats. Amphibia-Reptilia 34, 437–449 (2013). [Google Scholar]
- 118.Weigl R., Longevity of crocodiles in captivity. Intern. Zool. News 61, 363–374 (2014). [Google Scholar]
- 119.J. B. Thorbjarnarson, Black Caiman Melanosuchus niger, in Crocodiles. Status Survey and Conservation Action Plan, S. C. Manolis, C. Stevenson. Eds. (Crocodile Specialist Group, Darwin, ed. 3, 2010), pp. 29–39. [Google Scholar]
- 120.H. Kruuk, Otters: Ecology, Behaviour and Conservation (Oxford Univ. Press, Oxford, 2006). [Google Scholar]
- 121.M. L. Rheingantz, C. S. Trinca, Lontra longicaudis The IUCN Red List of Threatened Species 2015: e.T12304A21937379, http://dx.doi.org/10.2305/IUCN.UK.2015-2.RLTS.T12304A21937379.en (2015).
- 122.Parera A., Las nutrias verdaderas de la Argentina. Bol. Téc. Fund. Vida Silv. 21, (1996). [Google Scholar]
- 123.Mayor P., Bodmer R. E., López-Béjar M., López-Plana C., Reproductive biology of the wild red brocket deer (Mazama americana) female in the Peruvian Amazon. Anim. Reprod. Sci. 128, 123–128 (2011). [DOI] [PubMed] [Google Scholar]
- 124.Mayor P., Bodmer R. E., Lopez-Bejar M., Reproductive performance of the wild white-lipped peccary (Tayassu pecari) female in the Peruvian Amazon. Eur. J. Wildl. Res. 55, 631–634 (2009). [Google Scholar]
- 125.Mayor P., Guimarães D. A., Le Pendu Y., Da Silva J. V., Jori F., López-Béjar M., Reproductive performance of captive collared peccaries (Tayassu tajacu) in the eastern Amazon. Anim. Reprod. Sci. 102, 88–97 (2007). [DOI] [PubMed] [Google Scholar]
- 126.Mones A., Ojasti J., Hydrochoerus hydrochaeris. Mammalian Species 264, 1–7 (1986). [Google Scholar]
- 127.US Inflation Calculator. Consumer Price Index Data from 1913 to 2016, www.usinflationcalculator.com (2016).
- 128.Doughty R. W., Myers N., Notes on the Amazon wildlife trade. Biol. Conserv. 3, 293–297 (1971). [Google Scholar]
- 129.S. Benchimol, Amazônia, um pouco antes e além depois (Editora Umberto Calderaro, Manaus, 1977). [Google Scholar]
- 130.Pebesma E. J., Bivand R. S., Classes and methods for spatial data in R. R. News 5 (2005). [Google Scholar]
- 131.R. S. Bivand, E. Pebesma, V. Gomez-Rubio, Applied Spatial Data Analysis with R (Springer, New York, 2013). [Google Scholar]
- 132.R. J. Hijmans, J. van Etten, raster: Geographic analysis and modeling with raster data (2012). [Google Scholar]
- 133.Instituto Brasileiro de Geografia e Estatística (IBGE), Enciclopédia dos Municípios Brasileiros (Oficinas do Serviço Gráfico do IBGE, Rio de Janeiro, 1957), vol. 1. [Google Scholar]
- 134.Instituto Brasileiro de Geografia e Estatística (IBGE), Enciclopédia dos Municípios Brasileiros (Oficinas do Serviço Gráfico do IBGE, Rio de Janeiro, 1957), vol. 14. [Google Scholar]
- 135.Instituto Brasileiro de Geografia e Estatística (IBGE), Carta do Brasil ao Milionésimo (IBGE, Rio de Janeiro, 1972). [Google Scholar]
- 136.QGIS Development Team. QGIS Geographic Information System, http://qgis.osgeo.org (2015).
- 137.Maffei L., Noss A. J., Cuéllar E., Rumiz D. I., Ocelot (Felis pardalis) population densities, activity, and ranging behaviour in the dry forests of eastern Bolivia: Data from camera trapping. J. Trop. Ecol. 21, 349–353 (2005). [Google Scholar]
- 138.L. Maffei, A. J. Noss, S. C. Silver, M. J. Kelly, Abundance/density case study: Jaguars in the Americas, in Camera Traps in Animal Ecology: Methods and Analyses, A. F. O’Connell, J. D. Nichols, K. U. Karanth, Eds. (Springer Science & Business Media, Japan, 2011), pp. 119–144. [Google Scholar]
- 139.S. Benchimol, Manáos-do-Amazonas. Memória Empresarial (Governo do Estado, Manaus, 1994). [Google Scholar]
- 140.Associação Comercial do Amazonas, Boletim da Associação Comercial do Amazonas (1942).
- 141.Associação Comercial do Amazonas, Relatório da Diretoria da Associação Comercial do Amazonas - 1943 (1944).
- 142.Amazonas (State), Exposição ao senhor doutor Getúlio Vargas, presidente da República, por Álvaro Maia (Interventoria Federal no Estado do Amazonas, Manaus, 1943). [Google Scholar]
- 143.S. Benchimol, Amazônia, um Pouco Antes e Além Depois (Editora Umberto Calderaro, Manaus, 1977). [Google Scholar]
- 144.Instituto Brasileiro de Geografia e Estatística (IBGE), Estatísticas do Século XX (IBGE, Rio de Janeiro, 2006). [Google Scholar]
- 145.Instituto Brasileiro de Geografia e Estatística (IBGE), Séries históricas, http://seculoxx.ibge.gov.br (2015).
- 146.Associação Comercial do Amazonas (ACA), Documentário Comemorativo do Primeiro Centenário da Associação Comercial do Amazonas (Editora Umberto Calderaro, Manaus, 1971). [Google Scholar]
- 147.Associação Comercial do Amazonas, Boletim da Associação Comercial do Amazonas (1977).
- 148.Parry L., Day B., Amaral S., Peres C. A., Drivers of rural exodus from Amazonian headwaters. Popul. Environ. 32, 137–176 (2010). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/10/e1600936/DC1
text S1. Historical documents list.
text S2. Comparing the impacts of contemporary subsistence hunting versus historical commercial hunting in Amazonia.
text S3. International demand for Amazonian hides through time.
fig. S1. Rural population in the central-western Brazilian Amazon.
fig. S2. Central-western Brazilian Amazon yields (U.S. dollars in 2015 currency equivalence) for foremost 20th century products.
table S1. Average hide weights of commercially hunted species.
table S2. Intrinsic rate of natural increase (Rmax) for game species and parameters required for its calculation by the Cole equation.
table S3. Area of terrestrial and aquatic habitats, and their accessibility by hunters, under two hunting catchment area scenarios (buffers of 5 and 10 km around all settlements) in the central-western Brazilian Amazon.
table S4. Comparison between the Robinson-Redford production index and commercial harvests at two historical peaks for terrestrial species.
table S5. Comparison of the minimum refuge area required for maximum sustainable harvests (AMSY) according to the Joshi and Gadgil model (α = 1/λ) to actual refuge area (Arefuge) in the central-western Brazilian Amazon.


