Abstract
Aim:
Controversies and disagreement exist on conventional treatment strategies of hemorrhoids due to relapse, inefficacy, and complications. We intend to evaluate the role of individualized homeopathic treatment in hemorrhoids.
Materials and Methods:
In this prospective, open, observational trial, hemorrhoids patients were treated using five standardized scales measuring complaints severity and anoscopic score. It was conducted at two homeopathic hospitals in India, during from mid-July 2014 to mid-July 2015. Patients were intervened as per individualized homeopathic principles and followed up every month up to 6 months.
Results:
Total 73 were screened, 52 enrolled, 38 completed, 14 dropped out. Intention to treat population (n: = 52) was analyzed in the end. Statistically significant reductions of mean bleeding (month 3: −21.8, 95% confidence interval [CI]: −30.3, −13.3, P: < 0.00001, d = 0.787; month 6: −25.5, 95% CI −35.4, −15.6, P: < 0.00001, d = 0.775), pain (month 3: −21.3, 95% CI −28.6, −14.0, P: < 0.00001, d = 0.851; month 6: −27.6, 95% CI −35.6, −19.6, P: < 0.00001, d = 1.003), heaviness visual analog scales (VASs) (month 3: −8.1, 95% CI −13.9, −2.3, P: = 0.008, d = 0.609; month 6: −12.1, 95% CI −19.1, −5.1, P: = 0.001, d = 0.693), and anoscopic score (month 3: −0.4, 95% CI −0.6, −0.2, P: < 0.0001, d = 0.760; month 6: −0.5, 95% CI −0.7, −0.3, P: < 0.0001, d = 0.703) were achieved. Itching VASs reduced significantly only after 6 months (−8.1, 95% CI −14.6, −1.6, P: = 0.017, d = 0.586). No significant lowering of discharge VASs was achieved after 3 and 6 months.
Conclusion:
Under classical homeopathic treatment, hemorrhoids patients improved considerably in symptoms severity and anoscopic scores. However, being observational trial, our study cannot provide efficacy data. Controlled studies are required. Trial Reg. CTRI/2015/07/005958.
KEY WORDS: Hemorrhoids, homeopathy, observational trial, visual analog scales
INTRODUCTION
Hemorrhoids are distal displacement and prolapse of the hemorrhoidal cushions, distension of the hemorrhoidal arterio-venous anastomoses, or dilation of the veins of the internal hemorrhoidal venous plexus resulting from deterioration of anchoring connective tissue [1]. Common symptoms include pain, itching, swelling, anal discomfort, and rectal bleeding; all of which severely affect patient quality of life [2]. The condition affects 39-52% of adults [3,4]. The prevalence of hemorrhoids is extremely high in Western and other industrialized societies, with millions affected worldwide [3,5]. However, the true burden of disease is difficult to capture as many patients are reluctant to seek medical suggestions for various personal, cultural, and socio-economic reasons [2,6,7]. The prevalence of hemorrhoids in India according to recent surveys is around 40 million [8]. Several risk factors have been claimed to be the etiologies of hemorrhoid development including aging, obesity, depression, pregnancy, chronic constipation and diarrhea, low-fiber diet, spicy foods, and alcohol intake [9]. Hemorrhoids are generally classified by their location; internal (originates above the dentate line and covered by anal mucosa), external (originates below the dentate line and covered by anoderm), and mixed type. Internal hemorrhoids are further graded based on their appearance and degree of prolapse [10]:
Grade I: Non-prolapsing hemorrhoids;
Grade II: Prolapsing hemorrhoids on straining but reduce spontaneously;
Grade III: Prolapsing hemorrhoids requiring manual reduction; and
Grade IV: Non-reducible prolapsing hemorrhoids which include acutely thrombosed, incarcerated hemorrhoids.
Treatment options mainly depend on the type and severity of hemorrhoids, patient’s preference, and the expertise of physicians. The current therapies can be grouped into conservative management, office-based procedures, and surgical treatment [5]. Increased fiber intake, medical therapies, and lifestyle changes are included in the conservative treatment options for non-thrombosed hemorrhoids [5,11,12]. The main goal of medical treatment is to control acute symptoms of hemorrhoids rather than to cure the underlying hemorrhoids [13]. Office-based modalities include rubber-band ligation, injection sclerotherapy, laser photocoagulation, bipolar diathermy, cryotherapy, Doppler-guided hemorrhoidal artery ligation and infrared coagulation [14]; still, they are not suitable for all grades of hemorrhoids and have recognized complications. When an office-based therapy is still ineffective, patients may consider further intervention such as hemorrhoidectomy, thrombectomy of external hemorrhoids, and stapled hemorrhoidectomy; however, no single technique has been universally accepted as superior [15]. Based on clinical practice, it is assumed that surgery is effective for severe prolapsing hemorrhoids, but it is difficult to deal with the post-operative complications such as relapse, pain, prolonged convalescence, fecal urgency, and anal stenosis [16,17]. Thus, controversies and lack of agreement still exist on treatment strategies.
Homeopathy ranks the most popular among the traditional, complementary, or alternative medicines. Homeopathy, according to its “law of similar,” treats patients with a remedy that, in a healthy human, causes similar symptoms. Thus, the same diagnosis can be treated with different remedies in different patients (“individualization”), depending on “totality of symptoms,” and consideration of different complex issues (e.g. “susceptibility,” “miasm,” etc.) [18,19]. Homeopathic literature shows anecdotal data on the usefulness of homeopathic medicines in hemorrhoids. Although remarkable cure of hemorrhoids with homeopathic medicines in casual clinical experiences has been noted, research evidence remains seriously compromised [20,21]. Only until recently, there is a single-blind, randomized, placebo-controlled trial of the homeopathic use of 50 millesimal potencies in acute attacks of hemorrhoidal disorders has been published substantiating efficacy of individualized homeopathic treatment [22]. Another recent paper reported hemorrhoids as one of the most frequently encountered clinical conditions in a homeopathic hospital surgery and medicine outpatient in West Bengal, India and is treated with a considerable success rate of 60.3-82.3% [23]. The present study was aimed at investigating the role of individualized homeopathic medicines in the treatment of hemorrhoids in an open observational study in real practice settings.
MATERIALS AND METHODS
This study was conducted in the medicine and surgery outpatients of two Government homeopathic hospitals in West Bengal, India – Midnapore Homeopathic Medical College and Hospital (MHMCH) and Mahesh Bhattacharyya Homoeopathic Medical College and Hospital (MBHMCH). The study was started in mid-July 2014, enrollment continued until December 2014 and ended in mid-July 2015. In this prospective multicenter, open observational trial, patients were included consecutively on their first consultation with the participating physicians and followed up for 6 months using standardized scales. Before enrollment, written informed consent and approval by institutional ethics committees were obtained (IEC no. MHMCH/19/2015-16 and MBHMCH/370), and each patient was provided with a patient information sheet in local vernacular Bengali detailing the objectives, methods, risks and benefits of participating, and confidentiality issues. The study protocol (MHMCH/19/2015-16 and MBHMCH/70; version 1.0; date 28.05.2014) was registered with the Clinical Trials Registry, India, vide CTRI/2015/07/005958 on July 02, 2015, and has Universal Trial No. (UTN) U1111-1169-7497. The study was performed under the constant supervision of the independent ethics committees of the respective institutions.
Inclusion criteria were male and female patients between 25 and 60 years suffering from internal idiopathic/primary hemorrhoids presenting with any of the symptoms, namely, bleeding, pain (including discomfort and tenesmus during defecation or any other time), heaviness, pruritus, and mucus discharge with or without anitis. Patients with known but controlled diabetes (glycated hemoglobin <8%) and controlled hypertension and thyroid disorders were also eligible for the study. Patients using topical agents for hemorrhoids were included after a washout period of 1-week, subject to persistence of symptoms and signs of hemorrhoids.
Exclusion criteria were Grade IV piles, anal fissure and fistula, hereditary piles, inflammatory bowel disease, enlarged prostate, chronic alcoholism, recreational drug abuse, coagulation disorders, external hemorrhoids, previous history of surgery for hemorrhoids, hypertrophic anal papillae, rectal malignancies, history of leukemic disorders, patients with obstruction of portal circulation, pregnancy leading to obstruction of the portal circulation, lactating mothers, patients with psychiatric diseases.
Before treatment (at baseline), patients independently rated their severity on five 100 mm visual analog scales (VAS; 0 = no complaints; 100 = maximum severity) measuring intensity of symptoms of hemorrhoids – bleeding, pain, heaviness, discharge, and itching; and anoscopic examination by the treating physicians on a scale of 0 to 2 as follows: 0 = No signs of inflammation, 1 = a rather active grade, hemorrhoids without overt inflammatory findings (mild anitis), and 2 = an actively or easily bleeding hemorrhoids with overt signs of inflammation and edema (severe anitis). We planned to measure the outcomes at baseline, every month, up to 6 months. Two conventionally trained surgeons performed the anoscopic assessments for each patient at each time point.
We postulated the null hypothesis (H0) as pre-treatment score = post-treatment score; and alternative hypothesis (HA) was pre-treatment score ≠ post-treatment score. The study design was open-label, observational, single arm, non-randomized, non-controlled, and interventional. We targeted to achieve a sample size around 50 conveniently within the stipulated time frame of 1 year, from mid-July 2014 to mid-July 2015.
Interventions were planned as administering indicated remedies in centesimal or 50 millesimal potencies as appropriate. In centesimal scale, each dose consisted of four cane sugar globules medicated with a single drop of the indicated medicine, preserved in 88% v/v ethanol. In 50 millesimal scale, a single medicated cane sugar globules of poppy seed size (no.10) dissolved in 50 ml distilled water with addition of 2 drops of 88% v/v ethanol, 10 doses marked on the vial, each dose of 5 ml to be taken after 10 uniformly forceful downward strokes to the vial in 45 ml normal water in a clean cup, to stir well, to take 5 ml of this liquid orally, and to discard rest of the liquid in the cup. Repetition 24, 12, or 8 hourly or even oftener, depended on the individual requirement of the case. All medicines were procured from a Good Manufacturing Practice-certified firm.
Following recruitment, selection of the single individualized medicine was based on the presenting symptom totality, repertorization and consultation with Materia Medica, and individualized dose on the judgment of susceptibility of the patients. As per individual requirement of the cases, aid of different repertories (RADAR® software, Archibel, Belgium) was taken with due consultation of Materia Medica. Overall decision making was influenced by consensus among the physicians. Subsequent prescriptions were generated according to Kent’s observations and second prescription. Thus results of this study adhered to the criteria for reporting individualization in homeopathy [24].
All the participants were encouraged to have sitz bath as and when needed, maintain local hygiene, correct constipation, and unhealthy defecation habits such as ignoring the need to pass stools, irregular meals, spending a long time in the lavatory, straining, and lack of exercise. They were also advised about the importance of a fiber-rich diet in health and encouraged to consume food rich in natural fiber such as unpeeled fruits, vegetables, and whole-grain bread and will be prohibited from high consumption of spices.
Statistical analysis followed the intention-to-treat (ITT) approach; i.e., every included patient entered final analyses. Descriptive data (categorical and continuous) were presented in terms of absolute values, percentages, mean, and standard deviations (SD) as appropriate. Missing values were replaced by the last value carried forward method. To compare longitudinally obtained data measured repeatedly in the same patients at different points of time, paired t-test was performed comparing baseline data with that recorded after 3rd and 6th month. The significance level was set at P < 0.05 two-tailed. For within-subjects studies, dependence among means was corrected to make direct comparisons to effect sizes from between-subjects studies. To do this, correlations (Pearson’s r) between the two means were calculated first, and then Cohen’s d was estimated by the Morris and DeShon’s (2002) Equation (8) [25]. Effect sizes were classified: As |d|>0.8, large; |d|>0.5, medium; |d|>0.2, small [26].
Adverse or serious adverse event(s), if any, was planned to be treated accordingly as per homeopathic principles, or if non-responding, then the patient should be referred for surgery.
RESULTS
Total 73 patients suffering from hemorrhoids were screened for the project; 52 satisfied the pre-specified eligibility criteria; 21 were excluded for varied reasons. Since enrollment, each patient received classical homeopathic treatment for a period of 6-month. Total 38 continued till the end, 14 dropped out. Finally, the ITT population, i.e. 52 patients entered the analysis [Figure 1].
Figure 1.
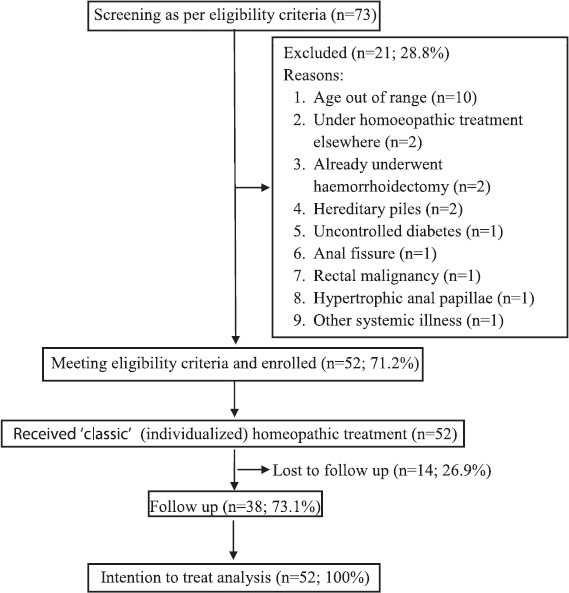
Study flow diagram
Baseline Characteristics
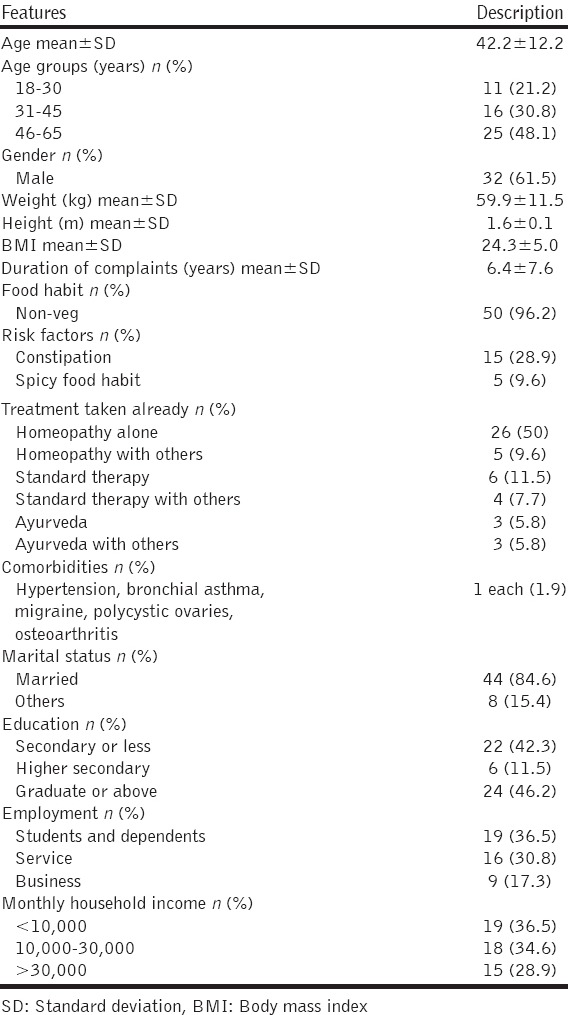
Mean age of the patients was 42.2 years (SD 12.2); spanned all age groups, but majority belonged to the group of 46-65 years (n = 25; 48.1%). Most of the patients were male (n = 32; 61.5%). Mean body mass index was 24.3 (SD 5.0). Non-vegetarian food habit was mostly prevalent (n = 50; 96.2%); constipation (n = 15; 28.9%), and spicy food habit (n = 5; 9.6%) as probable risk factors. Homeopathy treatment alone was already availed for treatment of hemorrhoids by 26 (50%) patients and along with other therapies by 5 (9.6%) patients. Associated comorbidities identified were hypertension, bronchial asthma, migraine, polycystic ovaries, and osteoarthritis; each condition was found in single patients. Majority of the patients were married (n = 44; 84.6%); had qualification of graduate and above (n = 24; 46.2%); employment status of students and dependents (n = 19; 36.5%); but having monthly household income of Rs. 10,000 or less (n = 19; 36.5%) [Table 1].
Table 1.
Socio-demographics and baseline status (n=52)
Bleeding VASs (mm)
The reductions achieved with time were as follows: Baseline 42.9 ± 28.9, 1st month 31.1 ± 20.4, 2nd month 25.8 ± 20.2, 3rd month 21.1 ± 20.8, 4th month 18.4 ± 21.5, 5th month 16.4 ± 20.6, and 6th month 17.4 ± 20.6. Mean reductions (mm) from baseline over month 3 (−21.8, 95% confidence interval [CI]: −30.3, −13.3; t = −5.141, P < 0.00001; paired t-test) and month 6 (−25.5, 95% CI −35.4, −15.6; t = −5.120, P < 0.00001; paired t-test) were statistically significant with almost large effect size (Cohen’s d) of 0.787 (Pearson’s r = 0.379) and 0.775 (Pearson’s r = 0.117), respectively [Table 2 and Figure 2].
Table 2.
Changes in outcomes over 3 and 6 months (n=52)
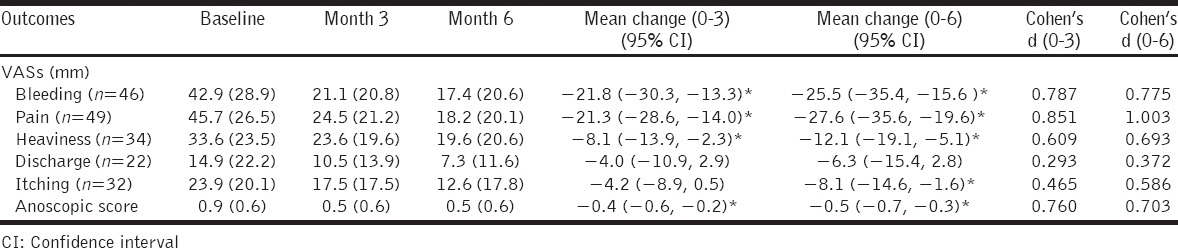
Figure 2.
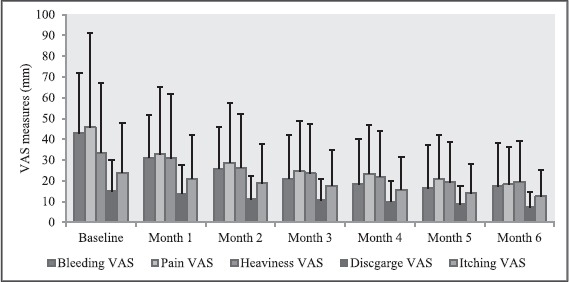
Changes in visual analog scales measures (mm) over months
Pain VASs (mm)
The decrease achieved with time were as follows: Baseline 45.7 ± 26.5, 1st month 32.7 ± 21.0, 2nd month 28.7 ± 22.1, 3rd month 24.5 ± 21.2, 4th month 23.3 ± 20.8, 5th month 21.1 ± 20.2, and 6th month 18.2 ± 20.1. Mean decrease (mm) from baseline over month 3 (−21.3, 95% CI: −28.6, −14.0; t = −5.815, P < 0.00001; paired t-test) and month 6 (−27.6, 95% CI: −35.6, −19.6; t = −6.839, P < 0.00001; paired t-test) were statistically significant with definitely large effect size (Cohen’s d) of 0.851 (Pearson’s r = 0.455) and 1.003 (Pearson’s r = 0.307), respectively [Table 2 and Figure 2].
Heaviness VASs (mm)
It declined with time as follows: Baseline 33.6 ± 23.5, 1st month 30.9 ± 22.4, 2nd month 26.2 ± 22.4, 3rd month 23.6 ± 19.6, 4th month 21.9 ± 19.4, 5th month 19.3 ± 19.7, and 6th month 19.6 ± 20.6. Mean decline (mm) from baseline over month 3 (−8.1, 95% CI: −13.9, −2.3; t = −2.794, P = 0.008; paired t-test) and month 6 (−12.1, 95% CI: −19.1, −5.1; t = −3.459, P = 0.001; paired t-test) were statistically significant with medium effect size (Cohen’s d) of 0.609 (Pearson’s r = 0.710) and 0.693 (Pearson’s r = 0.581), respectively [Table 2 and Figure 2].
Discharge VASs (mm)
It was reduced with time as follows: Baseline 14.9 ± 22.2, 1st month 13.7 ± 18.4, 2nd month 11.1 ± 15.4, 3rd month 10.5 ± 13.9, 4th month 10 ± 13.6, 5th month 8.7 ± 12.1, and 6th month 7.3 ± 11.6; however, mean reduction (mm) achieved from baseline over month 3 (−4.0, 95% CI: −10.9, 2.9; t = −1.181, P = 0.250; paired t-test) and month 6 (−6.3, 95% CI: −15.4, 2.8; t = −1.403, P = 0.174; paired t-test) were statistically non-significant with medium effect size (Cohen’s d) of 0.293 (Pearson’s r = 0.654) and 0.372 (Pearson’s r = 0.269), respectively [Table 2 and Figure 2].
Itching VASs (mm)
It declined with time as follows: Baseline 23.9 ± 20.1, 1st month 21 ± 17.8, 2nd month 18.9 ± 18.1, 3rd month 17.5 ± 17.5, 4th month 15.7 ± 18.0, 5th month 14.1 ± 18.7, and 6th month 12.6 ± 17.8. Mean reduction (mm) achieved from baseline over month 3 (−4.2, 95% CI: −8.9, 0.5; t = −1.764, P = 0.086; paired t-test) was non-significant with medium effect size (Cohen’s d) of 0.465 (Pearson’s r = 0.732). Reduction over month 6 (−8.1, 95% CI: −14.6, −1.6; t = −2.499, P = 0.017; paired t-test) was statistically significant with medium effect size of 0.586 (Pearson’s r = 0.483) [Table 2 and Figure 2].
Anoscopic Score
The scores obtained at baseline, and every month up to 6 months were 0.9 ± 0.6, 0.8 ± 0.5, 0.6 ± 0.6, 0.5 ± 0.6, 0.5 ± 0.6, 0.4 ± 0.6, and 0.5 ± 0.6, respectively. When the score of baseline was compared with that of month 3 and month 6, the reductions achieved of −0.4 (95% CI: −0.6, −0.2; t = −4.655, P < 0.0001; paired t-test) and −0.5 (95% CI: −0.7, −0.3; t = −4.728, P < 0.0001; paired t-test) were statistically significant with almost large effect sizes of 0.760 (Pearson’s r = 0.615) and 0.703 (Pearson’s r = 0.550), respectively [Table 2 and Figure 3].
Figure 3.
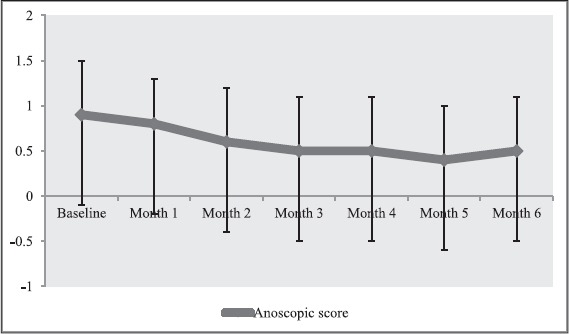
Changes in anoscopic score over months
Medicines Prescribed
The most frequently prescribed medicines were sulfur (n = 14; 26.9%), nux vomica (n = 9; 17.3%), calcarea phosphorica and natrum muriaticum (n = 4 each; 7.7%), and causticum (n = 3; 5.8%). Other prescribed medicines and their indications are enlisted in Tables 3 and 4. Aesculus hippocastanum, Collinsonia canadensis, muriatic acid, ratanhia, millefolium, and Hamamelis virginiana were used in lower centesimal potencies (6C, 30C) and mother tinctures as “acute” (rescue) remedies as and when required. Baseline prescriptions contained both centesimal (n = 44; 84.6%) and 50 millesimal (n = 6; 11.5%) potencies.
Table 3.
Medicines prescribed at baseline
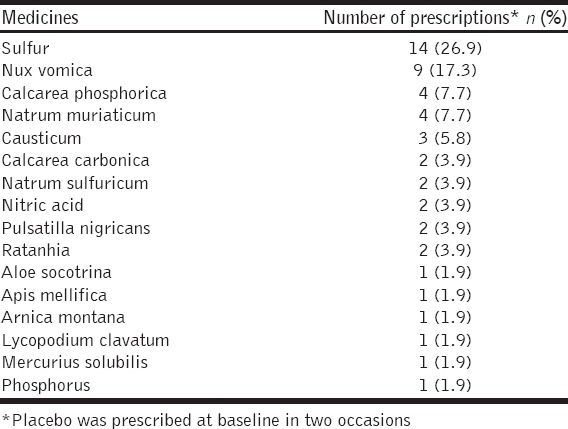
Table 4.
Indications of the most frequently prescribed medicines

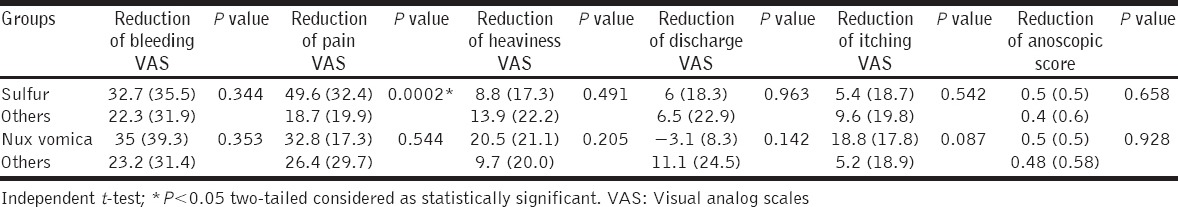
To detect medicine-specific treatment effects, if any, independent t-test was run to compare the reductions achieved on VASs and anoscopic scores over 3rd and 6th months by the two most frequently prescribed medicines – Sulfur and nux vomica. Analyses revealed significant reductions of pain VASs by sulfur only, both after 3 months (sulfur: 42.1 ± 32.2 vs. others: 12.9 ± 15.5; t = 4.197; P = 0.0001 two-tailed) and 6 months (sulfur: 49.6 ± 32.4 vs. others: 18.7 ± 19.9; t = 3.964; P = 0.0002 two-tailed) in comparison with other medicines. No other significant differences were observed under any occasion [Tables 5 and 6].
Table 5.
Medicine-specific treatment effects observed after 3 months
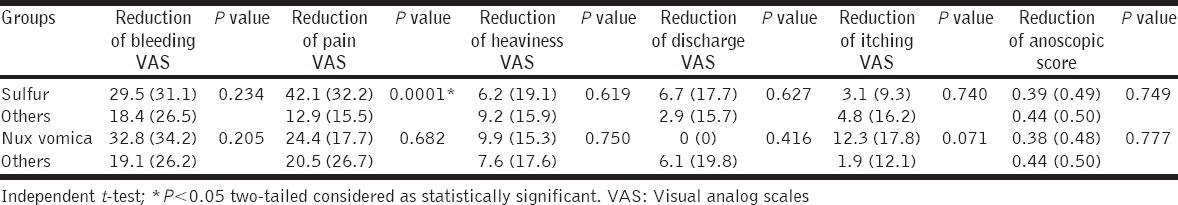
Table 6.
Medicine-specific treatment effects observed after 6 months
Some minor intercurrent illnesses were encountered, e.g., fever, indigestion, cough and cold, vertigo, frozen shoulder, diarrhea, dysentery, sore throat, hyperacidity, rheumatic complaints, and trauma; and all were treated successfully with homeopathic medicines.
DISCUSSION
After individualized homeopathic treatment of the patients suffering from symptomatic hemorrhoids, there was statistically significant lowering of mean VAS intensity measures of bleeding, pain, and heaviness and anoscopic score over 3rd and 6th months. The discharge VAS also reduced but that was statistically non-significant. Improvement was also observed in itching VAS but over a 6th month only. The effect sizes of the severity ratings after 3 and 6 months were moderate to almost large (except discharge VASs). Assessments of disease severity consistently showed substantial improvements, although the disease was long-standing, chronic, and mostly pre-treated. This may be partly explained by placebo and/or regression to the mean effects that our study was not designed to control. We also cannot rule out overestimation of the treatment effect and undisclosed use of concurrent therapeutic modalities, if any.
This prospective multicenter observational study was aimed to reflect the contemporary homeopathic health care in real practice settings and its outcome in 52 adult hemorrhoids patients. The methodological strengths of our study include the consecutive patient enrollment, the participation of qualified and experienced homeopathic physicians schooled in and practicing “classical” homeopathy, and use of standardized and already validated outcome scales. Our study is representative of individualizing (“classical”) homeopathy only. In a broader interpretation of the law of similar, remedies are selected for symptoms both typical of the diagnoses and outside the predominating pathologies (“constitutional”). In contrast to randomized trials, our study describes patients from everyday practice with multiple morbidities and a large variety of life styles. This ensures a high degree of external validity that allows extrapolation to usual medical care. We used VASs that are validated, often used and allowed for assessments of a specific complaint as well as for generalization and interpretation across various diagnoses.
Randomized controlled trials (RCTs) are known to be the gold standard for medical research investigating the efficacy of new interventions. However, this is also not an unquestionable truth. Whereas RCTs unquestionably hold many advantages over observational studies, it should be recognized that they also have many flaws that render them fallible under certain circumstances. RCTs may suffer from low external validity, i.e., rigid design control could reduce the ability to generalize the results. Another issue relating to RCTs is the fact that recruitment, randomization, and blinding are not always possible because of technical or ethical issues. So, a well-designed observational trial can be a better alternative in this situation, and they, in fact, do not systematically overestimate the magnitude of treatment effects as compared with those in RCTs [27,28]. So, RCTs and observational trials can be used synergistically to obtain more and better information. For example, observational trials can be used to test the external validity of RCTs by expanding the settings to a more representative population and formulate hypotheses for RCTs to test [29]; identify structures, processes, and outcomes to study and help establish the appropriate sample size for an RCT [30]. The main strength of observational studies is their greater proximity to “real life situations” by capturing large amount of uneven data since RCTs have stricter inclusion criteria and rigid protocols that may not reflect clinical practice. Other advantages of observational trials are that they are usually cheaper than RCTs, can be used to investigate rare outcomes, and easy to perform. Observational trials are also important for creating new hypotheses, proving the external validity of RCTs already performed, and establishing the sample size for RCT. In this way, observational trials can be complementary to RCTs in spite of their evidence level being lower than that of RCTs. This kind of investigation is crucial for elucidating many scientific questions [31]. Observational trials cannot replace RCTs nor do RCTs make non-experimental studies unnecessary or undesirable. When both experimental and non-experimental studies address the same question, both can contribute usefully to answering the question. Thorough consideration of both RCTs and observational trials will enhance the interpretation of the totality of the evidence and lead to stronger overall inferences about clinical questions and decision-making [32]. The advantages of including both in a meta-analysis could outweigh the disadvantages in many situations and is consistent with an evidence-based approach [33]. “Every research strategy within a discipline contributes importantly relevant and complementary information to a totality of evidence on which rational clinical decision-making and public policy can be reliably based. In this context, observational evidence has provided and will continue to make unique and important contributions to this totality of evidence on which to support a judgment of proof beyond a reasonable doubt in the evaluation of interventions” [34].
Comparing to the efficacy study by Chakraborty et al. [22], noticeable drop of VAS intensity measures of pain, bleeding, heaviness, itching, and anoscopic score was observed; however, there was no significant reduction of discharge, a similar finding of the earlier study [22]. Thus, the exact role of individualized homeopathic treatment in controlling discharges of hemorrhoids becomes questionable in two different study designs and needs further evaluation.
Although specific statistical analyses were run to detect specific treatment effects of individual remedies (sulfur and nux vomica), the study was never designed to assess the same; rather only to assess the role of individualized mode of homeopathic treatment. It may be only a play of chance or mere coincidence that in the representative sample of hemorrhoids patients, higher symptom similarities were observed with sulfur or nux vomica symptoms, hence prescribed in higher frequencies than other medicines; or significant pain reduction was attributed by sulfur group of patients; but these findings, in no way, undermine the potential of other medicines in improving hemorrhoids. Rather, these findings should be subjected to controlled efficacy trials for confirmation in future.
Our study does not support conclusions as to the effectiveness of the homeopathic remedies because no methodology for this purpose (control group, randomization, blinding, etc.) was built into its design. The aim of the investigation was to explore the effects of homeopathic medical care in treating some forms of hemorrhoids. Any improvement observed in this study should not be extrapolated to other forms of hemorrhoids. The data may also be helpful in the planning of further research projects on homeopathy. It would require specific instruments for more detailed assessment and effect size comparability, and a longer observation period.
CONCLUSION
Under “classical” or “individualized” homeopathic treatment of 6 months, the severity of hemorrhoidal symptoms – pain, bleeding, heaviness, and itching improved substantially; however, there was no significant lowering of discharge. Overall, homeopathic treatment appeared promising but needs further rigorous exploration in different designs for arriving at a confirmatory conclusion.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Nikhil Saha, Acting Principal, MBHMCH; Dr. Chapal Kanti Bhattacharjee, Acting Principal and Administrator, MHMCH, Dr. Byomkesh Bera, Head, Department of Materia Medica, MHMC&H, and Dr. Mamata Eyasmin, House Staff, MHMC&H, for their sincere cooperation. The authors will also remain grateful to the patients for their participation.
Footnotes
Source of Support: The study was funded by the two respective institutions – MHMCH and MBHMCH, but the funder has no role to play regarding authorship, contents, and/or publication of this article,
Conflict of Interest: None declared.
REFERENCES
- 1.Ganz RA. The evaluation and treatment of hemorrhoids: A guide for the gastroenterologist. Clin Gastroenterol Hepatol. 2013;11:593–603. doi: 10.1016/j.cgh.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 2.Riss S, Weiser FA, Riss T, Schwameis K, Mittlböck M, Stift A. Haemorrhoids and quality of life. Colorectal Dis. 2011;13:e48–52. doi: 10.1111/j.1463-1318.2010.02480.x. [DOI] [PubMed] [Google Scholar]
- 3.Riss S, Weiser FA, Schwameis K, Riss T, Mittlböck M, Steiner G, et al. The prevalence of hemorrhoids in adults. Int J Colorectal Dis. 2012;27:215–20. doi: 10.1007/s00384-011-1316-3. [DOI] [PubMed] [Google Scholar]
- 4.Johanson JF, Sonnenberg A. Constipation is not a risk factor for hemorrhoids: A case-control study of potential etiological agents. Am J Gastroenterol. 1994;89:1981–6. [PubMed] [Google Scholar]
- 5.Lohsiriwat V. Hemorrhoids: From basic pathophysiology to clinical management. World J Gastroenterol. 2012;18:2009–17. doi: 10.3748/wjg.v18.i17.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part II: Lower gastrointestinal diseases. Gastroenterology. 2009;136:741–54. doi: 10.1053/j.gastro.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Acheson AG, Scholefield JH. Management of haemorrhoids. BMJ. 2008;336:380–3. doi: 10.1136/bmj.39465.674745.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tripathi RK, Bolegave SS, Shetty PA, Uchil DA, Rege NN, Chawda MB, et al. Efficacy and safety of a polyherbal formulation in hemorrhoids. J Ayurveda Integr Med. 2015;6:225–32. doi: 10.4103/0975-9476.172382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pigot F, Siproudhis L, Allaert FA. Risk factors associated with hemorrhoidal symptoms in specialized consultation. Gastroenterol Clin Biol. 2005;29:1270–4. doi: 10.1016/s0399-8320(05)82220-1. [DOI] [PubMed] [Google Scholar]
- 10.Clinical Practice Committee, American Gastroenterological Association. American Gastroenterological Association medical position statement: Diagnosis and treatment of hemorrhoids. Gastroenterology. 2004;126:1461–2. doi: 10.1053/j.gastro.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Hall JF. Modern management of hemorrhoidal disease. Gastroenterol Clin North Am. 2013;42:759–72. doi: 10.1016/j.gtc.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Song SG, Kim SH. Optimal treatment of symptomatic hemorrhoids. J Korean Soc Coloproctol. 2011;27:277–81. doi: 10.3393/jksc.2011.27.6.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lohsiriwat V. Treatment of hemorrhoids: A coloproctologist’s view. World J Gastroenterol. 2015;21:9245–52. doi: 10.3748/wjg.v21.i31.9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ASGE Technology Committee Siddiqui UD. Barth BA, Banerjee S, Bhat YM, Chauhan SS, et al. Devices for the endoscopic treatment of hemorrhoids. Gastrointest Endosc. 2014;79:8–14. doi: 10.1016/j.gie.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Giamundo P. Advantages and limits of hemorrhoidal dearterialization in the treatment of symptomatic hemorrhoids. World J Gastrointest Surg. 2016;8:1–4. doi: 10.4240/wjgs.v8.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Argov S, Levandovsky O, Yarhi D. Milligan-Morgan hemorrhoidectomy under local anesthesia - An old operation that stood the test of time. A single-team experience with 2,280 operations. Int J Colorectal Dis. 2012;27:981–5. doi: 10.1007/s00384-012-1426-6. [DOI] [PubMed] [Google Scholar]
- 17.Wang ZG, Zhang Y, Zeng XD, Zhang TH, Zhu QD, Liu DL, et al. Clinical observations on the treatment of prolapsing hemorrhoids with tissue selecting therapy. World J Gastroenterol. 2015;21:2490–6. doi: 10.3748/wjg.v21.i8.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahnemann CF. Organon of Medicine. 5th, 6th ed. New Delhi: B. Jain Publishers Pvt., Ltd; 2002. [Google Scholar]
- 19.Hahnemennn CF. The Chronic Diseases, their Peculiar Nature and their Homoeopathic Cure. I, II. New Delhi: B. Jain Publishers Pvt., Ltd; 2009. [Google Scholar]
- 20.Bodman F. Haemorrhoids. Homoeopathy. 1990;40:143–5. [Google Scholar]
- 21.Sudarshan SR. A case of bleeding piles cured with Pulsatilla. Homoeopathic Herit. 1991;16:195–6. [Google Scholar]
- 22.Chakraborty PS, Varanasi R, Majumdar AK, Banoth K, Prasad S, Ghosh MS, et al. Effect of homoeopathic LM potencies in acute attacks of haemorrhoidal disease: A multicentric randomized single-blind placebo-controlled trial. Indian J Res Homoeopathy. 2013;7:72–80. [Google Scholar]
- 23.Saha S, Koley M, Ghosh S, Giri M, Das A, Goenka R. Documentation of prescriptions and clinical outcomes in a homeopathic hospital setting in West Bengal, India. J Evid Based Complement Altern Med. 2015;20:180–5. doi: 10.1177/2156587214568459. [DOI] [PubMed] [Google Scholar]
- 24.Saha S, Koley M, Ganguly S, Rath P, Roy Chowdhury P, Hossain SI. Developing the criteria for evaluating quality of individualization in homeopathic clinical trial reporting: A preliminary study. J Integr Med. 2014;12:13–9. doi: 10.1016/S2095-4964(14)60009-1. [DOI] [PubMed] [Google Scholar]
- 25.Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods. 2002;7:105–25. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- 26.Cohen J. Statistical Power Analysis for the Behavioural Sciences. 2nd ed. USA: Lawrence Erlbaum Associates Publishers; 1988. [Google Scholar]
- 27.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887–92. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovesdy CP, Kalantar-Zadeh K. Observational studies versus randomized controlled trials: Avenues to causal inference in nephrology. Adv Chronic Kidney Dis. 2012;19:11–8. doi: 10.1053/j.ackd.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stables RH. Observational research in the evidence based environment: Eclipsed by the randomised controlled trial? Heart. 2002;87:101–2. doi: 10.1136/heart.87.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Black N. Why we need observational studies to evaluate the effectiveness of health care. BMJ. 1996;312:1215–8. doi: 10.1136/bmj.312.7040.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mariani AW, Pêgo-Fernandes PM. Observational studies: Why are they so important? Sao Paulo Med J. 2014;132:1–2. doi: 10.1590/1516-3180.2014.1321784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sørensen HT, Lash TL, Rothman KJ. Beyond randomized controlled trials: A critical comparison of trials with nonrandomized studies. Hepatology. 2006;44:1075–82. doi: 10.1002/hep.21404. [DOI] [PubMed] [Google Scholar]
- 33.Shrier I, Boivin JF, Steele RJ, Platt RW, Furlan A, Kakuma R, et al. Should meta-analyses of interventions include observational studies in addition to randomized controlled trials? A critical examination of underlying principles. Am J Epidemiol. 2007;166:1203–9. doi: 10.1093/aje/kwm189. [DOI] [PubMed] [Google Scholar]
- 34.Hennekens CH, Buring JE. Observational evidence. Doing more good than harm: The evaluation of health care interventions. Ann NY Acad Sci. 1994;703:22. [Google Scholar]


