Abstract
Aim:
To evaluate the phenolics composition and inhibitory effect of the leaves extracts of Ocimum basilicum and Ocimum gratissimum on two key enzymes (pancreatic lipase [PL] and angiotensin 1-converting enzyme [ACE]) involved in obesity and hypertension in vitro.
Materials and Methods:
The phenolics (flavonoids and phenolic acids) were quantified using high-performance liquid chromatography coupled with diode array detection. PL and ACE inhibitory effects; DPPH* and ABTS*+ scavenging activities of the extracts were tested using spectrophotometric methods.
Results:
O. basilicum had the following major phenolics: Rutin, quercetin, and quercitrin (flavonoids); caffeic, chlorogenic, and gallic acids (phenolic acids); while O. gratissimum had the following major phenolics: Rutin, quercitrin, and luteolin (flavonoids); ellagic and chlorogenic acids (phenolic acids). “Extracts of both plants inhibited PL and ACE; scavenged DPPH* in a dose-dependent manner”. O. gratissimum extract was more potent in inhibiting PL (IC50: 20.69 µg/mL) and ACE (IC50: 29.44 µg/mL) than O. basilicum (IC50: 52.14 µg/mL and IC50: 64.99 µg/mL, against PL and ACE, respectively). O. gratissimum also scavenged DPPH* and ABTS*+ more than O. basilicum.
Conclusion:
O. basilicum and O. gratissimum leaves could be used as functional foods for the management of obesity and obesity-related hypertension. However, O. gratissimum may be more effective than O. basilicum.
KEY WORDS: Angiotensin I-converting enzyme, hypertension, obesity, Ocimum species, oxidative stress, pancreatic lipase
INTRODUCTION
Obesity, especially visceral obesity, is the most important risk factor for the development of hypertension and other cardiovascular diseases [1]. Population-based risk estimates studies have indicated that no less than two-thirds of the prevalence of hypertension can be directly attributed to obesity [2]. Obesity and obesity-related hypertension have become a global public health burden; their incidence being comparable with those of diabetes mellitus and chronic kidney diseases [3].
The pancreatic lipase (PL) (EC: 3.1.1.3) plays a pivotal role in the effective digestion of lipids, as it is responsible for the hydrolysis of the bulk (50-70%) of total dietary fats [4]. It is the key enzyme that hydrolyzes triglyceride to produce glycerol and fatty acids, thereby facilitating its uptake [5]. Thus, it is widely used as an index to evaluate the potential efficacy of natural products as anti-obesity agents [6]. Due to the vital catalytic function of PL, orlistat, currently used clinically for obesity management, has been designed to inhibit PL activity; thereby reducing the absorption of triglyceride. However, this drug like other PL inhibitors has some hepatic and gastrointestinal adverse effects [7,8].
The activation of the rennin-angiotensin system (RAS) has been reported as one of the possible mechanisms through which obesity could lead to hypertension and higher cardiovascular risk [3]. In fact, experimental evidence has shown that the RAS is activated in obesity; its involvement in the pathophysiology of obesity-related hypertension has also been reported [9]. In the RAS, angiotensin 1-converting enzyme (ACE) (EC: 3.4.15.1) plays a key role in the regulation of blood pressure and normal cardiovascular function. It catalyzes the conversion of angiotensin I to angiotensin II, which is known to increase the blood pressure. Hence, inhibition of ACE is an important strategy for the treatment and management of obesity-related hypertension [10,11]. Chemically synthesized ACE inhibitors including captopril, ramipril, enalapril and fosinopril, comprise a class of drugs frequently used clinically for the treatment of hypertension [12]. However, as with the PL inhibitors, these drugs have been reported to have some adverse effects such as cough, skin rashes, hypotension, and proteinuria [13,14].
Against the backdrop of the side effects associated with the synthetic inhibitors of both PL and ACE, research has shown that plant-derived inhibitors of these two enzymes have some potential for preventing or ameliorating obesity and hypertension, without noticeable side effects [15,16]. In this regard, plant polyphenolics have been reported by several studies to inhibit either one of these two enzymes (PL and ACE) or both [4,5,17,18].
The genus, Ocimum (Lamiaceae), comprises 65 aromatic species, distributed in tropical and subtropical regions of the world including Africa and Asia [19]. Various Ocimum species, including Ocimum basilicum and Ocimum gratissimum, have important culinary and pharmacological uses. Their culinary and pharmacological values are attributed mainly to their aromatic compounds [20]. Hence, the leaves are used as a fragrance and flavoring agent in a variety of products including food, beverages, condiments, and oral care products [21]. O. gratissimum leaves extract possesses anti-arthritic [22], hypoglycemic [19], anticonvulsant and anxiolytic activities [23]. On the other hand, O. basilicum leaves are used as anti-spasmodic, carminative, galactagogue, and stomachic in folk medicine [24]. To further explore the nutraceutical benefits of O. basilicum and O. gratissimum, this study evaluated the phenolics composition and inhibitory effects of their leaves extracts on two key enzymes (PL and ACE) involved in obesity and hypertension in vitro.
MATERIALS AND METHODS
Samples Collection and Preparation
About 500 g of fresh leaves samples of each of O. basilicum and O. gratissimum were collected from a local farm settlement in Akingbile area of Moniya, Ibadan, Nigeria. The samples were botanically identified and authenticated at the herbarium of the Department of Botany, University of Ibadan, Nigeria. Subsequently, they were air-dried for 7 days and later ground finely to a particle size of 0.5 mm. The powdery samples were used for the extracts preparation.
Chemicals and Reagents
Methanol, formic acid, gallic acid, chlorogenic acid, caffeic acid, and ellagic acid were purchased from Merck (Darmstadt, Germany). Catechin, epicatechin, quercetin, rutin, apigenin, and luteolin; porcine PL, Hippuryl-histidyl-leucine, Rabbit lung ACE, DPPH, ABTS, and Trolox were acquired from Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals used for the analysis were of analytical grade.
Preparation of Polyphenolics Extract
Polyphenolics extracts of O. basilicum and O. gratissimum leaves were prepared as described by Kuo et al. [25]. A portion of the leaves powder (100 g) was extracted three successive times with 300 mL of methanol at 50°C for 3 h, and the samples were filtered after each extraction with Whatman (No. 2) filter paper. Lipids and some pigments were removed by partitioning the extract with 200 mL hexane in a separatory funnel. The aqueous phase was extracted three times with 180 mL ethyl acetate and evaporated to dryness at 45°C under reduced pressure in a rotary evaporator. The residues obtained were used for the assays.
Quantification of Flavonoids and Phenolic Acids using High Performance Liquid Chromatography Coupled with Diode Array Detection (HPLC-DAD)
Extracts of the leaves were injected by means of auto-sampler (Shimadzu, model SIL-20A) at a concentration of 15 mg/mL. Separations were carried out using phenomenex C18 column (4.6 mm × 250 mm × 5 mm particle size). The mobile phase comprised solvent A (water:formic acid [98:2, v/v]) and solvent B (acetonitrile), at a flow rate of 0.6 mL/min and an injection volume of 40 µL. Gradient program was started with 95% of A and 5% of B until 2 min and changed to obtain 25%, 40%, 50%, 70% and 80% B at 10, 20, 30, 50 and 70 min, respectively, according to the method described by Boligon et al. [26], with slight modifications. The extracts and mobile phase were filtered through a 0.45 µm membrane filter (Millipore) and then degassed in ultrasonic bath before use. Standards references stock solutions were prepared in the HPLC mobile phase at a concentration range of 0.025-0.300 mg/mL. Quantifications of the flavonoids and phenolic acids in the extracts were carried out by integration of the peaks using the external standard method at the following wavelengths: 254 nm for gallic acid and ellagic acid; 280 nm for catechin and epicatechin; 325 nm for caffeic acid and chlorogenic acid; 366 nm for quercetin, quercitrin, kaempferol, luteolin and rutin. The individual chromatography peaks were confirmed by comparing their respective retention time with those of reference standards and by DAD spectra (200-600 nm). Calibration curves used were gallic acid: Y = 13581x + 1195.7 (r = 0.9997), catechin: Y = 12609x + 1187.4 (r = 0.9996), epicatechin: Y = 12719x + 1356.9 (r = 0.9995), caffeic acid: Y = 12750x + 1352.9 (r = 0.9999), chlorogenic acid: Y = 11825x + 1383.6 (r = 0.9998), ellagic acid: Y = 13192x + 1176.5 (r = 0.9997), quercitrin: Y = 12641x + 1295.7 (r = 0.9999), kaempferol: Y = 11846x + 1283.9 (r = 0.9994), rutin: Y = 11792x + 1305.8 (r = 0.9995), luteolin: Y = 13894x + 1267.1 (r = 0.9998), and quercetin: Y = 12618x + 1196.3 (r = 0.9996). All chromatography operations were carried out at ambient temperature and in triplicate.
The limit of detection (LOD) and limit of quantification (LOQ) of flavonoids and phenolic acids were calculated based on the standard deviation of the responses and the slope using three independent analytical curves, as previously defined by Khaliq et al. [27]. LOD and LOQ were calculated as 3.3 and 10 s/S, respectively, where s is the standard deviation of the response and S is the slope of the calibration curve.
PL Inhibition Assay
The ability of the extracts to inhibit porcine PL was determined using the method earlier reported by Eom et al. [28] with a slight modification. In this assay, PL hydrolyzes p-nitrophenyl butyrate to form p-nitrophenol which is measured spectrophotometrically. The enzyme solution was prepared by adding 30 µL (10 units) of porcine PL (Sigma, USA) in 10 mM morpholine propane sulfonic acid and 1 mM EDTA (pH 6.8) to 850 µL of Tris buffer (100 mM Tris-HC1 and 5 mM CaCl2, pH 7.0). Thereafter, 100 µL of appropriate concentrations (20, 40, 60, 80 µg/mL) of the extracts or orlistat (positive control) were mixed with 880 µL of the enzyme solution and incubated for 10 min at 37°C. After incubation, 20 µL of the substrate solution (10 mM of p-nitrophenyl butyrate in dimethylformamide) was added to initiate the hydrolytic reaction, which was allowed to proceed for 20 min at 37°C. A reference test (without the extract) was included in the assay. The absorbance of the p-nitrophenol produced from the hydrolysis of p-nitrophenylbutyrate was measured at 405 nm. The inhibition of PL activity by the extracts was expressed as % inhibition thus:
% Inhibition = [(A405reference–A405sample)÷A405reference]×100.
Where A405reference is the absorbance of the reference without the extract, and A405sample is the absorbance of test containing the extract.
ACE Inhibition Assay
ACE inhibition was assayed by a spectrophotometric method described by Cushman and Cheung [29]. ACE from rabbit lung (EC: 3.4.15.1) and the substrate (Hippuryl-histidyl-leucine [Bz-Gly-His-Leu]) were used. In this assay, the Bz-Gly-His-Leu is cleaved by ACE to form hippuric acid, which is measured spectrophotometrically. A portion (50 µL) of appropriate concentrations of the extracts (20, 40, 60, 80 µg/mL) and 50 µL ACE solution (4 mU/mL) were pre-incubated at 37°C for 15 min. After pre-incubation, 150 µL of 8.33 mM of the Bz-Gly-His-Leu in 125 mM Tris-HCl buffer (pH8.3) was added to the mixture to initiate the enzymatic reaction, and this was incubated at 37°C for 30 min. Next, the reaction was terminated by adding 250 µL of 1 M HCl. The hippuric acid produced by the reaction was extracted with 1.5 mL ethyl acetate. Subsequently, the ethyl acetate layer was separated from the mixture by centrifugation, and 1 mL of it was transferred to a clean test tube and evaporated to dryness in a hot-air oven. The resulting residue was redissolved in distilled water and its absorbance was measured at 228 nm. A reference test (without the extract) and a positive control test (containing 64 nmol/L of captopril) were carried out simultaneously with the test extract. 1 unit (U) of ACE activity is defined as the amount of enzyme required to catalyze the formation of 1 µmol of hippuric acid from hippuryl-histidyl-leucine per minute at 37°C. The ability of the extracts to inhibit ACE activity was expressed as percentage inhibition thus:
% Inhibition=[(A228reference–A228sample)÷A228reference]×100.
Where A228reference is the absorbance of the reference without the extract, and A228sample is the absorbance of test containing the extract.
Determination of DPPH* Scavenging Ability
DPPH* scavenging ability of the extracts was determined as described by Cervato et al. [30]. Appropriate dilutions (30, 60, 90, 120 µg/mL) of the extracts amounting to 1.0 mL were mixed with 3.0 mL of DPPH* (60 µM). The test mixture was kept in the dark for 30 min, after which the absorbance was measured at 517 nm. A reference test containing the DPPH* solution without the extract was carried simultaneously. The DPPH* percentage scavenging ability of the extract was calculated as follows:
% scavenging ability= [(Abs517reference–Abs517sample)÷Abs517reference]×100
Where Abs517reference is the absorbance of the reference test, and Abs517sample is the absorbance of the test containing the extract.
Determination of ABTS*+ Scavenging Ability
ABTS*+ scavenging ability of the extracts was determined according to the method described by Re et al. [31]. To generate the ABTS*+, an equal volume of 7 mM ABTS*+ aqueous solution was incubated with 2.45 mM K2S2O8 for 16 h at room temperature in the dark; then its absorbance (at 734 nm) was adjusted to 0.7 ± 0.02 with 95% ethanol. Thereafter, appropriate dilution of the extract amounting to 0.2 mL was mixed with 2.0 mL ABTS*+ solution. The test mixture was kept in the dark for 15 min, after which its absorbance was measured at 734 nm. The ABTS*+ scavenging ability of the extracts was subsequently calculated and expressed in trolox equivalent (TE).
Statistical Analysis
Results of replicate experiments were expressed as mean ± standard deviation (SD). T-test for independent samples was carried out on the result data at 95% confidence level using SPSS statistical software package, version 17. Half-maximal inhibitory and scavenging concentrations (IC50 and SC50) were calculated from the % inhibition versus extract concentration non-linear regression curve of each extract.
RESULTS
The HPLC chromatograms of O. basilicum and O. gratissimum [Figure 1a and b] revealed the presence of gallic acid (retention time - Rt = 10.19 min; peak 1), chlorogenic acid (Rt = 22.35 min; peak 3), caffeic acid (Rt = 26.97 min; peak 4), epicatechin (Rt = 37.41 min; peak 6), rutin (Rt = 43.86 min; peak 7), quercitrin (Rt = 48.13 min; peak 8), quercetin (Rt = 52.01 min; peak 9), and kaempferol (Rt = 57.69 min; peak 10). In addition to these, catechin (Rt = 16.07 min; peak 2), ellagic acid (Rt = 29.83 min; peak 5), and luteolin (Rt = 64.15 min; peak 11) were also present in O. gratissimum [Figure 1b].
Figure 1.
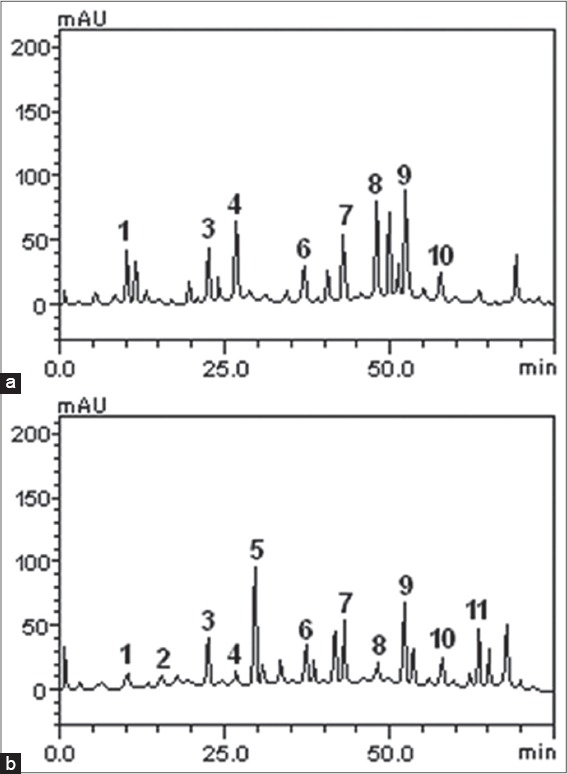
Representative high performance liquid chromatrography chromatograms of Ocimum basilicum and Ocimum gratissimum extracts; detection ultraviolet was at 325 nm. Gallic acid (peak 1), catechin (peak 2), chlorogenic acid (peak 3), caffeic acid (peak 4), ellagic acid (peak 5), epicatechin (peak 6), rutin (peak 7), quercitrin (peak 8), quercetin (peak 9), kaempferol (peak 10), and luteolin (peak 11). (a) O. basilicum, (b) O. gratissimum
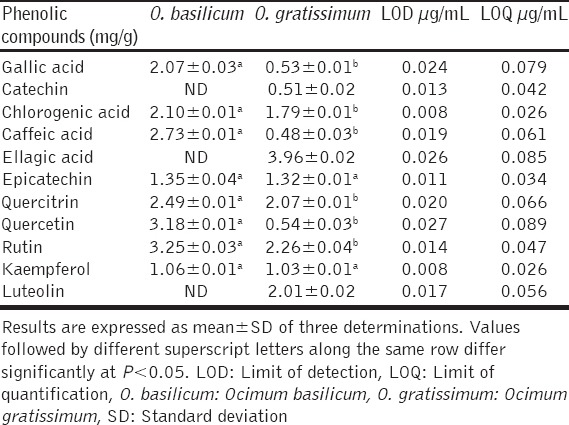
The quantitative composition of the phenolics (flavonoids and phenolic acids) is shown in Table 1. In O. basilicum, the flavonoids were in the order of rutin > quercetin > quercitrin > epicatechin > kaempferol, whereas the phenolic acids were in the order of caffeic acid > chlorogenic acid > gallic acid. In O. gratissimum, the flavonoids were in the order of rutin > quercitrin > luteolin > epicatechin > kaempferol > quercetin > catechin, while the phenolic acids were in the order of ellagic acid > chlorogenic acid > gallic acid > caffeic acid. Thus, the major flavonoids in O. basilicum were rutin, quercetin, and quercitrin; the major phenolic acids were caffeic, chlorogenic and gallic acids. But in O. gratissimum rutin, quercitrin and luteolin were the major flavonoids, whereas ellagic and chlorogenic acids were the major phenolic acids.
Table 1.
Flavonoids and phenolics acids composition of O. basilicum and O. gratissimum leaves extracts
The abilities of the O. basilicum and O. gratissimum leaves extracts to inhibit porcine PL and ACE were expressed in terms of their half-maximal inhibitory concentrations (IC50), and the results are presented in Table 2. The IC50 of O. gratissimum against PL (20.69 ± 2. 14 µg/mL) was significantly (P < 0.05) lower than that of O. basilicum (52.14 ± 3.96 µg/mL). However, the IC50 (3.48 ± 0.13 µmol/g) of orlistat, the reference PL inhibitor, was lower than those of O. gratissimum and O. basilicum. Similarly, the IC50 of O. gratissimum against ACE (29.44 ± 3.98 µg/mL) was significantly (P < 0.05) lower than that of O. basilicum (64.99 ± 5.04 µg/mL) but that of captopril (19.32 ± 2.23 µmol/g), the reference ACE inhibitor, was lower than both. The patterns of PL and ACE inhibition by O. basilicum and O. gratissimum were both dose-dependent as depicted by their % inhibition versus extract concentration curves [Figures 2 and 3] respectively.
Table 2.
IC50 of O. basilicum and O. gratissimum leaves extracts against PL and ACE activities
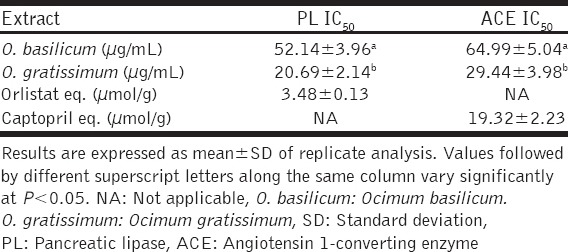
Figure 2.
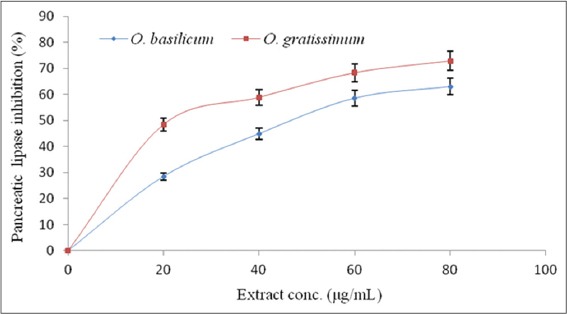
Pancreatic lipase % inhibition - extract concentration curve of Ocimum basilicum and Ocimum gratissimum leaves extracts
Figure 3.
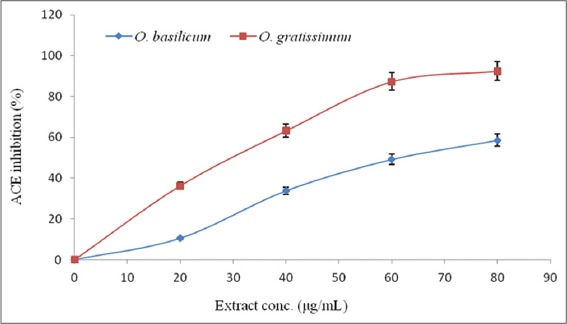
Angiotensin I-converting enzyme % inhibition - extract concentration curve of Ocimum basilicum and Ocimum gratissimum leaves extracts
The free radical scavenging activities of O. basilicum and O. gratissimum leaves extracts, as determined using DPPH* and ABTS*+ scavenging assays, are presented in Table 3. O. gratissimum had a significantly (P < 0.05) stronger DPPH* scavenging ability (SC50: 84.76 ± 1.06 µg/mL) than O. basilicum (SC50: 104.72 ± 1.85 µg/mL). However, ascorbic acid, the reference antioxidant, had much stronger DPPH* scavenging ability (SC50: 7.29 ± 0.34 µg/mL) than both O. gratissimum and O. basilicum. However, both leaves extracts scavenged DPPH* in a dose-dependent [Figure 4]. Similarly, O. gratissimum had a stronger ABTS*+ scavenging ability (1.82 ± 0.02 mmol TE/g) than O. basilicum extract (0.52 ± 0.01 mmol TE/g).
Table 3.
DPPH* SC50 and ABTS*+ scavenging ability of O. basilicum and O. gratissimum leaves extracts
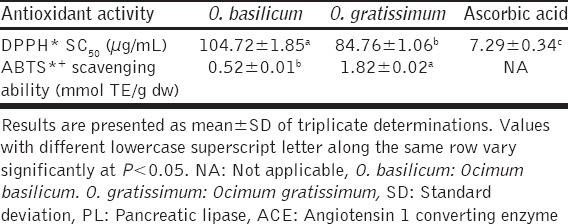
Figure 4.
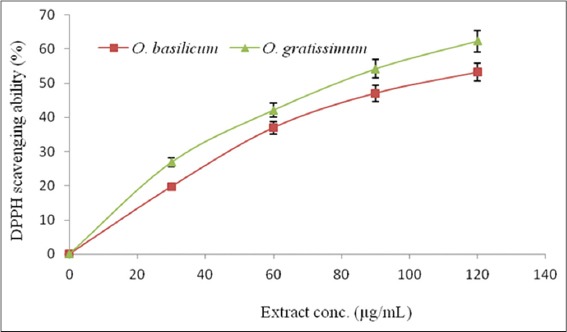
DPPH* % scavenging ability-extract concentration curve of Ocimum basilicum and Ocimum gratissimum leaves extracts
DISCUSSION
The various health benefits of medicinal plants are attributed to the presence of secondary metabolites such as phenolic acids and flavonoids [32]. In this study, therefore, we quantified the flavonoids and phenolic acids constituents of O. basilicum and O. gratissimum leaves, and evaluated their inhibitory effects against PL and ACE, as well as their free radicals scavenging abilities in vitro. Flavonoids and phenolic acids are prominent for their diverse health benefits including anti-diabetic [33], anti-hypertensive and antioxidative [34]; hence, they are regarded as being pharmacologically important [35]. Interestingly, the level of caffeic acid (2.73 mg/g) we quantified in O. basilicum in this study is about 40% higher than the 1.65 mg/g reported earlier by Harnafi et al. [36].
The inhibition of PL, the most important enzyme in the digestion of dietary triglycerides, is one of the possible approaches to retard the uptake of fat, and consequently, reduce weight and obesity [37]. Polyphenols, including flavonoids and phenolic acids, from natural origin are regarded as a major class of the PL inhibitors [38,39]. However, flavonoids, especially the catechin-types, are presumed to possess more PL inhibitory and anti-obesity effect. The presence of galloyl moiety in the structural backbone of the polyphenolics plays an important role in their ability to inhibit PL. Possibly, the more the galloyl moieties, the stronger the PL inhibitory effect, as Nakai et al. [40] demonstrated that the PL IC50 values of (−)-epigallocatechin 3,5-digallate with two galloyl groups, epigallocatechin gallate with one galloyl group and (−)-epigallocatechin with no galloyl group were 0.098, 0.349 and 20 µM, respectively, in their study in which they evaluated the inhibitory activities of 54 tea polyphenols against PL. The galloyl moieties may exert their inhibitory effect on PL by competing with its substrate for the active site, at least, at a substrate concentration below the Km value [38]. Therefore, it is possible that the presence of catechin in O. gratissimum accounted for its stronger ability to inhibit PL than O. basilicum that had no catechin [Table 1].
ACE catalyzes the cleavage of angiotensin I (a decapeptide) to angiotensin II (an octapeptide) and inactivates bradykinin, a vasodilator and hypotensive peptide [41]. Angiotensin II stimulates vasoconstriction, increases sodium and water reabsorption, and elevates blood pressure. Consequently, the excessive action of ACE leads to hypertension [42]; for this reason, inhibition of ACE has emerged as an important treatment and management strategy for hypertension [10,11]. Plant extracts rich in flavonoids and phenolic acids have been shown to inhibit ACE activity by previous studies [18,34]. The flavonoids are the largest group of polyphenolic compounds found in higher plants [43] and are regarded as an excellent source of functional anti-hypertensive products. Guerrero et al. [44] observed this in their study in which they evaluated the structure-activity relationship of inhibition of ACE activity by flavonoids. The combination of sub-structures on the flavonoid skeleton, including the catechol group in the B-ring, the double bond between C2 and C3 at the C-ring, and the cetone group in C4 at the C-ring, increases the ACE inhibitory activity of the flavonoids. Similarly, the phenolic acids are effective ACE inhibitors. Their ability to inhibit ACE has been attributed to the overall contribution of their functional groups, including carboxyl and hydroxyl groups; their ability to form charge-charge interactions with the zinc ion present in the active site of ACE, through the oxygen atom of their carboxylate moiety; and their interaction with the amino acids residues at the active site of ACE, to give a stable complex between the phenolic acid molecule and ACE. Thus, the ACE inhibitory activity of phenolic acids may be due the effect of this interaction with the zinc ion and the subsequent stabilization by other interactions with amino acids in the active site [45]. In this study, O. gratissimum had stronger ACE inhibitory activity than O. basilicum [Table 2]. This may be attributed to the presence of luteolin (flavonoid) in O. gratissimum (2.01 mg/g) and ellagic acid (phenolic acid), which were not detected in O. basilicum [Table 1]. This is supported by the findings of Guerrero et al. [44], who reported that luteolin had the highest ACE inhibitory activity, relative to other sixteen flavonoids in their study.
Systemic oxidative stress is a common feature of obesity [46], and by extension, obesity-related hypertension. Oxidative stress arises due to increased generation of free radicals and reactive oxygen species (ROS), with attendant reduction in the antioxidant defense system, both enzymic and non-enzymic, in the obese. Angiotensin II also induces oxidative stress, which plays a key role in the development of hypertension [47,48]. Free radicals and ROS are notorious for their ability to damage both intra- and extra-cellular macromolecules, including the DNA, lipids, and protein [49]. However, several previous studies have demonstrated that plant extracts rich in flavonoids and phenolic acids can scavenge free radicals [18,34]. The efficiency of flavonoids and phenolic acids as antioxidant polyphenols is attributable to their unique molecular structures. The hydroxyl groups of flavonoids, especially at the 3’ OH and 4’ OH of their three-carbon chain, make them potent electrons donors and terminators of chain reactions [50,51]; hence their antioxidant effect. On the other hand, the antioxidant activity of phenolic acids has been attributed to the ability of the phenolic ring in their structure to stabilize and delocalize unpaired electrons [52]. The ability of the O. basilicum and O. gratissimum leaves extracts to scavenge free radicals (DPPH* and ABTS*+) is an indication that they could help prevent and/or alleviate oxidative stress in obesity and obesity-related hypertension. This may complement the overall antihypertensive activity of the extracts, as Rodriguez-Rodriguez and Simonsen [53] reported that the anti-oxidative effects of ACE inhibitors contributed to their therapeutic effects in patients suffering from cardiovascular complications.
CONCLUSION
The leaves extracts of O. basilicum and O. gratissimum inhibited PL and ACE; and scavenged DPPH* and ABTS*+. These bioactivities could be due to the combined effects of the flavonoids and phenolic acids present in the leaves. Hence, O. basilicum and O. gratissimum leaves could be used as functional foods for the management of obesity and obesity-related hypertension, and preventing the oxidative stress that characterizes both disorders. However, O. gratissimum may be more effective due to its stronger inhibitory effect on PL and ACE.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Sironi AM, Gastaldelli A, Mari A, Ciociaro D, Positano V, Buzzigoli E, et al. Visceral fat in hypertension: Influence on insulin resistance and beta-cell function. Hypertension. 2004;44:127–33. doi: 10.1161/01.HYP.0000137982.10191.0a. [DOI] [PubMed] [Google Scholar]
- 2.Krauss RM, Winston M, Fletcher BJ, Grundy SM. Obesity: Impact on cardiovascular disease. Circulation. 1998;98:1472–6. [PubMed] [Google Scholar]
- 3.Narkiewicz K. Obesity and hypertension - The issue is more complex than we thought. Nephrol Dial Transplant. 2006;21:264–7. doi: 10.1093/ndt/gfi290. [DOI] [PubMed] [Google Scholar]
- 4.Birari RB, Bhutani KK. Pancreatic lipase inhibitors from natural sources: Unexplored potential. Drug Discov Today. 2007;12:879–89. doi: 10.1016/j.drudis.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Sakulnarmrat K, Konczak I. Composition of native Australian herbs polyphenolic-rich fractions and in vitro inhibitory activities against key enzymes relevant to metabolic syndrome. Food Chem. 2012;134:1011–9. doi: 10.1016/j.foodchem.2012.02.217. [DOI] [PubMed] [Google Scholar]
- 6.Sugiyama H, Akazome Y, Shoji T, Yamaguchi A, Yasue M, Kanda T, et al. Oligomeric procyanidins in apple polyphenol are main active components for inhibition of pancreatic lipase and triglyceride absorption. J Agric Food Chem. 2007;55:4604–9. doi: 10.1021/jf070569k. [DOI] [PubMed] [Google Scholar]
- 7.Filippatos TD, Derdemezis CS, Gazi IF, Nakou ES, Mikhailidis DP, Elisaf MS. Orlistat-associated adverse effects and drug interactions: A critical review. Drug Saf. 2008;31:53–65. doi: 10.2165/00002018-200831010-00005. [DOI] [PubMed] [Google Scholar]
- 8.Viner RM, Hsia Y, Tomsic T, Wong IC. Efficacy and safety of anti-obesity drugs in children and adolescents: Systematic review and meta-analysis. Obes Rev. 2010;11:593–602. doi: 10.1111/j.1467-789X.2009.00651.x. [DOI] [PubMed] [Google Scholar]
- 9.Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension: New insights into mechanisms. Hypertension. 2005;45:9–14. doi: 10.1161/01.HYP.0000151325.83008.b4. [DOI] [PubMed] [Google Scholar]
- 10.Sharma AM. Is there a rationale for angiotensin blockade in the management of obesity hypertension? Hypertension. 2004;44:12–9. doi: 10.1161/01.HYP.0000132568.71409.a2. [DOI] [PubMed] [Google Scholar]
- 11.Villiger A, Sala F, Suter A, Butterweck V. In vitro inhibitory potential of Cynara scolymus Silybum marianum Taraxacum officinale and Peumus boldus on key enzymes relevant to metabolic syndrome. Phytomedicine. 2015;22:138–44. doi: 10.1016/j.phymed.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Lan X, Liao D, Wu S, Wang F, Sun J, Tong Z. Rapid purification and characterization of angiotensin converting enzyme inhibitory peptides from lizard fish protein hydrolysates with magnetic affinity separation. Food Chem. 2015;182:136–42. doi: 10.1016/j.foodchem.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Thurman JM, Schrier RW. Comparative effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on blood pressure and the kidney. Am J Med. 2003;114:588–98. doi: 10.1016/s0002-9343(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 14.Vyssoulis GP, Karpanou EA, Papavassiliou MV. Side effects of anti-hypertensive treatment with ACE inhibitors. Am J Hypertens. 2001;14:14–5. [Google Scholar]
- 15.Mayer MA, Höcht C, Puyó A, Taira CA. Recent advances in obesity pharmacotherapy. Curr Clin Pharmacol. 2009;4:53–61. doi: 10.2174/157488409787236128. [DOI] [PubMed] [Google Scholar]
- 16.Tanzadehpanah H, Asoodeh A, Saberi MR, Chamani J. Identification of a novel angiotensin-I converting enzyme inhibitory peptide from ostrich egg white and studying its interactions with the enzyme. Innov Food Sci Emerging Technol. 2013;18:212–9. [Google Scholar]
- 17.Slanc P, Doljak B, Kreft S, Lunder M, Janes D, Strukelj B. Screening of selected food and medicinal plant extracts for pancreatic lipase inhibition. Phytother Res. 2009;23:874–7. doi: 10.1002/ptr.2718. [DOI] [PubMed] [Google Scholar]
- 18.Irondi EA, Agboola SO, Oboh G, Boligon AA, Athayde ML, Shode FO. Guava leaves polyphenolics-rich extract inhibits vital enzymes implicated in gout and hypertension in vitro . J Intercult Ethnopharmacol. 2016;5:122–30. doi: 10.5455/jice.20160321115402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casanova LM, da Silva D, Sola-Penna M, Camargo LM, Celestrini Dde M, Tinoco LW, et al. Identification of chicoric acid as a hypoglycemic agent from Ocimum gratissimum leaf extract in a biomonitoring in vivo study. Fitoterapia. 2014;93:132–41. doi: 10.1016/j.fitote.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 20.Bhuvaneshwari K, Gokulanathan A, Jayanthi M, Govindasamy V, Milella L, Lee S, et al. Can Ocimum basilicum L. and Ocimum tenuiflorum L. in vitro culture be a potential source of secondary metabolites? Food Chem. 2016;194:55–60. doi: 10.1016/j.foodchem.2015.07.136. [DOI] [PubMed] [Google Scholar]
- 21.Al Abbasy DW, Pathare N, Al-Sabahi JN, Khan SA. Chemical composition and antibacterial activity of essential oil isolated from Omani basil (Ocimum basilicum Linn.) Asian Pac J Trop Dis. 2015;5:645–9. [Google Scholar]
- 22.Madhu KD, Harindran J. Antiarthritic potential of Ocimum gratissimum L. in collagen induced arthritic Sprague-Dawley rats. Biomed Aging Pathol. 2014;4:191–6. [Google Scholar]
- 23.Okoli CO, Ezike AC, Agwagah OC, Akah PA. Anticonvulsant and anxiolytic evaluation of leaf extracts of Ocimum gratissimum a culinary herb. Pharmacognosy Res. 2010;2:36–40. doi: 10.4103/0974-8490.60580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sajjadi SE. Analysis of the essential oils of two cultivated basil (Ocimum basilicum L.). from Iran. Daru. 2006;14:128–30. [Google Scholar]
- 25.Kuo C, Kao E, Chan K, Lee H, Huang T, Wang C. Hibiscus sabdariffa L. extracts reduce serum uric acid levels in oxonate-induced rats. J Funct Foods. 2012;4:375–81. [Google Scholar]
- 26.Boligon AA, Piana M, Kubiça TF, Mario DN, Dalmolin TV, Bonez PC, et al. HPLC analysis and antimicrobial, antimycobacterial and antiviral activities of Tabernaemontana catharinensis A. DC. J Appl Biomed. 2015;13:7–18. [Google Scholar]
- 27.Khaliq A, Sabir SM, Ahmad SD, Boligon AA, Athayde ML, Jabbar A, et al. Antioxidant activities and phenolic composition of olive (Olea europaea) leaves. J Appl Bot Food Qual. 2015;88:16–21. [Google Scholar]
- 28.Eom SH, Lee MS, Lee EW, Kim YM, Kim TH. Pancreatic lipase inhibitory activity of phlorotannins isolated from Eisenia bicyclis. Phytother Res. 2013;27:148–51. doi: 10.1002/ptr.4694. [DOI] [PubMed] [Google Scholar]
- 29.Cushman DW, Cheung HS. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol. 1971;20:1637–48. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- 30.Cervato G, Carabelli M, Gervasio S, Cittera A, Cazzola R, Cestaro B. Antioxidant properties of oregano (Origanum vulgare) leaf extracts. J Food Biochem. 2000;24:453–65. [Google Scholar]
- 31.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 32.Ajila CM, Brar SK, Verma M, Tyagi RD, Godbout S, Valéro JR. Extraction and analysis of polyphenols: Recent trends. Crit Rev Biotechnol. 2011;31:227–49. doi: 10.3109/07388551.2010.513677. [DOI] [PubMed] [Google Scholar]
- 33.Irondi EA, Oboh G, Akindahunsi AA, Boligon AA, Athayde ML. Phenolics composition and antidiabetic property of Brachystegia eurycoma seed flour in high-fat diet, low-dose streptozotocin-induced type 2 diabetes in rats. Asian Pac J Trop Dis. 2015;5(Suppl 1):S159–65. [Google Scholar]
- 34.Oboh G, Ademiluyi AO, Akinyemi AJ, Henle T, Saliu JA, Schwarzenbolz U. Inhibitory effect of polyphenol-rich extracts of Jute leaf (Corchorus olitorius) on key enzyme linked to type-2 diabetes (a-amylase and a-glucosidase) and hypertension (angiotensin I converting) in vitro. J Funct Food. 2012;4:450–8. [Google Scholar]
- 35.Irondi AE, Oboh G, Akindahunsi AA, Boligon AA, Athayde ML. Phenolic composition and inhibitory activity of mango and horse-eye bean seeds extracts against key enzymes linked to the pathology and complications of type 2 diabetes. Asian Pac J Trop Biomed. 2014;4:903–10. [Google Scholar]
- 36.Harnafi H, Ramchoun M, Tits M, Wauters J, Frederich M, Angenot L, et al. Phenolic acid-rich extract of sweet basil restores cholesterol and triglyceridesmetabolism in high fat diet-fed mice: A comparison with fenofibrate. Biomed Prev Nutr. 2013;3:393–7. [Google Scholar]
- 37.Kaisoon O, Konczak I, Siriamornpun S. Potential health enhancing properties of edible flowers from Thailand. Food Res Int. 2012;46:563–71. [Google Scholar]
- 38.Rahim AT, Takahashi Y, Yamaki K. Mode of pancreatic lipase inhibition activity in vitro by some flavonoids and non-flavonoid polyphenols. Food Res Int. 2015;75:289–94. doi: 10.1016/j.foodres.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 39.Lunagariya NA, Patel NK, Jagtap SC, Bhutani KK. Inhibitors of pancreatic lipase: State of the art and clinical perspectives. EXCLI J. 2014;13:897–921. [PMC free article] [PubMed] [Google Scholar]
- 40.Nakai M, Fukui Y, Asami S, Toyoda-Ono Y, Iwashita T, Shibata H, et al. Inhibitory effects of oolong tea polyphenols on pancreatic lipase in vitro. J Agric Food Chem. 2005;53:4593–8. doi: 10.1021/jf047814+. [DOI] [PubMed] [Google Scholar]
- 41.Eriksson U, Danilczyk U, Penninger JM. Just the beginning: Novel functions for angiotensin-converting enzymes. Curr Biol. 2002;12:R745–52. doi: 10.1016/s0960-9822(02)01255-1. [DOI] [PubMed] [Google Scholar]
- 42.Lee BH, Lai YS, Wu SC. Antioxidation, angiotensin converting enzyme inhibition activity, nattokinase, and antihypertension of Bacillus subtilis (natto)-fermented pigeon pea. J Food Drug Anal. 2015. pp. 1–8. Available from: http://www.dx.doi.org/10.1016/j.jfda.2015.06.008 . [DOI] [PMC free article] [PubMed]
- 43.Croft KD. The chemistry and biological effects of flavonoids and phenolic acids. Ann N Y Acad Sci. 1998;854:435–42. doi: 10.1111/j.1749-6632.1998.tb09922.x. [DOI] [PubMed] [Google Scholar]
- 44.Guerrero L, Castillo J, Quiñones M, Garcia-Vallvé S, Arola L, Pujadas G, et al. Inhibition of angiotensin-converting enzyme activity by flavonoids: Structure-activity relationship studies. PLoS One. 2012;7:e49493. doi: 10.1371/journal.pone.0049493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al Shukor N, Van Camp J, Gonzales GB, Staljanssens D, Struijs K, Zotti MJ, et al. Angiotensin-converting enzyme inhibitory effects by plant phenolic compounds: A study of structure activity relationships. J Agric Food Chem. 2013;61:11832–9. doi: 10.1021/jf404641v. [DOI] [PubMed] [Google Scholar]
- 46.Mohamed S. Functional foods against metabolic syndrome (obesity, diabetes, hypertension and dyslipidemia) and cardiovasular disease. Trends Food Sci Technol. 2014;35:114–28. [Google Scholar]
- 47.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78:539–52. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Virdis A, Duranti E, Taddei S. Oxidative stress and vascular dam-age in hypertension: Role of angiotensin II. Int J Hypertens. 2011. 2011. Available from: http://www.dx.doi.org/10.4061/2011/916310 . [DOI] [PMC free article] [PubMed]
- 49.Takemoto K, Tanaka M, Iwata H, Nishihara R, Ishihara K, Wang DH, et al. Low catalase activity in blood is associated with the diabetes caused by alloxan. Clin Chim Acta. 2009;407:43–6. doi: 10.1016/j.cca.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 50.Kim JK, Lee SY, Chu SM, Lim SH, Suh SC, Lee YT, et al. Variation and correlation analysis of flavonoids and carotenoids in Korean pigmented rice (Oryza sativa L.) cultivars. J Agric Food Chem. 2010;58:12804–9. doi: 10.1021/jf103277g. [DOI] [PubMed] [Google Scholar]
- 51.Cho JG, Song NY, Nam TG, Shrestha S, Park HJ, Lyu HN, et al. Flavonoids from the grains of C1/R-S transgenic rice, the transgenic Oryza sativa spp. japonica, and their radical scavenging activities. J Agric Food Chem. 2013;61:10354–9. doi: 10.1021/jf403072c. [DOI] [PubMed] [Google Scholar]
- 52.Goufo P, Trindade H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, y-oryzanol, and phytic acid. Food Sci Nutr. 2014;2:75–104. doi: 10.1002/fsn3.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodriguez-Rodriguez R, Simonsen U. Measurement of nitric oxide and reactive oxygen species in the vascular wall. Curr Anal Chem. 2012;8:1–10. [Google Scholar]


