Abstract
Aim:
The objective of this study was to estimate the effect of orlistat alone and in combination with Garcinia cambogia on visceral adiposity index (VAI) in obese patients.
Patients and Methods:
A total of 99 obese male patients were recruited with aged range between 37 and 46 years. They were randomized into three equal groups, first group treated with orlistat 120 mg/day, second group treated with G. cambogia 166 mg/day, and third group treated with orlistat 120 mg/day plus G. cambogia 166 mg/day. The duration of the treatments was three consecutive months. Body mass index (BMI), VAI, blood pressure, blood glucose, total lipid profile, atherogenic index, and cardiac risk ratio were recorded at baseline and after 3 months.
Results:
The treatment with G. cambogia leads to reduction in VAI P < 0.05, whereas orlistat has a beneficial effect on cardiometabolic profiles without a reduction in VAI P > 0.05. Combined therapy of G. cambogia plus orlistat showed the more significant effect in reduction of VAI P < 0.05, cardiometabolic profiles and anthropometric measures P < 0.01 compared to pretreatment period.
Conclusion:
Combination of G. cambogia with orlistat lead to more significant effect than orlistat alone in amelioration of cardiometabolic profile and VAI in obese patients.
KEY WORDS: Garcinia cambogia, obesity, orlistat
INTRODUCTION
Obesity is a body fat excess as a result of positive energy balance or it is an excess of body fat mass that linked with frequent metabolic disorders such as ischemic heart diseases, dyslipidemia, type 2 diabetes mellitus, and hypertension [1,2].
Thus, the principal that been measures taken to manage the obesity like exercise and dietary restriction are often insufficient alone, so anti-obesity pharmacotherapy is needed as an adjuvant in the management of obesity [3].
Most of anti-obesity agents acting centrally leading to appetite suppression and reduction in food intake such as fenfluramine, sibutramine, and amphetamine which causes serious sympathetic activation and cardiovascular adverse effects that limit and restrict their uses chiefly in patients with cardiovascular disease [4]. Therefore, peripherally acting agents effects such as orlistat and Garcinia cambogia are recommended.
Orlistat is a potent and specific 47-91% of gastric lipase, 51-82% of pancreatic lipase) without any action on amylase, phospholipase and trypsin, this lead to inhibition of triglyceride (TG) hydrolysis, absorption of fatty acids, and monoglycerides from enterocytes consequently; the ingested fat absorption is diminished by about 36% [5].
Multicenter randomized trials on the efficacy of orlistat in weight reduction showed a favorable weight reduction and improvement in obesity profiles such as a reduction in insulin resistance, anthropometric measure, and improvement in lipid profiles in diabetic obese patients [6].
Orlistat was approved by Food and Drug Administration on 1999 as anti-obesity agent for long-term therapy; it is not absorbed so it’s not produced any systemic manifestation, thus, it causes local gastrointestinal disorders which include reduction of fat soluble vitamin absorption (vitamin A, D, E, K), oily spot, flatulence, and increases frequency of defecations and rarely it may cause a severe hepatic damage [7].
On the other hand, G. cambogia contains an active constituent called hydroxycitric acid (HCA) that plays an important role in weight reduction, which is a competitive inhibitor of citrate-lyase enzyme that catalyze extra-mitochondrial citrate into acetyl coenzyme A and oxaloacetate causing a significant reduction of acetyl coenzyme A pools that limiting the availability of substrate for lipogenesis through activation of glycogenesis [8]. A study has been revealed that HCA inhibits de novo fatty acid and cholesterol biosynthesis leading to significant reduction of subcutaneous and visceral fat accumulations subsequently; long-term therapy with G. cambogia leading to significant weight reduction [9].
Regarding the cardiometabolic risk profile in obesity, visceral adiposity index (VAI) has been recognized as a clinical marker for adipose tissue dysfunction before it expands into an evident metabolic syndrome. VAI is highly associated with cardiovascular complications and insulin resistance [10], while body mass index (BMI) is incorrectly considered as a reasonable predictor for body fat percentage regardless of gender because of BMI is not always linked with cardiovascular mortalities [11]. Moreover, waist circumference (WC) has been regarded as an applicable index in the evaluation of adipose tissue distribution that is highly linked to visceral and abdominal obesity, but cannot distinguish between subcutaneous and visceral adipose tissue in the abdominal area [12].
Therefore, the objective of this study was evaluating the effect of orlistat alone or with G. cambogia on VAI in obese patients.
PATIENTS AND METHODS
This randomized, population-based controlled study was done and carried out at Department of Clinical Pharmacology, College of Medicine, Al-Mustansiriya University, from June to September 2015, Baghdad-Iraq. The study was approved according to the guide of the Declaration of Helsinki and by the Institutional Review Board and Ethical Committee in College of Medicine. The procedures of the study were completely explained to all patients, and all patients provided a written consent before the contribution in this study according to the ethical resolution 33AT/2015.
Study Design
In this study, 99 obese male patients were recruited; age range was 37-46 years. The patients were selected according to the European Society of Cardiology [13]. Appropriate patients, identified from a direct interview and according to the routine investigations while, obese patients with diabetes mellitus, renal failure, liver disorders, severe cardiovascular disorders, cerebrovascular disorders and severe anemia were excluded from this study. Obese patients were randomized into three equal groups, first group treated with orlistat 120 mg/day (xenical capsule, Roch), second group treated with G. cambogia 166 mg/day (Garcinia capsule, himalaya), and third group treated with both orlistat and G. cambogia. The duration of treatments was three consecutive months. The baseline data were recorded, then after the end of the duration of therapy; the biochemical and anthropometric measures were estimated. The patients were allocated to receive G. cambogia and orlistat in addition to their current treatment like antihypertensive agents. All medications were supplied free of charge. The patients were asked to avoid treatment with mineral and vitamins during the study. In addition, the patients have been advised for standard diet and avoidance of sedentary lifestyle.
Assessment of Anthropometric and Cardio-Metabolic Measures
BMI = body weight (kg)/height (m2) [14].
VAI is calculated according the specific formula which depends on WC, TG, and high-density lipoprotein (HDL) in mmol/L [15].
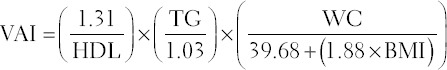
Assessment of Lipid Profile
TG, total cholesterol (TC), and HDL were assessed by specific ELISA kits; from this profile, we can measure the followings: Atherogenic index (AI) = log (TG/HDL), when TG and HDL measured in mmol/l., atherogenic coefficient (AC) = (TC-HDL)/HDL, low density lipoprotein (LDL) = (TC)-(HDL)-(TG)/5, very LDL (VLDL) = TG/5, and cardiac risk ratio (CRR) = TC/HDL [16].
Moreover, blood pressure measurements and other routine investigations such as fasting and random blood glucose were performed.
Statistical Analysis
Statistical estimation of data was done through using the statistical package for social sciences software version 21.0 (SPSS). Data were expressed as mean ± standard error. Continuous variables were compared via analysis of variance (ANOVA), which also evaluated the significance between groups. Paired t-test was used to compare results before and after administration of treatments, P value for all statistical analysis regarded significant when it was <0.05.
RESULTS
A total number of 99 obese male patients were enrolled in this study, of these 89 patients completed the study, 30 (26.7%) was allocated in orlistat group, 30 (26.7%) was allocated in G. cambogia and 29 (25.81%) was allocated in combined orlistat plus G. cambogia group.
A total number of 10 patients did not complete the study due to drug side effects, non-compliance and other reasons. At the end the duration of treatment, the study withdrawal rate was three (0.99%) [Figure 1].
Figure 1.
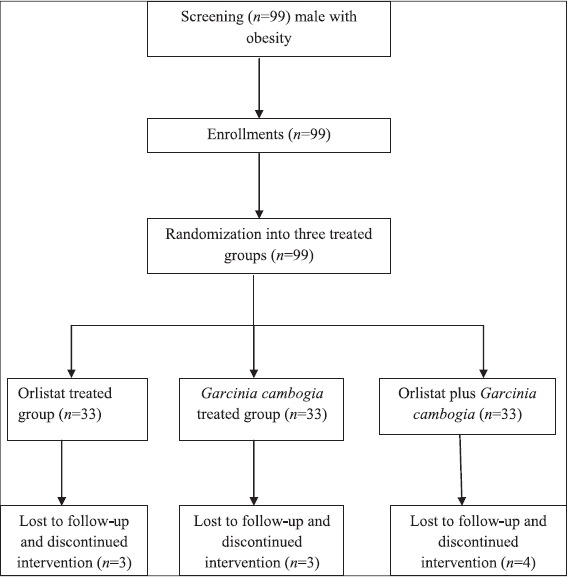
Flow chart of the present study
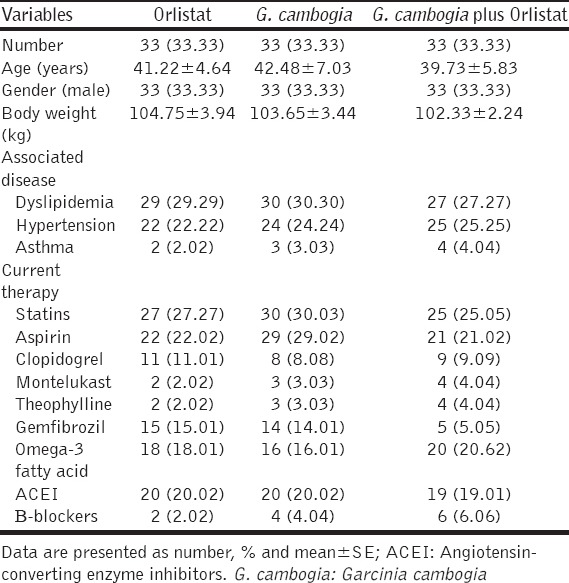
Most of the enrolled patients were hypertensive with dyslipidemia and other concomitant diseases that were currently treated with antihypertensive, antiplatelet, and hypolipidemic agents. The patient characteristics of the present study are shown in Table 1.
Table 1.
Characteristics of the study
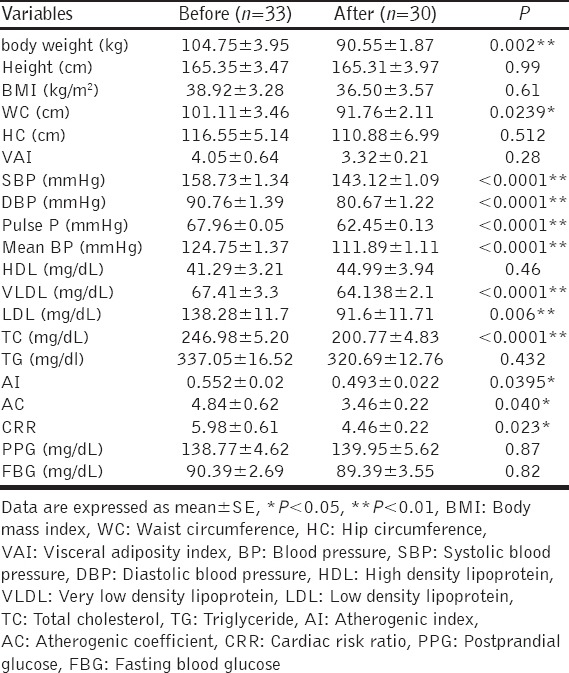
Orlistat produced significant reduction in body weight P < 0.01 and WC P < 0.05 compared to baseline parameters. Moreover, orlistat produced significant blood pressure lowering effect (P < 0.01). Regarding the effects of orlistat on lipid profile effect, it produced significant reductions in TC level P < 0.0001, VLDL P < 0.0001, LDL P = 0.006, AI P = 0.0395, AC P = 0.040, and CRR P = 0.023. The descriptive effects of orlistat showed in Table 2.
Table 2.
Effects of orlistat on cardiometabolic indices in obese patients
G. cambogia treatment led to significant body weight reduction (P < 0.05) and more significant reduction in WC (P < 0.01) compared with baseline data. G. cambogia therapy led to significant reduction in VAI P = 0.0051. Furthermore, G. cambogia produced a more significant effect on blood pressure, TC, and LDL (P < 0.01). Furthermore, AI (P = 0.039), AC (P = 0.0381), and CRR (P = 0.0032) are decreased significantly [Table 3].
Table 3.
Effects of G. cambogia on cardiometabolic indices in obese patients
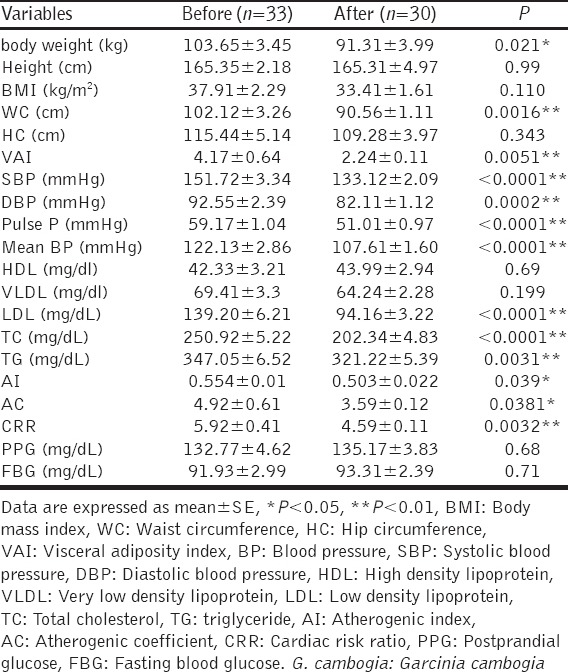
Our study also revealed that combination of G. cambogia and orlistat showed a significant reduction in cardiometabolic indices; body weight and BMI (P < 0.01 and 0.05), respectively. Moreover, the combined effect demonstrated significant reductions in WC P < 0.0001, VAI P = 0.023, significant reduction in all lipid profile without significant elevation in HDL cholesterol (P > 0.05). Furthermore, combination treatment lowered blood pressure significantly (P < 0.01), [Table 4].
Table 4.
Combined effect of G. cambogia plus orlistat on cardiometabolic indices in obese patients
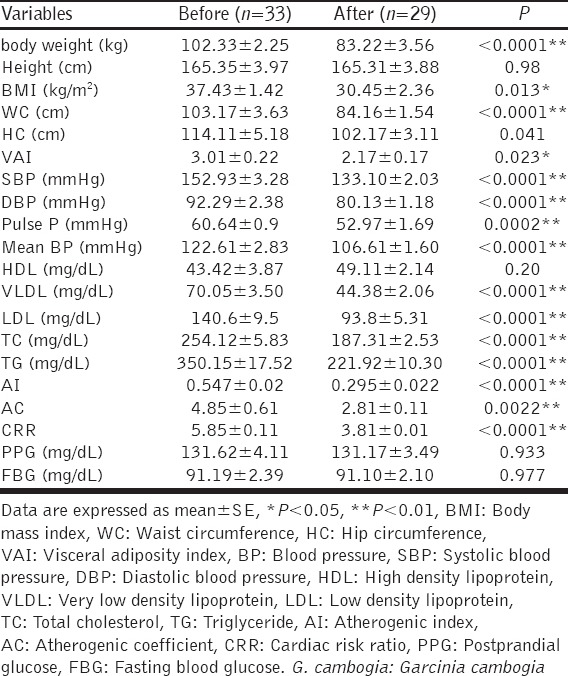
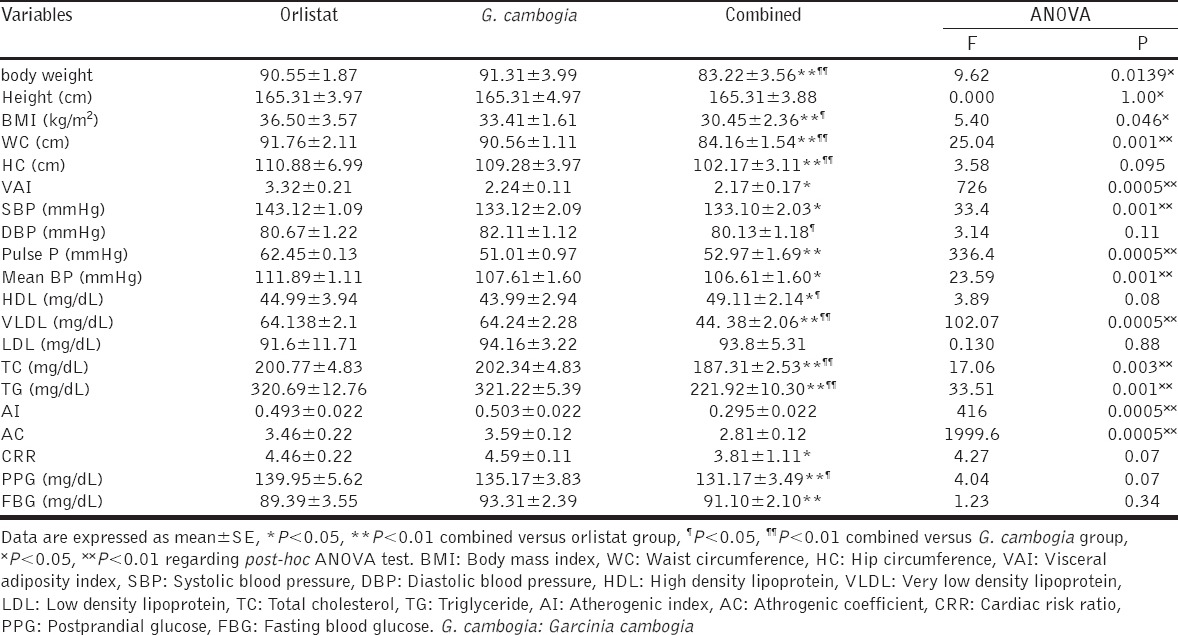
One-way ANOVA test analysis, demonstrated significant differences in most cardiometabolic indices among orlistat, G. cambogia and orlistat plus G. cambogia except on height, LDL, HDL, CRR, fasting and postprandial blood glucose when P > 0.05, [Table 5].
Table 5.
Variations of post-treatment effects among orlistat, G. cambogia and orlistat plus G. cambogia (intergroup comparisons)
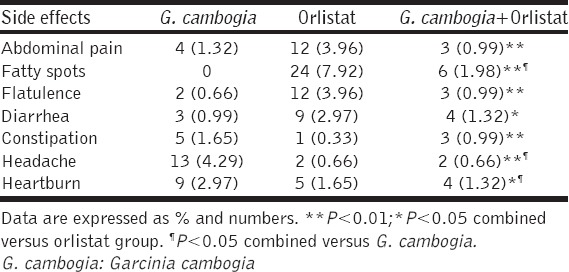
The compliance to the treatment was good, but some drug adverse effects were reported during the duration of the study among treated groups; fatty spot was the most reported side effect 7.92% in orlistat group which was decreased to the 0.99% in combined G. cambogia plus orlistat group, [Table 6].
Table 6.
Side effects of treatment during the study
DISCUSSION
This study revealed a new modality in the treatment of obesity through combination of drug and herbal medicine. Orlistat therapy in the current study produced a significant reduction in BMI, TC, WC without significant effects on VAI compared to baseline values. These findings corresponded with other studies which demonstrated that orlistat therapy produced a significant body weight reductions in obese patients with additional amelioration on lipid profile, anthropometric measures, inflammatory markers (tumor necrosis factor a [TNF-a] and interleukin 6 [IL-6]), and cardiometabolic risk factors [17,18].
Orlistat leads to dose-dependent inhibition of intestinal fat absorption through suppression of intestinal lipase activity [19] which per se explains the improvement in TC levels, VLDL, LDL AI, AC, and CRR.
TG serum levels were not decreased during orlistat therapy in the present study since; TGs hydrolyzed before inhibition of intestinal lipase by orlistat [20] or due to small sample size that may affects the statistical results, patients non-compliance or small dose that may not affect high TG levels while; a reduction in serum cholesterol levels in orlistat-treated group may be through inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase, decreasing in cholesterol intestinal absorption through decreasing of bilirubin blood concentration (via augmentations of bilirubin turnover and fecal excretion). Orlistat also decreases postprandial free fatty acid levels leading to the reduction in TG and cholesterol biosynthesis with a significant increment in insulin sensitivity since; free fatty acids interfere with insulin action [21]. Unfortunately, insulin resistance was not measured in the present study.
Furthermore, orlistat in the present study led to significant reduction in systolic and diastolic blood pressure, since treatment with orlistat in obese hypertensive patients, leads to the improvement in endothelial functions and significant vasodilatation that causing significant reductions in blood pressur [22].
As well, the presence of vascular endothelial dysfunction in obesity favor the development of cardiovascular disorders due to the ability of adipocytes for synthesis and secretion of adipocytokines - such as TNF-a, IL-6, and resistin - which play a potential role in induction of low-grade inflammatory changes at vascular endothelium causing obesity-induced vascular dysfunction [23]. Therefore, administration of orlistat leads to direct effect via reduction in adipocytes-induced inflammatory markers and indirect effect through augmentation of postprandial glucagon like peptide-1 that inhibits adipose tissue inflammatory mediators [24]. Moreover, orlistat produced significant homeostatic effects through reduction in plasma fibrinogen and P-selectin levels in obese patient [25]. All of these findings indicating a vascular protective effect of orlistat, which per se explaining the blood pressure lowering effect in the current study. Unluckily, these biomarkers and endothelial function were not measured in the present study.
Therefore, in spite of weight reduction effects of orlistat on body weight it failed in the reduction of VAI, this finding is incompatible with findings of Smith et al. study that revealed a significant reduction in visceral adiposity following orlistat therapy [26].
Regarding the effect of G. cambogia on obesity indices, it reduced body weight, BMI, WC and VAI; these findings correspond with a study that showed a significant reduction in anthropometric indices with G. cambogia treatment [27] but other studies reported non-significant differences between G. cambogia and placebo during 12th weeks of treatment on anthropometric indices [28].
Furthermore, this study revealed a significant reduction in TC, total TG, AI, AC, CRR, and blood pressure lowering effect significantly due to the treatment with G. cambogia that led to a reduction in the visceral and subcutaneous fat accumulations regardless of gender effect [29]. This potential effect of G. cambogia was due to the effects of the active ingredient, HCA that inhibits lipogenesis and reduction in LDL levels that causing amelioration in vascular and visceral functions [30]. These observations may explain the significant reduction in VAI in the present study.
Several animal and human studies reported that treatment of obesity with G. cambogia led to significant body weight loss compared with placebo, but these studies were conducted on small groups and for short duration <12th weeks [31] while in the present study the duration of therapy reached the duration of 3 months with minimal adverse effects.
In addition to the peripheral effect, G. cambogia has a central appetite suppressant effect through upregulation of serotonin neurotransmission [32]. Therefore, Semwal et al. study pointed out that G. cambogia acts in the dual pathway through central and peripheral suppressant effects causing a more significant reduction in visceral adiposity compared to orlistat alone [33].
Furthermore, effects of combined treatment with G. cambogia plus orlistat on obesity indices demonstrated a significant reduction in most of the obesity and cardiometabolic risk scores mainly on hip circumference and VAI.
VAI is a mathematical model that depends on BMI, WC, HDL and TG serum levels, VAI is gender dependent that reflect fat distribution, it linked with cardiometabolic risk scores and visceral adiposity-induced complications such as hypertension and diabetes mellitus [34]. Thus, combined therapy of G. cambogia plus orlistat led to a more significant reduction in VAI which pointed out to the potential additive effect of G. cambogia plus orlistat on weight reduction in obesity and obesity-induced complications.
Phytochemical analysis of G. cambogia detected polyphenolic flavonoids that inhibit pancreatic lipase activity which may explain the additive effect of G. cambogia with orlistat on weight reduction in obese patients [35]. Moreover, HCA isomer (2s, 3r) block pancreatic amylase and intestinal a-glucosidase leading to a reduction in the carbohydrate metabolism and absorption with significant reduction in blood glucose in diabetic patients [36]; this effect was not observed in our study since; all of the enrolled patients were non-diabetic. Consequently, G. cambogia potentiates the effect of orlistat in obese patients.
Regarding the reported side effects in the present study, combined treatment significantly showed less adverse effects compared to either G. cambogia or orlistat since, both drugs are well tolerated and G. cambogia induces feeling of satiety that decreases food intake and gastrointestinal disturbances [37,38].
CONCLUSION
Combinations of G. cambogia and orlistat have a more favorable effect than orlistat alone on cardiometabolic profile and VAI in obese patients.
LIMITATIONS OF THE STUDY
This study had many limitations. First, the total daily calorie intake was not determined and collected. Second, lifestyle and exercise were not documented. Finally, 3 months duration of treatment may be short for estimation the long-term effects of G. cambogia and orlistat.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Dansethakul P, Chuedoung A, Worachartcheewan A, Panichanapan P, Pidetcha P. Coincidence of obesity associated with cardio-renal abnormalities in Thais obese population. Diabetes Metab Syndr. 2015;20 doi: 10.1016/j.dsx.2015.09.018. pii: S1871-4021(15)30016-3. [DOI] [PubMed] [Google Scholar]
- 2.Bador KM, Wee LD, Halim SA, Fadi MF, Santhiran P, Rosli NF, et al. Serum osteocalcin in subjects with metabolic syndrome and central obesity. Diabetes Metab Syndr. 2015;9 doi: 10.1016/j.dsx.2015.09.009. pii: S1871-4021(15)30032-1. [DOI] [PubMed] [Google Scholar]
- 3.Krentz AJ, Fujioka K, Hompesch M. Anti-obesity pharmacotherapy: The intercontinental regulatory divide. Drugs. 2015;75:931–3. doi: 10.1007/s40265-015-0404-z. [DOI] [PubMed] [Google Scholar]
- 4.Joo JK, Lee KS. Pharmacotherapy for obesity. J Menopausal Med. 2014;20:90–6. doi: 10.6118/jmm.2014.20.3.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filippatos TD, Derdemezis CS, Gazi IF, Nakou ES, Mikhailidis DP, Elisaf MS. Orlistat-associated adverse effects and drug interactions: A critical review. Drug Saf. 2008;31:53–65. doi: 10.2165/00002018-200831010-00005. [DOI] [PubMed] [Google Scholar]
- 6.Lacey LA, Wolf A, O’shea D, Erny S, Ruof J. Cost-effectiveness of orlistat for the treatment of overweight and obese patients in Ireland. Int J Obes (Lond) 2005;29:975–82. doi: 10.1038/sj.ijo.0802947. [DOI] [PubMed] [Google Scholar]
- 7.Halpern B, Halpern A. Safety assessment of FDA-approved (orlistat and lorcaserin) anti-obesity medications. Expert Opin Drug Saf. 2015;14:305–15. doi: 10.1517/14740338.2015.994502. [DOI] [PubMed] [Google Scholar]
- 8.Márquez F, Babio N, Bulló M, Salas-Salvadó J. Evaluation of the safety and efficacy of hydroxycitric acid or Garcinia cambogia extracts in humans. Crit Rev Food Sci Nutr. 2012;52:585–94. doi: 10.1080/10408398.2010.500551. [DOI] [PubMed] [Google Scholar]
- 9.Clouatre DL, Preuss HG. Hydroxycitric acid does not promote inflammation or liver toxicity. World J Gastroenterol. 2013;19:8160–2. doi: 10.3748/wjg.v19.i44.8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wanderley Rocha DR, Jorge AR, Braulio VB, Arbex AK, Marcadenti A. Visceral adiposity measurements, metabolic and inflammatory profile in obese patients with and without type 2 diabetes mellitus: A cross-sectional analysis. Curr Diabetes Rev. 2015 doi: 10.2174/1573399812666151015115924. [DOI] [PubMed] [Google Scholar]
- 11.Fatima SS, Rehman R, Chaudhry B. Body mass index or body fat!Which is a better obesity scale for Pakistani population? J Pak Med Assoc. 2014;64:1225–8. [PubMed] [Google Scholar]
- 12.So ES. Waist circumference and health-related quality of life by sex in the Korean elderly. J Aging Health. 2014;26:887–99. doi: 10.1177/0898264314531618. [DOI] [PubMed] [Google Scholar]
- 13.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, et al. European guidelines on cardiovascular disease prevention in clinical practice (Version 2012). The fifth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2012;33:1635–701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 14.Lisko I, Stenholm S, Raitanen J, Hurme M, Hervonen A, Jylhä M, et al. Association of body mass index and waist circumference with physical functioning: The vitality 90 study. J Gerontol A Biol Sci Med Sci. 2015;70:885–91. doi: 10.1093/gerona/glu202. [DOI] [PubMed] [Google Scholar]
- 15.Mazzuca E, Battaglia S, Marrone O, Marotta AM, Castrogiovanni A, Esquinas C, et al. Gender-specific anthropometric markers of adiposity, metabolic syndrome and visceral adiposity index (VAI) in patients with obstructive sleep apnea. J Sleep Res. 2014;23:13–21. doi: 10.1111/jsr.12088. [DOI] [PubMed] [Google Scholar]
- 16.Idris S, Sunitha S. Assessment of BMI, serum leptin levels and lipid profile in patients with skin tags. J Clin Diagn Res. 2014;8:CC01–3. doi: 10.7860/JCDR/2014/10350.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain SS, Ramanand SJ, Ramanand JB, Akat PB, Patwardhan MH, Joshi SR. Evaluation of efficacy and safety of orlistat in obese patients. Indian J Endocrinol Metab. 2011;15:99–104. doi: 10.4103/2230-8210.81938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anagnostis P, Selalmatzidou D, Sapranidis M, Panagiotou A, Polyzos SA, Slavakis A, et al. Comparative effects of sibutramine and orlistat on weight loss, glucose metabolism and leptin levels in non-diabetic obese patients: A prospective study. Indian J Endocrinol Metab. 2012;16:146–7. doi: 10.4103/2230-8210.91214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauptman J. Orlistat: Selective inhibition of caloric absorption can affect long-term body weight. Endocrine. 2000;13:201–6. doi: 10.1385/ENDO:13:2:201. [DOI] [PubMed] [Google Scholar]
- 20.Carrière F, Renou C, Ransac S, Lopez V, De Caro J, Ferrato F, et al. Inhibition of gastrointestinal lipolysis by orlistat during digestion of test meals in healthy volunteers. Am J Physiol Gastrointest Liver Physiol. 2001;281:G16–28. doi: 10.1152/ajpgi.2001.281.1.G16. [DOI] [PubMed] [Google Scholar]
- 21.Mahmoud RH, Elnour WA. Comparative evaluation of the efficacy of ginger and orlistat on obesity management, pancreatic lipase and liver peroxisomal catalase enzyme in male albino rats. Eur Rev Med Pharmacol Sci. 2013;17:75–83. [PubMed] [Google Scholar]
- 22.Kiortsis DN, Filippatos TD, Elisaf MS. The effects of orlistat on metabolic parameters and other cardiovascular risk factors. Diabetes Metab. 2005;31:15–22. doi: 10.1016/s1262-3636(07)70161-1. [DOI] [PubMed] [Google Scholar]
- 23.Bougoulia M, Triantos A, Koliakos G. Effect of weight loss with or without orlistat treatment on adipocytokines, inflammation, and oxidative markers in obese women. Hormones (Athens) 2006;5:259–69. doi: 10.14310/horm.2002.11190. [DOI] [PubMed] [Google Scholar]
- 24.Prieto D, Contreras C, Sánchez A. Endothelial dysfunction, obesity and insulin resistance. Curr Vasc Pharmacol. 2014;12:412–26. doi: 10.2174/1570161112666140423221008. [DOI] [PubMed] [Google Scholar]
- 25.Olszanecka-Glinianowicz M, Dabrowski P, Kocelak P, Janowska J, Smertka M, Jonderko K, et al. Long-term inhibition of intestinal lipase by orlistat improves release of gut hormones increasing satiety in obese women. Pharmacol Rep. 2013;65:666–71. doi: 10.1016/s1734-1140(13)71044-2. [DOI] [PubMed] [Google Scholar]
- 26.Smith SR, Stenlof KS, Greenway FL, McHutchison J, Schwartz SM, Dev VB, et al. Orlistat 60 mg reduces visceral adipose tissue: A 24-week randomized, placebo - Controlled, multicenter trial. Obesity (Silver Spring) 2011;19:1796–803. doi: 10.1038/oby.2011.143. [DOI] [PubMed] [Google Scholar]
- 27.Vasques CA, Rossetto S, Halmenschlager G, Linden R, Heckler E, Fernandez MS, et al. Evaluation of the pharmacotherapeutic efficacy of Garcinia cambogia plus Amorphophallus konjac for the treatment of obesity. Phytother Res. 2008;22:1135–40. doi: 10.1002/ptr.2323. [DOI] [PubMed] [Google Scholar]
- 28.Hayamizu K, Ishii Y, Kaneko I, Shen M, Okuhara Y, Shigematsu N, et al. Effects of Garcinia cambogia (hydroxycitric acid) on visceral fat accumulation: A double-blind, randomized, placebo-controlled trial. Curr Ther Res Clin Exp. 2003;64:551–67. doi: 10.1016/j.curtheres.2003.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayer MA, Finlayson G, Fischman D, de Paz C, Telleriarte MR, Ferrero AJ, et al. Evaluation of the satiating properties of a nutraceutical product containing Garcinia cambogia and Ascophyllum nodosum extracts in healthy volunteers. Food Funct. 2014;5:773–9. doi: 10.1039/c3fo60631g. [DOI] [PubMed] [Google Scholar]
- 30.Badmaev V, Majeed M, Conte AA. Garcinia cambogia for weight loss. JAMA. 1999;282:233–4. doi: 10.1001/jama.282.3.233. [DOI] [PubMed] [Google Scholar]
- 31.Leonhardt M, Hrupka B, Langhans W. Effect of hydroxycitrate on food intake and body weight regain after a period of restrictive feeding in male rats. Physiol Behav. 2001;74:191–6. doi: 10.1016/s0031-9384(01)00547-9. [DOI] [PubMed] [Google Scholar]
- 32.Kim JE, Jeon SM, Park KH, Lee WS, Jeong TS, McGregor RA, et al. Does Glycine max leaves or Garcinia cambogia promote weight-loss or lower plasma cholesterol in overweight individuals: A randomized control trial. Nutr J. 2011;10:94. doi: 10.1186/1475-2891-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semwal RB, Semwal DK, Vermaak I, Viljoen A. A comprehensive scientific overview of Garcinia cambogia. Fitoterapia. 2015;102:134–48. doi: 10.1016/j.fitote.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Ohia SE, Opere CA, LeDay AM, Bagchi M, Bagchi D, Stohs SJ. Safety and mechanism of appetite suppression by a novel hydroxycitric acid extract (HCA-SX) Mol Cell Biochem. 2002;238:89–103. doi: 10.1023/a:1019911205672. [DOI] [PubMed] [Google Scholar]
- 35.Amato MC, Pizzolanti G, Torregrossa V, Misiano G, Milano S, Giordano C. Visceral adiposity index (VAI) is predictive of an altered adipokine profile in patients with type 2 diabetes. PLoS One. 2014;9:e91969. doi: 10.1371/journal.pone.0091969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buchholz T, Melzig MF. Polyphenolic compounds as pancreatic lipase inhibitors. Planta Med. 2015;81:771–83. doi: 10.1055/s-0035-1546173. [DOI] [PubMed] [Google Scholar]
- 37.Yamada T, Hida H, Yamada Y. Chemistry, physiological properties, and microbial production of hydroxycitric acid. Appl Microbiol Biotechnol. 2007;75:977–82. doi: 10.1007/s00253-007-0962-4. [DOI] [PubMed] [Google Scholar]
- 38.Yonei Y, Takahashi Y, Hibino S, Watanabe M, Yoshioka T. Effects on the human body of a dietary supplement containing L-Carnitine and Garcinia cambogia extract: A study using double-blind tests. J Clin Biochem Nutr. 2008;42:89–103. doi: 10.3164/jcbn.2008014. [DOI] [PMC free article] [PubMed] [Google Scholar]


