Abstract
Objectives:
This study was aimed to examine the anti-epileptic activity of leaf extracts of Punica granatum in experimental models of epilepsy in Swiss albino mice.
Materials and Methods:
Petroleum ether leaf extract of P. granatum (PLPG), methanolic LPG (MLPG), and aqueous LPG (ALPG) extracts of P. granatum leaves was initially evaluated against 6-Hz-induced seizure model; the potent extract was further evaluated against maximal electroshock (MES) and pentylenetetrazole (PTZ)-induced convulsions. Further, the potent extract was evaluated for its influence on Gamma amino butyric acid (GABA) levels in brain, to explore the possible mechanism of action. In addition, the potent extract was subjected to actophotometer test to assess its possible locomotor activity deficit inducing action.
Results:
In 6-Hz seizure test, the MLPG has alleviated 6-Hz-induced seizures significantly and dose dependently at doses 50, 100, 200, and 400 mg/kg. In contrast, PLPG and ALPG did not show any protection, only high dose of ALPG (400 and 800 mg/kg, p.o.) showed very slight inhibition. Based on these observations, only MLPG was tested in MES and PTZ models. Interestingly, the MLPG (50, 100, 200 and 400 mg/kg) has offered significant and dose-dependent protection against MES (P < 0.01) and PTZ-induced (P < 0.01) seizures in mice. Further, MLPG showed a significant increase in brain GABA levels (P < 0.01) compared to control and showed insignificant change in locomotor activity in all tested doses (100, 200 and 400 mg/kg). Interestingly, higher dose of MLPG (400 mg/kg, p.o.) and Diazepam (5 mg/mg, p.o.) have completely abolished the convulsions in all the anticonvulsant tests.
Conclusion:
These findings suggest that MLPG possesses significant anticonvulsant property, and one of the possible mechanisms behind the anticonvulsant activity of MLPG may be through enhanced GABA levels in the brain.
KEY WORDS: 6-Hz seizures, anticonvulsant, diazepam, epilepsy, herbal medicine, maximal electroshock-induced seizures, pentylenetetrazole-induced seizures, Punica granatum leaves
INTRODUCTION
Epilepsy is a serious neurological disease characterized by the transient occurrence of abnormal, excessive and/or synchronous neuronal activity in the brain, associated with various neurobiological, cognitive and psychological signs and/or symptoms [1]. Conventionally various chemical classes of drugs such as benzodiazepines (Diazepam), barbiturates (Phenobarbitone), gamma amino butyric acid (GABA) analogs, succinimides (ethosuximide), hydantoins (Phenytoin), carbamazepine, etc., were extensively used in the management of epilepsy [2]. Recently, many newer class of drugs such as vigabatrin, levetiracetam, topiramate, lamotrigine, zonisamide, lacosamide, rufinamide, and stiripentol have been developed, and they are considered to be comparatively safe [3]. However, despite of copious efforts, all the currently available drugs have one or more inherent side/adverse effects such as dizziness, mental slowing, ataxia, impaired concentration, mental confusion, sleep disturbance, anorexia, somnolence, and aggression [3]. Hence, there is great scope for safe and potent drug for the management of epilepsy, in his context herbal drugs are considered to have better edge over synthetic drugs, therefore many researchers are focusing on herbal remedies, to discover better and safe medicine for epilepsy. In this context, in literature, many plant-based medicines such as Melanthera scanden [4], Myrtus communis [5], Abies webbiana Lindl. [6], Crocus sativus [7], and Dodonaea viscosa (Linn.) [8] have been scientifically proved to possess potent anticonvulsant property in various experimental models of epilepsy. Similarly, in traditional system of medicine various parts of Punica granatum L. (belongs to family Punicaceae) has been used to treat varieties of ailments, and various parts of the plant have been scientifically proved for diverse biological activities such as antioxidant [9], hepatoprotective [10], antidiarrheal [11], antiulcer [12], anti-inflammatory [13], antimalarial [14], atherosclerosis and thyroid dysfunction [15], antimutagenic [16], immunomodulatory [17], memory enhancing [18], wound healing [19], and anticancer [20] activities; thus, P. granatum is one of the most common and potent plant-based medicine in the management of various ailments. Considering the strong literature reports on antioxidant and neuroprotective actions of P. granatum, this study was undertaken to evaluate the anticonvulsant activity of leaf extracts of P. granatum in various experimental models of epilepsy.
MATERIALS AND METHODS
Drugs and Chemicals
Phenytoin was purchased from Sun Pharmaceutical Industries Ltd., Mumbai, India, diazepam was procured from Ranbaxy Laboratories, New Delhi, India, and pentylenetetrazole (PTZ) and GABA was purchased from Sigma-Aldrich, Bengaluru, India. All other solvents and chemicals used were of analytical grade purchased from Hi-Media Laboratories Pvt., Ltd., Bengaluru, India.
Collection of Plant Material
The leaves of P. granatum L. were collected from Doddathekahalli, Sidlaghatta (T), Chikkaballapura (D) (Karnataka, India) in the month of May-June 2015 and it was identified and authenticated by Dr. K. Madhava Chetty, Professor of Botany, Sri Venkateshwara University, Tirupati, Andhra Pradesh, India. A voucher specimen of the plant material is preserved in the department with Voucher specimen number 1213.
Plant Material Preparation
The shade dried leaves of P. granatum were powdered, sieved and subjected to extraction process as follows. The plant material was extracted with various organic solvents successively in the ascending order of their polarity (petroleum ether, methanol, and water). In brief, 100 g of the plant material was initially defatted with 1 L of petroleum ether in a round bottom flask at room temperature for 24 h; the marc obtained was completely dried and extracted with 1 L of methanol at 60°C for 48 h in Soxhlet apparatus, subsequently, the marc obtained was extracted with 1 L of double distilled water in a round bottom flask, at room temperature for 48 h. Extracts obtained were concentrated at room temperature under reduced pressure, using Rota-evaporator (Rotavap-Remi instruments).
Formulation of Extracts for Administration
Various concentrations of petroleum ether leaf extract of P. granatum (PLPG), methanolic LPG (MLPG), and aqueous LPG (ALPG) were prepared in 3% tween 80 in distilled water. In short, required quantity of the extract was weighed and suspended in tween 80 and distilled water was added slowly with continuous shaking and volume was made up with distilled water, the concentration of tween 80 in the final formulation would be 3%. All the preparations were freshly prepared and administered by oral route at 10 ml/kg dose volume.
Experimental Protocol
Experimental animals
Inbred Swiss albino mice (25-30 g) were used for the study. The animals were maintained in polypropylene cages at a temperature of 25°C ± 1°C and relative humidity of 45-55% in a clean environment under 12 h light/dark cycle. The animals had free access to standard rat/mice pellets (Pranav Agro Industry, Bengaluru, India) and purified water ad libitum.
All the experimental protocols were approved by Institutional Animal Ethics Committee (IAEC) (Protocol No. IAEC/KMC/10/2016) and were conducted according to the principles and guidelines of the Committee for the Purpose of Control and Supervision of Experimentation on Animals, India.
Acute Oral Toxicity Studies
Acute oral toxicity of leaf extracts of P. granatum was determined by as per the OECD Guideline No. 425 - up and down procedure. In brief, nulliparous and non-pregnant female mice weighing between 25 and 30 g were fasted for 3 h and they were administered with single dose of extract, and the animals were observed for the toxic signs with special emphasis to changes in skin and fur, eyes and mucous membranes, and also respiratory, circulatory, autonomic and central nervous systems, and somatomotor activity and behavior pattern. Attention should be directed to observations of tremors, convulsions, salivation, diarrhea, lethargy, sleep and coma and mortality initially for 48 h (short term toxicity), animals found alive were further observed for 14 days (long-term outcomes). Lethal dose 50% was calculated using AOT425 stat program (OECD Guidelines No. 425, 2001) [21].
6 Hz Seizure Test
This test was carried out as per the previously described procedure [22,23]. In brief, mice were pretreated with extracts (doses ranging from 50 to 800 mg/kg, p.o.) for 3 days, the dose selection was done based on acute toxicity study, 100 mg/kg dose was selected and based on the response observed at 100 mg/kg, further dose levels were selected to establish dose-response correlation. Hence, in the process of establishing the dose-response correlation minimum dose was started with 50 mg/mg and saturation was observed after 200 mg/kg.
On Day-3, 1 h after the last dose, tetracaine ophthalmic solution (0.5% tetracaine hydrochloride) was applied, 30 min later the corneal electrodes were wetted with saline and electrical stimulation was given for 3 s (200 µs-duration, 32-mA mono polar rectangular pulses at 6 Hz) to all animal, using a constant current device ECT Unit 5780 (Ugo Basile, Comerio, Italy). In general, after the electrical stimulation, a stunned posture will be seen along with rearing and repeated movements that lasted from 60 to 120 s in control animals. After the seizures, animals will be resumed to normal exploratory behavior. The end point in this model is defined as protection against the seizures, the animals which gain their normal exploratory behavior within 10 s of stimulation are considered as protected [24].

Maximal Electroshock (MES)-induced Convulsions
In bred, male Swiss albino mice (25-30 g weight) were randomized and assigned in six groups, each group consisting of eight animals (n = 8). The respective group animals were treated with vehicle (3% tween 80) or phenytoin (25 mg/kg) or MLPG (50, 100, 200 and 400 mg/kg) orally for 3 days. On the 3rd day, exactly 1 h after the assigned treatment, tetracaine ophthalmic solution (0.5% tetracaine hydrochloride) was applied to cornea. Immediately after the electrical stimulation, individually all the animals were observed in an open top plastic cage for 30 min. The parameters such as duration of hind limb flexion, hind limb extensor, stupor, and death/survival were recorded during the observation, and the percentage protection is calculated using survival rate using previously provided equation [25-27].
PTZ-induced Convulsions
In bred, male Swiss albino mice (25-30 g) were divided into six groups each group consisting of eight animals (n = 8). The respective group animals were treated with either vehicle or diazepam (3 mg/kg) or MLPG (50, 100, 200 and 400 mg/kg) orally for 3 days. On the 3rd day, 1 h after the assigned treatment PTZ (80 mg/kg) was intraperitoneally administered to all the experimental animals, and they were individually observed in a plastic cage initially for 30 min and the animals survived were observed later up to 24 h. During the individual observation, the parameters such as onset of clonus, onset of tonic convulsions, and death/survival were recorded and expressed as percentage protection using the equation given previously [28].
Effect of MLPG on GABA levels in the Brain
Male Swiss albino mice (25-30 g) were treated with MLPG (200 and 400 mg/kg, p.o.) and diazepam (3 mg/kg, p.o.) for 1 day (single dose), and 3 days (three doses) in two different experimental sets. The first experimental set animals were sacrificed on day 1, 1 h after the dosing, while the second set animals were sacrificed on day 3, 1 h after the assigned treatment. The brain was collected and freezed, and subsequently, the cerebellum and whole brain were separated using microtome blade. These tissues were weighed and transferred to 10 ml of ice-cold 0.1 M perchloric acid containing 15 µg/ml of valine (internal standard). Under ice-cold conditions the contents were homogenized and centrifuged at 5000 rpm for 10 min at 4°C, the supernatant obtained was subjected to GABA estimation as per Suher et al. [29,30]. In short, 100 µl of each supernatant of the samples or the standard GABA is added to a micro-tube containing 100 µl of 0.1 M potassium carbonate solution, to induced dansylation reaction. These solutions were vortexed thoroughly and centrifuged at 10000 rpm for 10 min. Later, 100 µl of supernatant from each sample was transferred into a Pyrex tube containing 100 µl of 0.1 M sodium hydrogen carbonate solution, to this mixture 400 µl of working dansyl chloride solution (1.25 mg/ml anhydrous acetone) was added. The tubes were vortexed for 30 s and incubated at 90°C in benchtop oven for 30 min, during the incubation period the tubes were not capped to allow most of the solvent to get evaporate. Subsequently, the tubes taken out of the oven, and allowed to cool to room temperature. The dansylated derivatives obtained were transferred to 1.5 ml microtubes and subjected to high-performance liquid chromatography (HPLC) analysis. For HPLC analysis, C8 reversed-phase HPLC columns (5 µm, 250 × 3.2 mm) was used, mobile phase consisting of deionized and helium degassed water: Acetonitrile (HPLC grade) mixture (65:35 v/v) containing 0.15% v/v phosphoric acid, and flow rate was kept at 0.5 ml/min. The fluorescence detector with excitation and emission wavelength of 333 nm and 532 nm were adjusted, respectively. Exactly, 25 µl of the dansylated GABA samples were transferred to HPLC micro-sample vials and injected into the column. The retention time of GABA and internal standard were determined, and peak ratios of the samples were calculated with reference to the internal standard. The quantification of GABA in the unknown biological sample is done using the calibration curve of standard GABA and expressed as ng/g of tissue [29]. The calibration curve for standard GABA is established by subjecting various concentrations (25-6400 ng/ml) of standard GABA stock solutions to dansylation process similar to unknown sample supernatants.
Actophotometer Test
The actophotometer test was performed to evaluate the effect of MLPG on locomotor activity. In short, 48 male Swiss albino mice (25-30 g) were assigned into six groups (n = 8), and they were treated similar to previous tests. The locomotor activity was measured as a number of photocell counts by placing the animals individually in an actophotometer for 10 min, before and exactly at 60 min after the respective treatment [31].
The change in the locomotor activity was derived using photocell counts recorded before and after the assigned treatments, and reduction in locomotor activity is calculated using the following equation.

Statistical Analysis
The data obtained was expressed as mean ± SEM. The locomotor activity results were statistically analyzed by paired t-test. Remaining all experimental findings were statistically analyzed by one-way analysis of variance followed by Dunnett’s multiple comparison test using GraphPad Prism software version 5.0 for Windows (GraphPad Software, San Diego California USA). The P < 0.05 was considered as statistically significant.
RESULTS
Extraction of Plant Material
The extractive values of PLPG, MLPG and ALPG extracts were found to be 0.69%, 12.52%, and 7.41% w/w (gram by gram), respectively.
Acute Oral Toxicity Study
Acute oral toxicity study for the plant extracts was performed as per OECD Guidelines No. 425 up and down method. The outcome of the study showed that PLPG, MLPG, and ALPG were safe upto 2000 mg/kg, p.o. Further, no signs of toxicity were observed during short-term (48 h) and long-term (14 days) observation period.
Anticonvulsant Activity
6 Hz seizure test
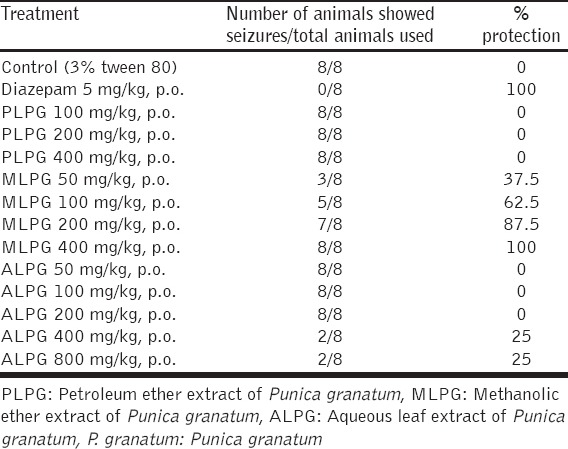
In 6 Hz seizure test, PLPG (100, 200 and 400 mg/kg), MLPG (50, 100, 200, 400 mg/kg), and ALPG (50, 100, 200, 400 and 800 mg/kg) were evaluated. In this model, the MLPG (50, 100, 200, 400 mg/kg) has alleviated 6-Hz-induced seizures significantly and dose dependently. In contrast, PLPG and ALPG did not show any protection, only high dose of ALPG (400 and 800 mg/kg, p.o.) showed very slight inhibition [Table 1].
Table 1.
Effect of Punica granatum leaf extracts on 6-Hz induced seizures in mice
MES-induced Convulsions
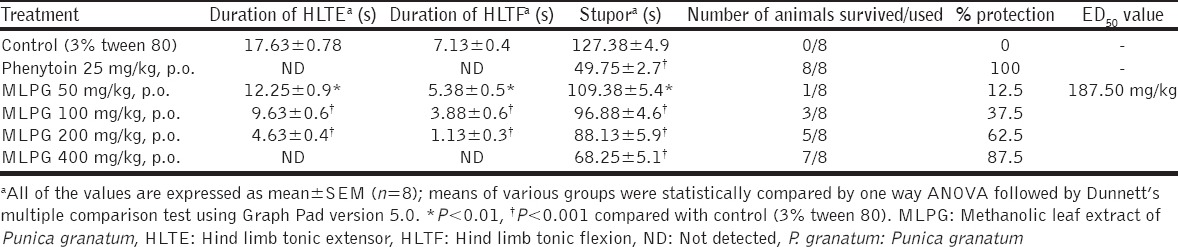
Based on the outcomes of 6-Hz seizure test, only MLPG was further evaluated in MES and PTZ models. Interestingly, the MLPG (50, 100, 200 and 400 mg/kg) has offered significant and dose-dependent protection against MES-induced HLTF (P < 0.01), HLTE (P < 0.01) and stupor (P < 0.01) compared to control. Further, the high dose of MLPG has completely abolished hind limb tonic extensor and hind limb tonic flexion. Noteworthy, the reference phenytoin (25 mg/kg) has completely abolished MES-induced convulsions and mortality, and the effective dose (ED50) value of MLPG was found to be 187.50 mg/kg [Table 2].
Table 2.
Effect of methanolic leaf extract of Punica granatum on MES-induced convulsions in mice
PTZ-induced Convulsions
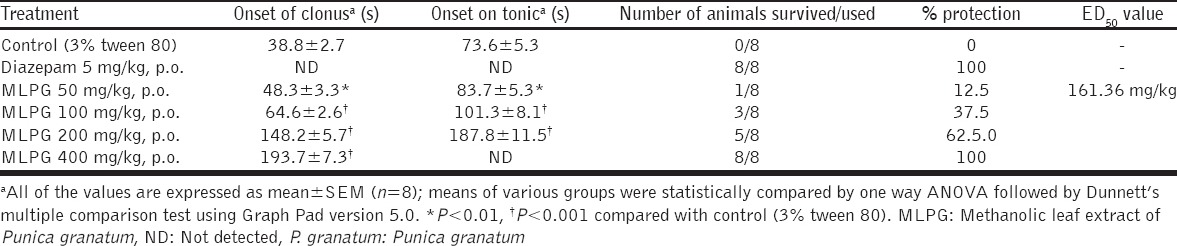
In continuation with MES model, MLPG (50, 100, 200 and 400 mg/kg) was evaluated against PTZ-induced seizures in mice. On administration of PTZ (80 mg/kg, i.p.) the control group animals have showed clonic and tonic convulsions and death. Remarkably, MLPG (50, 100, 200 and 400 mg/kg) has prolonged the PTZ-induced onset of clonus, onset of tonic, and reduced the mortality rate dose dependently compared to control. Exceptionally, a high dose of MLPG (400 mg/kg) has completely inhibited the PTZ-induced tonic convulsions and mortality, and the ED50 value of MLPG was found to be 161.36 mg/kg [Table 3].
Table 3.
Effect of methanolic leaf extract of Punica granatum on PTZ-induced convulsions in mice
Effect of MLPG on GABA Levels in the Brain
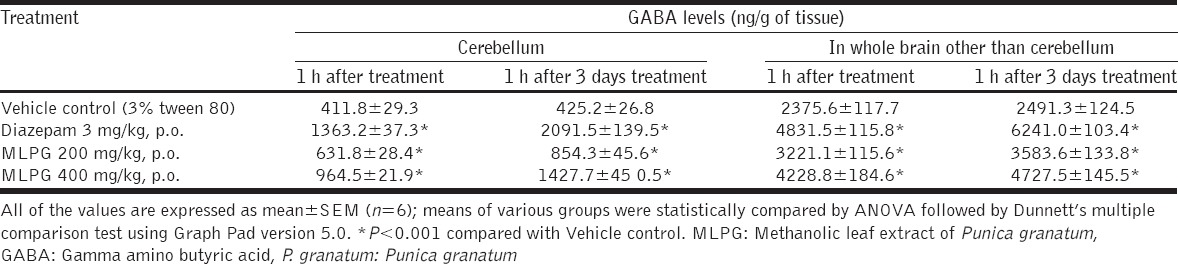
In this study, MLPG has evolved as a good anticonvulsant extract in all models (6 Hz Seizure Test, MES and PTZ models), further to explore the mechanism behind the anticonvulsant activity, various doses of MLPG (200 and 400 mg/kg) were evaluated for its influence on GABA levels in various parts of brain. The outcomes of the study showed significant elevation of GABA levels in cerebellum (P < 0.01) and whole brain other than cerebellum (P < 0.01), in both single dose (Day-1)and multiple doses (3 days) administration of MLPG compared to control. In similar lines, the reference standard diazepam (3 mg/kg) also showed significant elevation of GABA compared to control (P < 0.01) [Table 4].
Table 4.
Effect of methanolic leaf extract of Punica granatum on GABA levels in brain
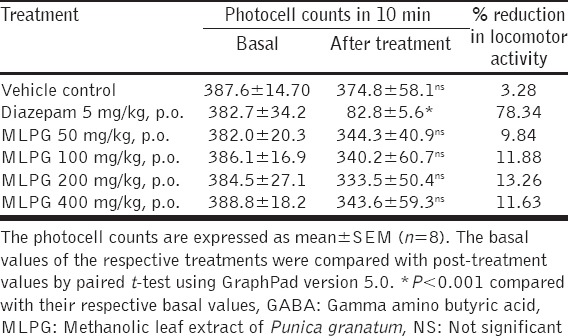
Actophotometer Test
Currently many potent anticonvulsant drugs are available in the market, however, most of these drugs have adverse effects related to musculoskeletal functions, with this hypothesis we thought to evaluate the MLPG for its influence on normal locomotor activity by actophotometer test, using diazepam (5 mg/kg) as a reference drug. Diazepam has significantly reduced the photocell counts and thereby decreased the locomotor activity (78.34%) significantly compared to before treatment. Furthermore, the MLPG has shown slight reduction in photocell counts compared to their basal values. However, no significant reduction in locomotor activity was observed in MLPG treatment compared to diazepam [Table 5].
Table 5.
Effect of methanolic leaf extract of Punica granatum on locomotor activity (Actophotometer test)
DISCUSSION
Epilepsy is one of the chronic and most common neurological disorders, affecting approximately 50 million people worldwide [32]. The basic and major mechanisms associated with epilepsy are increased synaptic connectivity of neurons (such as excitatory glutaminergic neurons), channelopathies (weekening of potassium channels and/or more persistent sodium channels, changes in voltage-gated ion channels), perturbance in synaptic receptors (suppressed GABAergic receptors, altered nicotinic receptors), decrease in inhibitory neurotransmission (decreased GABA levels), enhanced excitatory neurotransmission (enhanced glutamate levels) [33].
In this study, anticonvulsant activity of leaf extracts of P. granatum was evaluated against 6 Hz Seizure test, MES-induced convulsions and PTZ-induced convulsions in Swiss albino mice.
The 6 Hz seizure test is reported to involve a minimal, clonic phase, followed by stereotyped and automatistic behaviors which mimic the partial or limbic epilepsy in humans [34]. At present, the 6 Hz seizure test is preferred for early identification of anticonvulsant activity of new compounds which are effective against therapy-resistant epilepsy [35]. As per the recent antiepileptic discovery guidelines and recommendations, the test drugs which fail in conventional models (MES, PTZ, etc.,) are better to screen against 6 Hz seizure test, because 6 Hz seizure model is known as very sensitive and useful model, which is known to facilitate in identifying a very mild agent also against drug-resistant epilepsies [34,35]. Hence, this model is considered to be highly useful in identifying a new anticonvulsant drug, and also it is useful in avoiding false negatives. Therefore, all the extracts of P. granatum leaves were initially evaluated against 6 Hz seizure test. In this test, only MLPG (50, 100, 200, 400 mg/kg) has showed promising anticonvulsant effect. In contrast, PLPG and ALPG did not show any protection, only high dose of ALPG (400 and 800 mg/kg, p.o.) showed very slight inhibition.
Based on the results obtained in 6 Hz seizure test, the MLPG was further evaluated against MES and PTZ-induced convulsions in Swiss albino mice.
In principle, the electroshock delivered in MES model is well known to potentiate the sodium influx through opening of sodium channels, and also increases glutamate levels, glutamate is an excitatory neurotransmitter, which binds with NMDA receptors and induces the symptoms that exactly mimic the petit mal epilepsy in humans [36]. Based on the underlying mechanism of MES convulsions, it can be understood that the agents which could block the voltage-dependent sodium channels (phenytoin, sodium valproate,) and/or the agents that decrease the levels of excitatory amino acids and/or antagonize their actions are proved to be effective in MES-induced epilepsy model (e.g., felbamate) [31].
Furthermore, PTZ is a potent GABA receptor antagonist, it is well known to decrease the GABA levels, and density of GABA-A receptors in various parts of the brain [37], this leads to continuous stimulation of cortical neurons and results in convulsions similar to absence seizures in humans [38]. Hence, it thought that the agents which enhance GABA levels, and/or enhances GABA-A receptor density, and/or GABA-A receptor agonists (like diazepam), and/or the agents behave like GABA are thought to be useful in abolishing PTZ-induced convulsions [31].
In both MES and PTZ models, the MLPG (50, 100, 200, and 400 mg/kg) showed significant and dose-dependent protection. Exceptionally, high dose of MLPG has completely abolished MES-induced Hind limb Tonic extensor and Hind limb Tonic flexion, also high dose (400 mg/kg) of MLPG has completely inhibited the PTZ-induced tonic convulsions and mortality. Further, the effect of MLPG was evaluated for its possible locomotor deficits; however, the MLPG did not show significant influence on locomotor activity.
With the above finding, it can be hypothesized that the MLPG is eliciting potent anticonvulsant activity by increasing the inhibitory neurotransmitters (GABA) and/or, decreasing the excitatory neurotransmission and/or, by blocking the sodium channels and/or by neutralizing the PTZ binding site. To explore the possible mechanism of action, the effect of MLPG was evaluated on GABA levels in brain. Interestingly pretreatment with MLPG showed a significant increase in GABA levels in cerebellum and whole brain other than cerebellum, in both single dose (Day-1) and multiple dose (3 days) administration of MLPG compared to control. Thus, we can conclude that one of the possible mechanisms behind the anticonvulsant effect of MLPG may be through enhanced GABA levels in the brain. All the findings in this study are in mutual relation and supports that MLPG possesses potent anticonvulsant activity and it could be a useful agent in treating both petit mal and grand mal epilepsy. Interestingly, many plant derived constituents such as aconitine, berberine, piperine, baicalin, vitexin, rutin, apigenin, chrysin, eugenol, ellagic acid, gallic acid, quercetin, kaemferol, abietic acid, and α-terpineol are well proved to possess anticonvulsant activity in various experimental models [39]. In these lines, some of the these phytocompounds such as gallic acid, ellagic acid, rutin, corilagin, kaempferol, luteolin, myricetin, quercetin, and quercimetrine were previously isolated from P. granatum [40]; based on these observations, we can conclude that the MLPG is eliciting its anticonvulsant activity by synergestic interaction of some these compounds, through enhanced GABA levels in the brain.
CONCLUSION
These findings suggest that the MLPG possesses significant anticonvulsant property, further one of the possible mechanism/s behind the anticonvulsant activity of MLPG may be due to enhanced GABA levels in the brain. Indeed, there is a scope for further studies to explore molecular mechanism and identify the phytoconstituent responsible for the anticonvulsant activity. Hence, further studies are in the pipe line to explore the molecular mechanism of anticonvulsant action, and phytochemical studies are under progress to characterize and isolate the component responsible for the anticonvulsant activity of MLPG.
Acknowledgement
The authors greatly acknowledge Ms. Radiant Research Services Pvt Ltd, Bangalore for, Dr. Yoganand Moolemath, Novo Catalyz, Bangalore Bioinnocation Centre, Bangalore and Dr. Nandakumar, Manical college of Pharmaceutical sciences, Manipal for providing the facilities and valuable technical support to carry out the research work.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Trinka E, Cock H, Hesdorffer D, Rossetti A, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus - Report of the ILAE task force on classification of status epilepticus. Epilepsia. 2015;56:1515–23. doi: 10.1111/epi.13121. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg MM. Overview of drugs used for epilepsy and seizures: Etiology, diagnosis, and treatment. P T. 2010;35:392–415. [PMC free article] [PubMed] [Google Scholar]
- 3.Aneja S, Sharma S. Newer anti-epileptic drugs. Indian Pediatr. 2013;50:1033–40. doi: 10.1007/s13312-013-0284-9. [DOI] [PubMed] [Google Scholar]
- 4.Twinomujuni SS, Oloro J, Alele PE. Anticonvulsant and anxiolytic activity of the leaf aqueous and ethanolic extracts of Melanthera scandens in a rat model. Afr J Pharm Pharmacol. 2016;10:216–222. doi: 10.5897/AJPP2015.4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hajiaghaee R, Faizi M, Shahmohammadi Z, Abdollahnejad F, Naghdibadi H, Najafi F, et al. Hydroalcoholic extract of Myrtus communis can alter anxiety and sleep parameters: A behavioural and EEG sleep pattern study in mice and rats. Pharm Biol. 2016:1–8. doi: 10.3109/13880209.2016.1148175. [DOI] [PubMed] [Google Scholar]
- 6.Parkash O, Kumar D, Kumar S. Screening of methanol extract and ethyl acetate fraction of Abies webbiana Lindl. for neuropharmacological activities. Indian J Pharm Sci. 2015;77:536–41. doi: 10.4103/0250-474x.169039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khazdair MR, Boskabady MH, Hosseini M, Rezaee R, Tsatsakis M A. The effects of Crocus sativus (saffron) and its constituents on nervous system: A review. Avicenna J Phytomed. 2015;5:376–91. [PMC free article] [PubMed] [Google Scholar]
- 8.Karim N, Irshad S, Khan I, Mohammad A, Anis I, Shah MR, et al. GABA(A) receptor modulation and neuropharmacological activities of viscosine isolated from Dodonaea viscosa (Linn) Pharmacol Biochem Behav. 2015;136:64–72. doi: 10.1016/j.pbb.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Basiri S. Evaluation of antioxidant and antiradical properties of Pomegranate (Punica granatum L.) seed and defatted seed extracts. J Food Sci Technol. 2015;52:1117–23. doi: 10.1007/s13197-013-1102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celik I, Temur A, Isik I. Hepatoprotective role and antioxidant capacity of pomegranate (Punica granatum) flowers infusion against trichloroacetic acid-exposed in rats. Food Chem Toxicol. 2009;47:145–9. doi: 10.1016/j.fct.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Das AK, Mandal SC, Banerjee SK, Sinha S, Das J, Saha BP, et al. Studies on antidiarrhoeal activity of Punica granatum seed extract in rats. J Ethnopharmacol. 1999;68:205–8. doi: 10.1016/s0378-8741(99)00102-6. [DOI] [PubMed] [Google Scholar]
- 12.Ajaikumar KB, Asheef M, Babu BH, Padikkala J. The inhibition of gastric mucosal injury by Punica granatum L. (pomegranate) methanolic extract. J Ethnopharmacol. 2005;96:171–6. doi: 10.1016/j.jep.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Chia JL, Lih GC, Wen LL, Ching CW. Antiinflammatory effects of Punica granatum Linne in vitro and in vivo. Food Chem. 2010;118:315–22. [Google Scholar]
- 14.Dell’Agli M, Galli GV, Corbett Y, Taramelli D, Lucantoni L, Habluetzel A, et al. Antiplasmodial activity of Punica granatum L. fruit rind. J Ethnopharmacol. 2009;125:279–85. doi: 10.1016/j.jep.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 15.Parmar HS, Kar A. Protective role of Citrus sinensis, Musa paradisiaca, and Punica granatum peels against diet-induced atherosclerosis and thyroid dysfunctions in rats. Nutr Res. 2007;27:710–8. [Google Scholar]
- 16.Zahin M, Aqil F, Ahmad I. Broad spectrum antimutagenic activity of antioxidant active fraction of Punica granatum L. peel extracts. Mutat Res. 2010;703:99–107. doi: 10.1016/j.mrgentox.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Gracious Ross R, Selvasubramanian S, Jayasundar S. Immunomodulatory activity of Punica granatum in rabbits – A preliminary study. J Ethnopharmacol. 2001;78:85–7. doi: 10.1016/s0378-8741(01)00287-2. [DOI] [PubMed] [Google Scholar]
- 18.Adiga S, Trivedi P, Ravichandra V, Deb D, Mehta F. Effect of Punica granatum peel extract on learning and memory in rats. Asian Pac J Trop Med. 2010;3:687–90. [Google Scholar]
- 19.Hayouni EA, Miled K, Boubaker S, Bellasfar Z, Abedrabba M, Iwaski H, et al. Hydroalcoholic extract based-ointment from Punica granatum L. peels with enhanced in vivo healing potential on dermal wounds. Phytomedicine. 2011;18:976–84. doi: 10.1016/j.phymed.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Lansky EP, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol. 2007;109:177–206. doi: 10.1016/j.jep.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 21.OECD. Guideline on Acute Oral Toxicity, (AOT), Environmental Health and Safety Monograph Series on Testing and Adjustment No. 425. 2001. [Last accessed on 2016 Aug 23]. Available from: http://www.oecd.org/chemicalsafety/risk-assessment/1948378.pdf .
- 22.Brown WC, Schiffman DO, Swinyard EA, Goodman LS. Comparative assay of an antiepileptic drugs by psychomotor seizure test and minimal electroshock threshold test. J Pharmacol Exp Ther. 1953;107:273–83. [PubMed] [Google Scholar]
- 23.Kaminski RM, Livingood MR, Rogawski MA. Allopregnanolone analogs that positively modulate GABA receptors protect against partial seizures induced by 6-Hz electrical stimulation in mice. Epilepsia. 2004;45:864–7. doi: 10.1111/j.0013-9580.2004.04504.x. [DOI] [PubMed] [Google Scholar]
- 24.Florek-Luszczki M, Wlaz A, Kondrat-Wrobel MW, Tutka P, Luszczki JJ. Effects of WIN 55,212-2 (a non-selective cannabinoid CB1 and CB 2 receptor agonist) on the protective action of various classical antiepileptic drugs in the mouse 6 Hz psychomotor seizure model. J Neural Transm (Vienna) 2014;121:707–15. doi: 10.1007/s00702-014-1173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chroscinska-Krawczyk M, Jargiello-Baszak M, Andres-Mach M, Luszczki JJ, Czuczwar SJ. Influence of caffeine on the protective activity of gabapentin and topiramate in a mouse model of generalized tonic-clonic seizures. Pharmacol Rep. 2016;68:680–5. doi: 10.1016/j.pharep.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Joshi R, Reeta KH, Sharma SK, Tripathi M, Gupta YK. Pharmacodynamic and pharmacokinetic interaction of Panchagavya Ghrita with phenytoin and carbamazepine in maximal electroshock induced seizures in rats. Ayu. 2015 Jun;36:196–202. doi: 10.4103/0974-8520.175538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishra A, Punia JK, Bladen C, Zamponi GW, Goel RK. Anticonvulsant mechanisms of piperine, a piperidine alkaloid. Channels (Austin) 2015;9:317–23. doi: 10.1080/19336950.2015.1092836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Showraki A, Emamghoreishi M, Oftadegan S. Anticonvulsant effect of the aqueous extract and essential oil of Carum carvi L. Seeds in a Pentylenetetrazol model of seizure in mice. Iran J Med Sci. 2016;41:200–8. [PMC free article] [PubMed] [Google Scholar]
- 29.Aburawi SM, Elhuwuegi AS, Ahmed SS, Saad SF, Attia AS. Effects of acute and chronic triazolam treatments on brain GABA levels in albino rats. Acta Neurobiol Exp (Wars) 2000;60:447–55. doi: 10.55782/ane-2000-1364. [DOI] [PubMed] [Google Scholar]
- 30.Viswanatha GL, Mohan CG, Shylaja H, Yuvaraj HC, Sunil V. Anticonvulsant activity of 1,2,3,4,6-penta-O-galloyl-ß-D-glucopyranose isolated from leaves of Mangifera indica. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:599–604. doi: 10.1007/s00210-013-0858-z. [DOI] [PubMed] [Google Scholar]
- 31.Fisher RS, Van Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: Definitions proposed by the International League against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46:470–2. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 32.Jefferys JG. Advances in understanding basic mechanisms of epilepsy and seizures. Seizure. 2010;19:638–46. doi: 10.1016/j.seizure.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 33.Wojda E, Wlaz A, Patsalos PN, Luszczki JJ. Isobolographic characterization of interactions of levetiracetam with the various antiepileptic drugs in the mouse 6 Hz psychomotor seizure model. Epilepsy Res. 2009;86:163–74. doi: 10.1016/j.eplepsyres.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Löscher W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure. 2011;20:359–68. doi: 10.1016/j.seizure.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Jarogniew JŁ, Aleksandra W, Ewa M, Dominika P, Dariusz D, Magdalena F. Isobolographic characterization of interaction of levetiracetam with clobazam in the mouse 6 Hz psychomotor seizure model. J Pre Clin Clin Res. 2012;6:25–30. [Google Scholar]
- 36.Snead OC, 3rd, Banerjee PK, Burnham M, Hampson D. Modulation of absence seizures by the GABA(A) receptor: A critical rolefor metabotropic glutamate receptor 4 (mGluR4) J Neurosci. 2000;20:6218–24. doi: 10.1523/JNEUROSCI.20-16-06218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Psarropoulou C, Matsokis N, Angelatou F, Kostopoulos G. Pentylenetetrazol-induced seizures decrease gamma-aminobutyric acid-mediated recurrent inhibition and enhance adenosine-mediated depression. Epilepsia. 1994;35:12–9. doi: 10.1111/j.1528-1157.1994.tb02906.x. [DOI] [PubMed] [Google Scholar]
- 38.Huang RQ, Bell-Horner CL, Dibas MI, Covey DF, Drewe JA, Dillon GH. Pentylenetetrazole-induced inhibition of recombinant gamma-aminobutyric acid type A (GABA(A)) receptors: Mechanism and site of action. J Pharmacol Exp Ther. 2001;298:986–95. [PubMed] [Google Scholar]
- 39.Zhu HL, Wan JB, Wang YT, Li BC, Xiang C, He J, et al. Medicinal compounds with antiepileptic/anticonvulsant activities. Epilepsia. 2014;55:3–16. doi: 10.1111/epi.12463. [DOI] [PubMed] [Google Scholar]
- 40.Jain V, Viswanatha GL, Manohar D, Shivaprasad HN. Isolation of antidiabetic principle from fruit rinds of Punica granatum. Evid Based Complement Alternat Med. 2012;2012:147202. doi: 10.1155/2012/147202. [DOI] [PMC free article] [PubMed] [Google Scholar]


