Abstract
Background:
Brazilian propolis has many biological activities including the ability to help prevent thrombotic diseases, but this particular effect has not been proven. Plasma levels of plasminogen activator inhibitor-1 (PAI-1), an inhibitor of fibrinolysis, increase under inflammatory conditions such as infection, obesity and atherosclerosis and such elevated levels predispose individuals to a risk of developing thrombotic diseases.
Aim:
This study aimed to determine the effects of a diet containing Brazilian propolis on lipopolysaccharide (LPS)-induced increases in plasma PAI-1 levels.
Materials and Methods:
Mice were fed with a diet containing 0.5% (w/w) Brazilian propolis for 8 weeks. Thereafter, the mice were subcutaneously injected with saline containing 0.015 mg/kg of LPS and sacrificed 4 h later.
Results:
Orally administered Brazilian propolis significantly suppressed the LPS-induced increase in PAI-1 antigen and its activity in mouse plasma.
Conclusion:
This study indicated that Brazilian propolis contains natural products that can decrease thrombotic tendencies in mice.
KEY WORDS: Dietary supplement, mouse, plasminogen activator inhibitor-1, thrombosis
INTRODUCTION
Prothrombotic factors play a key role in the development of thrombotic diseases [1]. For instance, plasminogen activator inhibitor-1 (PAI-1) is the main physiological inhibitor of the fibrinolytic system, where it plays an important role in disease prevention by removing thrombi from the vascular system [2]. It is also the primary physiological inhibitor of tissue-type plasminogen activator, a key protease of the fibrinolytic system [2], through forming irreversible 1:1 complexes. An increase in plasma PAI-1 levels suppresses the normal fibrinolytic system and leads to prothrombotic states. Many factors such as blood lipids, glucose, and insulin influence habitual PAI-1 levels. Therefore, elevated PAI-1 levels are intimately related to prothrombotic states such as hypertension, obesity, insulin resistance, and diabetes as well as aging [3-6]. Increased levels of plasma PAI-1 comprise a risk factor for thrombotic diseases and serve as a marker of the onset of such diseases [2]. Maintaining physiological plasma levels and activities of PAI-1 might thus represent a promising intervention for treating and preventing thrombotic diseases [7].
Many compounds that affect coagulation and platelet function are derived from natural sources such as garlic, ginger, gingko, and mushrooms [8,9]. Furthermore, the reports indicate that some products derived from natural sources inhibit PAI-1 production in vitro and in experimental animals in vivo. For example, Liu et al. reported that green tea polyphenols inhibit PAI-1 expression and secretion in endothelial cells [10], and Zhou et al. reported that salvianolic acid B attenuates PAI-1 production in human umbilical vein endothelial cells (HUVEC) incubated with tumor necrosis factor a (TNF-a) [11]. A citrus extract containing flavones represses PAI-1 expression in human colon fibroblasts [12] and xanthoangelol isolated from Angelica keiskei inhibits PAI-1 increases in mice plasma induced by lipopolysaccharide (LPS) and in culture media of HUVEC induced by TNF-a [13].
Propolis is a hive product comprising resinous materials collected by honey bees from plants and it includes over 300 chemical compounds [14]. Propolis is a feature of folk medicines and health supplements worldwide, and various biological activities have been indicated [14,15]. The composition and biological activities of propolis greatly depend on the location of the honey bees and the plant source from which it is derived [16]. Brazilian propolis contains various biologically active organic compounds in abundance such as artepillin C [17]. The effect of Brazilian propolis on various pathological conditions such as tumors, inflammation, diabetes, and immunocompromised patients have mainly been investigated in vitro and in experimental animals [18-22], inflammatory conditions alter the coagulation and fibrinolytic system, frequently leading to a procoagulant state [23]. Proinflammatory cytokines and endotoxins play a central role in the effects on the coagulation and fibrinolysis pathways [24]. Brazilian propolis inhibits increases in PAI-1 antigen induced by TNF-a in culture media of HUVEC [25]. Anti-inflammatory properties of Brazilian propolis have been demonstrated in mouse models of inflammation and in cultured activated macrophages [20,26,27]. This study aimed to determine the effects of a diet containing Brazilian propolis on LPS-induced plasma PAI-1 increases in model mice.
MATERIALS AND METHODS
Materials
Brazilian propolis (Institute for Bee products and Health Science, Yamada Bee Company Inc., Okayama, Japan) is an ethanol extract comprising 55% (w/w) solids. Samples of propolis were collected from colonies of Scaptotrigona bees between February 2007 and December 2008 in Minas Gerais, Brazil. Insoluble matter was removed by passage through diatomaceous earth and filter paper. LPSs from Escherichia coli 0111:B4 were obtained from Sigma Chemical Co. (St. Louis, MO, USA). All other materials were commercial products of the highest grade available.
HPLC Analysis of Extract
The ethanol extract of Brazilian was analyzed by HPLC [28] using a Cosmosil 5C18-ARII column (Nacalai Tesque, Kyoto, Japan) and a gradient CH3CN in 0.1% trifluoroacetic acid at a flow rate of 1.0 mL/min. Compounds were detected at 260 nm.
Animal Experiments
About 7-week-old male kwl ICR mice (Tokyo Laboratory Animals Science Co. Ltd., Tokyo, Japan) were housed at 24 ± 2°C and provided with water and the MF diet (Oriental yeast Co. Ltd., Tokyo, Japan) ad libitum. The mice were subcutaneously injected with saline containing 0.015 mg/kg of LPS, sacrificed at indicated times, and then, blood was collected into plastic tubes containing 3.2% sodium citrate. The effect of propolis was evaluated by providing the mice with water and the MF diet (Oriental yeast Co. Ltd., Tokyo, Japan) with or without 0.5% (w/w) of propolis ethanol extract for 2, 4 and 8 weeks ad libitum. The mice were then subcutaneously injected with saline containing 0.015 mg/kg of LPS. Blood specimens collected from the inferior vena cava using a plastic syringe and needle under pentobarbital (40 mg/kg i.p.) anesthesia 4 h later was mixed with 0.2 volumes of 3.2% sodium citrate. Platelet-poor plasma prepared by centrifugation at 7000 rpm (3800 ×g) for 10 min at 4°C in an MX-100 micro-centrifuge (TOMY, Tokyo, Japan) was stored at −80°C. All animal experiments proceeded in accordance with the Guide for the Care and Use of Laboratory Animal at Teikyo University and were approved by the Animal Care and Use Committee at Teikyo University (Permission No. 12-013).
Measurement of Plasma PAI-1 Levels and Activity
Levels of total PAI-1 and PAI-1 activity in mouse plasma and corresponding active PAI-1 antigen levels were measured using relevant ELISA kits (Molecular Innovations Inc., Southfield, MI, USA) according to the manufacturer’s instructions.
Statistics
Data are expressed as means ± standard deviation. Statistical significance was determined using Mann–Whitney U tests. P < 0.05 was considered to represent significance.
RESULTS
The effects of subcutaneous injection of LPS on plasma PAI-1 levels in mice were observed. The mice were subcutaneously injected with 0.015 mg/kg LPS, blood was collected at the indicated times, and then total PAI-I antigen in plasma was determined. Figure 1 shows the time course of PAI-1 antigen levels in plasma after LPS injection. The LPS caused a significant increase in plasma PAI-1 levels that peaked 4 h after injection. The LPS-induced PAI-1 increase in plasma was statistically significant at 3 and 4 h compared with control mice (3 h control [means = 1.06, (95% confidence interval [CI], 0.43-1.69), n = 4] vs. 3 h LPS [means = 12.43, (95% CI, 7.87-17.00), n = 3], P = 0.034; 4 h control [means = 1.34, (95% CI, −0.26-2.94), n = 4] vs. 4 h LPS [means = 12.98, (95% CI, 8.12-17.84), n = 3], P = 0.034).
Figure 1.
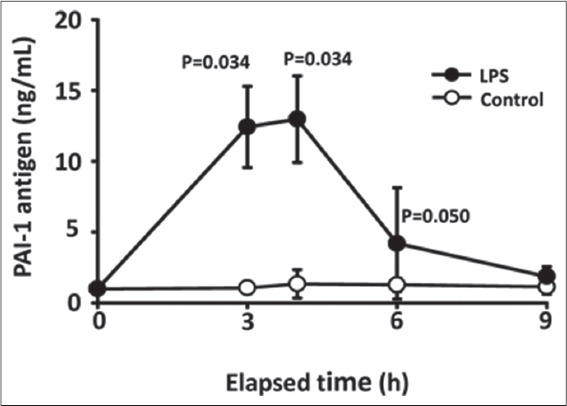
Time course of plasminogen activator inhibitor-1 (PAI-1) antigen levels in plasma after lipopolysaccharide (LPS) injection. Mice were subcutaneously injected with 0.015 mg/kg LPS, blood was collected at indicated times and then total PAI-1 antigen in plasma was determined using ELISA kits. Data are expressed as means ± standard deviation (LPS, n = 3; control n = 4)
The inhibitory effects of dietary propolis on LPS-induced PAI-1 production were assessed in mice fed with a diet containing 0.5% (w/w) Brazilian propolis for 8 weeks. Thereafter, the mice were subcutaneously injected with saline containing 0.015 mg/kg of LPS and sacrificed 4 h later because PAI-1 levels peaked at this point [Figure 1]. Stimulation with LPS (LPS [+]) significantly increased levels of PAI-1 antigen in plasma compared with control LPS (−) mice (LPS [−] control [means = 1.49 (95% CI, 0.78-2, 21), n = 8] vs. LPS (+) control [means = 14.87 (95% CI, 12.36-17.38, n = 11], P < 0.001). Orally administered propolis significantly suppressed the LPS-induced increase of PAI-1 antigen in mouse plasma (LPS (+) control vs. LPS (+) propolis, [means = 7.74 (95% CI, 4.66-10.81, n = 12], P = 0.002) [Figure 2].
Figure 2.
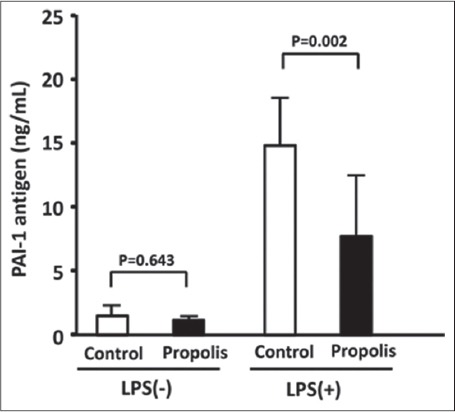
Effects of dietary propolis on lipopolysaccharide (LPS)-induced increase in plasminogen activator inhibitor-1 (PAI-1) antigen in mouse plasma. Mice were fed with a diet containing 0.5% (w/w) Brazilian propolis for 8 weeks. Thereafter, the mice were subcutaneously injected with saline containing 0.015 mg/kg of LPS and sacrificed 4 h later. Total PAI-1 antigen in plasma was determined using ELISA kits. Data are expressed as means ± standard deviation (n = 8~12)
The plasma levels of PAI-1 activity were then measured using an ELISA that detects active PAI-1 antigen. Since the active form spontaneously converts to the latent form, understanding levels of the active form are important for evaluating plasma PAI-1 activity. Figure 3 shows that LPS also increased the level of active PAI-1 (PAI-1 activity) in plasma. Dietary propolis for 8 weeks significantly decreased the LPS-induced increase in PAI-1 activity (LPS [+] control [means = 13.07 (95% CI, 5.40-20.75), n = 6] vs. LPS (+) propolis, [means = 4.47 (95% CI, −0.09-6.40, n = 6], P = 0.014) [Figure 3]. The oral administration of propolis for 8 weeks did not affect plasma PAI-1 antigen levels in non-stimulated mice (LPS [−] control vs. LPS [−] propolis, [means = 1.16 (95% CI, 0.93-1.39), n = 9], P = 0.643) [Figure 2]. These results show that oral propolis administered for 8 weeks does not affect spontaneous plasma PAI-1 levels.
Figure 3.
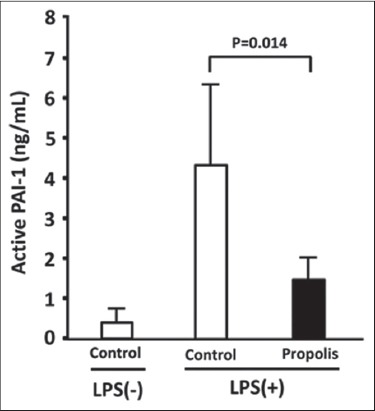
Effect of dietary propolis on lipopolysaccharide (LPS)-induced increase in plasminogen activator inhibitor-1 (PAI-1) activity in mouse plasma. Mice were fed with a diet containing 0.5% (w/w) Brazilian propolis for 8 weeks. Thereafter, the mice were subcutaneously injected with saline containing 0.015 mg/kg of LPS and sacrificed 4 h later. PAI-1 activity in plasma was determined using active PAI-1 ELISA kits. Data are expressed as means ± standard deviation (n = 5~6)
DISCUSSION
PAI-1 inhibits the normal fibrinolytic system and the increase in plasma are involved in the onset of thrombotic diseases. PAI-1 is a powerful acute-phase reactant, reflected by rapid, large increases in plasma levels during the acute-phase response [29]. Thus, the suppression of increased PAI-1 expression during the acute phase would improve prothrombotic conditions. This study aimed to determine the effects of diets containing Brazilian propolis on LPS-induced plasma PAI-1 increases in model mice. The amount of LPS used was considerably less than that applied in the LPS-induced disseminated intravascular coagulation model [30]. These findings confirmed that the present mouse model has a slight thrombotic tendency without clot formation, despite having increased plasma PAI-1 levels.
This study showed that orally administered Brazilian propolis significantly suppressed LPS-induced increases of PAI-1 antigen and activity in mice. PAI-1 production was significantly suppressed in mice that consumed Brazilian propolis for 8 weeks. This time frame is appropriate to explore anti-thrombotic and anti-inflammatory effects in mice [13,31,32].
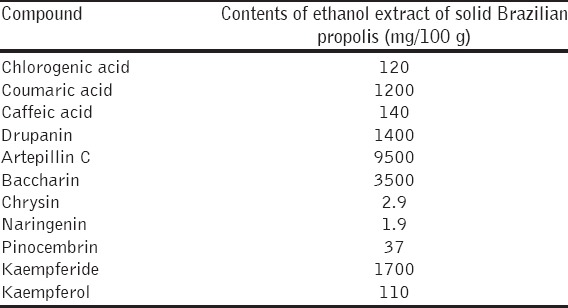
Brazilian propolis contains many natural components [33,34] and Table 1 lists some of the components of the Brazilian propolis used in the present study. Among many natural compounds such as cinnamic acid derivatives (drupanin, artepillin C, and baccarin), benzoic acid derivatives (caffeic acid and coumaric acid), chlorogenic acid, and flavonoids (chrysin, naringenin, pinocembrin, kaempferide, and kaempferol) [Table 1]; cinnamic acid derivatives are likely to have anti-thrombotic activities. The commercially-produced cinnamic acid derivative, artepillin C, the major cinnamic acid derivative in Brazilian propolis, tended to inhibit PAI-1 production in a previous study [25]. Konishi et al. have described the absorption and bioavailability of artepillin C in rats after oral administration [35]. The other cinnamic acid derivatives, drupanin and baccharin, are abundant in Brazilian propolis [Table 1] and have various biological activities [18,36-39]. Thus, one or more cinnamic acid derivatives in Brazilian propolis might contribute to the inhibition of PAI-1 production. Chrysin inhibits TNF-a -induced increases in PAI-1 antigen in culture media of human HUVEC, and LPS induces increases in PAI-1 antigen in mouse plasma [25]. Chrysin is abundant in European propolis [40], but the Brazilian propolis used in the present study contains only trace amounts (2.9 mg/100 g propolis extract). Thus, the contribution of chrysin in Brazilian propolis to inhibiting PAI-1 production might be minimal. Detailed information is needed about specific molecules in Brazilian propolis that suppress increases in PAI-1.
Table 1.
Contents of cinnamic acid derivatives, benzoic acid derivatives, chlorogenic acid and flavonoids in ethanol extract of Brazilian propolis
CONCLUSION
The present findings indicated that Brazilian propolis contains some natural products that can decrease thrombotic tendencies in mice by suppressing increased plasma PAI-1 levels.
ACKNOWLEDGMENTS
We thank Yoshiko Kobayashi and Asami Haga for providing helpful comments and valuable information.
Footnotes
Source of Support: AH and TT are employed by Yamada Apiculture Center, Inc. The other authors have no conflicts of interest to declare. A part of this study was supported by Yamada Research Grants during 2009 and 2010 (to N. Ohkura),
Conflict of Interest: This study was supported in part by a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) 25350144 (to N. Ohkura).
REFERENCES
- 1.Samad F, Ruf W. Inflammation, obesity, and thrombosis. Blood. 2013;122:3415–22. doi: 10.1182/blood-2013-05-427708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van De Craen B, Declerck PJ, Gils A. The biochemistry, physiology and pathological roles of PAI-1 and the requirements for PAI-1 inhibition in vivo. Thromb Res. 2012;130:576–85. doi: 10.1016/j.thromres.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 3.Kohler HP, Grant PJ. Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med. 2000;342:1792–801. doi: 10.1056/NEJM200006153422406. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto K, Saito H. A pathological role of increased expression of plasminogen activator inhibitor-1 in human or animal disorders. Int J Hematol. 1998;68:371–85. doi: 10.1016/s0925-5710(98)00094-2. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto K, Takeshita K, Saito H. Plasminogen activator inhibitor-1 in aging. Semin Thromb Hemost. 2014;40:652–9. doi: 10.1055/s-0034-1384635. [DOI] [PubMed] [Google Scholar]
- 6.Kruithof EK. Regulation of plasminogen activator inhibitor type 1 gene expression by inflammatory mediators and statins. Thromb Haemost. 2008;100:969–75. [PubMed] [Google Scholar]
- 7.Brown NJ. Therapeutic potential of plasminogen activator inhibitor-1 inhibitors. Ther Adv Cardiovasc Dis. 2010;4:315–24. doi: 10.1177/1753944710379126. [DOI] [PubMed] [Google Scholar]
- 8.Mousa SA. Antithrombotic effects of naturally derived products on coagulation and platelet function. Methods Mol Biol. 2010;663:229–40. doi: 10.1007/978-1-60761-803-4_9. [DOI] [PubMed] [Google Scholar]
- 9.Vilahur G, Badimon L. Antiplatelet properties of natural products. Vascul Pharmacol. 2013;59:67–75. doi: 10.1016/j.vph.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Ying C, Meng Y, Yi W, Fan Z, Zuo X, et al. Green tea polyphenols inhibit plasminogen activator inhibitor-1 expression and secretion in endothelial cells. Blood Coagul Fibrinolysis. 2009;20:552–7. doi: 10.1097/MBC.0b013e32832e05f0. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Z, Liu Y, Miao AD, Wang SQ. Salvianolic acid B attenuates plasminogen activator inhibitor type 1 production in TNF-alpha treated human umbilical vein endothelial cells. J Cell Biochem. 2005;96:109–16. doi: 10.1002/jcb.20567. [DOI] [PubMed] [Google Scholar]
- 12.Giménez-Bastida JA, Martínez-Florensa M, Espín JC, Tomás-Barberán FA, García-Conesa MT. A citrus extract containing flavanones represses plasminogen activator inhibitor-1 (PAI-1) expression and regulates multiple inflammatory, tissue repair, and fibrosis genes in human colon fibroblasts. J Agric Food Chem. 2009;57:9305–15. doi: 10.1021/jf901983g. [DOI] [PubMed] [Google Scholar]
- 13.Ohkura N, Nakakuki Y, Taniguchi M, Kanai S, Nakayama A, Ohnishi K, et al. Xanthoangelols isolated from angelica keiskei inhibit inflammatory-induced plasminogen activator inhibitor 1 (PAI-1) production. Biofactors. 2011;37:455–61. doi: 10.1002/biof.187. [DOI] [PubMed] [Google Scholar]
- 14.Khalil ML. Biological activity of bee propolis in health and disease. Asian Pac J Cancer Prev. 2006;7:22–31. [PubMed] [Google Scholar]
- 15.Banskota AH, Tezuka Y, Kadota S. Recent progress in pharmacological research of propolis. Phytother Res. 2001;15:561–71. doi: 10.1002/ptr.1029. [DOI] [PubMed] [Google Scholar]
- 16.Bankova V. Chemical diversity of propolis and the problem of standardization. J Ethnopharmacol. 2005;100:114–7. doi: 10.1016/j.jep.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Salatino A, Teixeira EW, Negri G, Message D. Origin and chemical variation of Brazilian propolis. Evid Based Complement Alternat Med. 2005;2:33–38. doi: 10.1093/ecam/neh060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akao Y, Maruyama H, Matsumoto K, Ohguchi K, Nishizawa K, Sakamoto T, et al. Cell growth inhibitory effect of cinnamic acid derivatives from propolis on human tumor cell lines. Biol Pharm Bull. 2003;26:1057–9. doi: 10.1248/bpb.26.1057. [DOI] [PubMed] [Google Scholar]
- 19.Dib LH, Ortega MT, Fleming SD, Chapes SK, Melgarejo T. Bone marrow leptin signaling mediates obesity-associated adipose tissue inflammation in male mice. Endocrinology. 2014;155:40–6. doi: 10.1210/en.2013-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machado JL, Assunção AK, da Silva MC, Dos Reis AS, Costa GC, Arruda Dde S, et al. Brazilian green propolis: Anti-inflammatory property by an immunomodulatory activity. Evid Based Complement Alternat Med. 2012;2012:157652. doi: 10.1155/2012/157652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukuda T, Fukui M, Tanaka M, Senmaru T, Iwase H, Yamazaki M, et al. Effect of Brazilian green propolis in patients with type 2 diabetes: A double-blind randomized placebo-controlled study. Biomed Rep. 2015;3:355–60. doi: 10.3892/br.2015.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao W, Wu J, Wei J, Pu L, Guo C, Yang J, et al. Brazilian green propolis improves immune function in aged mice. J Clin Biochem Nutr. 2014;55:7–10. doi: 10.3164/jcbn.13-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esmon CT. Inflammation and thrombosis. J Thromb Haemost. 2003;1:1343–8. doi: 10.1046/j.1538-7836.2003.00261.x. [DOI] [PubMed] [Google Scholar]
- 24.Levi M, van der Poll T, Schultz M. Infection and inflammation as risk factors for thrombosis and atherosclerosis. Semin Thromb Hemost. 2012;38:506–14. doi: 10.1055/s-0032-1305782. [DOI] [PubMed] [Google Scholar]
- 25.Ohkura N, Takata Y, Ando K, Kanai S, Watanabe E, Nohira T, et al. Propolis and its constituent chrysin inhibit plasminogen activator inhibitor 1 production induced by tumour necrosis factor-a and lipopolysaccharide. J Apicult Res. 2012;51:179–84. [Google Scholar]
- 26.Paulino N, Teixeira C, Martins R, Scremin A, Dirsch VM, Vollmar AM, et al. Evaluation of the analgesic and anti-inflammatory effects of a Brazilian green propolis. Planta Med. 2006;72:899–906. doi: 10.1055/s-2006-947185. [DOI] [PubMed] [Google Scholar]
- 27.Szliszka E, Kucharska AZ, Sokół-Łętowska A, Mertas A, Czuba ZP, Król W. Chemical composition and anti-inflammatory effect of ethanolic extract of Brazilian green propolis on activated J774A.1 macrophages. Evid Based Complement Alternat Med. 2013;2013:976415. doi: 10.1155/2013/976415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tani H, Hasumi K, Tatefuji T, Hashimoto K, Koshino H, Takahashi S. Inhibitory activity of Brazilian green propolis components and their derivatives on the release of cys-leukotrienes. Bioorgan Med Chem. 2010;18:151–7. doi: 10.1016/j.bmc.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Kluft C, Verheijen JH, Jie AF, Rijken DC, Preston FE, Sue-Ling HM, et al. The postoperative fibrinolytic shutdown: A rapidly reverting acute phase pattern for the fast-acting inhibitor of tissue-type plasminogen activator after trauma. Scand J Clin Lab Invest. 1985;45:605–10. doi: 10.3109/00365518509155267. [DOI] [PubMed] [Google Scholar]
- 30.Asakura H, Aoshima K, Suga Y, Yamazaki M, Morishita E, Saito M, et al. Beneficial effect of the active form of vitamin D3 against LPS-induced DIC but not against tissue-factor-induced DIC in rat models. Thromb Haemost. 2001;85:287–90. [PubMed] [Google Scholar]
- 31.Hollinger K, Shanely RA, Quindry JC, Selsby JT. Long-term quercetin dietary enrichment decreases muscle injury in mdx mice. Clin Nutr. 2015;34:515–22. doi: 10.1016/j.clnu.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Saini A, Harjai K, Mohan H, Punia RP, Chhibber S. Long-term flaxseed oil supplementation diet protects BALB/c mice against Streptococcus pneumoniae infection. Med Microbiol Immunol. 2010;199:27–34. doi: 10.1007/s00430-009-0132-7. [DOI] [PubMed] [Google Scholar]
- 33.Tazawa S, Warashima T, Noro T, Miyase T. Studies on the constituents of Brazilian propolis. Chem Pharm Bull. 1998;46:1477–9. [Google Scholar]
- 34.Tazawa S, Warashima T, Noro T. Studies on the constituents of Brazilian propolis. II. Chem Pharm Bull. 1999;47:1388–92. [Google Scholar]
- 35.Konishi Y, Hitomi Y, Yoshida M, Yoshioka E. Absorption and bioavailability of artepillin C in rats after oral administration. J Agric Food Chem. 2005;53:9928–33. doi: 10.1021/jf051962y. [DOI] [PubMed] [Google Scholar]
- 36.Hattori H, Okuda K, Murase T, Shigetsura Y, Narise K, Semenza GL, et al. Isolation, identification, and biological evaluation of HIF-1-modulating compounds from Brazilian green propolis. Bioorg Med Chem. 2011;19:5392–401. doi: 10.1016/j.bmc.2011.07.060. [DOI] [PubMed] [Google Scholar]
- 37.Nakajima Y, Shimazawa M, Mishima S, Hara H. Water extract of propolis and its main constituents, caffeoylquinic acid derivatives, exert neuroprotective effects via antioxidant actions. Life Sci. 2007;80:370–7. doi: 10.1016/j.lfs.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Nakajima Y, Tsuruma K, Shimazawa M, Mishima S, Hara H. Comparison of bee products based on assays of antioxidant capacities. BMC Complement Altern Med. 2009;9:4. doi: 10.1186/1472-6882-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mishima S, Ono Y, Araki Y, Akao Y, Nozawa Y. Two related cinnamic Acid derivatives from Brazilian honey bee propolis, baccharin and drupanin, induce growth inhibition in allografted sarcoma S-180 in mice. Biol Pharm Bull. 2005;28:1025–30. doi: 10.1248/bpb.28.1025. [DOI] [PubMed] [Google Scholar]
- 40.Bankova V, Popova M, Bogdanov S, Sabatini AG. Chemical composition of European propolis: Expected and unexpected results. Z Naturforsch C. 2002;57:530–3. doi: 10.1515/znc-2002-5-622. [DOI] [PubMed] [Google Scholar]


