Comparative morphology in living and extinct platypuses revealed that there was a shift in feeding behavior and sensory efficiency.
Keywords: Monotremes, ornithorhynchids, evolution, trigeminal nerve
Abstract
The modern platypus, Ornithorhynchus anatinus, has an eye structure similar to aquatic mammals; however, platypuses also have a “sixth sense” associated with the bill electro- and mechanoreception that they use without opening their eyes underwater. We hypothesize that Ornithorhynchus and the Miocene taxon Obdurodon have different sensory capacities, which may have resulted from differences in foraging behavior. To estimate differences in foraging, sensory systems, and anatomical divergence between these monotremes, we compared their skull morphologies. Results indicate that the bill of Obdurodon is more dorsally deflected than that of Ornithorhynchus, suggesting a pelagic foraging behavior in Obdurodon compared to the bottom-feeding behavior in Ornithorhynchus. The infraorbital foramen of Obdurodon, through which the maxillary nerve passes sensory data from the bill to the brain, is relatively less developed than that of Ornithorhynchus. Whereas bill-focused sensory perception was likely shared among Mesozoic monotremes, the highly developed electrosensory system of Ornithorhynchus may represent an adaptation to foraging in cloudy water. Computed tomography imagery indicates that the enlarged infraorbital canal of Ornithorhynchus restricts the space available for maxillary tooth roots. Hence, loss of functional teeth in Ornithorhynchus may possibly have resulted from a shift in foraging behavior and coordinate elaboration of the electroreceptive sensory system. Well-developed electroreceptivity in monotremes is known at least as far back as the early Cretaceous; however, there are differences in the extent of elaboration of the feature among members of the ornithorhynchid lineage.
INTRODUCTION
Extant platypuses (Ornithorhynchus anatinus) forage at the bottom of streams with their eyes closed, using only electro- and mechanoreception (1–3). The Miocene monotreme Obdurodon dicksoni has a larger bill than Or. anatinus and a seemingly well-developed trigeminal nerve, similar to the extant platypus (4–6). Morphological similarities in bill structure between these species suggest that they may have filled similar ecological niches. However, there are also several notable morphological differences. For example, Ornithorhynchus has no teeth as adults in contrast to Obdurodon, which had fully functional cheek teeth. Many mammals have lost their teeth as a result of evolution, including echidnas, anteaters, and baleen whales, but none of these edentulous animals actually masticate their prey with their jaw moving (7). In contrast, the adult extant platypus still masticates its prey by using horny pads, located in the same position as the cheek teeth of Obdurodon, to crush items being consumed (2). Therefore, the loss of teeth in Ornithorhynchus is unlikely to be attributable to the lack of their necessity given that commutation of food remains an important component of feeding in the living animal. The cause of the loss of teeth is still unclear.
Or. anatinus swims under water with its eyes shut. However, its eyes show morphological similarities to those of other aquatic and semiaquatic mammals, such that the lens is adapted to underwater vision with a steeply curved posterior surface in relation to the flatter anterior surface (8, 9). This at least raises the possibility that Obdurodon may have kept its eyes open while foraging for food. However, the difference of sensory efficiency between Ornithorhynchus and Obdurodon is also still unclear.
The skull morphology of Ob. dicksoni has been examined in detail (4–6). However, there has been relatively little attention paid to comparing features that may relate specifically to differences in feeding behaviors and sensory efficiency with modern platypuses. Here, we report several skull differences between the two species that would appear to reflect differences in how these two species operated within their respective habitats, and a potentially correlated difference that may have led to loss of teeth in adult Or. anatinus.
RESULTS
Morphological measurements and angles of the skulls of Ob. dicksoni and Or. anatinus (Fig. 1) are shown in Table 1. Regression results suggest that all variables are significantly correlated to skull size (P < 0.05; Fig. 2). Because mainland and Tasmanian Ornithorhynchus were plotted along the same regression line, they were analyzed together (Fig. 2). Regression lines and corresponding prediction intervals for Ornithorhynchus and the plot of Obdurodon are shown in Fig. 2.
Fig. 1. Photographs showing measurements on the skulls of Ornithorhynchus in left lateral (A, left side) and dorsal (B) views, and Obdurodon in right lateral view (A, right side).
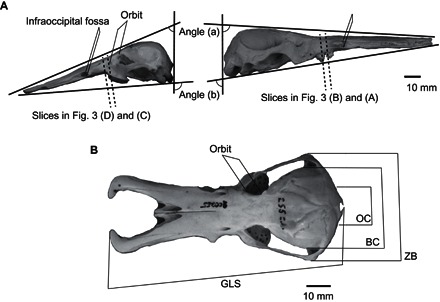
GLS, greatest length of the skull; OC, occipital condyle; BC, braincase; ZB, zygomatic breadth.
Table 1. Skull measurements of Obdurodon and Ornithorhynchus.
Data are means ± SD. n, number of specimens.
| Measurements | Obdurodon | Ornithorhynchus | n |
| GLS | 131.20 | 92.80 ± 8.31 | 32 |
| Angle (a) | 87.06 | 69.15 ± 3.43 | 25 |
| Angle (b) | 80.59 | 93.33 ± 3.55 | 25 |
| Infraorbital foramen | 3.47 | 3.13 ± 0.38 | 32 |
| Braincase | 35.86 | 32.18 ± 2.19 | 32 |
| Zygomatic breadth | 50.63 | 42.20 ± 3.98 | 32 |
| Occipital breadth | 27.45 | 23.76 ± 2.36 | 32 |
| Orbit | 11.85 | 9.17 ± 0.84 | 32 |
| Geometric mean | 25.41 | 21.02 ± 1.87 | 32 |
Fig. 2. Bivariate plots of skull measurements.
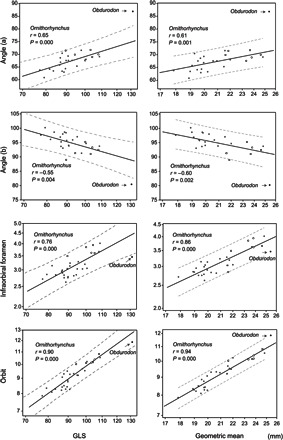
Regression lines and 95% prediction intervals of the measurements and angles plotted by GLS and geometric mean in the Ornithorhynchus compared to Obdurodon. Scales are log-transformed for linear measurements. Mainland platypuses are shown as gray circles, whereas Tasmanian platypuses are shown as black open circles.
Obdurodon has a higher angle (a) and lower angle (b) than Ornithorhynchus (Table 1 and Fig. 1). When the effect of size is removed by allometric comparison, the result is not different (Fig. 2). Together, these results suggest that the bill of Obdurodon is deflected more dorsally relative to the basicranium (dorsal flexion of the face) than that of Ornithorhynchus, which exhibits ventral deflection (ventral flexion of the face).
Obdurodon is bigger than Ornithorhynchus in all skull measurements, but the proportions of the measurements are also different (Table 1). On the basis of the allometric comparison, the size of the infraorbital foramen for Obdurodon falls on the regression line of Ornithorhynchus (Fig. 2). In addition, in the regression of infraorbital foramen on the greatest length of the skull (GLS), Obdurodon plots below the lower prediction interval for Ornithorhynchus (Fig. 2). When geometric mean was used as the independent variable, the results indicated that the size of the infraorbital foramen was just smaller than the lower prediction interval for Ornithorhynchus (Fig. 2). In contrast, the orbit size of Obdurodon seems to be greater than the lower prediction interval (that is, within prediction intervals) for Ornithorhynchus when using GLS as an independent variable (Fig. 2). In addition, measures for Obdurodon are greater than or just on the higher prediction interval of Ornithorhynchus when geometric mean was used as an independent variable (Fig. 2). These results indicate that Obdurodon had a relatively smaller infraorbital foramen (and maxillary nerve) but an identical or a larger orbit (and eye) than Ornithorhynchus in relation to size (for both GLS and geometric mean). Although the result above is based on intraspecific allometry of Ornithorhynchus, previous reports on the interspecific allometry of the eye also support our finding of larger eyes in Obdurodon in relation to its skull size (fig. S1).
Cross-sectional images of Obdurodon and Ornithorhynchus indicate a narrower infraorbital canal in Obdurodon, compared to Ornithorhynchus, and further reveal the complete lack of space for what might otherwise have been normal mammalian tooth roots in the maxilla of Ornithorhynchus (Fig. 3).
Fig. 3. CT slices along the anteroposterior axis of Obdurodon and Ornithorhynchus skulls at the tooth or horny pad position (see Fig. 1).
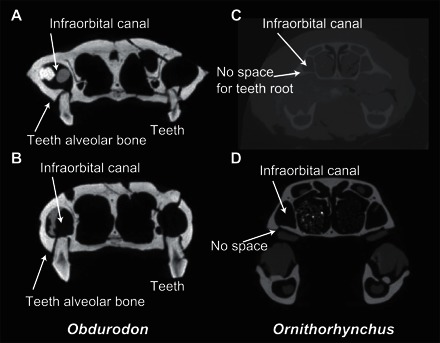
(A and B) Ob. dicksoni (from Digimorph; Queensland Museum F20568). (C) Or. anatinus (YAMA M-1). (D) Or. anatinus (from Digimorph; American Museum of Natural History M200255).
DISCUSSION
Possible behavioral change over time in feeding strategies
Obdurodon exceeds the range of Ornithorhynchus in terms of cranial deflection (Fig. 2), indicating that the rostrum of the extinct taxon was more dorsally deflected with respect to the basicranium than that of Ornithorhynchus (Fig. 1). Interspecific and intraspecific differences in the cranial deflection with respect to the basicranium have been reported in other mammals [for example, in previous studies (10–17)]. Cranial deflection is relatively “plastic” in mammals. For example, investigations of canids have revealed significant intraspecific variation in this trait, which is determined by the physical relationships of the splanchnocranium and neurocranium to each other (11–17). However, Tasmanian and mainland Ornithorhynchus, which became isolated from each other around 0.7 to 0.94 million years ago (Ma) (18), are plotted along the same regression line (Fig. 2). Thus, there may be purifying selection for the trait; that is, the degree of cranial deflection is functionally important and maintained by natural selection in Ornithorhynchus. Therefore, the difference in the angle of cranial deflection between modern and fossil ornithorhynchids may reflect differences in behaviors, rather than being a product of neutral evolution.
In other mammals, interspecific differences in cranial deflection are often considered to reflect differences in sensory efficiency [such as width of visual field in lagomorphans (17) or vocalization in chiropterans (10)]. However, Ornithorhynchus depends less on vision and sound than do many mammals (2, 19–21), and sensory efficiency probably does not explain the ventrally deflected bill in the skull of Ornithorhynchus.
Another possible explanation for a ventrally deflected bill is feeding behavior. If the rostrum is deflected ventrally, it should optimize bottom feeding by aquatic mammals, as seen in, for example, dugongids feeding on sea grasses and fossil cetaceans on benthic prey (22). Ornithorhynchus is known to forage on the bottom of rivers and lakes (2). Therefore, the ventrally deflected bill of Ornithorhynchus likely represents an adaptation to this kind of foraging. In contrast, the more dorsally deflected bill of Obdurodon suggests that this taxon might not have been a bottom feeder but rather may have foraged in the water column (above the bottom).
Adaptive changes in sensory system
Allometric comparison of the skull measurements indicates that Obdurodon has a relatively larger orbit (and eyes) and a narrower infraorbital foramen (and maxillary nerve) than Ornithorhynchus (Fig. 2). A well-developed trigeminal nerve, including the maxillary nerve, is the most prominent anatomical feature responsible for relaying “bill sense” (electro- and mechanoreception) to the brain of the platypus (2, 3). Although Obdurodon had a bigger bill than Ornithorhynchus (7, 8), the trigeminal nerve should be a direct index of bill sensitivity. Therefore, our findings regarding the infraorbital foramen suggest that the bill of Obdurodon was less electro- or mechanoreceptive than that of Ornithorhynchus. The results regarding orbit size suggest that Obdurodon had greater visual acuity than Ornithorhynchus. It suggests that within the ornithorhynchid lineage, there was an evolutionary transition from dependence on vision to dependence on electro- and mechanoreception during foraging. This transition probably involved a concomitant reduction in eye size and expansion of the trigeminal nerve (Fig. 2).
One possible cause of the transition in dependence from eyes to electro- and mechanoreception may have been a change in foraging behavior as discussed above. Vision might have been effective for Obdurodon foraging in the water column. However, Ornithorhynchus forages on the stream bottom, where mud can be stirred up by the moving bill and subsequently cloud the field of vision. In such a situation, vision becomes less effective, and electro- and mechanoreception of the bill are more important. This behavioral difference may be responsible for the sensory transition from vision to bill sense.
Cause of tooth loss in Ornithorhynchus
The enlargement of the trigeminal nerve and infraorbital canal, immediately above the base of the maxillary cheek teeth in ornithorhynchids, could well have resulted in increasing space constraints to maintain tooth roots required to support cheek teeth. Our computed tomography (CT) slice images indicate that Obdurodon had tooth alveoli in the upper jaw (Fig. 3) (7, 8). In contrast, the extant Ornithorhynchus maxilla is very thin and unable to house tooth roots. This change in the maxilla, as well as increased dependence on electro- and mechanoreception in Ornithorhynchus associated with bottom foraging, correlates with hypertrophy of the infraorbital canal and the associated maxillary nerve (Fig. 3). Ornithorhynchus is unique among mammals that have lost their teeth in that it still masticates using horny pads, which do not require alveoli for support (Fig. 3). It should be mentioned that Obdurodon barely managed to sustain teeth because it had a substantially developed infraorbital canal, albeit slightly less developed than that of Ornithorhynchus (Figs. 2 and 3).
Well-developed trigeminal nerves and electroreception are found in monotremes at least as far back as the early Cretaceous (23, 24). However, there are differences in the extent of elaboration of these features within the group. Toothed monotremes, such as the species of Obdurodon that lived during the Miocene, may have had less-developed electroreceptive capacities than Ornithorhynchus. Ornithorhynchus is sometimes called a living fossil, yet this species is characterized by autapomorphies that distinguish it from its extinct relatives. The loss of teeth and extremely well-developed electroreceptivity in Ornithorhynchus may have emerged relatively recently.
MATERIALS AND METHODS
We examined 32 skulls of Or. anatinus in the mammalogy collections of the U.S. National Museum of Natural History (Washington, DC). We obtained comparative data about the skull of Ob. dicksoni from Digimorph (http://digimorph.org). The holotype, Queensland Museum F20568, is a skull from the middle Miocene Ringtail Site from the Riversleigh World Heritage Area in northwestern Queensland. Ringtail Site has been radiometrically dated at 13.56 ± 0.66 Ma (25). To compare the internal morphology of the infraorbital canal, we CT-imaged a fluid specimen (preserved in 50% ethanol) of Or. anatinus in the Department of Acupuncture and Moxibustion, Tokyo Ariake University of Medical and Health Sciences, Tokyo (YAMA M-1), using the TXS320-ACTIS industrial microfocus CT scanner (Tesco Corp.) at the National Museum of Nature and Sciences, Tokyo. Slice images were obtained from the three-dimensional (3D) data from Digimorph and the fluid specimen. We used ImageJ software (National Institutes of Health) for 3D image processing.
Caliper measurements (Fig. 1) were taken from skulls of Or. anatinus and a 3D-printed replica of the skull of Ob. dicksoni (using DMM.com service, Japan; acrylic plastic, 0.2-mm resolution). In addition, photographs were taken from the left side of the specimens of the two species, and the two angles were measured (shown in Fig. 1) using ImageJ. The angles were determined on the basis of the intersections of the lateral and dorsoventral planes. The lateral plane was defined on the basis of how the specimen lays when placed on a flat surface. The dorsoventral plane was defined as the plane connecting the occipital condyle and the dorsal ridge of the foramen magnum, which determines the orientation of the cranium from the neck.
To eliminate the effect of size, allometric regressions were used to compare skull measurements for both species. We used the GLS and the geometric mean of all linear measurements as independent variables in this analysis. All statistical analyses were performed using Minitab 14 (Minitab Inc.).
Supplementary Material
Acknowledgments
We thank L. Gordon, E. Langan, D. Lunde, and K. Helgen (U.S. National Museum of Natural History) for arranging specimens. We thank T. Rowe for providing CT data from the skull of Obdurodon. We thank S. Fujiwara, T. Kuramochi, and R. Kono for CT scans of the specimens. Funding: This study was financially supported by the Mishima Kaiun Memorial Foundation, the Kyoto University Foundation, Grants-in-Aid from Japan Society for the Promotion of Science (11J01149 and 16K18601), Narishige Zoological Science Award (to M. Asahara), and the Australian Research Council (to M. Archer and S.J.H.). Author contributions: M. Asahara contributed conceptualization, investigation, and writing. M.K., T.E.M., S.J.H., and M. Archer contributed resources, discussions, and writing. Competing interests: The authors declare that they have no competing interests. Data and materials availability: Measurements data are listed in Table 1. All materials are available as explained in Materials and Methods. All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/10/e1601329/DC1
fig. S1. Relationship between body mass and orbit size (anteroposterior diameter) for Ornithorhynchus and Obdurodon.
Reference (26)
REFERENCES AND NOTES
- 1.H. Burrell, The Platypus (Angus and Robertson, 1927). [Google Scholar]
- 2.T. Grant, Platypus (CSIRO Publishing, ed. 4, 2007). [Google Scholar]
- 3.K. W. S. Ashwell, C. D. Hardman, in Neurobiology of Monotremes, K. W. S. Ashwell, Ed. (CSIRO Publishing, 2013), pp. 179–218. [Google Scholar]
- 4.M. Archer, F. A. Jenkins Jr., S. J. Hand, P. Murray, H. Godthelp, in Platypus and Echidnas, M. L. Augee, Ed. (Royal Zoological Society of New South Wales, 1992). [Google Scholar]
- 5.Musser A. M., Archer M., New information about the skull and dentary of the Miocene platypus Obdurodon dicksoni, and a discussion of ornithorhynchid relationships. Philos. Trans. R. Soc. B 353, 1063–1079 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macrini T. E., Rowe T., Archer M., Description of a cranial endocast from a fossil platypus, Obdurodon dicksoni (Monotremata, Ornithorhynchidae), and the relevance of endocranial characters to monotreme monophyly. J. Morphol. 267, 1000–1015 (2006). [DOI] [PubMed] [Google Scholar]
- 7.T. A. Vaugham, J. M. Ryan, N. J. Czaplewski, Mammalogy (Jones and Bartlett Publishers, ed. 5, 2011). [Google Scholar]
- 8.Sivak J. G., Accommodation in vertebrates: A contemporary survey. Curr. Top. Eye Res. 3, 281–330 (1980). [PubMed] [Google Scholar]
- 9.Pettigrew J. D., Manger P. R., Fine S. L. B., The sensory world of the platypus. Philos. Trans. R. Soc. B 353, 1199–1210 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedersen S. C., Cephalometric correlates of echolocation in the Chiroptera. J. Morphol. 218, 85–98 (1993). [DOI] [PubMed] [Google Scholar]
- 11.Fondon J. W. III, Garner H. R., Detection of length-dependent effects of tandem repeat alleles by 3-D geometric decomposition of craniofacial variation. Dev. Genes Evol. 217, 79–85 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Drake A. G., Klingenberg C. P., Large-scale diversification of skull shape in domestic dogs: Disparity and modularity. Am. Nat. 175, 289–301 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Milenković M., Šipetić V. J., Blagojević J., Tatović S., Vujošević M., Skull variation in Dinaric–Balkan and Carpathian gray wolf populations revealed by geometric morphometric approaches. J. Mammal. 91, 376–386 (2010). [Google Scholar]
- 14.Drake A. G., Dispelling dog dogma: An investigation of heterochrony in dogs using 3D geometric morphometric analysis of skull shape. Evol. Dev. 13, 204–213 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Asahara M., Shape variation in the skull and lower carnassial in a wild population of raccoon dog (Nyctereutes procyonoides). Zool. Sci. 30, 205–210 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Asahara M., Shape variation in the skull within and between wild populations of the raccoon dog (Nyctereutes procyonoides) in Japan. Mamm. Study 39, 105–113 (2014). [Google Scholar]
- 17.Kraatz B. P., Sherratt E., Bumacod N., Wedel M. J., Ecological correlates to cranial morphology in Leporids (Mammalia, Lagomorpha). PeerJ 3, e844 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gongora J., Swan A. B., Chong A. Y., Ho S. Y. W., Damayanyi C. S., Kolomyjec S., Grant T., Miller E., Blair D., Furlan E., Gust N., Genetic structure and phylogeography of platypuses revealed by mitochondrial DNA. J. Zool. 286, 110–119 (2012). [Google Scholar]
- 19.K. W. S. Ashwell, in Neurobiology of Monotremes, K. W. S. Ashwell, Ed. (CSIRO Publishing, 2013), pp. 161–178. [Google Scholar]
- 20.K. W. S. Ashwell, in Neurobiology of Monotremes, K. W. S. Ashwell, Ed. (CSIRO Publishing, 2013), pp. 219–234. [Google Scholar]
- 21.Krubitzer L., Manger P., Pettigrew J., Calford M., Organization of somatosensory cortex in monotremes: In search of the prototypical plan. J. Comp. Neurol. 351, 261–306 (1995). [DOI] [PubMed] [Google Scholar]
- 22.Bianucci G., Gingerich P. D., Aegyptocetus tarfa, n. gen. et sp. (Mammalia, Cetacea), from the middle Eocene of Egypt: Clinorhynchy, olfaction, and hearing in a protocetid whale. J. Vertebr. Paleontol. 31, 1173–1188 (2011). [Google Scholar]
- 23.Rowe T., Rich T. H., Vickers-Rich P., Springer M., Woodburne M. O., The oldest platypus and its bearing on divergence timing of the platypus and echidna clades. Proc. Natl. Acad. Sci. U.S.A. 105, 1238–1242 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rich T. H., Hopson J. A., Gill P. G., Trusler P., Rogers-Davidson S., Morton S., Cifelli R. L., Pickering D., Kool L., Siu K., Burgmann F. A., Senden T., Evans A. R., Wagstaff B. E., Seegets-Villiers D., Corfe I. J., Flannery T. F., Walker K., Musser A. M., Archer M., Pian R., Vickers-Rich P., The mandible and dentition of the Early Cretaceous monotreme Teinolophos trusleri. Alcheringa 40, 1–27 (2016). [Google Scholar]
- 25.Woodhead J., Hand S. J., Archer M., Graham I., Sniderman K., Arena D. A., Black K. H., Godthelp H., Creaser P., Price E., Developing a radiometrically-dated chronologic sequence for Neogene biotic change in Australia, from the Riversleigh World Heritage Area of Queensland. Gondwana Res. 29, 153–167 (2016). [Google Scholar]
- 26.Howland H. C., Merola S., Basarab J. R., The allometry and scaling of the size of vertebrate eyes. Vision Res. 44, 2043–2065 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/10/e1601329/DC1
fig. S1. Relationship between body mass and orbit size (anteroposterior diameter) for Ornithorhynchus and Obdurodon.
Reference (26)


