Abstract
Importance
Poor continuity of care may contribute to high healthcare spending and adverse patient outcomes in dementia.
Objective
To examine the association between medical provider continuity and healthcare utilization, testing, and spending in older adults with dementia.
Design
Observational retrospective cohort from 2012 using inverse probability weighted analysis.
Setting
National sample in fee-for-service Medicare.
Participants
1,416,369 continuously enrolled, community dwelling, fee-for-service Medicare beneficiaries age ≥ 65 with a claims-based dementia diagnosis and at least 4 ambulatory visits in 2012.
Exposure
Continuity of care score measured on patient visits across physicians over 12 months. A higher continuity score is assigned to visit patterns in which a larger share of the patient’s total visits are with fewer providers. Score range from 0 to 1 was examined in low, medium, and high continuity tertiles.
Main Outcomes and Measures
Outcomes include all-cause hospitalization, ambulatory care sensitive condition hospitalization, emergency department visit, imaging and lab testing (CT head, chest x-ray, urinalysis, and urine culture), and healthcare spending (overall, hospital and skilled nursing facility, and physician).
Results
Beneficiaries with dementia who had lower levels of continuity of care were younger, higher income, and had more comorbid medical conditions. Almost 50% of patients had at least one hospitalization and emergency department visit during the year. Utilization was lower with increasing level of continuity. Specifically comparing the highest versus lowest continuity groups, annual rates per beneficiary of hospitalization (0.83 vs 0.88), emergency department visits (0.84 vs 0.99), CT head (0.71 vs 0.83), urinalysis (0.72 vs 1.09), and healthcare spending (total spending $22,004 vs $24,371) were higher with lower continuity even after accounting for sociodemographic factors and comorbidity burden (all p values < 0.001). The rate of ambulatory care sensitive condition hospitalization was similar across continuity groups.
Conclusions and Relevance
Among older fee-for-service Medicare beneficiaries with a dementia diagnosis, lower continuity of care is associated with higher rates of hospitalization, emergency department visits, testing, and healthcare spending. Further research into these relationships, including potentially relevant clinical, provider, and systems factors, can inform whether improving continuity of care in this population may benefit patients and the wider health system.
INTRODUCTION
The growing population of older adults with dementia poses a unique challenge to the United States healthcare system.1 The cost of caring for this population is on par or higher than the financial burden of heart disease and cancer.2, 3 Individuals with dementia have high rates of hospitalization, including potentially preventable hospitalization,4–7 and are frequently evaluated in outpatient or emergency department settings.6, 7 Hospitalization and emergent evaluations increase medical costs6 and more importantly, may subject persons with dementia, who frequently have multiple chronic conditions, to distressing and potentially harmful interventions given risk of adverse outcomes in this population.8–12 Understanding key factors affecting medical care in dementia is required to promote more efficient, effective, and patient-centered healthcare delivery.
The majority of persons with dementia receive treatment for this complex neuropsychiatric illness and medical comorbidities in the ambulatory setting.13, 14 Ambulatory care in the United States is often fragmented. The average Medicare beneficiary sees seven physicians in four different practices annually, and communication and coordination between physicians is generally suboptimal.15, 16 Quality of care in dementia is also threatened by other factors, including provider time constraints, provider comfort, impaired patient communication and insight, and overwhelmed caregivers.16–18 Continuity of care (COC) at the level of the medical provider may be particularly important for building provider-patient-family relationships, addressing goals and expectations of care over time, understanding patients’ cognition and stage of dementia, and recognizing and appropriately managing acute and chronic conditions in this population.
To our knowledge, continuity of care in dementia has not previously been studied despite its potentially crucial role in healthcare for persons with this complex, expensive illness. In other populations, higher continuity of care is associated with decreased hospitalization, medical procedure overuse, and cost.19–23 Greater continuity may also improve patient-provider trust,24 quality of communication,25 and patient satisfaction,23, 26, 27 factors that may alleviate barriers to high quality care in dementia.16–18 Recent healthcare reforms promote continuity of care through accountable care organizations and Patient Centered Medical Homes, models of care which emphasize care coordination.22, 28, 29 The anticipated effects of such reforms on improving care in dementia are not fully understood. The objective of our study was to examine and understand the association of continuity of care with healthcare utilization and spending in the vulnerable population of community-dwelling older adults with dementia.
METHODS
Study population
We used the complete national sample of fee-for-service patients insured by Medicare in 2012. We first identified Medicare beneficiaries who were age 65 or older on January 1 and had 12 months of continuous Parts A and B coverage from the 2012 Medicare Beneficiary Summary file. We then searched their 2012 inpatient (Medicare Provider Analysis and Review file, MedPAR) and outpatient (Physician/Supplier Part B file and Outpatient file for rural or federally qualified health centers) claim records to identify diagnoses of dementia (see eTable 1 for diagnostic codes). Patients needed one outpatient or inpatient claim with dementia in 2012 to be considered to have the condition.30, 31 We restricted study patients to those who had a claims diagnosis of dementia (N = 2,657,648 patients), lived in the community (631,544 patients excluded with greater than 100 nursing home days according to Minimum Dataset), survived the entire year (207,028 patients excluded who died), and had at least 4 outpatient visits in 2012 to allow for calculation of the continuity measure (402,707 patients excluded with less than 4 visits). All remaining patients were included in the study (1,416,369 patients).
Continuity of Care Measurement
Continuity of care reflects the degree to which patient visits are concentrated among providers. The continuity of care score measures the concentration of a patient’s visit pattern, assigning a higher score to visit patterns in which a larger share of the patient’s total visits are with fewer providers (see eTable 2 for example score derivations).32, 33 The COC score was calculated using a patient’s ambulatory evaluation and management visits with physicians or nurse practitioners in 2012. The formula, (∑ni – N) / N(N-1), where ni = number of visits that the patient has with the ith physician and N = total visits, has been used previously.22, 32, 34 Each National Provider Identifier in outpatient claims was considered a unique medical provider. The COC index is less meaningful with few visits as it is easy to attain a minimum score of 0 or maximum score of 1 so we restricted analyses to patients with ≥ 4 visits,34–36 which represented approximately 85% of all older beneficiaries with a dementia diagnosis. COC scores were converted from a continuous value to low, medium, and high tertiles based on the distribution of scores within the sample for analyses and ease of interpretation as the actual score lacks inherent clinical meaning.19, 35
Outcome Measures
We measured four categories of health utilization and spending in 2012: hospitalizations, emergency department (ED) visits, imaging and lab testing, and healthcare spending.
(1) Hospitalizations
We identified acute, non-observation, short-stay hospitalizations from acute care or critical access hospitals from inpatient claims. We further measured hospitalizations for ambulatory care sensitive conditions (ACSC) defined by the Agency for Healthcare Research and Quality as prevention quality indicators. ACSC hospitalizations represent conditions for which hospitalization could be avoided if the patient receives timely and adequate outpatient care, such as diabetes complications, chronic obstructive pulmonary disease (COPD) or asthma, hypertension, congestive heart failure (CHF), dehydration, pneumonia, and urinary tract infection. We examined composite ACSC hospitalizations as well as acute and chronic ACSC hospitalizations subcategories.37
(2) Emergency department visits
We identified ED visits that did not result in inpatient hospitalization from outpatient claims. ED visits leading to hospitalization were captured in the hospitalization outcomes.
(3) Imaging and lab testing
While persons with dementia and other chronic conditions undergo the same routine lab tests and imaging as individuals without dementia, certain tests may be overused in this population to evaluate changes in mental status or behavioral symptoms. Specifically, computed tomography (CT) of the brain, chest x-ray, urinalysis, and urine culture may be ordered to evaluate for an infection or acute neurologic event in the absence of a suggestive history or localizing signs/symptoms.38, 39 We identified these specific imaging and lab tests from inpatient and outpatient claims (see eTable 3 for CPT codes).
(4) Healthcare spending
We examined Medicare spending in three categories. Hospital and skilled nursing facility spending from the MedPAR file reflects inpatient spending, while physician spending was derived from Medicare Part B spending. Total spending includes MedPAR, Part B claims, home health, hospice, durable medical equipment, and other facility spending. We standardized spending to adjust for differences in Medicare reimbursement due to cost-of-living, disproportionate share, graduate medical education, and hospital payments.40
Covariates
From the denominator file, we obtained beneficiaries’ age, sex, race/ethnicity, Medicare-Medicaid dual eligibility and ZIP code of residence. We linked 9-digit ZIP codes to the 2010 Census Tract to obtain median household income. Zip code was also linked to hospital referral region (HRR) to consider regional market-related characteristics.41 We accounted for illness burden using the Hierarchical Condition Categories (HCC) score, a Medicare risk adjustment system that gives more weight to comorbidities, and severe manifestations of comorbidities, that have greater effect on healthcare utilization.42 We also examined the total number of ambulatory visits, primary care (internal/family medicine, geriatrics, nurse practitioners) and specialist (all other specialties) visits, unique outpatient physicians seen, and predominant ambulatory provider in 2012 to better understand COC score components; these variables were not included in statistical models given their representation within the COC score.
Statistical Analysis
We compared characteristics of the study population by COC tertile to determine if there were significant differences amongst the three groups. To account for observed differences between each of the COC tertiles, we applied propensity weighting methods. Inverse probability weighting was used to balance the differences in patient characteristics across continuity levels. We used a multinomial logistic regression model to estimate the probability of each beneficiary belonging to their actual COC tertile based on their observed characteristics. Age, sex, race, HCC score, dual eligible status, and median household income were included as covariates in the model based upon their potential to be predictive of COC level. In sensitivity analysis, HRR was removed from the final model given it did not add significantly to balancing covariates between tertiles. The inverse propensity score, or inverse of the probability of each patient being in their actual COC level, was then calculated. These weights were applied to the characteristics to obtain weighted means and counts to assess the balance of covariates after risk adjustment; no more than 10% absolute difference between the three COC groups was required. For each outcome, the inverse probability weight was then applied to generate a weighted mean or rate. Based on the weighted results, differences between COC tertiles were evaluated using analysis of variance.
Analyses were conducted using SAS V9.3 (SAS Institute Inc., Cary, NC). The Dartmouth College Institutional Review Board approved this study.
RESULTS
Characteristics of the 1,416,369 community-dwelling Medicare beneficiaries who had a claims diagnosis of dementia, survived the year, and had 4 or more ambulatory visits in 2012 are shown in Table 1. Mean age was 81 years, with 63.3% female and 82.9% white beneficiaries. The sample had a mean of 13.6 outpatient visits with 4.8 unique providers in the year. With increasing levels of continuity, age, proportions of females and non-white were higher and median household income was lower. Individuals with low continuity had more chronic conditions and higher HCC scores; they had higher proportions of coronary artery disease (CAD), CHF, and COPD compared to high continuity. The low continuity group had a mean of 15.6 visits to 7.1 unique providers, compared to 14.8 visits to 4.8 providers and 10.5 visits to 2.5 providers in medium and high continuity groups, respectively.
Table 1.
Characteristics of Medicare beneficiaries with dementia by continuity of care tertile before propensity weightinga
| Continuity of Care Index | ||||
|---|---|---|---|---|
| Characteristic | Overall | Low | Medium | High |
| Beneficiaries, No. (%) | 1416369 (100) | 486276 (34.3) | 445710 (31.5) | 484383 (34.2) |
| Age, mean (SD)b | 81.0 (7.51) | 79.9 (7.31) | 80.9 (7.44) | 82.3 (7.60) |
| Sex, No. (%) | ||||
| Female | 896010 (63.3) | 279423 (57.5) | 279344 (62.7) | 337243 (69.6) |
| Race/ethnicity, No. (%) | ||||
| White | 1174538 (82.9) | 418030 (86.0) | 370264 (83.1) | 386244 (79.7) |
| Black | 118168 (8.3) | 35333 (7.3) | 36551 (8.2) | 46284 (9.6) |
| Hispanic | 78244 (5.5) | 21636 (4.5) | 24822 (5.6) | 31786 (6.6) |
| Other | 45419 (3.2) | 11277 (2.3) | 14073 (3.2) | 20069 (4.1) |
| Dual eligible, No. (%) | 291020 (20.6) | 77011 (15.8) | 90950 (20.4) | 123059 (25.4) |
| Median household income, mean (SD), $c |
57934 (27522) | 60466 (28424) | 57766 (27293) | 55549 (26575) |
| Chronic conditions, mean (SD) | 2.16 (1.90) | 2.40 (2.04) | 2.32 (1.91) | 1.78 (1.69) |
| Chronic condition, No. (%) | ||||
| Coronary artery disease | 131917 (9.3) | 54112 (11.1) | 45899 (10.3) | 31906 (6.6) |
| Congestive heart failure | 291661 (20.6) | 107114 (22.0) | 100102 (22.5) | 84445 (17.4) |
| Diabetes mellitus | 400309 (28.3) | 136761 (28.1) | 132369 (29.7) | 131179 (27.1) |
| Chronic obstructive pulmonary disease |
242085 (17.1) | 87691 (18.0) | 81176 (18.2) | 73218 (15.1) |
| HCC score, mean (SD) | 1.64 (1.21) | 1.77 (1.32) | 1.73 (1.23) | 1.44 (1.03) |
| Health utilization, mean (SD) | ||||
| Ambulatory visits | 13.6 (9.17) | 15.6 (10.04) | 14.8 (8.92) | 10.5 (7.52) |
| Visits to primary care providers | 7.6 (6.03) | 6.8 (5.58) | 8.3 (6.25) | 7.9 (6.16) |
| Visits to specialty providers | 5.5 (6.10) | 8.2 (6.72) | 6.0 (5.59) | 2.3 (4.15) |
| Unique doctors seen | 4.8 (2.91) | 7.1 (3.04) | 4.8 (1.87) | 2.5 (1.3) |
| Continuity of care scored | 0.35 (0.25) | 0.13 (0.05) | 0.28 (0.05) | 0.64 (0.22) |
| Predominant provider, No. (%) | ||||
| Primary Care | 1083454 (76.5) | 310427 (63.8) | 343098 (77.0) | 429929 (88.8) |
| Specialist | 332915 (23.5) | 175849 (36.2) | 102612 (23.0) | 54454 (11.2) |
| Proportion of visits to predominant provider, mean (SD) |
53.7 (22.2) | 32.5 (8.4) | 49.2 (7.5) | 79.0 (14.2) |
SD = standard deviation; HCC = hierarchical condition categories score
2012 characteristics of fee-for-service beneficiaries with 4 or more ambulatory visits who survived the entire year.
Age on January 1, 2012
Based on 2010 tract median household income.
Range 0 to 1, higher score indicates higher continuity.
The inverse probability weighted sample included 1,416,344 beneficiaries (25 beneficiaries excluded due to missing median household income). After inverse probability weighting, key characteristics became more similar between individuals with different levels of continuity (Table 2). Mean age, HCC score, income, and gender, race, and dual eligible proportions were comparable between groups. Mean chronic conditions and CAD and CHF proportions also became more similar. Mean outpatient visits remained different, with almost 15 visits annually in the low continuity group versus 11 visits in the high continuity group. The low continuity group saw more unique physicians.
Table 2.
Characteristics of Medicare beneficiaries who had dementia by continuity of care tertile after propensity weightinga
| Continuity of Care Index | |||
|---|---|---|---|
| Characteristic | Low | Medium | High |
| Beneficiaries, No. (%) | 486272 (34.3) | 445701 (31.5) | 484371 (34.2) |
| Age, mean (SD)b | 81.0 (12.55) | 81.0 (13.3) | 80.9 (13.36) |
| Sex | |||
| Female, % | 63.3 | 63.2 | 62.9 |
| Race/ethnicity, % | |||
| White | 82.6 | 82.9 | 82.6 |
| Black | 8.6 | 8.3 | 8.6 |
| Hispanic | 5.6 | 5.5 | 5.6 |
| Other | 3.2 | 3.2 | 3.2 |
| Dual eligible, % | 20.9 | 20.6 | 21.0 |
| Median household income, mean (SD), $c |
57805 (45938) | 57934 (49093) | 57797 (48767) |
| Chronic conditions, mean (SD) | 2.23 (3.32) | 2.22 (3.32) | 2.1 (3.31) |
| HCC score, mean (SD) | 1.65 (2.05) | 1.65 (2.1) | 1.71 (2.38) |
| Health utilization, mean (SD) | |||
| Ambulatory visits | 15.0 (16.57) | 14.6 (15.62) | 11.2 (14.32) |
| Unique doctors seen | 6.9 (5.03) | 4.8 (3.29) | 2.6 (2.39) |
SD = standard deviation; HCC = hierarchical condition categories score
2012 characteristics of fee-for-service beneficiaries with 4 or more ambulatory visits who survived the entire year. Age, sex, race, dual eligible status, HCC score, and median household income included in propensity weighting. Twenty five beneficiaries were dropped during propensity weighting due to missing median household income values.
Age on January 1, 2012
Based on 2010 tract median household income.
Table 3 shows unweighted proportions of health services utilization by continuity level in 2012. Overall, 47% of the sample had at least one hospitalization, and 47% had at least one ED visit in the year. 47% also had a CT head performed. 13% of the sample experienced at least one ACSC hospitalization. Crude rates of hospitalization, ED visits, testing and mean healthcare spending were highest in the low continuity group, followed by the medium and high continuity groups respectively. Mean healthcare spending per beneficiary in 2012 was $22,740. Approximately 55% of total spending per beneficiary was due to hospital or skilled nursing facility spending.
Table 3.
Unweighted health services utilization and spending by continuity of care tertile
| Continuity of Care Index | ||||
|---|---|---|---|---|
| Outcome | Overall (N=1416369) |
Low (N=486276) |
Medium (N=445710) |
High (N=484383) |
| Any health services utilization or testing, No. (%) | ||||
| Hospitalization | 668824 (47.2) | 250273 (51.5) | 225523 (50.6) | 193028 (39.9) |
| ACSC hospitalization |
189809 (13.4) | 66892 (13.8) | 64654 (14.5) | 58263 (12.0) |
| ED visit | 667589 (47.1) | 242890 (50.0) | 217784 (48.9) | 206915 (42.7) |
| CT Head | 668758 (47.2) | 246952 (50.8) | 222721 (50.0) | 199085 (41.1) |
| Chest X-ray | 896660 (63.3) | 325978 (67.0) | 296288 (66.5) | 274394 (56.7) |
| Urinalysis | 605541 (42.8) | 237329 (48.8) | 198560 (44.6) | 169652 (35.0) |
| Urine Culture | 270027 (19.1) | 99063 (20.4) | 89448 (20.1) | 81516 (16.8) |
| Healthcare spending per beneficiary, mean (SD), $ | ||||
| Total | 22740 (29597) | 26768 (33359) | 24454 (29971) | 17104 (23792) |
| Hospital & SNF | 12543 (22773) | 15259 (25974) | 13748 (23252) | 8697 (17822) |
| Physician | 4576 (5513) | 5657 (6002) | 4947 (5751) | 3144 (4349) |
ACSC = ambulatory care sensitive condition; ED = emergency department; CT= computed tomography; SD = standard deviation; SNF = skilled nursing facility
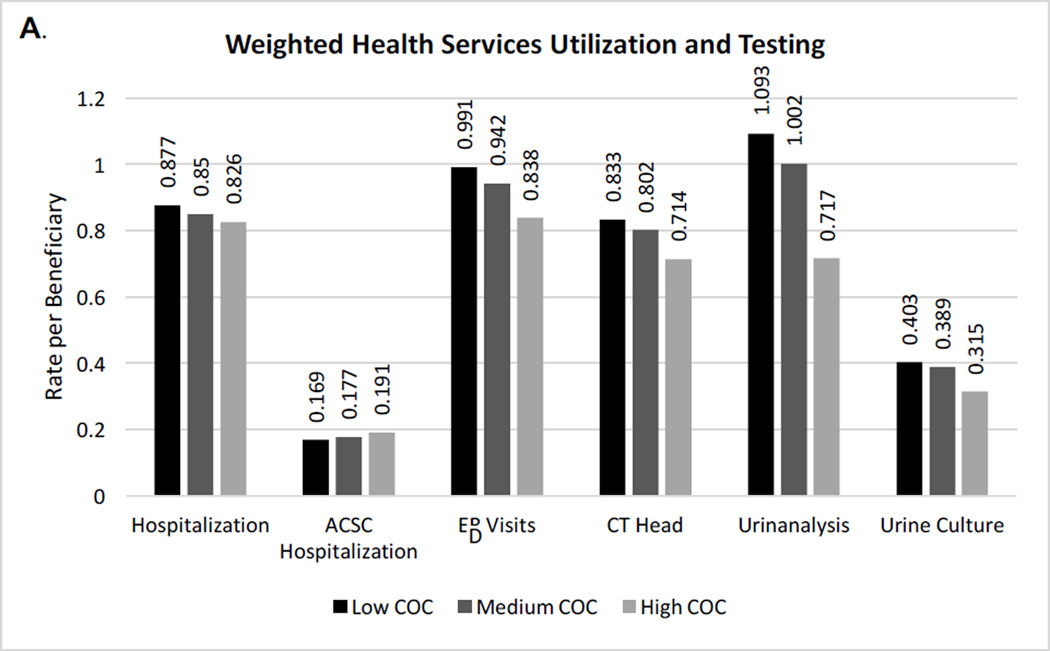
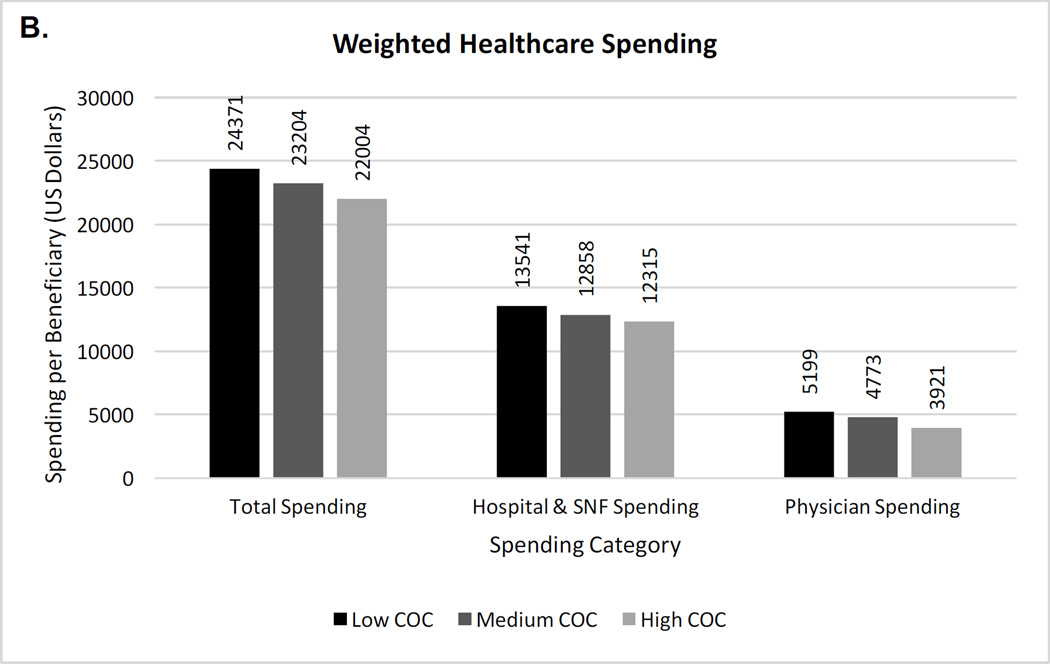
As shown in Figure 1a and eTable 4, even after inverse probability weighting, the use of most health services was higher with lower continuity. The annual rate of hospitalization per beneficiary was 5.8% higher and ED visits were 15.4% higher in the lowest continuity compared to highest continuity group. While CT head and urinalysis were performed in the low continuity group at higher rates, chest x-rays were performed at similarly high rates across groups, with over 2 chest x-rays per beneficiary at all continuity levels. ACSC hospitalizations were relatively similar across continuity groups, including when acute and chronic ACSC hospitalizations were examined separately. Significant differences in healthcare spending remained (Figure 1b). Mean yearly spending was $2,367 higher in the lowest versus highest continuity group, with higher inpatient and outpatient costs. Between group differences were statistically significant for all outcomes (p values < 0.001).
Figure 1. (A&B) Inverse probability weighted rates of health services, testing, and healthcare spending per beneficiary by continuity of care level.
Chest x-ray rates, not shown due to scale, were 2.13, 2.05, and 2.12 for low, medium, and high continuity tertiles, respectively. Differences between the three continuity of care groups for all outcomes were statistically significant (p < 0.001) using analysis of variance.
COC = continuity of care; ACSC = ambulatory care sensitive condition; ED = emergency department; CT= computed tomography; SNF = skilled nursing facility
We explored the possibility of interaction effects between predominant provider and COC tertile on each outcome (eTable 5) given potentially different effects of continuity in primary versus specialty predominant care. Using a two-way analysis of variance, the interaction was significant (p < 0.05) for all outcomes. Thus, effects of continuity differ between those whose predominant provider was primary care versus a specialist.
DISCUSSION
Low continuity of ambulatory care among community-dwelling older adults with a dementia diagnosis is associated with higher rates of hospitalization, ED visits, radiologic and lab testing, and greater healthcare spending. Lower continuity is not associated with more ACSC hospitalizations in this population, however. The overall volume of health services utilization and testing is striking, with almost half of the cohort experiencing hospitalization, an ED visit, and a CT head in the course of the year. Rates of testing translate to approximately two chest x-rays and one urinalysis per beneficiary per year. When considering differences in healthcare spending per beneficiary, individuals with the most fragmented care are associated with an additional $567 million to $1.1 billion in healthcare spending compared to those with medium or high continuity. Within care continuity, the balance of primary care and specialists may also be important given this population is particularly sick and complex.
These findings resemble patterns seen in studies of continuity of care in other populations; findings regarding potentially avoidable hospitalizations, however, have been mixed. Odds of hospitalization among older adults in Taiwan were 38% and 68% lower with medium and high continuity, respectively, compared to low continuity. The same study found a similar relationship for avoidable hospitalizations, which contradicts our finding of no clinically significant difference in ACSC hospitalizations.19 A study in younger Medicaid beneficiaries found that higher provider continuity was associated with lower likelihood of hospitalization, with an 8–11% absolute difference between the highest and lowest continuity groups in proportion of patients hospitalized; there was no significant association between continuity and acute ACSC hospitalization.20 Other studies in older adults, however, found that higher continuity was associated with lower rates of preventable hospitalization.34, 43 Higher continuity was also associated with a decrease in potentially overused procedures in Medicare beneficiaries.22 Imaging and lab tests examined in our study may similarly represent overused procedures in persons with dementia. Our results are also similar to findings in older veterans and other chronic conditions.21, 43 Medicare beneficiaries with CHF, COPD, or diabetes had lower odds of hospitalization, ED visits, complications, and lower cost with higher levels of continuity.21 Compared to those beneficiaries, mean HCC score in our cohort (1.64) was higher than median HCC scores for beneficiaries with CHF (0.7), COPD (0.6), and diabetes (0.5). Annual utilization was also higher in dementia compared to proportions with hospitalization or ED visits in CHF (10.5% and 44.6%) and COPD (6.8% and 35.9%).21
While our findings reflect the importance of continuity of care found in other populations and chronic conditions, the importance for older adults in dementia may extend beyond healthcare utilization. Quality of care in dementia has been noted to be inconsistent and often reactive.44 Persons with dementia are at risk of experiencing adverse events during the course of medical care.8, 10, 11, 45, 46 This population is also at risk of unnecessary testing, which can lead to patient burden through invasive tests and overtreatment.47, 48 Administration of sedative-hypnotic medications to complete a CT scan or catheterization for a urine sample are examples of clinical circumstances that may be particularly challenging in persons with dementia. Our results suggest the possibility that addressing care fragmentation could decrease hospitalizations, ED visits, unnecessary testing, and overtreatment in this at-risk population. Potential mechanisms include provider familiarity with patients’ cognitive and functional abilities, coexisting conditions, support systems, and goals of care. Decreased fragmentation may facilitate anticipatory guidance and communication/coordination between providers. Beneficiaries with greater continuity also had greater primary care involvement; for complex patients, balance between primary care and select specialists, with provider continuity, may prove most beneficial. Interestingly, rates of ACSC hospitalizations were similar across continuity levels. It may be that these conditions present differently in persons with dementia, as symptoms specific to the ACSC condition may be overshadowed by delirium, and early signs/symptoms are missed even with high continuity and a longstanding physician relationship. Conflicting results may also point to shortcomings in the ACSC construct, originally designed as a metric of area-level access to quality care.49
This study has several limitations. First, we used claims-based dementia diagnoses, which may not represent the entire dementia population.30, 31 Individuals with Medicare managed care, for example, were excluded, people with mild disease may be missed, and individuals being evaluated for dementia but not yet diagnosed may be included. In addition, we excluded individuals who died during the year or who resided in a nursing home, as the pattern of continuity of care and health utilization would likely be different in these groups. Exclusion of beneficiaries with less than 4 outpatient visits, who may have less utilization and spending, also limits the generalizability of our findings. Because our study was cross-sectional, we also cannot make inferences about whether lower continuity of care causes higher healthcare utilization and spending. Though we accounted for multiple variables that may affect this relationship, there could also be reverse causality, with acute events such as hospitalization leading to low continuity. There may also be additional unmeasured confounding factors that influence both continuity and healthcare utilization. For example, clinical factors leading to specialty referral may also drive utilization. Additionally, the COC index is one of several continuity measures; available measures are highly correlated, however, such that using alternatives is unlikely to significantly alter results.21, 50 Lastly, we could not consider additional factors related to dementia, such as disease severity, or to continuity, such as relationship duration, trust, and patient perceptions.51
Lower continuity of care, measured as increased fragmentation of care across healthcare providers, is associated with greater healthcare utilization, including hospitalization, ED visits, and testing, and higher healthcare costs in community-dwelling older adults with a dementia diagnosis. Additional research disentangling the relationship between continuity, types of providers seen, and outcomes is indicated as decreasing fragmentation at the provider level may be a mechanism to reduce hospitalization and unnecessary testing in these older adults at high risk of adverse events. Even within new models of care, emphasis on continuity of care with providers may be necessary to improve quality and cost of care for this growing, complex patient population.
Supplementary Material
Acknowledgments
Funding/Support: This study was funded by grants from the John A. Hartford Foundation and the National Institute of Aging (P01 AG19783). Dr. Amjad is supported by fellowship grants from HRSA (D01HP08789) and the Pearl M. Stetler Research Fund.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr. Bynum had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Amjad, Austin, Chang, Bynum
Acquisition, analysis or interpretation of data: All authors
Drafting of the manuscript: Amjad
Critical revision of the manuscript for important intellectual content: Austin, Chang, Bynum
Statistical analysis: Carmichael, Austin
Obtained funding: Bynum
Administrative, technical, or material support: All authors
Study supervision: Bynum
Conflict of Interest Disclosures: None.
REFERENCES
- 1.Bynum JP. The long reach of Alzheimer’s disease: Patients, practice, and policy. Health Aff (Millwood) 2014;33(4):534–540. doi: 10.1377/hlthaff.2013.1247. [DOI] [PubMed] [Google Scholar]
- 2.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelley AS, McGarry K, Gorges R, Skinner JS. The burden of health care costs for patients with dementia in the last 5 years of life. Ann Intern Med. 2015;163(10):729–736. doi: 10.7326/M15-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bynum JP, Rabins PV, Weller W, Niefeld M, Anderson GF, Wu AW. The relationship between a dementia diagnosis, chronic illness, Medicare expenditures, and hospital use. J Am Geriatr Soc. 2004;52(2):187–194. doi: 10.1111/j.1532-5415.2004.52054.x. [DOI] [PubMed] [Google Scholar]
- 5.Phelan EA, Borson S, Grothaus L, Balch S, Larson EB. Association of incident dementia with hospitalizations. JAMA. 2012;307(2):165–172. doi: 10.1001/jama.2011.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Y, Kuo TC, Weir S, Kramer MS, Ash AS. Healthcare costs and utilization for Medicare beneficiaries with Alzheimer’s. BMC Health Serv Res. 2008;8 doi: 10.1186/1472-6963-8-108. 108-6963-8-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng Z, Coots LA, Kaganova Y, Wiener JM. Hospital and ED use among Medicare beneficiaries with dementia varies by setting and proximity to death. Health Aff (Millwood) 2014;33(4):683–690. doi: 10.1377/hlthaff.2013.1179. [DOI] [PubMed] [Google Scholar]
- 8.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 9.McCloskey RM. Caring for patients with dementia in an acute care environment. Geriatr Nurs. 2004;25(3):139–144. doi: 10.1016/j.gerinurse.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Mecocci P, von Strauss E, Cherubini A, et al. Cognitive impairment is the major risk factor for development of geriatric syndromes during hospitalization: Results from the GIFA study. Dement Geriatr Cogn Disord. 2005;20(4):262–269. doi: 10.1159/000087440. [DOI] [PubMed] [Google Scholar]
- 11.Pedone C, Ercolani S, Catani M, et al. Elderly patients with cognitive impairment have a high risk for functional decline during hospitalization: The GIFA study. J Gerontol A Biol Sci Med Sci. 2005;60(12):1576–1580. doi: 10.1093/gerona/60.12.1576. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan-Marx EM. Achieving restraint-free care of acutely confused older adults. J Gerontol Nurs. 2001;27(4):56–61. doi: 10.3928/0098-9134-20010401-11. [DOI] [PubMed] [Google Scholar]
- 13.Boustani M, Schubert C, Sennour Y. The challenge of supporting care for dementia in primary care. Clin Interv Aging. 2007;2(4):631–636. doi: 10.2147/cia.s1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geldmacher DS, Kerwin DR. Practical diagnosis and management of dementia due to Alzheimer’s disease in the primary care setting: An evidence-based approach. Prim Care Companion CNS Disord. 2013;15(4) doi: 10.4088/PCC.12r01474. Epub 2013 Aug 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pham HH, Schrag D, O’Malley AS, Wu B, Bach PB. Care patterns in Medicare and their implications for pay for performance. N Engl J Med. 2007;356(11):1130–1139. doi: 10.1056/NEJMsa063979. [DOI] [PubMed] [Google Scholar]
- 16.Hinton L, Franz CE, Reddy G, Flores Y, Kravitz RL, Barker JC. Practice constraints, behavioral problems, and dementia care: Primary care physicians’ perspectives. J Gen Intern Med. 2007;22(11):1487–1492. doi: 10.1007/s11606-007-0317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boustani M, Sachs G, Callahan CM. Can primary care meet the biopsychosocial needs of older adults with dementia? J Gen Intern Med. 2007;22(11):1625–1627. doi: 10.1007/s11606-007-0386-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris DP, Chodosh J, Vassar SD, Vickrey BG, Shapiro MF. Primary care providers’ views of challenges and rewards of dementia care relative to other conditions. J Am Geriatr Soc. 2009;57(12):2209–2216. doi: 10.1111/j.1532-5415.2009.02572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng SH, Chen CC, Hou YF. A longitudinal examination of continuity of care and avoidable hospitalization: Evidence from a universal coverage health care system. Arch Intern Med. 2010;170(18):1671–1677. doi: 10.1001/archinternmed.2010.340. [DOI] [PubMed] [Google Scholar]
- 20.Gill JM, Mainous AG., 3rd The role of provider continuity in preventing hospitalizations. Arch Fam Med. 1998;7(4):352–357. doi: 10.1001/archfami.7.4.352. [DOI] [PubMed] [Google Scholar]
- 21.Hussey PS, Schneider EC, Rudin RS, Fox DS, Lai J, Pollack CE. Continuity and the costs of care for chronic disease. JAMA Intern Med. 2014;174(5):742–748. doi: 10.1001/jamainternmed.2014.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romano MJ, Segal JB, Pollack CE. The association between continuity of care and the overuse of medical procedures. JAMA Intern Med. 2015;175(7):1148–1154. doi: 10.1001/jamainternmed.2015.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wasson JH, Sauvigne AE, Mogielnicki RP, et al. Continuity of outpatient medical care in elderly men. A randomized trial. JAMA. 1984;252(17):2413–2417. [PubMed] [Google Scholar]
- 24.Mainous AG, 3rd, Baker R, Love MM, Gray DP, Gill JM. Continuity of care and trust in one’s physician: Evidence from primary care in the United States and the United Kingdom. Fam Med. 2001;33(1):22–27. [PubMed] [Google Scholar]
- 25.Katz DA, McCoy K, Sarrazin MV. Does improved continuity of primary care affect clinician-patient communication in VA? J Gen Intern Med. 2014;29(Suppl 2):S682–S688. doi: 10.1007/s11606-013-2633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan VS, Burman M, McDonell MB, Fihn SD. Continuity of care and other determinants of patient satisfaction with primary care. J Gen Intern Med. 2005;20(3):226–233. doi: 10.1111/j.1525-1497.2005.40135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy A, Pollack CE, Asch DA, Canamucio A, Werner RM. The effect of primary care provider turnover on patient experience of care and ambulatory quality of care. JAMA Intern Med. 2015;175(7):1157–1162. doi: 10.1001/jamainternmed.2015.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bodenheimer T, Ghorob A, Willard-Grace R, Grumbach K. The 10 building blocks of high-performing primary care. Ann Fam Med. 2014;12(2):166–171. doi: 10.1370/afm.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longworth DL. Accountable care organizations, the patient-centered medical home, and health care reform: What does it all mean? Cleve Clin J Med. 2011;78(9):571–582. doi: 10.3949/ccjm.78gr.11003. [DOI] [PubMed] [Google Scholar]
- 30.Lin P, Kaufer DI, Maciejewski ML, Ganguly R, Paul JE, Biddle AK. An examination of Alzheimer’s disease case definitions using Medicare claims and survey data. Alzheimer’s & Dementia. 2010;6(4):334–341. doi: 10.1016/j.jalz.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Taylor DH, Jr, Ostbye T, Langa KM, Weir D, Plassman BL. The accuracy of Medicare claims as an epidemiological tool: The case of dementia revisited. J Alzheimers Dis. 2009;17(4):807–815. doi: 10.3233/JAD-2009-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bice TW, Boxerman SB. A quantitative measure of continuity of care. Med Care. 1977;15(4):347–349. doi: 10.1097/00005650-197704000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Shortell SM. Continuity of medical care: Conceptualization and measurement. Med Care. 1976;14(5):377–391. doi: 10.1097/00005650-197605000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Nyweide DJ, Anthony DL, Bynum JP, et al. Continuity of care and the risk of preventable hospitalization in older adults. JAMA Intern Med. 2013;173(20):1879–1885. doi: 10.1001/jamainternmed.2013.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christakis DA, Wright JA, Koepsell TD, Emerson S, Connell FA. Is greater continuity of care associated with less emergency department utilization? Pediatrics. 1999;103(4 Pt 1):738–742. doi: 10.1542/peds.103.4.738. [DOI] [PubMed] [Google Scholar]
- 36.Jee SH, Cabana MD. Indices for continuity of care: A systematic review of the literature. Med Care Res Rev. 2006;63(2):158–188. doi: 10.1177/1077558705285294. [DOI] [PubMed] [Google Scholar]
- 37.Agency for Healthcare Research and Quality. [Accessed July 1, 2015];Prevention quality indicators overview. http://www.qualityindicators.ahrq.gov/Modules/PQI_TechSpec.aspx.
- 38.Dufour AB, Shaffer ML, D’Agata E, Habtemariam D, Mitchell SL. Survival after suspected urinary tract infection in individuals with advanced dementia. J Am Geriatr Soc. 2015;63(12):2472–2477. doi: 10.1111/jgs.13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell SL, Shaffer ML, Loeb MB, et al. Infection management and multidrug-resistant organisms in nursing home residents with advanced dementia. JAMA Intern Med. 2014;174(10):1660–1667. doi: 10.1001/jamainternmed.2014.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gottlieb DJ, Zhou W, Song Y, Andrews KG, Skinner JS, Sutherland JM. Prices don’t drive regional Medicare spending variations. Health Aff (Millwood) 2010;29(3):537–543. doi: 10.1377/hlthaff.2009.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wennberg JE, Cooper MM, Birkmeyer J. The quality of medical care in the United States: A report on the Medicare program. The Dartmouth atlas of health care. 1999 [PubMed] [Google Scholar]
- 42.Pope GC, Kautter J, Ellis RP, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25(4):119–141. [PMC free article] [PubMed] [Google Scholar]
- 43.Katz DA, McCoy KD, Vaughan-Sarrazin MS. Does greater continuity of veterans administration primary care reduce emergency department visits and hospitalization in older veterans? J Am Geriatr Soc. 2015;63(12):2510–2518. doi: 10.1111/jgs.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Odenheimer G, Borson S, Sanders AE, et al. Quality improvement in neurology: Dementia management quality measures. J Am Geriatr Soc. 2014;62(3):558–561. doi: 10.1111/jgs.12630. [DOI] [PubMed] [Google Scholar]
- 45.Kelley AS, Siegler EL, Reid MC. Pitfalls and recommendations regarding the management of acute pain among hospitalized patients with dementia. Pain Med. 2008;9(5):581–586. doi: 10.1111/j.1526-4637.2008.00472.x. [DOI] [PubMed] [Google Scholar]
- 46.Mossello E, Pieraccioli M, Nesti N, et al. Effects of low blood pressure in cognitively impaired elderly patients treated with antihypertensive drugs. JAMA Intern Med. 2015;175(4):578–585. doi: 10.1001/jamainternmed.2014.8164. [DOI] [PubMed] [Google Scholar]
- 47.Gordon LB, Waxman MJ, Ragsdale L, Mermel LA. Overtreatment of presumed urinary tract infection in older women presenting to the emergency department. J Am Geriatr Soc. 2013;61(5):788–792. doi: 10.1111/jgs.12203. [DOI] [PubMed] [Google Scholar]
- 48.D’Agata E, Mitchell SL. Patterns of antimicrobial use among nursing home residents with advanced dementia. Arch Intern Med. 2008;168(4):357–362. doi: 10.1001/archinternmed.2007.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel KK, Vakharia N, Pile J, Howell EH, Rothberg MB. Preventable admissions on a general medicine service: Prevalence, causes and comparison with AHRQ prevention quality indicators-A cross-sectional analysis. J Gen Intern Med. 2016 doi: 10.1007/s11606-016-3615-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pollack CE, Hussey PS, Rudin RS, Fox DS, Lai J, Schneider EC. Measuring care continuity: A comparison of claims-based methods. Med Care. 2016;54(5):e30–e34. doi: 10.1097/MLR.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nyweide DJ. Concordance between continuity of care reported by patients and measured from administrative data. Med Care Res Rev. 2014;71(2):138–155. doi: 10.1177/1077558713505685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.