Abstract
Objective
The aim of the study was to determine the differential expression patterns of the wingless-type (Wnt) pathway inhibitors Dkk3 (Dickkopf 3), SFRP1 (secreted frizzled-related protein 1), and SFRP4 in normal müllerian tissue and endometrial endometrioid adenocarcinoma specimens.
Methods
Messenger RNA (mRNA) and protein levels of the Wnt pathway inhibitors Dkk3, SFRP1, and SFRP4 were evaluated by real-time reverse transcription–polymerase chain reaction and Western blot analysis. A total of 87 human tissue specimens were obtained from 60 women who participated in Gynecologic Oncology Group protocol 210. Twenty-seven normal müllerian tissues, 32 early-stage, and 28 advanced-stage endometrial endometrioid cancer specimens were analyzed.
Results
Median age for this cohort was 60 years, with median body mass index of 32 kg/m2. There was a difference in Dkk3 protein expression between normal müllerian tissues and primary endometrial endometrioid adenocarcinoma samples (P = 0.05). There was down-regulation of Dkk3, SFRP1, and SFRP4 mRNA expression in patients with high-grade disease (P = 0.08, 0.06, and 0.05, respectfully). Furthermore, a decrease in SFRP1 and SFPR4 mRNA expression was noted in patients with a diagnosis of locoregional and distant disease recurrence. Lastly, a trend toward decreased progression-free survival in patients with low Dkk3, SFRP1, and SFRP4 mRNA expression levels was noted.
Conclusions
Wnt pathway inhibitor (Dkk3, sFRP1, and/or sFRP4) expression was down-regulated in patients with high-grade disease and was associated with locoregional and distant disease recurrence. Despite sample size (power) limitations, these results support previous preclinical studies and may suggest a therapeutic role for Wnt signaling in endometrial cancer.
Keywords: Dkk3, SFRP1, SFRP4, Uterine cancer, Wnt pathway
Endometrial carcinoma (EC) is the most common gynecologic malignancy in the United States with an estimated 52,630 new cases and 8590 deaths projected in 2013.1 Most women (80%–85%) present with early-stage disease, and surgery in the form of hysterectomy is curative. Unfortunately, however, a proportion of patients present with advanced or recurrent disease, with associated poor survival. Current cytotoxic therapies for the treatment of recurrent disease have shown limited success, with 5-year survival rates of less than 15%.2 Although recent phase II trials have shown promise with novel biologic agents (mTOR inhibitors, bevacizumab), none have shown a response rate of greater than 25%. This trend represents an unmet need in EC care.
Recently, the wingless-type (Wnt) signaling pathway has been implicated in EC pathogenesis. The Wnt pathway normally participates in physiologic processes during both embryogenesis and in the maintenance of adult hemostasis. Aberrant regulation of the Wnt pathway has been associated with a variety of human malignancies.3 This pathway has been most extensively studied in colorectal cancer, where the mutational inactivation of adenomatous polyposis coli, an important component of the Wnt pathway, is an early event in carcinogenesis.4 This ultimately results in nuclear accumulation of β-catenin, with recruitment of TCF/LEF1 (T-cell factor/lymphoid enhancer factor 1) promoting transcription of target genes involved in tumorigenesis.3 Importantly, 14% to 45% of EC specimens have been identified to have activating β-catenin mutations, although no functional relationships have been recognized.5–10 These findings suggest that other mechanisms might result in deregulation of β-catenin and altered expression of TCF target genes in EC.
Several extracellular antagonists, including secreted frizzled-related proteins (SFRPs) and Dickkopf (Dkk), regulate Wnt signaling.11 The SFRP family of proteins can competitively displace Wnt ligands from their receptors, attenuating cancer growth. The Dkk family of proteins exerts its inhibitory effects by preventing Wnt ligands from binding a functional receptor complex. In humans, the Dkk protein family comprises 4 members (Dkk1-Dkk4) and a unique Dkk3-related protein, soggy (SGY1). Importantly, all Dkk members, except SGY1, share 2 conserved cysteine-rich domains, termed Cys1 and Cys2. In addition, WIF (Wnt inhibitory factor 1) has also been shown to regulate Wnt signaling via β-catenin transcriptional regulation by directly sequestering Wnt ligands.
These Wnt inhibitors, whose tumor-suppressive properties may represent a novel therapeutic target, have been investigated recently.12,13 Specifically, Dkk3, a Wnt pathway inhibitor is down-regulated in gastrointestinal, breast, prostate, and renal carcinoma, and its role as a tumor suppressor has been investigated in both non–small cell lung cancer and osteosarcoma.14–16 In addition, SFRP1 down-regulation has been shown in microsatellite unstable EC tissue specimens.17
In this article, we explore the differential expression patterns of the Wnt inhibitors Dkk3, SFRP1, and SFRP4 and their potential role as prognosticators of aggressive disease.
MATERIALS AND METHODS
Institutional review board (IRB) approval was obtained from the University of California, Irvine, prior to the initiation of research. Gynecologic Oncology Group (GOG) protocol 210 was approved by a centralized IRB, as well as the IRB at all participating sites. Patients provided written informed consent consistent with federal, state, and local institutional requirements.
Tissue Specimens
The tissue specimens analyzed in this study were provided by the GOG via protocol 210. Samples were selected based on tumor cell content and the availability of normal müllerian tissue to act as an internal control. None of the patients received chemotherapy or endocrine treatment prior to tumor excision. Hematoxylineosin staining was used for routine pathologic evaluation. All specimens underwent central pathologic review prior to distribution (NR). The content of tumor cells in all supplied specimens was required to be at least 70%. Specimens were stored in an isothermal liquid nitrogen freezer (V1500-AB; Custom Biogenic Systems, Romeo, MI), with lid temperature maintained at −178°C.
Protein Isolation and Western Blot Analysis
Under a fumed hood, while maintained on dry ice, specimens were fragmented using disposable surgical scalpels, weighed, and placed into a 14-mL polypropylene round-bottom tube with 500 mL of RIPA lysis buffer containing protease inhibitor tablets (Sigma, St Louis, MO). Samples were homogenized using a Bio-Gen PRO200 (PRO Scientific, Oxford, CT) at full speed for 30 seconds. Prior to the processing of each specimen, the homogenizer rotor was cleansed with sequential washes in 70% alcohol, followed by ddH2O × 3. The tissue lysates were centrifuged at 12,000g for 30 minutes, and the supernatant was collected. The DC protein assay (Bio-Rad, Hercules, CA) was utilized to determine protein concentrations per package insert protocol.
Volumes of clarified protein lysate containing equal amounts of protein (100 μg) were then separated on a 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and electrophoretically transferred (90 minutes at 100 V) to a Hybond-ECL membrane (GE Healthcare, Piscataway, NJ). Blots were then blocked for 1 hour in Tris-buffered saline with Tween 20 (10 mM Tris-HCl, pH 8.0, 150 mM NaCL, and 0.05% Tween-20) containing 5% blocking grade nonfat dry milk (Bio-Rad) and incubated overnight with primary antibody at 4°C. Antibodies for Dkk3 were obtained from Santa Cruz Biotechnology, Inc (Santa Cruz, CA) and diluted 1:400. Antibodies for SFRP1 and SFRP4 were obtained from Abcam (Cambridge, MA) and diluted 1:286 and 1:200, respectively. Blots were washed 3 times in Tris-buffered saline with Tween 20 and incubated for 1.5 hours at room temperature with horse-radish peroxidase–conjugated goat anti–rabbit or anti–mouse immunoglobulin G secondary antibody at dilution of 1:200 (Santa Cruz Biotechnology). Immunoreactive bands were visualized using an enhanced chemiluminescence detection system (Thermo Scientific, Rockford, IL). All membrane was exposed to blue devil autoradiography film (Genesee Scientific, San Diego, CA) for 10 seconds prior to image development. Glyceraldehyde phosphate dehydrogenase (GAPDH) (Cell Signaling Technology, Danvers, MA) was used as a loading control.
Densitometry was calculated after each film was scanned and loaded onto Adobe Photoshop 7.0 (Adobe Systems Inc, San Jose, CA). Film images were inverted, and each band was outlined using Adobe Photoshop tools. Histogram values, including mean and pixels, were obtained. Sample values were divided by GAPDH and reported. All samples were run in triplicate, with average densitometry calculated. Results were reported as presence or absence of immunoreactive bands.
RNA Extraction and Real-Time Reverse Transcription–Polymerase Chain Reaction
Tissue samples were processed as detailed above. Tissue lysates were vortexed briefly after addition of 0.4 mL of 99% chloroform and left to rest on ice for 10 minutes. Samples were transferred to 2.0-mL centrifuge tubes and centrifuged for 15 minutes at 12,000 revolutions/min (rpm). The clear supernatant was subsequently transferred to a new 2-mL centrifuge tube, and 1 mL of 99% 2-propanol was added. The sample was vortexed and incubated at room temperature for 10 minutes. Each sample was centrifuged for 15 minutes at 12,000 rpm in a 4°C cold room. The supernatant was aspirated, and 1 mL of 75% alcohol in DEPC was added to each tube. Samples were centrifuged at 7500 rpm for 10 minutes in a cold room. The supernatant was aspirated, and the pellet resuspended in 50 μL of RNAse-free water. cDNA was synthesized from 2 μg of total RNA using a high-capacity cDNA reverse transcription kit per protocol (Applied Biosystems, Foster City, CA). Real-time polymerase chain reaction (PCR) amplification reactions for Dkk3, SFRP1, and SFRP4 were carried out using the MyiQ system (Bio-Rad) as previously described by Tang et al.18 Dkk3, SFRP1, and SFRP4 miScript primer assays were obtained from Qiagen (Valencia, CA), with primer sequences available upon request. Using the qSTAR expression detection system (OriGene, Rockville, MD), a known quantitative PCR copy-number standard curve was created for each primer evaluated. Briefly, each quantitative PCR standard was provided as a dried pellet and suspended in 50 μL of ddH2O. The resuspended standard had a known copy number of 10,000,000 copy/μL. Serial dilutions were then prepared to a maximum dilution of 10 copies/μL. A standard curve was subsequently created, allowing for copy-number detection for each sample, per package insert instructions. Each 96-well PCR plate was run with positive control primers, as well as standards for copy-number calculations. Samples were run in triplicate. Total copy number was normalized to GAPDH copy-number expression to facilitate comparison. Each experiment was carried out in triplicate.
Statistical Analysis
Marker expression levels were dichotomized by the median for each marker (less than = low; greater than or equal to = high). Specimens with messenger RNA (mRNA) copy number outside the boundaries of the standard curve were excluded from analysis. Given variability in the site of normal tissue acquisition, control samples were stratified into 3 distinct categories: myometrium, cervix and other (ovary, fallopian tube, fibromuscular tissue).
This study was exploratory, and the sample size was based on institutional pilot data. Data analysis utilized SAS/STAT software version 9.1. One-way ANOVA, F test, and Fisher exact test were used to assessed log mRNA and protein expression respectively with known prognostic factors.19 Progression-free survival (PFS) was calculated as time in months from study enrollment to disease progression or death for noncensored observations (events) or to date of last contact for censored observations. Recurrence site was classified as local if within the pelvic field; locoregional if vagina, PA lymph nodes, or abdomen; and distant otherwise. Product limit estimates were computed by using the method of Kaplan and Meier, and PFS comparisons were evaluated by using the log-rank test.20 All statistical tests were 2-sided, and given the exploratory nature of the study, P < 0.1 was considered statistically significant.
RESULTS
Cohort Characteristics
The data analysis is based on 60 tumor and 27 normal tissue specimens from 60 randomly chosen patients with early-or advanced-stage endometrial endometrioid adenocarcinoma, enrolled on GOG protocol 210 with valid tissue parameters as detailed previously. The median age for this cohort was 60 years, with median body mass index (BMI) of 32 kg/m2. Eighty-one percent of patients had grade 1 or 2 tumors, 34.5% of patients had a BMI greater than 35 kg/m2, and 48.3% of patients had stage 3 or 4 disease. In addition, 35.7% and 29.6% of patients had lymphovascular space invasion and lymph node involvement, respectively (Table 1).
TABLE 1.
Clinical characteristics
| Characteristics | n | % | |
|---|---|---|---|
| Age, y | ≤54 | 10 | 17 |
| 55–69 | 38 | 66 | |
| ≥70 | 10 | 17 | |
| BMI, kg/m2 | ≤24 | 10 | 17 |
| 25–35 | 28 | 48 | |
| ≥35 | 20 | 34 | |
| Stage | Early | 30 | 52 |
| Advanced | 28 | 48 | |
| Tumor grade | Low | 47 | 81 |
| High | 11 | 19 | |
| Tumor penetration | Inner 1/2 | 27 | 50 |
| Outer 1/2 | 24 | 44 | |
| Serosa | 3 | 6 | |
| Cytology | Negative | 44 | 83 |
| Positive | 9 | 17 | |
| LVSI | Absent | 36 | 64 |
| Present | 20 | 36 | |
| Nodal status | Negative | 38 | 70 |
| Positive | 16 | 30 |
LVSI, Lymphovascular space involvement.
Dkk3
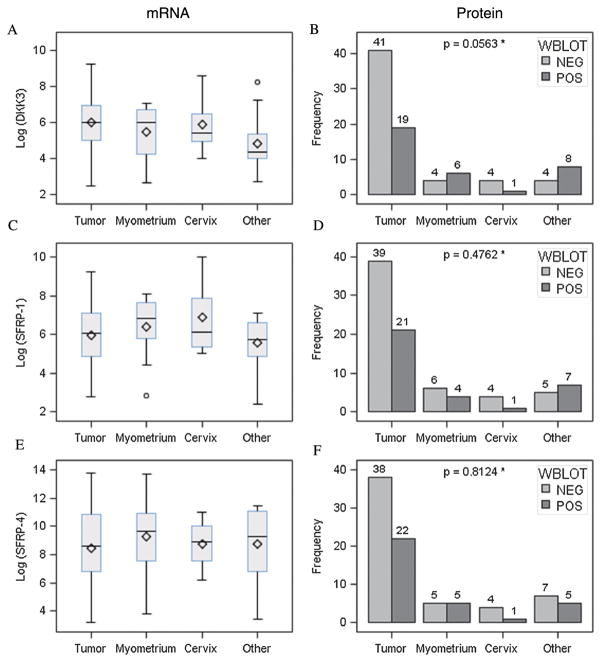
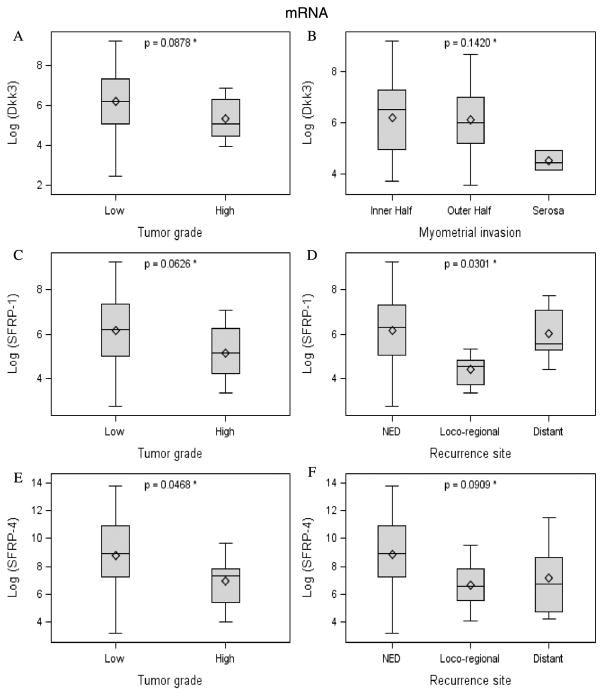
No significant differences in Dkk3 mRNA expression were noted between tumor specimens and matched normal tissue (Fig. 1A). Conversely, Western blot evaluation of protein expression did show decreased Dkk3 expression in tumor tissue samples compared with normal myometrium (P = 0.0563) (Fig. 1B). Among cancer tissue specimens, low Dkk3 expression was associated with higher tumor grade (P = 0.0878) (Fig. 2A) and obesity (BMI, 25–34 kg/m2; P = 0.05). These relationships were seen only for mRNA. No significant differences in Dkk3 protein expression were identified between early- and advanced-stage tumor samples using Western blot (data not shown).
FIGURE 1.
Dkk3, SFRP-1, and SFRP-4 expression (mRNA and protein) by tissue type.
FIGURE 2.
Dkk3, SFRP-1, and SFRP-4 expression (mRNA) by prognostic factors and recurrence site.
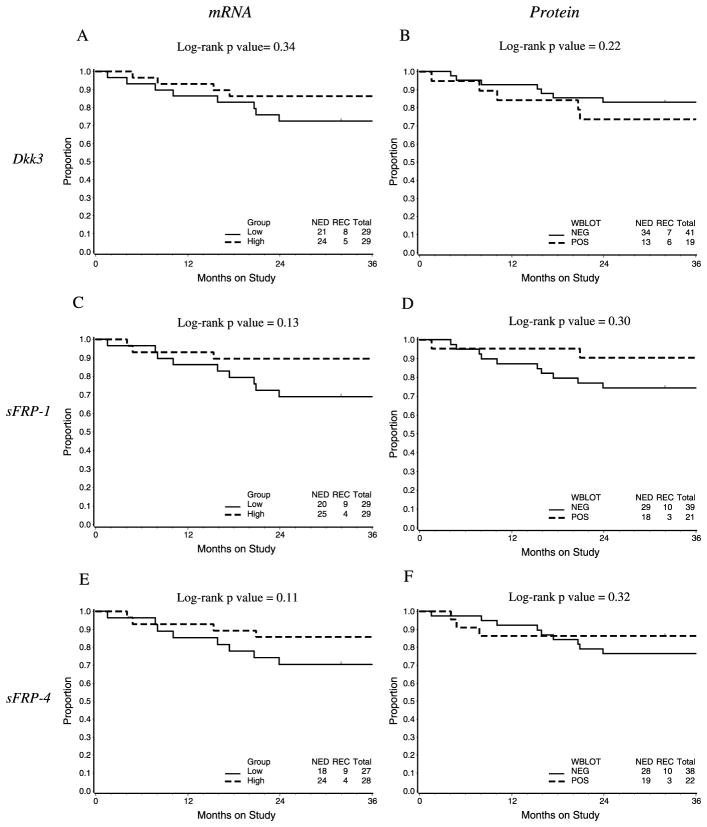
Lastly, when exploring the association between Dkk3 expression and PFS, a trend toward decreased PFS in patients with low Dkk3 mRNA expression levels was noted, although this did not reach statistical significance. This same relationship was not seen when stratified by protein expression (Figs. 3A, B).
FIGURE 3.
Progression-free survival by mRNA (categorized as below median [Low] or above median) and protein (WBLOT, negative [or positive] expression).
SFRP1
No significant differences in SFRP1 mRNA or protein expression levels were noted between tumor specimens and matched normal müllerian tissues (Figs. 1C, D). When looking specifically at tumor samples, low SFRP1 mRNA expression was associated with higher grade (P = 0.0626) and site of disease recurrence (P = 0.0301) (Figs. 2C, D).
When PFS was evaluated, decreased SFRP1 expression (both protein and mRNA) was associated with a nonsignificant decrease in PFS (Figs. 3C, D).
SFRP4
No significant differences in SFRP4 mRNA or protein expression levels were noted between tumor specimens and matched normal müllerian tissues (Figs. 1E, F). Within the cohort of cancer specimens, decreased SFRP4 mRNA expression was associated with higher tumor grade (P = 0.0468; Fig. 2E). Furthermore, a decrease in SFPR4 mRNA expression was identified in patients with a diagnosis of locoregional and distant disease recurrence (P = 0.0909; Fig. 2F). These relationships were not seen on a protein level.
In addition, evaluation of PFS in the cohort of EC patients illustrated a trend toward decreased PFS in patients with reduced SFP4 mRNA and protein expression, although this did not reach statistical significance (Figs. 3E, F).
DISCUSSION
The canonical Wnt signaling pathway has been associated with EC as indicated by the identification of β-catenin mutations in up to 45% of early-stage endometrial cancers. However, the implications of β-catenin mutations, as well as the contribution of Wnt pathway inhibitors in endometrial cancer, are poorly defined. Specifically, few studies exploring Dkk3, SFRP1, and SFRP4 expression in both tumor and matched normal tissues have been conducted.
The implication of Dkk3 in tumorigenesis was first described by Tsuji et al,21 who showed decreased Dkk3 expression in several human cancer cell lines. Since that time, several authors have detailed decreased Dkk3 expression in solid tumors including non–small cell lung, prostate, colon, and breast cancers, as well as hematologic malignancies.22–25 Data exploring Dkk3 expression specifically in gynecologic malignancies are limited. Jiang et al26 investigated serum Dkk3 expression using enzyme-linked immunosorbent assays in patients with cervical cancer, ovarian cancer, and EC. The serum levels of Dkk3 protein in patients with EC were higher than those in control subjects. The etiology behind the discrepancy in serum and tissue expression of Dkk3 in patients with solid malignancies is unclear at this time. While deregulation of Dkk family members is a common feature of several human tumors, suppression of Dkk3 appears to be shared alteration among many cancer types.27 More recently, Dkk3 gene expression was shown to be down-regulated in a cohort of early- and advanced-stage EC tissue specimens, with in vitro assays confirming the suppressive effects of Dkk3 expression on Wnt pathway throughput, tumor growth, and invasiveness.28
Our data illustrated similar Dkk3 mRNA expression levels in both normal and matched early-stage endometrial endometrioid adenocarcinoma tissue samples. However, there was a decrease in Dkk3 protein expression in tumor samples relative to control tissue (P = 0.05). Within the cohort of early- and advanced-stage specimens, decreased Dkk3 expression was associated with higher tumor grade, deep myometrial or serosal invasion, and obesity. These relationships were seen on an mRNA level.
The discrepancy between mRNA and protein expression is not uncommon and can be explained by posttranslational modification, differential splicing, intracellular localization, molecular association, and variable product half-lives.19 In eloquent studies carried out in yeast, similar mRNA expression levels resulted in protein concentrations varying by more than 20-fold.20 Thus, additional molecular studies are warranted to better understand posttranscriptional regulation of Wnt pathway inhibitors.
In an analogous manner, the impact of SFRPs on cancer progression and prognosis has been described. Secreted frizzled-related proteins are soluble mediators that bind Wnt ligands in the extracellular domain, thus inhibiting signal transduction and β-catenin accumulation.29 Abundant SFRP1 expression was initially identified in normal breast tissue.30 Since that time, SFRP1 silencing has been shown in colon, ovarian, bladder, prostate, and lung cancers and has been associated with decreased overall survival in patients with early-stage breast cancer.31–34 With respect to SFRP4, Hrzenjak et al35 reported reduced mRNA expression levels in both low-grade endometrial stromal sarcomas and undifferentiated endometrial sarcomas, when compared with normal endometrium. Additional in vitro data demonstrated decreased endometrial cancer cell growth following SFRP4 transfection and over-expression, mediated via inhibition of Wnt7a.36
Our data illustrated similar SFRP1 and SFRP4 mRNA and protein expression levels in both normal and matched early-stage endometrial endometrioid adenocarcinoma tissue samples. Conversely, within the cohort of early- and advanced-stage specimens, decreased SFRP1 and SFRP4 expression was associated with increased grade, as well as disease recurrence.
Given the number of samples evaluated and the proportion of patients with early-stage disease, correlation between Wnt inhibitor expression levels and overall survival was not feasible. When exploring the association between Wnt inhibitor expression and PFS, a trend toward decreased PFS in patients with low Dkk3, SFRP1, and SFRP4 mRNA expression levels was noted. Taken together, relative expression of these Wnt inhibitors may assist in risk stratification and prognostication of patients with EC.
Interestingly, there also appears to be cross-talk between the canonical Wnt pathway and mTOR signaling.37,38 In light of recent clinical trials suggesting modest activity of mTOR inhibitors in advanced and recurrent EC,39–43 a role for combined mTOR and Wnt pathway targeting may emerge. Currently, several agents designed to target the canonical Wnt pathway are in various stages of development.27 Potential therapeutic agents acting through canonical Wnt signaling have been suggested, including nonsteroidal anti-inflammatory drugs,44,45 vitamins,46,47 polyphenols, recombinant SFRP proteins,48 and molecular targeted drugs.49 Ultimately, exploration of Wnt inhibition in EC xenograft models may help clarify the therapeutic potential of these agents and shed light on the impact of combination treatment with cytotoxic chemotherapy. Currently, limited data exist on the use of Wnt inhibitors in combination with chemotherapy (5-fluorouracil) in various colorectal cancer cell lines.50
CONCLUSIONS
In summary, our data indicate a potential role for Wnt inhibitors in EC pathogenesis. Despite a lack of differential expression between early-stage tumor tissue and matched control müllerian tissue samples, association between decreased expression and several clinicopathological variables was shown. Although the sample size was small, the strengths of this study include the use of matched normal tissues, centralized pathologic review confirming the histologic diagnosis and tumor grade, multi-institutional collaboration, and detailed molecular analysis. It is unclear why discrepancies existed between mRNA and protein expression levels for all markers evaluated in this study. Future assessment of epigenetic modifications, such as promoter methylation and the contribution of gene silencing of Wnt pathway inhibitors on outcomes in EC, is warranted. The signals identified in this small patient cohort support future exploration into the role of Wnt inhibitors in EC, as both bio-markers and potential therapeutic targets.
Acknowledgments
This study was supported by an institutional NIH T-32 training grant (Ruth L. Kirschstein NRSA Institutional Training Research Grant, 2 T32 CA06039611), National Cancer Institute award P30CA062203, and was also supported in part by National Cancer Institute grants to the Gynecologic Oncology Group (GOG) Administrative Office (CA 27469) and the GOG Statistical and Data Center (CA 37517). The GOG Tissue Bank is supported by NCI U24 CA114793.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Obel JC, Friberg G, Fleming GF. Chemotherapy in endometrial cancer. Clin Adv Hematol Oncol. 2006;4:459–468. [PubMed] [Google Scholar]
- 3.Gatcliffe TA, Monk BJ, Planutis K, et al. Wnt signaling in ovarian tumorigenesis. Int J Gynecol Cancer. 2008;18:954–962. doi: 10.1111/j.1525-1438.2007.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda T, Yoshinaga K, Semba S, et al. Mutational analysis of the CTNNB1 (beta-catenin) gene in human endometrial cancer: frequent mutations at codon 34 that cause nuclear accumulation. Oncol Rep. 2000;7:323–326. doi: 10.3892/or.7.2.323. [DOI] [PubMed] [Google Scholar]
- 6.Machin P, Catasus L, Pons C, et al. CTNNB1 mutations and beta-catenin expression in endometrial carcinomas. Hum Pathol. 2002;33:206–212. doi: 10.1053/hupa.2002.30723. [DOI] [PubMed] [Google Scholar]
- 7.Moreno-Bueno G, Hardisson D, Sánchez C, et al. Abnormalities of the APC/beta-catenin pathway in endometrial cancer. Oncogene. 2002;21:7981–7990. doi: 10.1038/sj.onc.1205924. [DOI] [PubMed] [Google Scholar]
- 8.Nei H, Saito T, Yamasaki H, et al. Nuclear localization of beta-catenin in normal and carcinogenic endometrium. Mol Carcinog. 1999;25:207–218. [PubMed] [Google Scholar]
- 9.Schlosshauer PW, Pirog EC, Levine RL, et al. Mutational analysis of the CTNNB1 and APC genes in uterine endometrioid carcinoma. Mod Pathol. 2000;13:1066–1071. doi: 10.1038/modpathol.3880196. [DOI] [PubMed] [Google Scholar]
- 10.Scholten AN, Creutzberg CL, van den Broek LJ, et al. Nuclear beta-catenin is a molecular feature of type I endometrial carcinoma. J Pathol. 2003;201:460–465. doi: 10.1002/path.1402. [DOI] [PubMed] [Google Scholar]
- 11.You A, Fokas E, Wang LF, et al. Expression of the Wnt antagonist Dkk3 is frequently suppressed in sporadic epithelial ovarian cancer. J Cancer Res Clin Oncol. 2011;137:621–627. doi: 10.1007/s00432-010-0916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawasaki K, Watanabe M, Sakaguchi M, et al. REIC/Dkk-3 overexpression downregulates P-glycoprotein in multidrug-resistant MCF7/ADR cells and induces apoptosis in breast cancer. Cancer Gene Ther. 2009;16:65–72. doi: 10.1038/cgt.2008.58. [DOI] [PubMed] [Google Scholar]
- 13.Lee EJ, Jo M, Rho SB, et al. Dkk3, downregulated in cervical cancer, functions as a negative regulator of beta-catenin. Int J Cancer. 2009;124:287–297. doi: 10.1002/ijc.23913. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh SY, Hsieh PS, Chiu CT, et al. Dickkopf-3/REIC functions as a suppressor gene of tumor growth. Oncogene. 2004;23:9183–9189. doi: 10.1038/sj.onc.1208138. [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi M, Kataoka K, Abarzua F, et al. Overexpression of REIC/Dkk-3 in normal fibroblasts suppresses tumor growth via induction of interleukin-7. J Biol Chem. 2009;284:14236–14244. doi: 10.1074/jbc.M808002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuji T, Nozaki I, Miyazaki M, et al. Antiproliferative activity of REIC/Dkk-3 and its significant down-regulation in non–small-cell lung carcinomas. Biochem Biophys Res Commun. 2001;289:257–263. doi: 10.1006/bbrc.2001.5972. [DOI] [PubMed] [Google Scholar]
- 17.Risinger JI, Maxwell GL, Chandramouli GV, et al. Gene expression profiling of microsatellite unstable and microsatellite stable endometrial cancers indicates distinct pathways of aberrant signaling. Cancer Res. 2005;65:5031–5037. doi: 10.1158/0008-5472.CAN-04-0850. [DOI] [PubMed] [Google Scholar]
- 18.Tang Y, Simoneau AR, Xie J, et al. Effects of the kava chalcone flavokawain A differ in bladder cancer cells with wild-type versus mutant p53. Cancer Prev Res (Phila) 2008;1:439–451. doi: 10.1158/1940-6207.CAPR-08-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwanhäusser B, Busse D, Li N, et al. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- 20.Gygi SP, Rochon Y, Franza BR, et al. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19:1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuji T, Miyazaki M, Sakaguchi M, et al. A REIC gene shows down-regulation in human immortalized cells and human tumor-derived cell lines. Biochem Biophys Res Commun. 2000;268:20–24. doi: 10.1006/bbrc.1999.2067. [DOI] [PubMed] [Google Scholar]
- 22.Abarzua F, Sakaguchi M, Takaishi M, et al. Adenovirus-mediated overexpression of REIC/Dkk-3 selectively induces apoptosis in human prostate cancer cells through activation of c-Jun–NH2-kinase. Cancer Res. 2005;65:9617–9622. doi: 10.1158/0008-5472.CAN-05-0829. [DOI] [PubMed] [Google Scholar]
- 23.Chim CS, Pang R, Liang R. Epigenetic dysregulation of the Wnt signalling pathway in chronic lymphocytic leukaemia. J Clin Pathol. 2008;61:1214–1219. doi: 10.1136/jcp.2008.060152. [DOI] [PubMed] [Google Scholar]
- 24.Nozaki I, Tsuji T, Iijima O, et al. Reduced expression of REIC/ Dkk-3 gene in non-small cell lung cancer. Int J Oncol. 2001;19:117–121. doi: 10.3892/ijo.19.1.117. [DOI] [PubMed] [Google Scholar]
- 25.Veeck J, Bektas N, Hartmann A, et al. Wnt signalling in human breast cancer: expression of the putative Wnt inhibitor Dickkopf-3 (Dkk3) is frequently suppressed by promoter hypermethylation in mammary tumours. Breast Cancer Res. 2008;10:R82. doi: 10.1186/bcr2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang T, Huang L, Wang S, et al. Clinical significance of serum Dkk-3 in patients with gynecological cancer. J Obstet Gynaecol Res. 2010;36:769–773. doi: 10.1111/j.1447-0756.2010.01234.x. [DOI] [PubMed] [Google Scholar]
- 27.Veeck J, Dahl E. Targeting the Wnt pathway in cancer: the emerging role of Dickkopf-3. Biochim Biophys Acta. 2012;1825:18–28. doi: 10.1016/j.bbcan.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Dellinger TH, Planutis K, Jandial DD, et al. Expression of the Wnt antagonist Dickkopf-3 is associated with prognostic clinicopathologic characteristics and impairs proliferation and invasion in endometrial cancer. Gynecol Oncol. 2012;126:259–267. doi: 10.1016/j.ygyno.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veeck J, Niederacher D, An H, et al. Aberrant methylation of the Wnt antagonist SFRP1 in breast cancer is associated with unfavourable prognosis. Oncogene. 2006;25:3479–3488. doi: 10.1038/sj.onc.1209386. [DOI] [PubMed] [Google Scholar]
- 30.Finch PW, He X, Kelley MJ, et al. Purification and molecular cloning of a secreted, Frizzled-related antagonist of Wnt action. Proc Natl Acad Sci U S A. 1997;94:6770–6775. doi: 10.1073/pnas.94.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klopocki E, Kristiansen G, Wild PJ, et al. Loss of SFRP1 is associated with breast cancer progression and poor prognosis in early stage tumors. Int J Oncol. 2004;25:641–649. [PubMed] [Google Scholar]
- 32.Marsit CJ, Karagas MR, Andrew A, et al. Epigenetic inactivation of SFRP genes and TP53 alteration act jointly as markers of invasive bladder cancer. Cancer Res. 2005;65:7081–7085. doi: 10.1158/0008-5472.CAN-05-0267. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki H, Watkins DN, Jair KW, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36:417–422. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- 34.Takada T, Yagi Y, Maekita T, et al. Methylation-associated silencing of the Wnt antagonist SFRP1 gene in human ovarian cancers. Cancer Sci. 2004;95:741–744. doi: 10.1111/j.1349-7006.2004.tb03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hrzenjak A, Tippl M, Kremser ML, et al. Inverse correlation of secreted frizzled-related protein 4 and beta-catenin expression in endometrial stromal sarcomas. J Pathol. 2004;204:19–27. doi: 10.1002/path.1616. [DOI] [PubMed] [Google Scholar]
- 36.Carmon KS, Loose DS. Secreted frizzled-related protein 4 regulates two Wnt7a signaling pathways and inhibits proliferation in endometrial cancer cells. Mol Cancer Res. 2008;6:1017–1028. doi: 10.1158/1541-7786.MCR-08-0039. [DOI] [PubMed] [Google Scholar]
- 37.Boras-Granic K, Wysolmerski JJ. Wnt signaling in breast organogenesis. Organogenesis. 2008;4:116–122. doi: 10.4161/org.4.2.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ayyanan A, Civenni G, Ciarloni L, et al. Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc Natl Acad Sci U S A. 2006;103:3799–3804. doi: 10.1073/pnas.0600065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oza AM, Elit L, Tsao MS, et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J Clin Oncol. 2011;29:3278–3285. doi: 10.1200/JCO.2010.34.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mackay H, Welch S, Tsao MS, et al. Phase II study of oral ridaforolimus in patients with metastatic and/or locally advanced recurrent endometrial cancer: NCIC CTG IND 192. J Clin Oncol. 2011;29 Abstract 5013. [Google Scholar]
- 41.Slomovitz BM, Jiang Y, Yates MS, et al. A phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma. J Clin Oncol. 2015;33:930–936. doi: 10.1200/JCO.2014.58.3401. abstract 5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fleming GF, Filiaci VL, Hanjani P, et al. Hormone therapy plus temsirolimus for endometrial carcinoma (EC): Gynecologic Oncology Group trial #248. J Clin Oncol. 2011;29 abstract 5014. [Google Scholar]
- 43.Oza AM, Poveda A, Clamp AR, et al. A randomized phase II (RP2) trial of ridaforolimus (R) compared with progestin (P) or chemotherapy (C) in female adult patients with advanced endometrial carcinoma. J Clin Oncol. 2011;29 abstract 5009. [Google Scholar]
- 44.Boon EM, Keller JJ, Wormhoudt TA, et al. Sulindac targets nuclear beta-catenin accumulation and Wnt signalling in adenomas of patients with familial adenomatous polyposis and in human colorectal cancer cell lines. Br J Cancer. 2004;90:224–229. doi: 10.1038/sj.bjc.6601505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dihlmann S, Siermann A, von Knebel Doeberitz M. The nonsteroidal anti-inflammatory drugs aspirin and indomethacin attenuate beta-catenin/TCF-4 signaling. Oncogene. 2001;20:645–653. doi: 10.1038/sj.onc.1204123. [DOI] [PubMed] [Google Scholar]
- 46.Pendás-Franco N, Aguilera O, Pereira F, et al. Vitamin D and Wnt/beta-catenin pathway in colon cancer: role and regulation of DICKKOPF genes. Anticancer Res. 2008;28:2613–2623. [PubMed] [Google Scholar]
- 47.Pálmer HG, González-Sancho JM, Espada J, et al. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154:369–387. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeAlmeida VI, Miao L, Ernst JA, et al. The soluble Wnt receptor Frizzled8CRD-hFc inhibits the growth of teratocarcinomas in vivo. Cancer Res. 2007;67:5371–5379. doi: 10.1158/0008-5472.CAN-07-0266. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi-Yanaga F, Kahn M. Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clin Cancer Res. 2010;16:3153–3162. doi: 10.1158/1078-0432.CCR-09-2943. [DOI] [PubMed] [Google Scholar]
- 50.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]