A retrospective review of 382 patients with localized extremity or truncal soft tissue sarcoma who underwent limb-sparing surgery and radiotherapy was performed. There were no differences in the rates of local or distant recurrence or of any survival outcome on the basis of the quantitative width of the surgical margin, provided that it was negative.
Keywords: Soft tissue sarcoma, Extremity, Margin width, Radiation therapy, Outcome
Abstract
Background and Objectives.
It is unclear whether the quantitative width of the surgical margin influences outcomes in patients with extremity and truncal soft tissue sarcoma (STS) treated with radiotherapy (RT).
Methods.
We performed a retrospective review of 382 patients with localized extremity or truncal STS who underwent limb-sparing surgery and RT from 1983 to 2010, and we analyzed the significance of resection margin status and quantitative margin width on outcomes.
Results.
Surgical margins were positive in 68 (18%) patients and negative in 314 (82%) patients. For those patients with a reported quantitative margin width (n = 235), the width of the negative margin was ≤1 mm (n = 128), >1 mm and ≤5 mm (n = 79), and >5 mm (n = 28). At a median follow-up of 82 months, the local recurrence rates were 5.4% and 11.8% for margin-negative and margin-positive patients, respectively. There were no differences in the rates of local or distant recurrence nor of any survival outcome based on the quantitative width of the surgical margin, provided that it was negative.
Conclusions.
In patients undergoing RT and limb-sparing surgery for STS, achieving a negative margin is essential for optimizing both local control and survival. However, the absolute quantitative width of the negative margin does not significantly influence outcome, and so attempts at wide margins of resection appear to be unnecessary. Importantly, the conclusions drawn from this study must not be applied to those patients undergoing surgery alone as the local treatment of their STS, in which case wider margins of resection may be necessary.
Implications for Practice:
In patients undergoing radiation therapy and limb-sparing surgery for soft tissue sarcoma, the quantitative width of the negative margin does not influence outcome, and so attempts at wide margins of resection appear to be unnecessary, especially when such attempts compromise the functional outcome. Importantly, the conclusions drawn from this study must not be applied to those patients undergoing surgery alone as the local treatment of their soft tissue sarcoma, in which case wider margins of resection may be necessary.
Introduction
The primary treatment of localized extremity and truncal soft tissue sarcoma (STS) is wide excision of the tumor, together with a rim of normal tissue to ensure microscopically negative resection margins. Indeed, it is well established that the extent of resection influences the local control of STS, with several authors demonstrating that tumors resected with positive margins have a significantly higher rate of local recurrence [1–9]. In cases in which the margins of resection are anticipated to be narrow, if not microscopically positive, such as along critical neurovascular structures, radiation therapy (RT) is considered appropriate to optimize local control and to permit function-preserving, limb-sparing surgery. Adjuvant RT delivered by external beam or brachytherapy has been shown in prospective randomized trials to improve the rates of local control for extremity and truncal STS [6, 10]. Although some authors have reported that margin status does not influence the risk of local recurrence (LR) when RT is given [11], many others have found an increased risk of LR when RT is administered in conjunction with surgical resection with positive margins [4, 12–14]. Together, these findings suggest that although microscopic disease left behind at the time of surgery may in some cases be sterilized by RT, this may be incomplete, and LR is still a significant risk under such circumstances, and so achieving an optimal, negative surgical margin is paramount.
The real question is what constitutes an “optimal” margin? How wide a surgical margin is needed to ensure the lowest risk of LR while still preserving function? This question has never been satisfactorily addressed in the literature, such that the National Comprehensive Cancer Network guidelines for surgery for STS conclude the following: “The surgical procedure necessary to resect the tumor with appropriately negative margins should be used. Close margins may be necessary to preserve uninvolved critical neurovascular structures, bones, joints, etc.” [15]. What constitutes an “appropriate” negative margin? Is a 1-mm margin (or less) adequate, or is a 2-mm, 5-mm, or some other quantitative margin width most appropriate? Two groups to our knowledge have attempted to address this issue of the optimal quantitative margin width for STS. Dickinson et al. [16], in a single institution retrospective study, concluded that the widest surgical margin possible should be attempted to improve local control and overall survival. However, these authors did not include the distribution of the grades of the tumors included in their analysis nor other important treatment factors, such as which patients received adjuvant RT and/or chemotherapy. In another single-institution retrospective study, McKee et al. [17] found that margins ≥10 mm independently predicted a longer local recurrence-free interval and were thus deemed “optimal” for extremity STS resection. However, although most patients in this study had large, deep, high-grade tumors, few (38%) received RT, which we would argue is not in keeping with the standard approach to such sarcomas in most specialized centers today. Furthermore, it is virtually impossible to achieve a margin width of 10 mm or more for the vast majority of large, high-grade, deep-seated STS, and so defining an optimal margin width of 10 mm is not clinically feasible in most patients.
In this single-institution retrospective study, we sought to determine the prognostic significance of the surgical margin status and of the quantitative margin width on LR, distant recurrence (DR), and survival in a homogeneous cohort of patients with predominantly large, high-grade tumors treated with limb-sparing surgery and RT.
Materials and Methods
From our institutional sarcoma database, we identified 382 patients who underwent complete macroscopic (R0 or R1) resection and RT of a localized STS of the trunk or extremity at the Massachusetts General Hospital (MGH) from 1983 to 2010. Patients were excluded if they underwent their definitive surgical resection at a facility other than MGH, underwent an incomplete (R2) resection, or had an amputation. Patients were also excluded if they had metastatic disease at the time of presentation or if they did not receive RT as a component of their treatment. Patients who received some or all of their RT at a facility other than MGH were included. Patient follow-up was complete and was obtained from clinical chart review and tumor registry information. Each patient was thoroughly evaluated prior to treatment with a history and physical examination, routine blood tests, either computed tomography (CT) of the chest or chest x-ray to exclude the presence of pulmonary metastases, and magnetic resonance imaging of the primary site (at least for those patients treated in the more recent time period). The histologic diagnosis was established by a review of the pathology slides by a pathologist with expertise in soft tissue sarcomas. Note that the pathology slides were not rereviewed for the purpose of this study, such that the histologic classification of a sarcoma as a malignant fibrous histiocytoma (MFH) was not reclassified. Data concerning pathologic findings were obtained from the pathology reports, with special emphasis on the status of the microscopic margin, including the numerical width of the closest margin. Tumor size was measured as the maximum diameter of the fresh resected tumor specimen, and tumor grade was classified according to the National Cancer Institute grading system using three tiers, and tumors with overlapping grades were classified at the higher tier. Pathology reports of surgical specimens were evaluated in conjunction with operative reports to determine the type of resection and margin status. Resections were classified as being R0 (macroscopically complete with negative microscopic margins), R1 (macroscopically complete with positive microscopic margins), or R2 (macroscopically incomplete). A positive microscopic margin was defined as tumor present at the inked surface of the specimen. The closest identified margin of resection was classified as positive (tumor at ink), ≤1 mm, >1 mm and ≤5 mm, or >5 mm.
For those patients receiving neoadjuvant therapy, surgical resection was planned approximately 4–6 weeks after the completion of preoperative RT or preoperative chemoradiation therapy. Tumors were resected with the intent of limb salvage with negative margins (R0 resection). The biopsy site was excised en bloc with the definitive surgical specimen. The wounds were either closed primarily or reconstructed with rotational flaps, with or without a skin graft. For those patients receiving postoperative RT, this was typically initiated from 4 to 8 weeks after surgery.
Patients were routinely seen in follow-up at our multidisciplinary Connective Tissue Oncology Clinic. Patients were typically seen at 1 month and 3 months after the end of treatment, then every 3 months for the next 2 years, every 4 months during year 3, every 6 months for years 4 and 5, and then yearly thereafter, typically until year 10. Of course, the radiographic follow-up evolved over the long time period of this study such that in the more recent time period we routinely obtained imaging of the chest (typically by chest CT scan) at least every 6 months for the first 5 years of follow-up, and magnetic resonance imaging of the primary site was obtained as clinically indicated.
Data were analyzed for associations between microscopic margin status and numerical margin width and outcomes, including rates of LR, DR, local recurrence-free survival (LRFS), disease-free survival (DFS), and overall survival (OS). Local recurrence was defined as any tumor recurrence at the same tumor site as the primary tumor. All other tumor recurrences were defined as distant metastases. Dates of death for patients with social security numbers were obtained from the Social Security Death Index (SSDI). LRFS was calculated from the date of treatment initiation to the date of first local recurrence. DFS was calculated from the date of treatment initiation to the date of documented recurrence of any kind. OS was calculated from the date of treatment initiation to the date of documented death by SSDI. Censoring occurred at the earlier date of death or date of last contact. Estimates for LRFS, DFS, and OS rates were calculated by using the method of Kaplan and Meier. Unadjusted intergroup comparisons based on each outcome were made by using the log-rank test. Multivariate analyses of patient and tumor characteristics relative to survival and recurrence-free intervals were performed by the Cox Proportional Hazards Model. All reported p values are two sided, using a significance threshold of .05. Statistical analyses were performed by using SPSS 20.0 (IBM, Chicago, IL, http://www-01.ibm.com/support/docview.wss?uid=swg24029274).
Results
Patient, Tumor, and Treatment Characteristics
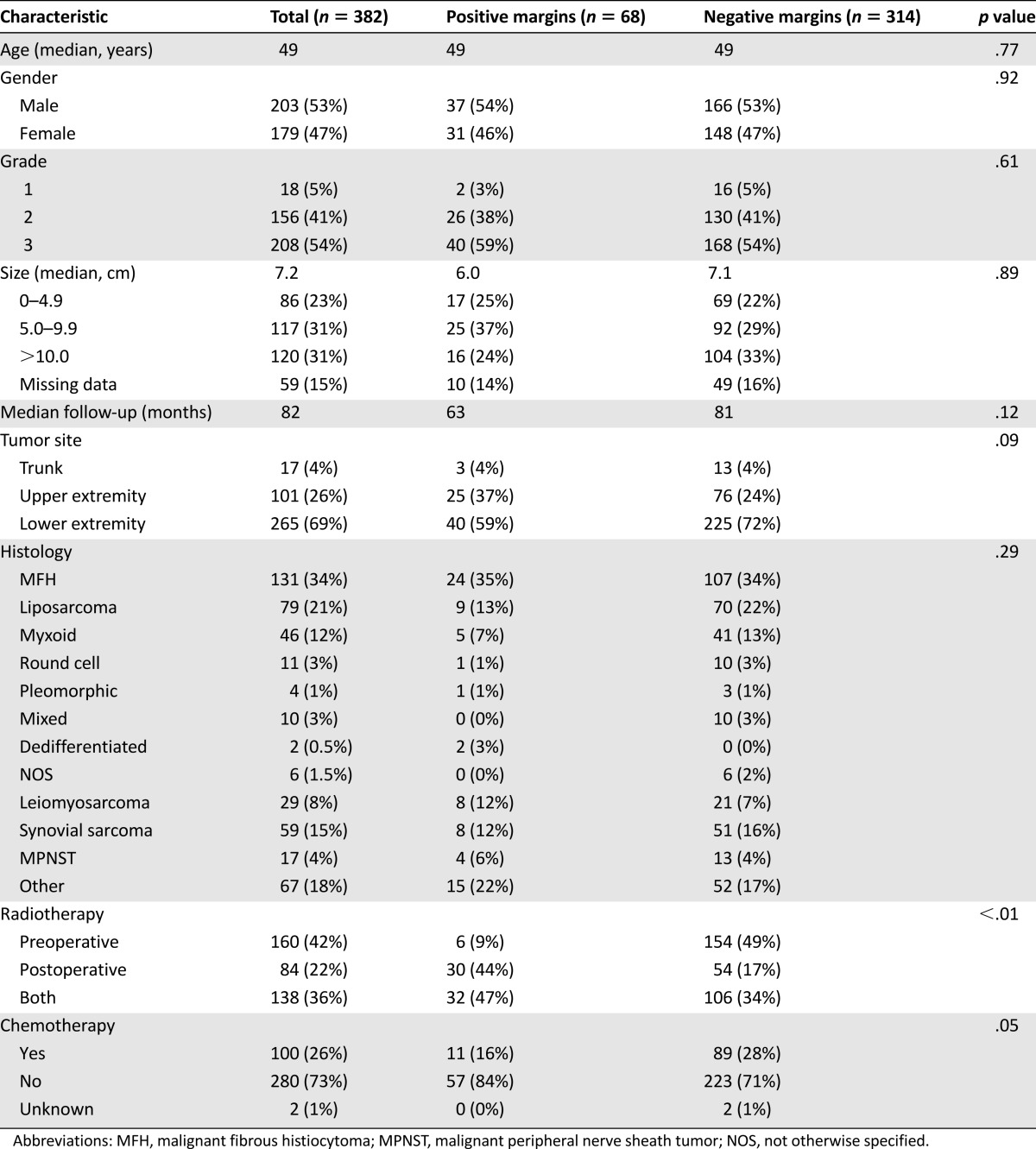
Patient, tumor, and treatment characteristics are detailed in Table 1. The median age of the cohort was 49 years, and 53% were male. The majority (69%) of the tumors were located in the lower extremity. The most common tumor histologies were MFH (34%), liposarcoma (21%), and synovial sarcoma (15%). The median tumor size was 7.2 cm, and 95% of the tumors were either grade 2 or 3.
Table 1.
Patient, tumor, and treatment characteristics according to resection margin status
All patients received radiotherapy, including 160 (42%) who underwent preoperative RT, 84 (22%) who received postoperative RT, and 138 (36%) who received both preoperative and postoperative RT. Although the treatment regimens varied slightly, the median total dose received was 59 Gy, and therapy was administered in a median number of 28 fractions. Those receiving preoperative RT received a median of 48.8 Gy, with 138 patients receiving a median of 18 Gy as additional postoperative therapy. Those receiving postoperative RT alone were administered a median of 64 Gy. Most therapy was delivered as external beam radiation, but 13 patients received brachytherapy via implants and 4 underwent intraoperative administration as part of their regimen. One hundred (26%) patients also received chemotherapy with a variety of regimens, although a majority of patients received both preoperative and postoperative mesna, doxorubicin, ifosfamide, and dacarbazine, according to a protocol carried out at our institution for many years [18].
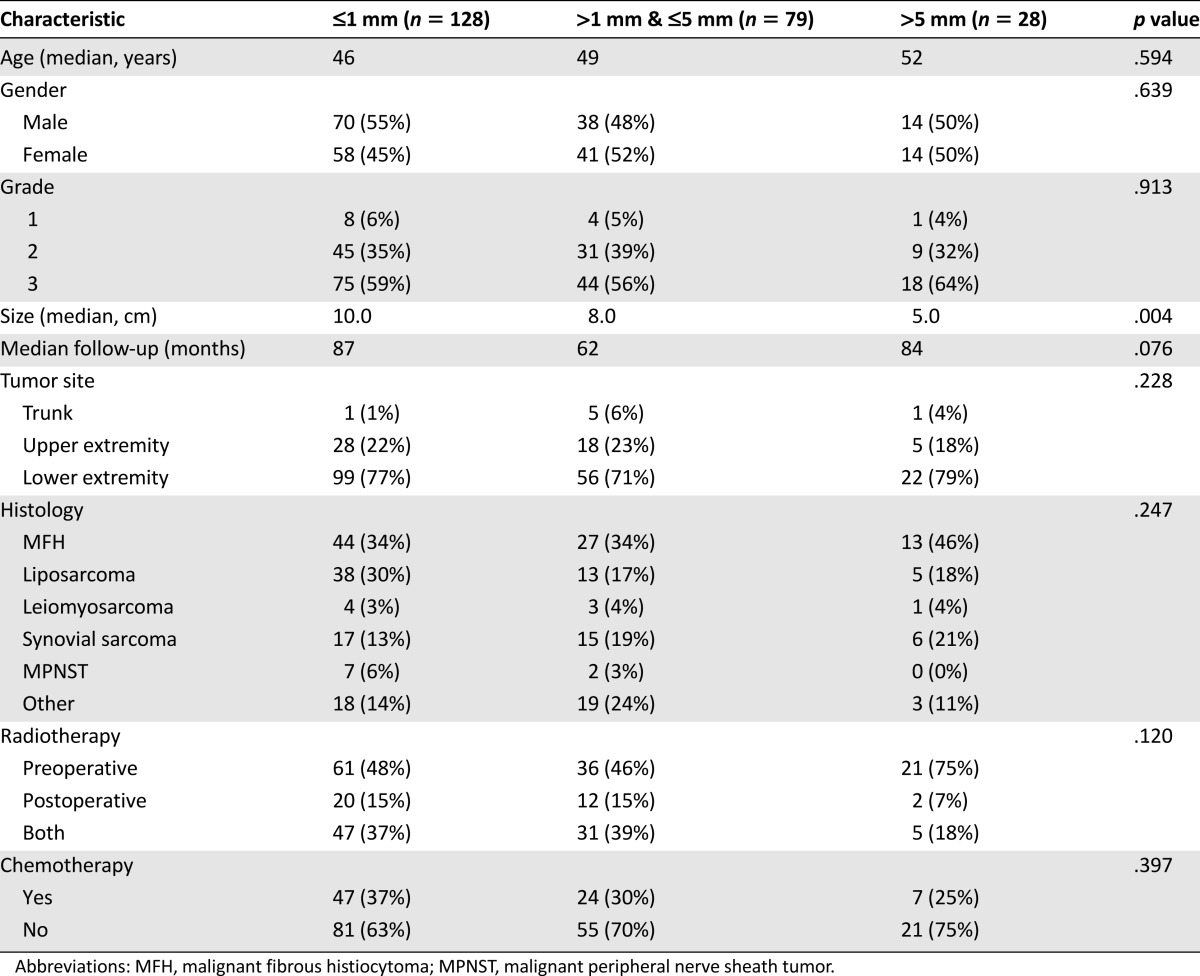
Overall, 82% of the patients had margin-negative (R0) resections, and 18% of the patients had margin-positive (R1) resections. Patients with positive margins did not differ significantly from those with negative margins in terms of demographic data or the histopathological characteristics of their tumors (Table 1). However, patients who had negative margins were significantly more likely to have received preoperative RT than were patients who had positive margins (83% vs. 56%; p < .01). The R1 resection rate for patients receiving all of their RT postoperatively was 39%, whereas those receiving some or all of their RT preoperatively had a much lower R1 resection rate of 13% (p < .01). A quantitative margin width was not available for 79 of the 314 (25%) patients who underwent negative-margin resections for the following reasons: 62 patients had no residual tumor in the resected specimen, and so calculating a margin width was obviously not possible in these cases; 8 patients had separately excised final margins from which an accurate numerical width could not be determined; and 9 patients had margins reported simply as “negative” without a numeric width. For those patients with a reported quantitative margin width (n = 235), the width of the negative margin was ≤1 mm in 128 (55%) patients, >1 mm and ≤5 mm in 79 (33%) patients, and >5 mm in 28 (12%) patients. The three quantitative margin width groups were similar in most respects, although the tumor size was significantly smaller for tumors in which wider margins were achieved (5.0 cm for margins >5 mm vs. 10.0 cm for margins ≤1 mm; p = .004) (Table 2).
Table 2.
Patient, tumor, and treatment characteristics according to resection margin width
Influence of Surgical Margins on Outcomes
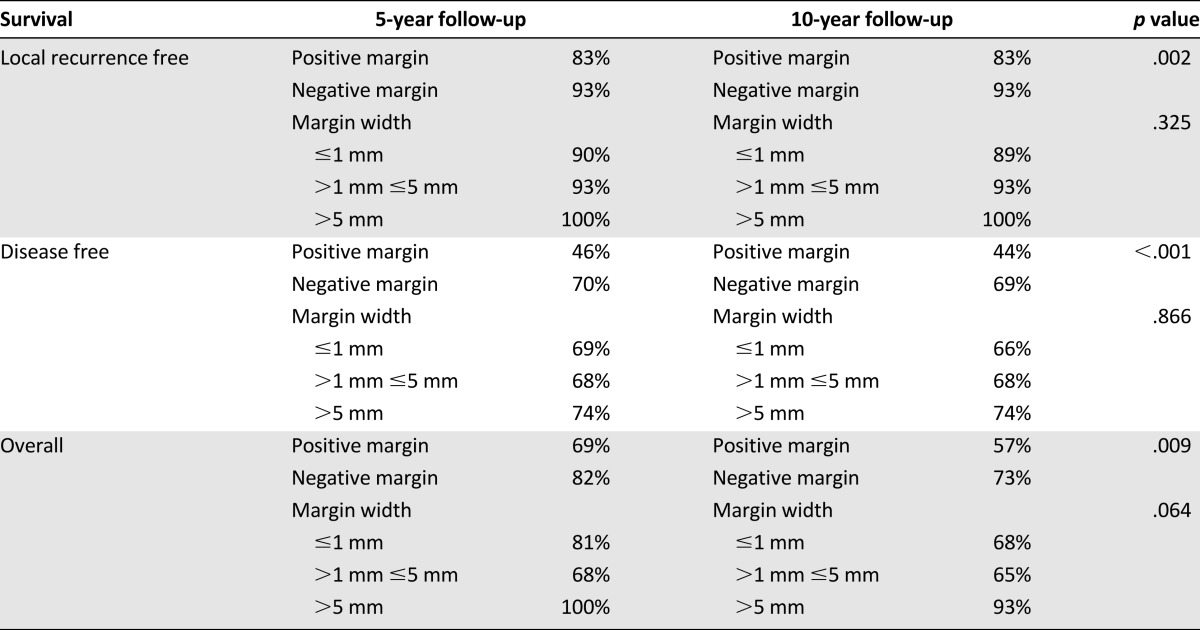
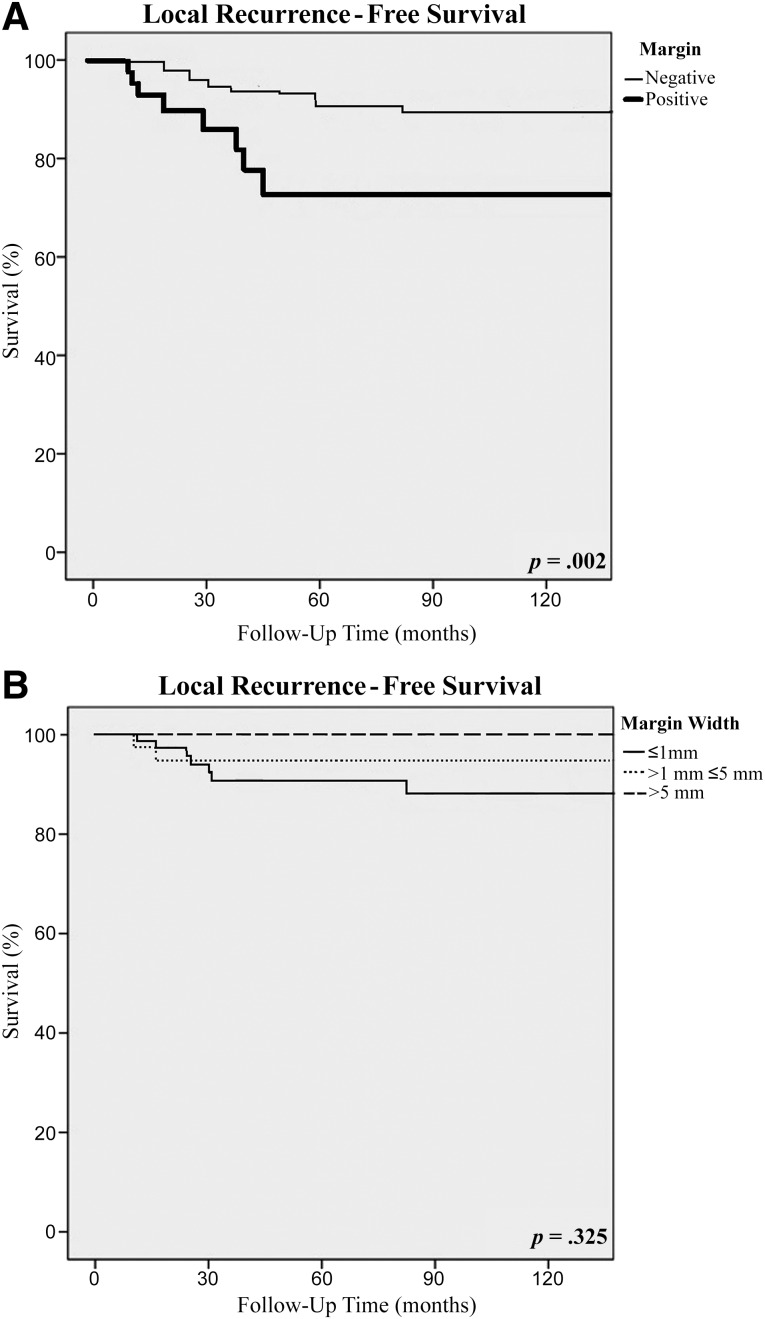
The median follow-up was 82 months (range: 1–328 months), and 60% of the patients were followed for at least 5 years. The overall recurrence rate was 37%, with a median disease-free survival of 53 months. Local recurrences occurred in 25 (6.5%) patients, including 17 (5.4%) patients with a negative margin and 8 (11.8%) patients with a positive margin, and the median time to LR was 54 months. Positive microscopic margins were associated with a significantly higher rate of local recurrence (hazard ratio [HR], 3.25; 95% confidence interval [CI], 1.00–10.59; p = .003) and an inferior 10-year LRFS rate (83% vs. 93%; p = .002) (Table 3; Fig. 1A). For patients with microscopically negative margins, the 10-year LRFS was 89% for patients with margins ≤1 mm, 93% for those with margins >1 mm and ≤5 mm, and 100% for those with margins >5 mm (Table 3; Fig. 1B). These differences were not statistically significant on multivariate analysis, controlling for pathologic variables, including tumor size, grade, and histology, and for treatment variables, such as chemotherapy and radiotherapy. The presence of a local recurrence did not lead to a significantly worse overall survival, because the overall survival rate was 52% for those patients who developed a local recurrence, in comparison with 61% for those who did not develop a local recurrence (p = .380). Distant metastases were diagnosed in 74 (19%) patients at a median disease-free survival interval of 52 months. The presence of a DR was associated with a significantly worse overall survival rate (18%) in comparison with those patients without a DR (76%) (p < .01). Positive microscopic margins were associated with a significantly higher rate of distant recurrence (HR 2.07; 95% CI, 1.08–3.97; p = .005).
Table 3.
Survival rates according to resection margin status overall and surgical margin width
Figure 1.
Local recurrence-free survival according to resection margin status (A) and the width of the surgical margin (B).
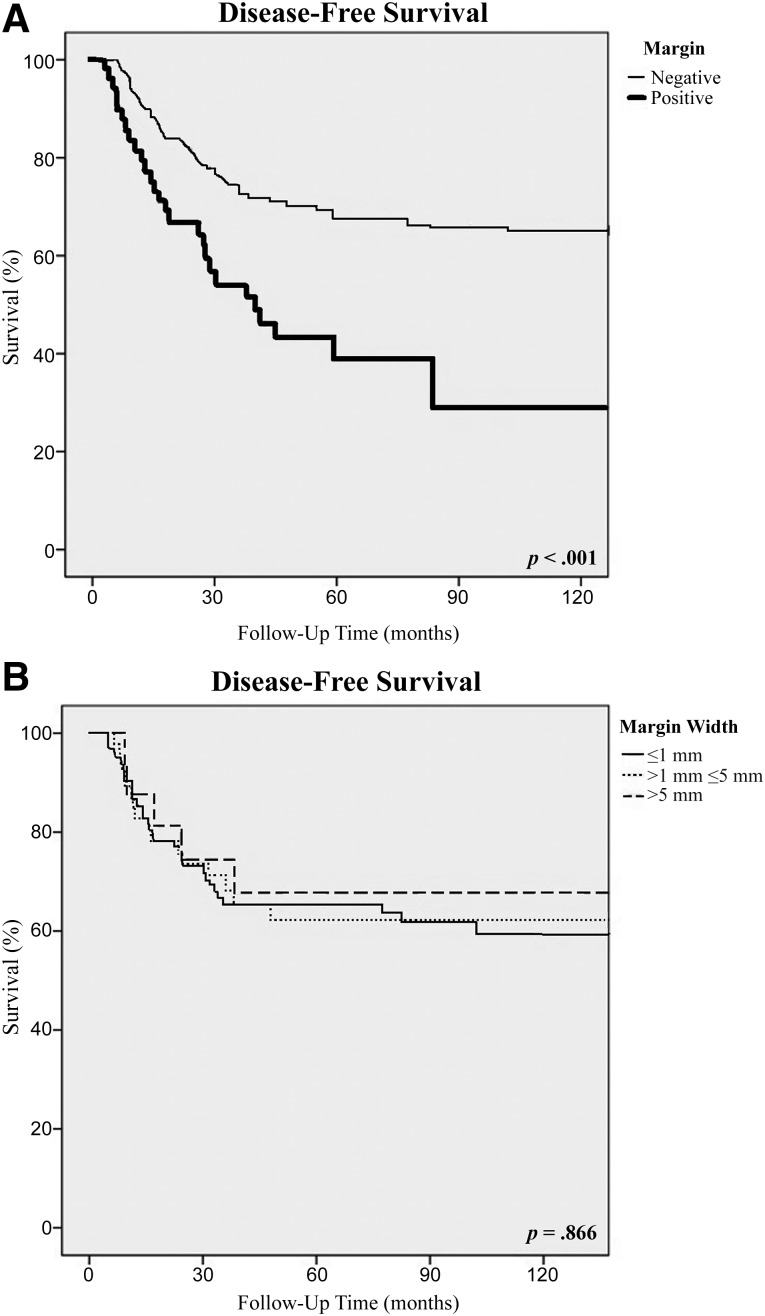
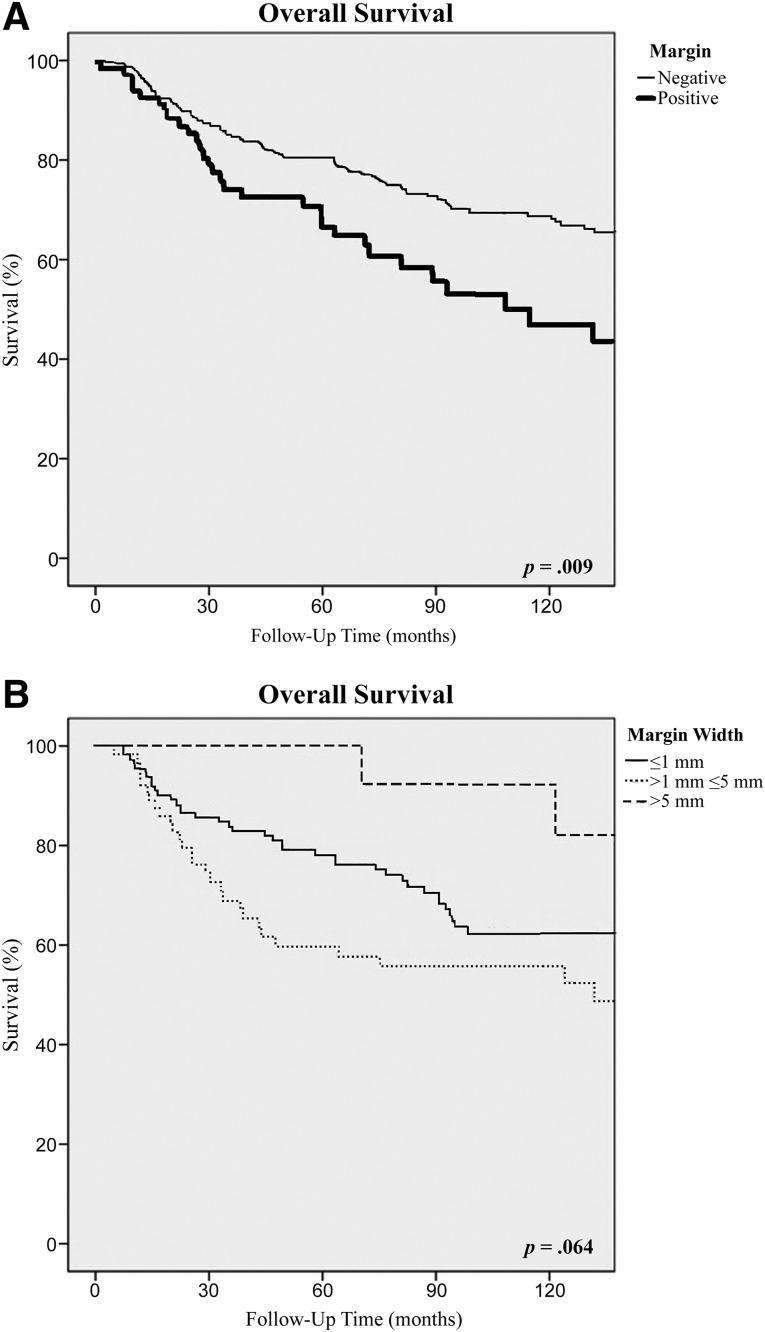
There were significant differences in the 10-year disease-free and overall survival rates between patients with positive and negative microscopic resection margins. Patients with positive margins had significantly worse 10-year DFS (44% vs. 69%; p < .001) and 10-year OS (57% vs. 73%; p = .009) rates than did patients with negative margins (Table 3; Figs. 2A, 3A). The median overall survival was 108 months in comparison with 180 months (p < .01), and the median disease-free survival was 27 months in comparison with 82 months (p = .01) for the positive and negative margin groups, respectively. However, for patients who had a negative-margin resection, the width of the surgical margin had no influence on 10-year DFS, which was 66% for those with ≤1 mm margins, 68% for those with >1 mm and ≤5 mm margins, and 74% for those with >5 mm margins (Table 3; Fig. 2B). Similarly, the width of the surgical margin had no influence on 10-year OS, which was 68% for those with ≤1 mm margins, 65% for those with >1 mm and ≤5 mm margins, and 93% for those with >5 mm margins (p = .064) (Table 3; Fig. 3B).
Figure 2.
Disease-free survival according to resection margin status (A) and the width of the surgical margin (B).
Figure 3.
Overall survival according to resection margin status (A) and the width of the surgical margin (B).
Discussion
Local control of extremity and truncal STS can be achieved in 90% or more of patients with the combination of limb-sparing surgery and RT [6, 19, 20]. The goal of limb-sparing surgery is to resect the STS with a cuff of normal tissue to ensure a R0 (margin-negative) resection while leaving intact the critical neurovascular and bony structures that enable preservation of a functional limb. In many circumstances, particularly in the case of a large, deep-seated STS, achieving a “cuff of normal tissue” around the tumor is difficult, if not impossible. In such cases, RT is added to the best surgical resection possible to enable limb salvage while still achieving excellent rates of local control [19, 20]. However, even with the addition of RT, patients with extremity STS resected with positive margins have higher rates of local failure [13, 19, 20]. Thus, resecting a STS with a negative margin is critically important for achieving the best rate of local control. The real question, especially from the standpoint of the surgeon, is how wide this negative margin needs to be in patients treated with RT. This question has yet to be satisfactorily addressed or answered in the literature, and so we sought to answer it with this study.
Other authors have studied the significance of resection margin width in STS, but these studies are limited by the heterogeneity in the characteristics of the patients included and in the treatments the patients received. McKee et al. [17] studied 111 patients with predominantly deep, high-grade tumors measuring ≥5 cm in size. However, only 38% received RT, and 34% received adjuvant chemotherapy. Remarkably, they achieved a resection margin width of ≥10 mm in 53 (47%) patients and of 1–9 mm in another 45 (41%) patients. They found that margins ≥10 mm independently predicted a longer local recurrence-free interval, although there was no difference in the distant metastasis-free interval nor overall survival according to margin width. They concluded that adjuvant RT should be considered for all STS resected with margins <10 mm. The major limitation of this study is the fact that very few patients received RT despite the high percentage of patients with large, high-grade tumors. Arguably, virtually all of these patients today receive RT, and most major sarcoma centers deliver RT preoperatively. The addition of RT almost certainly decreases the width of the surgical margin needed to achieve durable local control, and so this study fails to clarify what the optimal margin width is for such patients.
Dickinson et al. [16] also sought to assess the significance of surgical margin width on outcomes for STS. They studied 279 patients with STS and examined outcomes according to margin widths ranging from positive (“contaminated”) to ≥20 mm. They reported similar outcomes in terms of local recurrence, distant metastasis, and survival independent of margin width, provided that the margin was negative. However, the authors concluded that wider margins of resection improve local control and overall survival despite the lack of any statistical evidence to support this conclusion. Furthermore, this study is limited by a curious lack of important data, such as the distribution of the grades of the tumors included in their analysis and important treatment factors, such as which patients received adjuvant RT and/or chemotherapy.
In this study of patients with predominantly large, high-grade tumors treated in a fairly homogeneous fashion with limb-sparing surgery and RT, we demonstrate that achieving a negative margin is essential for optimizing local control and survival, but the absolute quantitative width of the negative margin does not influence outcome. Indeed, we found that resection of a sarcoma with a narrow margin (e.g., 1 mm or less) confers similar outcomes as does resection with a wider margin. The clinicopathologic and treatment characteristics of patients whose sarcomas were resected with margins ≤1 mm were the same as those whose tumors were resected with >1-mm and ≤5-mm margins and as those with >5-mm margins with only one exception: The median tumor size was significantly larger in the ≤1-mm margin group than in the wider-margin groups. Despite this fact, those patients whose tumors were resected with ≤1-mm margins had similar rates of LRFS, DFS, and OS as did those whose tumors were resected with wider margins. Importantly, one must note that all patients in this study received radiation therapy preoperatively and/or postoperatively. Accordingly, the conclusions drawn from this study must not be applied to those patients undergoing surgery alone as the local treatment of their STS, in which case wider margins of resection may be necessary.
Our finding that positive surgical margins predicted higher rates of both local and distant recurrence is consistent with what prior authors have reported [4, 9, 11, 19–22]. Notably, however, there are published series that demonstrate that even surgical resection with “planned” positive margins along critical structures after radiation therapy is not associated with worse outcomes in comparison with wide resection [23] and that the adverse effect of positive surgical margins on local recurrence is lost in patients who receive preoperative chemotherapy and radiation therapy [24]. The influence of surgical margins on survival, however, has been controversial. In this study, we found that patients with frankly positive margins had worse outcomes across the board, including worse 10-year LRFS, DFS, and OS rates. This finding is in keeping with that reported by several other groups [6, 7, 9, 12, 21, 22], yet it contradicts the findings of others [4, 17].
Our study has several limitations. First, 25% of our patients who underwent negative-margin resections did not have an available quantitative margin width, although in the vast majority of these cases it was owing to the fact that there was no residual tumor in the resection specimen. For the past several decades we have used a standardized pathology report template that includes the quantitative width of the negative surgical margin, perhaps explaining why truly only 3% of the patients who underwent R0 resection in this study did not have a margin width included in the pathology report. Second, the quality of the surgical margin could not be deduced for the majority of patients from the available records, and thus we could not comment on this factor. Indeed, it is believed that the quality of the margin is more important than the absolute width of the margin. For example, most sarcoma experts agree that a fascial margin of 1 mm is a superior margin to a fat or muscle margin of several millimeters, because fascia is an excellent barrier to tumor penetration [25, 26]. Third, although all patients included in this study received radiation treatment, the timing of such treatment was variable. Furthermore, there was heterogeneity among the patients in the receipt of chemotherapy as well as in its timing (neoadjuvant vs. adjuvant) and regimens. What impact these differences in treatment may have had on the results is not certain, but note that there were no statistical differences in the receipt of chemotherapy among the different margin-width groupings.
Conclusion
In summary, for patients undergoing RT and limb-sparing surgery for STS, achieving a negative margin is essential to optimizing both local control and survival. However, the absolute quantitative width of the negative margin does not significantly influence outcome, and so attempts at wide margins of resection appear to be less important than simply achieving a negative margin. Importantly, the conclusions drawn from this study must not be applied to those patients undergoing surgery alone as the local treatment of their STS, in which case wider margins of resection may be necessary.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgment
This study was presented at the Connective Tissue Oncology Society Annual Meeting, New York, NY, October 2013.
Author Contributions
Conception/Design: Rima Ahmad, Francis Hornicek, Alex B. Haynes, Edwin Choy, Gregory Cote, G. Petur Nielsen, Yen-Lin Chen, Thomas F. DeLaney, John T. Mullen
Provision of study material or patients: Alex Jacobson, Francis Hornicek, G. Petur Nielsen, Yen-Lin Chen, Thomas F. DeLaney, John T. Mullen
Collection and/or assembly of data: Rima Ahmad, Alex Jacobson, John T. Mullen
Data analysis and interpretation: Rima Ahmad, Alex B. Haynes, Gregory Cote, G. Petur Nielsen, Thomas F. DeLaney, John T. Mullen
Manuscript writing: Rima Ahmad, Alex B. Haynes, Gregory Cote, Thomas F. DeLaney, John T. Mullen
Final approval of manuscript: Rima Ahmad, Alex Jacobson, Francis Hornicek, Alex B. Haynes, Edwin Choy, Gregory Cote, G. Petur Nielsen, Yen-Lin Chen, Thomas F. DeLaney, John T. Mullen
Disclosures
Edwin Choy: Amgen (C/A). The other authors indicated no financial relationships.
References
- 1.Alho A, Alvegård TA, Berlin O, et al. Surgical margin in soft tissue sarcoma. The Scandinavian Sarcoma Group experience. Acta Orthop Scand. 1989;60:687–692. doi: 10.3109/17453678909149605. [DOI] [PubMed] [Google Scholar]
- 2.Bell RS, O’Sullivan B, Liu FF, et al. The surgical margin in soft-tissue sarcoma. J Bone Joint Surg Am. 1989;71:370–375. [PubMed] [Google Scholar]
- 3.Heslin MJ, Gaynor JJ, Newman E, et al. Effect of perioperative blood transfusion on recurrence and survival in 232 primary high-grade extremity sarcoma patients. Ann Surg Oncol. 1994;1:189–197. doi: 10.1007/BF02303523. [DOI] [PubMed] [Google Scholar]
- 4.Tanabe KK, Pollock RE, Ellis LM, et al. Influence of surgical margins on outcome in patients with preoperatively irradiated extremity soft tissue sarcomas. Cancer. 1994;73:1652–1659. doi: 10.1002/1097-0142(19940315)73:6<1652::aid-cncr2820730617>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 5.Singer S, Corson JM, Demetri GD, et al. Prognostic factors predictive of survival for truncal and retroperitoneal soft-tissue sarcoma. Ann Surg. 1995;221:185–195. doi: 10.1097/00000658-199502000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pisters PW, Leung DH, Woodruff J, et al. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–1689. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 7.Lewis JJ, Leung D, Casper ES, et al. Multifactorial analysis of long-term follow-up (more than 5 years) of primary extremity sarcoma. Arch Surg. 1999;134:190–194. doi: 10.1001/archsurg.134.2.190. [DOI] [PubMed] [Google Scholar]
- 8.Trovik CS, Bauer HC, Alvegård TA, et al. Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically-treated patients from the Scandinavian Sarcoma Group Register. Eur J Cancer. 2000;36:710–716. doi: 10.1016/s0959-8049(99)00287-7. [DOI] [PubMed] [Google Scholar]
- 9.Stojadinovic A, Leung DH, Hoos A, et al. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg. 2002;235:424–434. doi: 10.1097/00000658-200203000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 11.Kim YB, Shin KH, Seong J, et al. Clinical significance of margin status in postoperative radiotherapy for extremity and truncal soft-tissue sarcoma. Int J Radiat Oncol Biol Phys. 2008;70:139–144. doi: 10.1016/j.ijrobp.2007.05.067. [DOI] [PubMed] [Google Scholar]
- 12.Herbert SH, Corn BW, Solin LJ, et al. Limb-preserving treatment for soft tissue sarcomas of the extremities. The significance of surgical margins. Cancer. 1993;72:1230–1238. doi: 10.1002/1097-0142(19930815)72:4<1230::aid-cncr2820720416>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 13.Sadoski C, Suit HD, Rosenberg A, et al. Preoperative radiation, surgical margins, and local control of extremity sarcomas of soft tissues. J Surg Oncol. 1993;52:223–230. doi: 10.1002/jso.2930520405. [DOI] [PubMed] [Google Scholar]
- 14.Alektiar KM, Leung D, Zelefsky MJ, et al. Adjuvant radiation for stage II-B soft tissue sarcoma of the extremity. J Clin Oncol. 2002;20:1643–1650. doi: 10.1200/JCO.2002.20.6.1643. [DOI] [PubMed] [Google Scholar]
- 15.von Mehren M, Randall RL, Benjamin RS et al. NCCN Guidelines Soft Tissue Sarcoma Version 3.2012. Available at http://www.nccn.org/professionals/physician_gls/PDF/sarcoma.pdf. Accessed March 29, 2015.
- 16.Dickinson IC, Whitwell DJ, Battistuta D, et al. Surgical margin and its influence on survival in soft tissue sarcoma. ANZ J Surg. 2006;76:104–109. doi: 10.1111/j.1445-2197.2006.03615.x. [DOI] [PubMed] [Google Scholar]
- 17.McKee MD, Liu DF, Brooks JJ, et al. The prognostic significance of margin width for extremity and trunk sarcoma. J Surg Oncol. 2004;85:68–76. doi: 10.1002/jso.20009. [DOI] [PubMed] [Google Scholar]
- 18.Look Hong NJ, Hornicek FJ, Harmon DC, et al. Neoadjuvant chemoradiotherapy for patients with high-risk extremity and truncal sarcomas: A 10-year single institution retrospective study. Eur J Cancer. 2013;49:875–883. doi: 10.1016/j.ejca.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strander H, Turesson I, Cavallin-Ståhl E. A systematic overview of radiation therapy effects in soft tissue sarcomas. Acta Oncol. 2003;42:516–531. doi: 10.1080/02841860310014732. [DOI] [PubMed] [Google Scholar]
- 20.Jebsen NL, Trovik CS, Bauer HC, et al. Radiotherapy to improve local control regardless of surgical margin and malignancy grade in extremity and trunk wall soft tissue sarcoma: a Scandinavian sarcoma group study. Int J Radiat Oncol Biol Phys. 2008;71:1196–1203. doi: 10.1016/j.ijrobp.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Potter BK, Hwang PF, Forsberg JA, et al. Impact of margin status and local recurrence on soft-tissue sarcoma outcomes. J Bone Joint Surg Am. 2013;95:e151. doi: 10.2106/JBJS.L.01149. [DOI] [PubMed] [Google Scholar]
- 22.Novais EN, Demiralp B, Alderete J, et al. Do surgical margin and local recurrence influence survival in soft tissue sarcomas? Clin Orthop Relat Res. 2010;468:3003–3011. doi: 10.1007/s11999-010-1471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biau DJ, Weiss KR, Bhumbra RS, et al. Monitoring the adequacy of surgical margins after resection of bone and soft-tissue sarcoma. Ann Surg Oncol. 2013;20:1858–1864. doi: 10.1245/s10434-012-2863-8. [DOI] [PubMed] [Google Scholar]
- 24.Gronchi A, Verderio P, De Paoli A, et al. Quality of surgery and neoadjuvant combined therapy in the ISG-GEIS trial on soft tissue sarcomas of limbs and trunk wall. Ann Oncol. 2013;24:817–823. doi: 10.1093/annonc/mds501. [DOI] [PubMed] [Google Scholar]
- 25.Malawer MM, Sugarbaker PH. Musculoskeletal Cancer Surgery: Treatment of Sarcomas and Allied Diseases. Dortrecht, Netherlands: Kluwer Academic Publishers; 2001. [Google Scholar]
- 26.Trovik CS, Skjeldal S, Bauer H, et al. Reliability of margin assessment after surgery for extremity soft tissue sarcoma: The SSG experience. Sarcoma. 2012;2012:290698. doi: 10.1155/2012/290698. [DOI] [PMC free article] [PubMed] [Google Scholar]