Vismodegib and sonidegib are Hedgehog pathway inhibitors (HPIs) approved for treatment of patients with advanced basal cell carcinoma. The adverse events (AEs) associated with these therapies can impact clinical outcomes as a result of decreased quality of life and treatment discontinuation. The incidence, clinical presentation, putative mechanisms, and management strategies for AEs related to administration of HPIs are described in this article.
Keywords: Adverse events, Management, Vismodegib, Sonidegib, Advanced basal cell carcinoma, Hedgehog pathway
Abstract
Abnormal activation of hedgehog pathway signaling is a key driver in the pathogenesis of basal cell carcinoma (BCC). Vismodegib, a first-in-class small-molecule inhibitor of hedgehog pathway signaling, is approved by regulatory authorities for the treatment of adults who have metastatic BCC or locally advanced BCC that has recurred after surgery, or who are not candidates for surgery and who are not candidates for radiation. A second inhibitor, sonidegib, was also recently approved for the same patient group with locally advanced BCC. Adverse events (AEs) commonly observed in hedgehog pathway inhibitor (HPI)-treated patients include muscle spasms, ageusia/dysgeusia, alopecia, weight loss, and asthenia (fatigue). These AEs are thought to be mechanistically related to inhibition of the hedgehog pathway in normal tissue. Although the severity of the majority of AEs associated with HPIs is grade 1–2, the long-term nature of these AEs can lead to decreased quality of life, treatment interruption, and in some cases discontinuation, all of which might affect clinical outcome. The incidence, clinical presentation, putative mechanisms, and management strategies for AEs related to HPIs in advanced BCC are described. These observations represent the first step toward the development of mechanism-based preventive and management strategies. Knowledge of these AEs will allow health care professionals to provide appropriate counseling and supportive care interventions, all of which will contribute to improved quality of life and optimal benefit from therapy.
Implications for Practice:
The hedgehog pathway inhibitors (HPIs) vismodegib and sonidegib represent a therapeutic breakthrough for patients with advanced basal cell carcinoma. However, the nature of the low-grade adverse events (AEs) commonly observed in HPI-treated patients, including muscle spasms, ageusia/dysgeusia, alopecia, weight loss, and fatigue, can impact clinical outcomes as a result of decreased quality of life and treatment discontinuation. The incidence, clinical presentation, putative mechanisms, and management strategies for AEs related to administration of HPIs are described, with the goal of enabling health care professionals to provide appropriate counseling and supportive care interventions to their patients.
Introduction
Abnormal activation of hedgehog pathway signaling, observed in >90% of cases of basal cell carcinoma (BCC) [1, 2], is a key driver in the pathogenesis of BCC. Although the majority of BCCs can be successfully treated with surgery, topical agents, or radiation, hedgehog pathway inhibition has been a therapeutic breakthrough for patients with advanced disease (locally advanced BCC [laBCC] or metastatic BCC [mBCC]; it is estimated that metastatic disease develops in <1% of patients) [1, 2]. Vismodegib was the first hedgehog pathway inhibitor (HPI) to be approved for the treatment of adults who have mBCC or laBCC that has recurred after surgery or who are not candidates for surgery and are not candidates for radiation treatment (approval granted by the U.S. Food and Drug Administration [FDA] in January 2012 [3] and the European Medicines Agency [EM]) in July 2013 [4]). In 2015, a second HPI (sonidegib) was approved by both the FDA and the EMA for patients with laBCC, based on data from the Basal Cell Carcinoma Outcomes with LDE225 Treatment (BOLT) study [5].
The safety profile of vismodegib is consistent within clinical trials and general clinical practice [6–12]. Commonly observed adverse events (AEs) include muscle spasms, ageusia/dysgeusia, alopecia, weight loss, and asthenia (fatigue). Similarly, the most commonly observed AEs in sonidegib-treated patients in the BOLT study included muscle spasms, alopecia, dysgeusia, nausea, increased blood creatine kinase level, asthenia, and weight loss [5]. Many of these AEs are thought to be mechanistically related to inhibition of the hedgehog pathway. Although the majority of AEs associated with HPIs are grade 1–2, they tend to be long-term and interfere with patient quality of life (QOL), leading to treatment interruption and discontinuation that may preclude the intended therapeutic effect. Therefore, appropriate strategies for the prevention and management of AEs are essential to ensure that patients can continue to receive and benefit from HPI treatment.
The Hedgehog Signaling Pathway
The hedgehog signaling pathway is critical for normal embryonic development and patterning [2]. In normal cells, the canonical hedgehog signaling pathway is activated by the binding of hedgehog ligands to their receptor protein patched homolog 1 (Patched; PTCH1). Ligand binding relieves the inhibitory effects of PTCH1 on the Smoothened protein (SMO). Signal transduction by SMO leads to activation and nuclear translocation of Gli transcription factors and induction of target genes, many of which are involved in proliferation, survival, and differentiation. In addition to the canonical signaling pathway, several noncanonical hedgehog signaling pathways have been identified [13, 14]. Inappropriate reactivation of the hedgehog signaling pathway can result in tumorigenesis, and it was first described in basal cell carcinoma nevus syndrome (BCCNS), also known as Gorlin syndrome [15–17]. In BCC, the hedgehog signaling pathway is typically activated by mutations that lead to dysfunction of the PTCH1 gene, seen almost universally in patients with BCCNS and in most patients with sporadic BCC tumors. An estimated 80%–90% of sporadic BCC tumors have PTCH1 mutations, whereas 10% harbor SMO mutations. Both types of mutations lead to constitutive SMO signaling and BCC development [2].
Although hedgehog signaling is silenced in most normal adult tissues, it has been shown to play a role in the repair of damaged tissues, the promotion of stem cell proliferation, and the regulation of maintenance of various tissues, including muscle, hair, taste buds, and the reproductive system [1, 18]. After injury, sonic hedgehog (Shh) signaling is reactivated in adult skeletal muscle and plays a functionally important role in regeneration by regulating injury-induced angiogenesis and myogenesis [19]. Pathway inhibition impairs production of angiogenic factors, decreases muscle blood flow, and reduces capillary density after injury [19]. Hedgehog signaling has been shown to regulate development and maintenance of taste buds and seems to play a critical role in taste function integrity [20, 21]. Adult mice treated with vismodegib display significant reductions in taste-bud size and number of taste cells, and decreased behavioral responses to sweet and bitter stimuli [21]. Interestingly, hedgehog-responding cells were lost in fungiform papilla epithelium in sonidegib-treated mice, with taste-bud remnants entirely absent or severely decreased in >90% of aberrant papillae [22]. The hedgehog signaling pathway is also active in the hair follicle, transitioning hair from the telogen (resting) to the anagen (active growth) phase of the growth cycle [23–25]. Shh-null mice (in which the Shh gene has been knocked out) exhibit follicles arrested at the hair germ stage of development [23, 24]. In mice, transient overexpression of Shh by gene transfer or topical application of a hedgehog agonist induces resting hair follicles to enter anagen, resulting in hair growth [25, 26]. Antibodies that block the activity of Shh are able to prevent hair growth in adult mice [27].
HPI Safety Profile
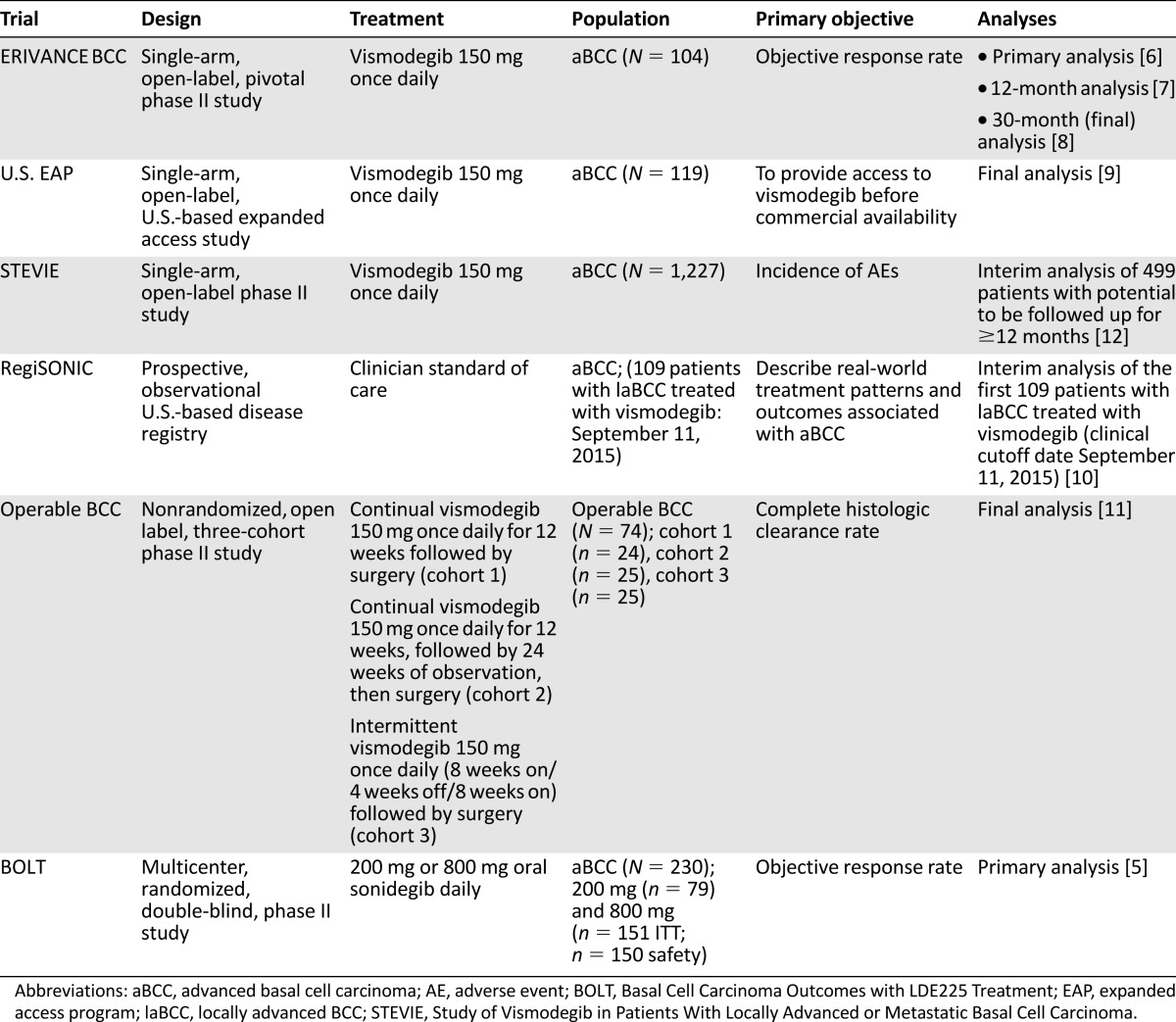
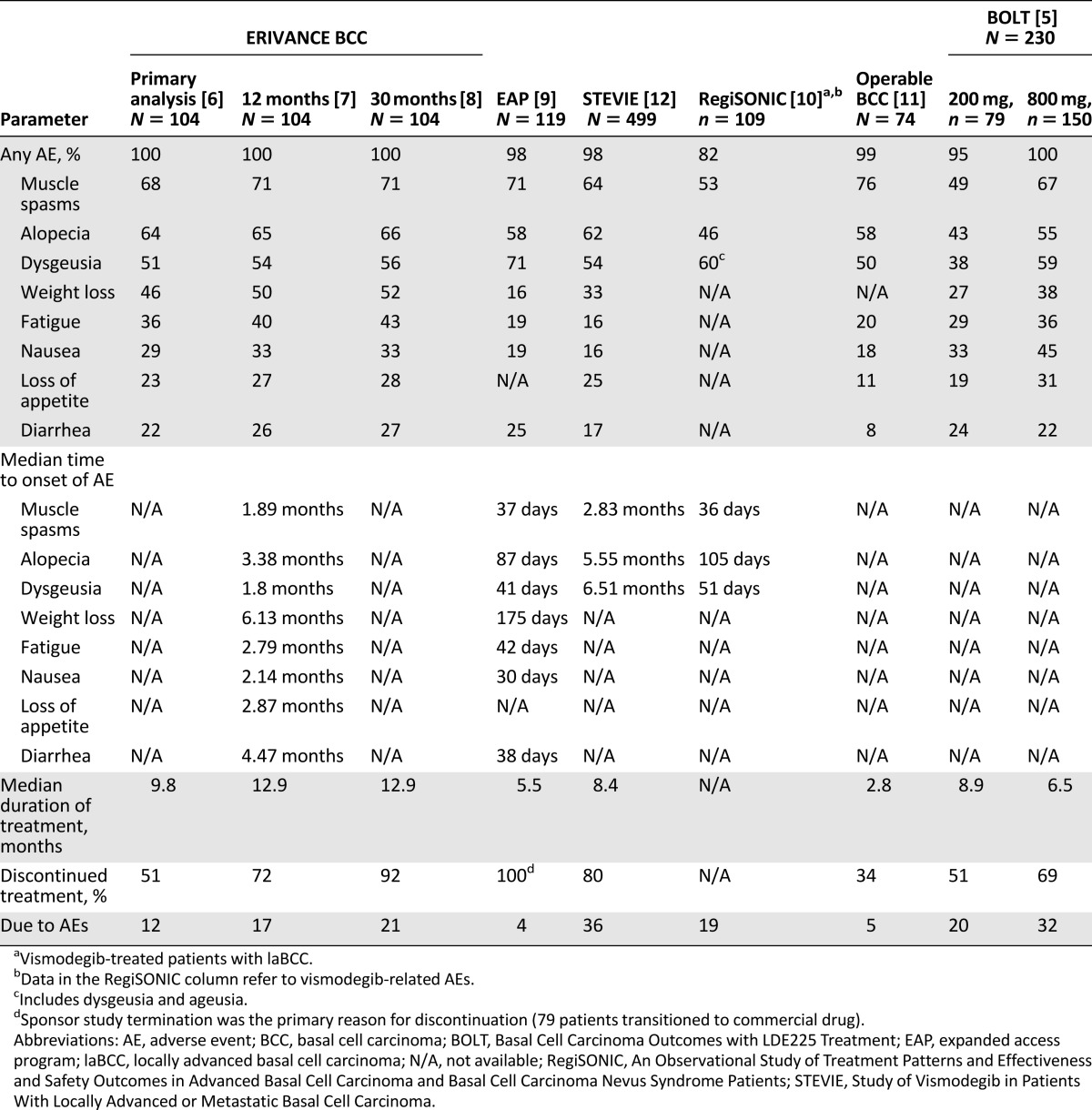
HPI safety data are available from a number of key studies (Table 1) [5–12]. Nearly all patients treated with HPIs experience at least one treatment-emergent AE (TEAE), with an incidence rate of 95%–100% observed across studies. In the RegiSONIC disease registry, protocol specified-related AEs were reported in 82% of the first 109 patients with laBCC given vismodegib [6–11]. The most frequent AEs observed with HPIs include muscle spasms, alopecia, dysgeusia, weight loss, asthenia, nausea, decreased appetite, and diarrhea (Table 2) [5–12]. The majority of AEs are mild or moderate (grade 1–2) and occur early in the course of treatment [6–11]. Reported rates of discontinuation because of all AEs vary across studies (e.g., 5% in the 12-week operable BCC study [11] and 36% in the Study of Vismodegib in Patients With Locally Advanced or Metastatic Basal Cell Carcinoma (STEVIE) [median duration of treatment 36.4 weeks] [12]). In addition, data from the ERIVANCE BCC 30-month analysis [8] and the STEVIE study [12] show that the severity of AEs did not worsen with longer treatment duration. Reasons for discontinuation vary depending on the type of advanced BCC; patients with laBCC are more likely to request discontinuation because of AEs, whereas patients with mBCC often discontinue because of disease progression [6]. However, it has been observed that patients who discontinued because of a grade 1 or 2 AE often experience complete or partial response before discontinuation [12]. It may be that some patients discontinue vismodegib when a response has been achieved and if the grade 1–2 AEs (considered “annoying”) are significantly impacting QOL. A similar safety profile was reported with sonidegib in the BOLT study [5] and in the subsequent 18-month follow-up [28]. There is a clear need to educate health care professionals (HCPs) and patients regarding AE management strategies that allow patients to continue treatment.
Table 1.
Overview of key hedgehog pathway inhibitor studies providing safety data
Table 2.
Summary of safety data from key hedgehog pathway inhibitor studies
Prevention and Management Strategies for HPI-Related Adverse Events
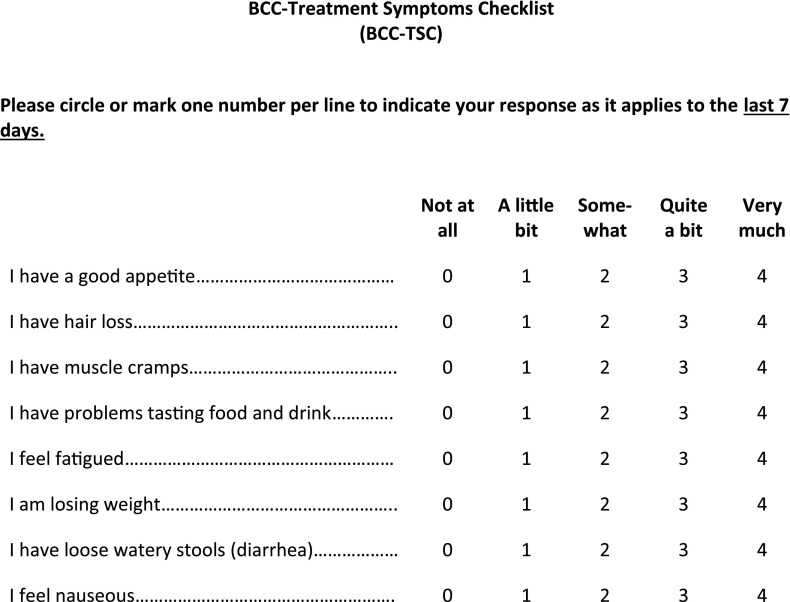
Because the majority of AEs emerge early in treatment, patient education is key in terms of what to expect and the possible management options. It is recommended that physicians follow their local prescribing information. Treatment interruption is a common approach, and HCPs and patients should be encouraged to continue supportive care interventions after re-exposure. A thorough baseline examination is also important, including an evaluation of comorbidities (i.e., alopecia, myopathies, diabetes, gastrointestinal/liver disease, and sensory and motor neuropathies) and concomitant medications that might contribute to tolerability issues (e.g., glucose-lowering or diarrhea-causing agents, or neurotoxic, hepatotoxic, or myotoxic medications). Finally, the AEs and their associated management strategies (Figs. 1, 2; Table 3) [29] should be considered in the context of the benefit of HPI treatment and the rapid clinical response. Because most of the AEs induced by HPIs have a significant subjective component, it would be important to include questions specific to these untoward events during clinical evaluations. Although a patient-reported outcomes questionnaire was recently developed for use in patients with BCC (Advanced Basal Cell Carcinoma Index) [30], it is not specific for AEs related to HPIs. Preliminary studies have yielded a questionnaire that, although it has not been validated, addresses some of the most common HPI toxicities and their impact on patient QOL (Fig. 3). This questionnaire would be useful in routine clinical care of patients treated with HPIs and in supportive care trials.
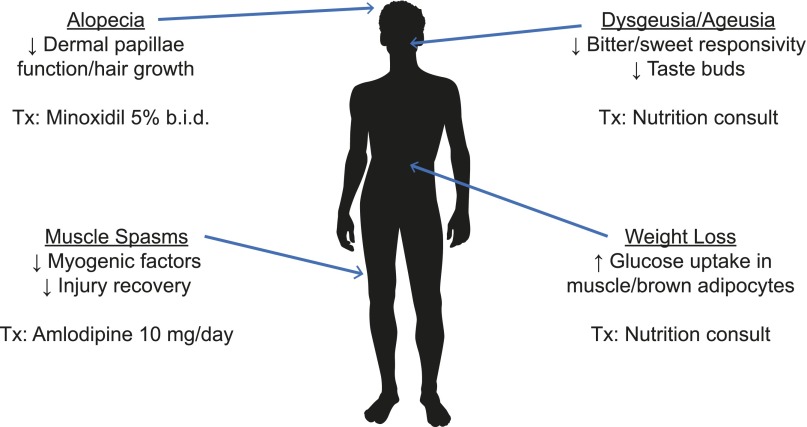
Figure 1.
Adverse events associated with hedgehog pathway inhibitors [19, 21, 26, 29].
Abbreviations: b.i.d., twice daily; Tx, treatment.
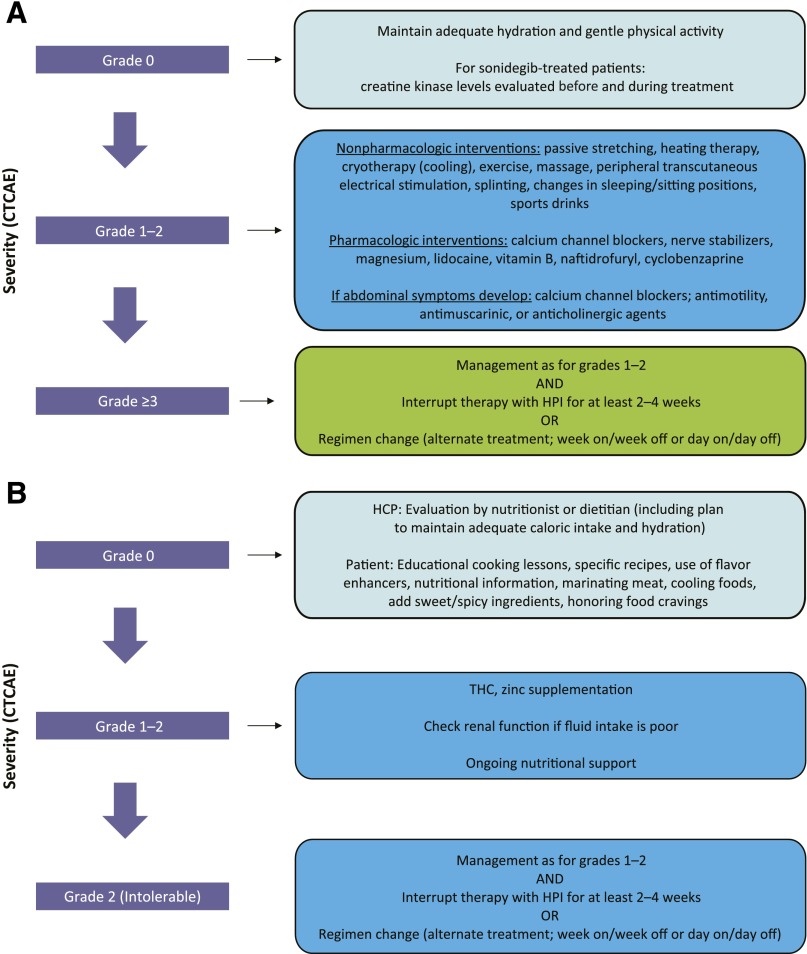
Figure 2.
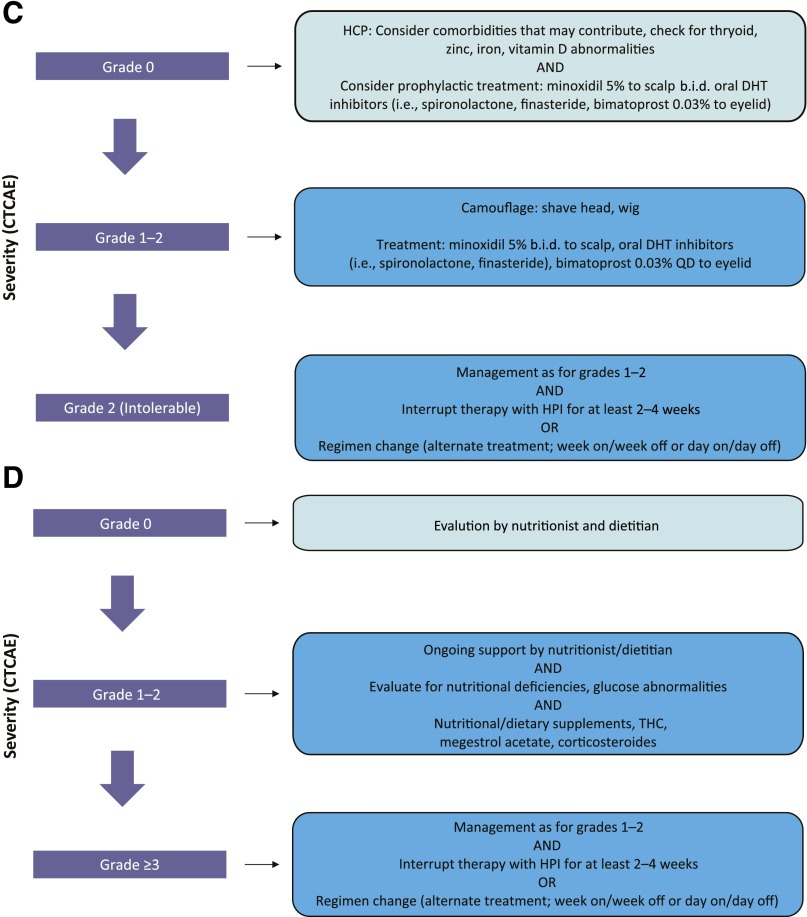
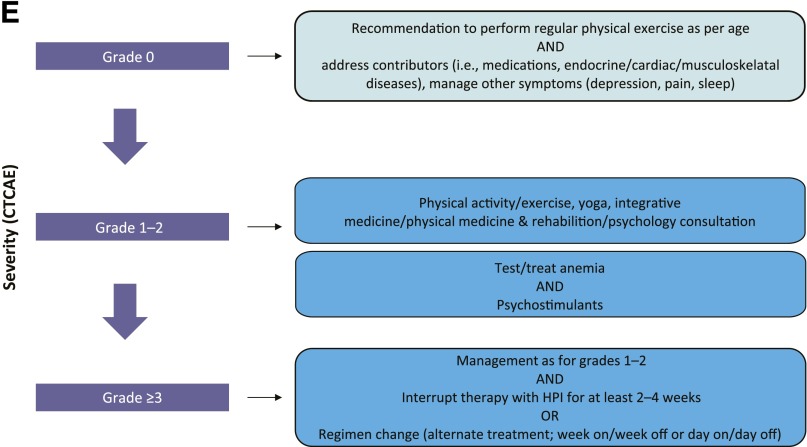
Proposed treatment algorithms based on anecdotal evidence discussed at the expert meeting. (A): Muscle spasms. (B): Taste disturbance. (C): Alopecia. (D): Weight loss. (E): Fatigue. Per CTCAE, for grade ≥3 events, at least 2 treatment interruptions (2–4 weeks) should be attempted before treatment discontinuation is considered.
Abbreviations: b.i.d., twice daily; CTCAE, Common Terminology Criteria for Adverse Events; DHT, dihydrotestosterone; HCP, health care professional; HPI, hedgehog pathway inhibitor; QD, once daily; THC, delta-9-tetrahydrocannabinol.
Table 3.
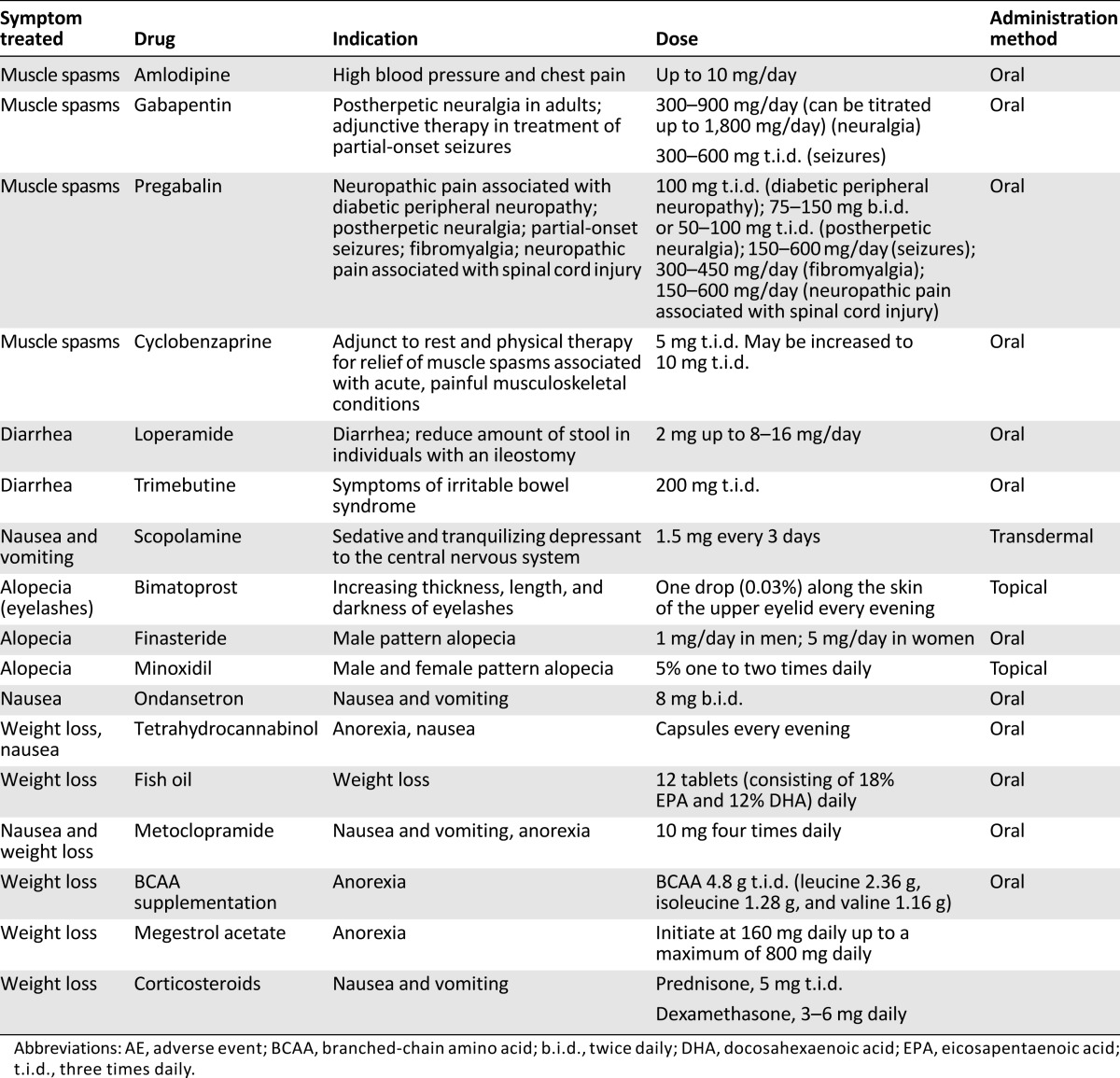
Summary of drugs and doses for prevention or treatment of some of the hedgehog pathway inhibitor-related AEs
Figure 3.
BCC treatment symptoms checklist for hedgehog pathway inhibitor-associated adverse events.
Abbreviation: BCC, basal cell carcinoma.
Muscle Spasms
In general, intensity of HPI-related muscle spasms is mild or moderate. Muscle spasms tend to occur more frequently at night and in the muscles of the lower leg and foot, although they can occur in any location. The impact of muscle spasms is compounded given their high incidence (50%) in elderly individuals [31]. They are thought to occur as a result of paradoxical activation of the noncanonical SMO/Ca2+/AMPK axis and inhibition of the canonical SMO signaling pathway, resulting in an influx of Ca2+ into muscle cells and, subsequently, immediate muscle contraction [29]. Muscle spasms are typically the most frequently observed AE during HPI treatment (49%–76% of all patients) and usually occur early in the course of treatment (Table 2).
The recommended management algorithm is presented in Figure 2A. Patients should be advised to maintain adequate hydration and gentle physical activity while taking an HPI. Laboratory testing is not required, according to the vismodegib label, although for sonidegib, creatine kinase (CK) levels should be assessed before treatment and periodically as clinically indicated. Any additional laboratory testing is at the discretion of the treating physician. A number of nonpharmacologic approaches can be used for grade 1–2 muscle spasms, with anecdotal benefit reported with passive stretching (these techniques can be easily taught to the patient by a physical therapist) [32], heating therapy, cryotherapy (ice) [33], exercise, massage, peripheral transcutaneous electrical stimulation (for localized cramps) [34], splinting of affected areas while sleeping, and changes in sleeping or sitting position [35]. Although hydration is frequently used, especially when cramps are associated with exercise, no formal evidence supports hydration as a mechanism to reduce cramping. A number of pharmacologic treatments may be used for initial grade 1–2 symptoms, including calcium channel blockers (i.e., amlodipine [36]), diltiazem [37], verapamil [38], sports drinks (those that replace electrolytes), and nerve stabilizers (e.g., gabapentin or pregabalin) [39]. Magnesium supplementation has also been studied, and only a small, nonsignificant difference has been reported in reducing the frequency of idiopathic cramping [40]. Lidocaine [41], levetiracetam [42], vitamin B complex [43], naftidrofuryl (not available in the United States) [44], and cyclobenzaprine [45] have also all demonstrated effectiveness. For symptoms that have spread to the abdomen, calcium channel blockers (i.e., amlodipine) and antimotility (e.g., loperamide), antimuscarinic (e.g., trimebutine), or anticholinergic agents (e.g., scopolamine) may be useful. Although a number of agents, including vitamin E, baclofen, riluzole, l-threonine, xaliproden, indinavir, memantine, carbamazepine, and oxcarbazepine, have been investigated in patients with amyotrophic lateral sclerosis/motor neuron disease, none has shown any significant benefit in reducing cramping [46]. The FDA has advised against the use of quinine for cramps because of potentially dangerous and life-threatening side effects (QT prolongation, thrombocytopenia, and hypersensitivity reactions) [47], although, in non-U.S. countries, cautious use for severe cramps is permitted. The American Academy of Neurology recommends that quinine could be considered in select patients (i.e., in those whom cramps are disabling, no other agents relieve symptoms) after potential AEs are evaluated [48]. No adverse interactions have been reported with the use of HPIs and quinine.
For muscle spasms grade ≥3 reported during vismodegib treatment, experience indicates that at least two cycles of vismodegib treatment interruption (2–4 weeks each time) should be attempted before treatment discontinuation is considered. Anecdotal experience with use of a change in the dosing regimen (e.g., week on/week off) to mitigate spasms has also been reported. In the operable BCC study, most muscle spasms resolved within 6 weeks of treatment discontinuation [11]. Currently, a randomized, double-blind crossover study is recruiting patients to assess the effects of levocarnitine (vs. placebo) on the incidence and severity of vismodegib-related muscle spasms (ClinicalTrials.gov identifier NCT01893892). Limited information is available on sonidegib in this aspect, and there are no ongoing trials. The Muscle and Joint Measure (available from: https://www.fredhutch.org/content/dam/public/labs-projects/clinical-research/biobehavioral/MJM_updated_1-15-10_Syrjala_et_al_PDF.pdf) is a patient-reported outcomes tool used to measure musculoskeletal symptoms in cancer survivors and patients with spinal cord injuries, and it might be useful in assessment of the impact of muscle spasms and response to therapy [49, 50].
Taste Disturbances (Dysgeusia/Ageusia)
Severity of the loss (ageusia) or distortion (dysgeusia) of the sense of taste can range and can affect oral intake; it may even cause anorexia and depression. Gustation is important for QOL and can be affected by age, medication, and comorbidity [51]. Approximately 5% of the general population reports taste alterations, which increases progressively to 27% in those older than 80 years [52]. In patients with cancer, dysgeusia affects 56.3% of those treated with chemotherapy, 66.5% of those treated with radiotherapy, and 76% of those treated with combined chemotherapy and radiation [53]. In patients receiving chemotherapy, increased thresholds for bitter, sweet, sour, and salt tastes have been reported, whereas no difference has been reported for umami taste [54].
Hedgehog pathway signaling has been found to regulate differentiation and maintenance of taste buds [20, 55]. Vismodegib decreases the number of Shh-expressing type IV taste cells [21], which have been shown to be critical for taste-bud differentiation in adults. Taste disturbances occur in 50%–71% of vismodegib-treated patients, typically developing within the first few months of treatment (Table 2). In the sonidegib BOLT study, dysgeusia was reported in 38% and 59% of patients in the 200-mg and 800-mg groups, respectively [5]. Assessing taste changes in patients treated with HPIs may be challenging given the subjective nature; interestingly, a Taste and Smell Survey was used to evaluate improvement in chemosensory perceptions in a study of delta-9-tetrahydrocannabinol [56], and it might be useful in patients treated with HPIs.
When managing this particular AE, it is important to recognize that the symptoms may be independent of observed weight loss. The recommended management algorithm is presented in Figure 2B. Access to a nutritionist or dietitian at baseline and throughout treatment is beneficial [57], and the patient should be reminded to maintain adequate caloric intake and hydration. Dietary counseling has resulted in reduction in the occurrence of both early-onset and long-term dysgeusia. Similar effects were found with audiotapes and written information that was mailed to patients [53]. In addition, specific recipes given to patients, information on the use of flavor enhancers, and detailed nutritional information have resulted in better reporting of taste perception in patients receiving chemotherapy [58]. Strategies such as adding spicy ingredients, marinating meat, cooling foods, and adding sweet ingredients to meat have also been helpful. Cold foods seem to lessen metallic taste. Other helpful advice for patients, based on experience of the experts, included using new recipes, honoring specific food cravings, eating candy before meals, cutting food with lemon, using sweetened drinks, using plastic utensils, drinking from a straw, brushing the teeth and tongue before meals, and using a baking soda-salt wash or an antibacterial mouthwash. Given the half-life of HPIs and the life span of taste cells (10–24 days), it is likely the interruptions caused by dysgeusia require at least 4 weeks for recovery of gustation, although, in some cases, it can take as long as 6 months to return. Patients should attempt to restart HPIs if interruption is necessary.
A pilot study investigating delta-9-tetrahydrocannabinol compared with placebo in 24 patients with idiopathic dysgeusia showed improved chemosensory perception, food taste, appetite, and calories consumed [56]. In patients with idiopathic dysgeusia, zinc gluconate supplementation resulted in improved taste (p < .05) [59]; however, contradictory results were seen in two studies of patients receiving radiotherapy for head and neck squamous cell carcinoma [60].
A pilot study investigating delta-9-tetrahydrocannabinol compared with placebo in 24 patients with idiopathic dysgeusia showed improved chemosensory perception, food taste, appetite, and calories consumed.
Alopecia
There are approximately 100,000 hairs on the scalp, of which approximately 90% are in the anagen (growth) phase; the remainder are in the catagen (involuting or resting) and telogen (resting or quiescent) phases. Hair plays a critical role in the psychosocial well-being of patients, and its loss has been associated with decreased QOL, depression, and isolation. Alopecia with HPIs is usually characterized by diffuse loss of hair density over the entire scalp and may affect the eyebrows, eyelashes, and beard. Hair texture and quality is also often changed.
Hedgehog pathway inhibitors are thought to induce alopecia by preventing follicles from transitioning to the anagen phase after shedding of hair in the telogen phase [61]. Alopecia occurs in 46%–66% of vismodegib-treated patients and has a longer time to onset than is observed with cytotoxic chemotherapy, developing after at least 2 months of treatment (Table 2). In the sonidegib BOLT study, alopecia was reported in 43% and 55% of patients in the 200-mg and 800-mg groups, respectively [5]. The severity of alopecia can be exacerbated because of the normal development of alopecia (40%–70% of individuals) as part of the aging process. The psychosocial impact of alopecia as reported by a patient can be captured using the Hairdex questionnaire [62]. In the interim analysis of the STEVIE study, treatment was discontinued because of alopecia in 4.2% of patients [12]. In the operable BCC study (cohort 2: 12 weeks of vismodegib and 24 weeks of observation), most cases of alopecia (13 of 17 patients) resolved within 24 weeks of treatment discontinuation [11]. Interestingly, alopecia with use of vismodegib might be more pronounced and longer lasting in patients with BCCNS [63]. Patients should be reminded that the alopecia is unlike that caused by traditional cytotoxic chemotherapy. Alopecia typically develops gradually, and, in some cases, can be delayed and actually occur after HPI treatment has been halted. Alopecia associated with HPI treatment can also be persistent and, in rare cases, might be permanent [64]. Supportive care includes the identification of comorbidities that might contribute to alopecia and use of minoxidil, oral dihydrotestosterone inhibitors (i.e., spironolactone, finasteride), and camouflaging methods (i.e., sprays, powders, hairpieces, and wigs) (Fig. 2C).
Weight Loss
Cancer-related weight loss occurs frequently and may be associated with the underlying disease, depression, or therapy. An international consensus defined cancer anorexia-cachexia as a multifactorial syndrome, with weight loss >5% over the past 6 months or weight loss >2% in individuals already showing depletion [65]. Weight loss was reported in approximately 50% of patients in the ERIVANCE BCC analyses [6–8], with lower incidence (33%) observed in the real-world STEVIE study [12]. In the BOLT study, weight loss was reported in 27% and 38% of patients in the 200-mg and 800-mg sonidegib groups, respectively [5]. Onset is generally seen 6 months after the start of treatment [7]. Although muscle mass is often lost, there does not seem to be a correlation between weight loss and taste disturbances, and body weight typically returns to previous levels after treatment interruption. Ongoing evaluations by a nutritionist can be helpful, and supplements can be useful for patients at risk for weight loss (including elderly individuals; those with low body mass index or significant comorbidities; or those who live alone). Vismodegib administration can be interrupted for up to 8 weeks, followed by discontinuation if necessary (Fig. 2D).
Asthenia
Asthenia (or fatigue) is defined by the National Comprehensive Cancer Network (NCCN) as a distressing, persistent, and subjective sense of physical, emotional, or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and that interferes with usual functioning [66]. In HPI-treated patients, asthenia can also be influenced by a number of factors, including interrupted sleep patterns because of muscle spasms, or decreased caloric intake because of taste disturbances. Patients should be assessed and treated appropriately for depression and questioned about general fatigue, physical fatigue, reduced activity, reduced motivation, and mental fatigue. NCCN guidelines recommend patient education (especially at the start of treatment), together with physical activity, yoga, other physical-based therapies, psychosocial interventions, nutritional consultation, sleep therapy, and management of comorbidities (e.g., pain, insomnia, depression) [66]. Provider-directed National Cancer Institute recommendations also include the use of psychostimulants, along with anemia treatment, cognitive behavioral therapy, and rest [67]. Although there is little evidence to support the use of pharmacologic intervention (e.g., methylphenidate) [68, 69], because depression and HPI-induced fatigue are concomitant factors in patients with advanced BCC, these agents might play a role. Self-report instruments to measure fatigue are available [70], and the American Society of Clinical Oncology published guidelines for the management of fatigue in cancer survivors that might be useful [71]. The proposed management algorithm is presented in Figure 2E.
Nausea
Nausea is often the first AE to be reported and it can be challenging to manage. Vismodegib and sonidegib are considered moderate to highly emetogenic oral antineoplastic agents by the NCCN, and NCCN guidelines for the prevention or oral chemotherapy-related antiemesis include the use of serotonin inhibitors, to be started before initiation and continued daily. For breakthrough emesis, recommendations are based on adding one agent from a different drug class to the current regimen and, if emesis is controlled, continuing on a schedule. Breakthrough agents include the atypical antipsychotic olanzapine, the benzodiazepine lorazepam, cannabinoids (such as dronabinol and nabilone), phenothiazines, steroids, and others, such as haloperidol, metoclopramide, and scopolamine [72]. Patient education is important so patients know what to expect during treatment. Nonmedical interventions include taking vismodegib at nighttime or with food. Gastrointestinal motility medications (e.g., domperidone) or antinausea medications (e.g., dimenhydrinate), are useful medical interventions. Drugs that alter the pH of the upper gastrointestinal tract (e.g., proton pump inhibitors, H2-receptor antagonists, and antacids) might alter the solubility of vismodegib and reduce bioavailability. However, results of a clinical study did not show clinically significant pharmacokinetic interaction between vismodegib and rabeprazole (a proton pump inhibitor) in healthy volunteers (unpublished data; Roche). If symptoms do not improve, treatment interruption or a change in dosing regimens should be the next step, with discontinuation as the final option.
Other AEs
Components of the hedgehog signaling pathway play a functional role in the ovaries of mature rodents [73, 74], and, in phase I and II trials, amenorrhea or irregular menses occurred in 10 of 35 premenopausal female patients (28.5%) treated with vismodegib [75]. Resolution of amenorrhea in some patients has been observed after discontinuation of vismodegib. HPIs can affect female fertility; consequently, fertility preservation strategies should be discussed with women of childbearing age before starting treatment with HPIs. HPIs are teratogenic in animals and, in humans, can result in embryo-fetal death or severe birth defects. Pregnancy status should be verified before treatment with HPIs in women of childbearing potential, and patients should be informed of the need for contraception.
HPIs are teratogenic in animals and, in humans, can result in embryo-fetal death or severe birth defects. Pregnancy status should be verified before treatment with HPIs in women of childbearing potential, and patients should be informed of the need for contraception.
For sonidegib-treated patients, CK levels should be assessed before treatment and periodically as clinically indicated. Additional laboratory testing is at the discretion of the treating physician. Elevated CK levels were observed in 29% and 37% of patients treated with sonidegib 200 mg and 800 mg, respectively [5]. Patients starting therapy with sonidegib must be informed of the risk for muscle-related AEs, including rhabdomyolysis. Patients should be carefully monitored during treatment and advised to promptly report any unexplained muscle pain, tenderness, or weakness. Patients with neuromuscular disorders and those in whom sonidegib is administered in combination with agents that can increase the risk for muscle toxicity (e.g., 3-hydroxy-3-methylglutaryl-coenzyme [HMG-CoA] reductase inhibitors) should be followed up carefully. According to the vismodegib label, laboratory testing for CK level is not necessary.
Conclusion
Hedgehog pathway inhibitors could be an important treatment option for patients with advanced BCC—a population for whom options were previously limited. The safety profile of vismodegib was consistent during long-term follow-up and across a number of clinical trials. Although the severity of the AEs typically associated with treatment is mostly grade 1 or 2, the long-term nature of these AEs can lead patients to discontinue treatment. Moreover, patients with complete response to HPIs tend to discontinue treatment because of these common AEs more frequently than patients with no response or with partial response, who might discontinue only because of more severe AEs. Evidence also suggests that changing HPIs will not lead to significantly different AEs, underscoring the importance of maintaining patients on therapy with the first HPI administered and providing sufficient support to manage AEs to maximize effectiveness.
Assessing the QOL of patients treated with HPIs is critical because discrepancies have been seen in how HCPs and patients grade the severity of AEs [76]. A patient-reported outcomes questionnaire has been developed for patients with advanced BCC and BCCNS; however, this questionnaire is not specifically designed to capture AEs unique to HPIs [30]. A symptom questionnaire has been developed for use in vismodegib-treated patients (Fig. 3), and its use might aid in ascertaining the impact of these events in patient QOL and aid in supportive care interventions. Studies in BCC populations are ongoing to further explore an optimal dosing regimen for vismodegib [77, 78]. In addition, data indicate that the safety profile of vismodegib is comparable in elderly patients and younger patients [79]. To ensure maximum benefit from therapy, use of the management recommendations presented here might enable HCPs to maintain QOL in patients taking HPIs.
Acknowledgments
We thank employees of F. Hoffmann-La Roche, Ltd., including Daniel Bergström, Damian Page, and Jeannie Hou, for comments on the manuscript before approval and submission. Medical editorial assistance in preparation for the expert meeting and this manuscript was provided by David Gibson, CMPP (ApotheCom, San Francisco, California), and was funded by F. Hoffmann-La Roche, Ltd. This work was supported by F. Hoffmann-La Roche, Ltd. Neither F. Hoffman-La Roche, Ltd., nor ApotheCom influenced the content of the manuscript. A National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748 supported Mario E. Lacouture. Research at the Melanoma Unit, Dermatology Department, Hospital Clinic in Barcelona, Spain, is partially funded by Spanish Fondo de Investigaciones Sanitarias grants 09/01393 and 12/00840; CIBER de Enfermedades Raras of the Instituto de Salud Carlos III, Spain; AGAUR 2009 SGR 1337 and AGAUR 2014_SGR_603 of the Catalan Government, Spain; European Commission under the 6th Framework Programme, Contract No. LSHC-CT-2006-018702 (GenoMEL); the European Commission under the 7th Framework Programme, Diagnoptics; the National Cancer Institute (NCI) of the U.S. National Institute of Health (NIH) (CA83115); and a grant from “Fundació La Marató de TV3, 201331-30”, Catalonia, Spain. Susan Puig's work was carried out at the Esther Koplowitz Center, Barcelona. The authors received no financial compensation for authoring the manuscript.
Author Contributions
Conception/Design: Mario E. Lacouture, Paolo Antonio Ascierto, Reinhard Dummer, Nicole Basset-Seguin, Scott Ernst, Rogerio I. Neves, Ketty Peris, Susana Puig, Jonas Sokolof
Provision of study material or patients: Mario E. Lacouture, Brigitte Dréno, Paolo Antonio Ascierto, Reinhard Dummer, Nicole Basset-Seguin, Kate Fife, Scott Ernst, Lisa Licitra, Rogerio I. Neves, Ketty Peris, Rainer Kunstfeld
Collection and/or assembly of data: Mario E. Lacouture, Brigitte Dréno, Paolo Antonio Ascierto, Reinhard Dummer, Nicole Basset-Seguin, Kate Fife, Scott Ernst, Lisa Licitra, Rogerio I. Neves, Ketty Peris, Jonas Sokolof, Rainer Kunstfeld
Data analysis and interpretation: Mario E. Lacouture, Brigitte Dréno, Paolo Antonio Ascierto, Reinhard Dummer, Kate Fife, Lisa Licitra, Rogerio I. Neves, Ketty Peris, Aleksandar Sekulic, Axel Hauschild
Manuscript writing: Mario E. Lacouture, Paolo Antonio Ascierto, Reinhard Dummer, Kate Fife, Susana Puig, Jonas Sokolof, Aleksandar Sekulic, Axel Hauschild
Final approval of manuscript: Mario E. Lacouture, Brigitte Dréno, Paolo Antonio Ascierto, Reinhard Dummer, Nicole Basset-Seguin, Kate Fife, Scott Ernst, Lisa Licitra, Rogerio I. Neves, Ketty Peris, Susana Puig, Jonas Sokolof, Aleksandar Sekulic, Axel Hauschild, Rainer Kunstfeld
Other (financial and administrative support): Mario E. Lacouture, Reinhard Dummer
Disclosures
Mario E. Lacouture: Roche-Genentech, Novartis, EMD Serono, Merck Sharp & Dohme (C/A), Berg, Bristol-Myers Squibb, Roche-Genentech (RF); Brigitte Dréno: Roche (C/A); Paolo Antonio Ascierto: Bristol-Myers Squibb, Roche-Genentech, Merck Sharp & Dohme, Novartis, Ventana, Amgen, Array (C/A), Bristol-Myers Squibb, Roche-Genentech, Ventana, Array (RF); Reinhard Dummer: Roche (C/A); Nicole Basset-Seguin: Roche, Leo, Galderma, Novartis (C/A); Kate Fife: Roche, Pfizer, Novartis (H), Roche (RF); Scott Ernst: Roche, Bristol-Myers Squibb, Merck, Novartis (C/A), Roche, Bristol-Myers Squibb, Merck (RF), Roche, Bristol-Myers Squibb, Novartis (H); Lisa Licitra: Eisai, Bristol-Myers Squibb, Merck Sharp & Dohme, Merck-Serono, Boehringer Ingelheim, Debiopharm, SOBI, Novartis, AstraZeneca, Bayer (C/A), Eisai, Merck Sharp & Dohme, Merck-Serono, Boehringer Ingelheim, Novartis, AstraZeneca, Roche (RF); Merck-Serono, Debiopharm, SOBI, Bayer (other); Rogerio I. Neves: Roche, Genentech, Amgen (C/A); Ketty Peris: International Advisory Board (C/A); Susana Puig: Roche (C/A), Roche, Almirall, Leti, ISDIN (H); La Roche Posay, Meda (RF); Axel Hauschild: Roche, Novartis (C/A, RF, H); Rainer Kunstfeld: F. Hoffmann-La Roche, Basel (C/A, H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Macha MA, Batra SK, Ganti AK. Profile of vismodegib and its potential in the treatment of advanced basal cell carcinoma. Cancer Manag Res. 2013;5:197–203. doi: 10.2147/CMAR.S45976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sekulic A, Mangold AR, Northfelt DW, et al. Advanced basal cell carcinoma of the skin: Targeting the hedgehog pathway. Curr Opin Oncol. 2013;25:218–223. doi: 10.1097/CCO.0b013e32835ff438. [DOI] [PubMed] [Google Scholar]
- Erivedge [package insert]. South San Francisco, CA: Genentech USA, Inc.; 2015.
- 4.Medicines.co.uk. Erivedge 150 mg hard capsules: Summary of product characteristics. Available at https://http://www.medicines.org.uk/emc/medicine/28107. Accessed April 4, 2016.
- 5.Migden MR, Guminski A, Gutzmer R, et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): A multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16:716–728. doi: 10.1016/S1470-2045(15)70100-2. [DOI] [PubMed] [Google Scholar]
- 6.Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sekulic A, Migden MR, Lewis K, et al. Pivotal ERIVANCE basal cell carcinoma (BCC) study: 12-month update of efficacy and safety of vismodegib in advanced BCC. J Am Acad Dermatol. 2015;72:1021–6.e8. doi: 10.1016/j.jaad.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Sekulic A, Migden MR, Basset-Seguin N, et al. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: Final update (30-month) of the pivotal ERIVANCE BCC study. J Clin Oncol. 2014;32(suppl):9013a. [Google Scholar]
- 9.Chang ALS, Solomon JA, Hainsworth JD, et al. Expanded access study of patients with advanced basal cell carcinoma treated with the Hedgehog pathway inhibitor, vismodegib. J Am Acad Dermatol. 2014;70:60–69. doi: 10.1016/j.jaad.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Lacouture M, Guillen J, Kudchadkar R et al. Management of frequent vismodegib-related adverse events in patients with locally advanced basal cell carcinoma: RegiSONIC Disease Registry Study. Poster presented at the 74th Annual Meeting of the American Academy of Dermatology; March 6, 2016; Washington, DC. [Google Scholar]
- 11.Sofen H, Gross KG, Goldberg LH, et al. A phase II, multicenter, open-label, 3-cohort trial evaluating the efficacy and safety of vismodegib in operable basal cell carcinoma. J Am Acad Dermatol. 2015;73:99–105.e1. doi: 10.1016/j.jaad.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Basset-Seguin N, Hauschild A, Grob J-J, et al. Vismodegib in patients with advanced basal cell carcinoma (STEVIE): A pre-planned interim analysis of an international, open-label trial. Lancet Oncol. 2015;16:729–736. doi: 10.1016/S1470-2045(15)70198-1. [DOI] [PubMed] [Google Scholar]
- 13.Teperino R, Aberger F, Esterbauer H, et al. Canonical and non-canonical Hedgehog signalling and the control of metabolism. Semin Cell Dev Biol. 2014;33:81–92. doi: 10.1016/j.semcdb.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brennan D, Chen X, Cheng L, et al. Noncanonical Hedgehog signaling. Vitam Horm. 2012;88:55–72. doi: 10.1016/B978-0-12-394622-5.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein EH. Basal cell carcinomas: Attack of the hedgehog. Nat Rev Cancer. 2008;8:743–754. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Göppner D, Leverkus M. Basal cell carcinoma: From the molecular understanding of the pathogenesis to targeted therapy of progressive disease. J Skin Cancer. 2011;2011:650258. doi: 10.1155/2011/650258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo Muzio L. Nevoid basal cell carcinoma syndrome (Gorlin syndrome) Orphanet J Rare Dis. 2008;3:32. doi: 10.1186/1750-1172-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gould SE, Low JA, Marsters JC, Jr, et al. Discovery and preclinical development of vismodegib. Expert Opin Drug Discov. 2014;9:969–984. doi: 10.1517/17460441.2014.920816. [DOI] [PubMed] [Google Scholar]
- 19.Straface G, Aprahamian T, Flex A, et al. Sonic hedgehog regulates angiogenesis and myogenesis during post-natal skeletal muscle regeneration. J Cell Mol Med. 2009;13(8B):2424–2435. doi: 10.1111/j.1582-4934.2008.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H-X, Ermilov A, Grachtchouk M, et al. Multiple Shh signaling centers participate in fungiform papilla and taste bud formation and maintenance. Dev Biol. 2013;382:82–97. doi: 10.1016/j.ydbio.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang H, Cong WN, Yoon JS, et al. Vismodegib, an antagonist of hedgehog signaling, directly alters taste molecular signaling in taste buds. Cancer Med. 2015;4:245–252. doi: 10.1002/cam4.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumari A, Ermilov AN, Allen BL, et al. Hedgehog pathway blockade with the cancer drug LDE225 disrupts taste organs and taste sensation. J Neurophysiol. 2015;113:1034–1040. doi: 10.1152/jn.00822.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.St-Jacques B, Dassule HR, Karavanova I, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058–1068. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- 24.Chiang C, Swan RZ, Grachtchouk M, et al. Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev Biol. 1999;205:1–9. doi: 10.1006/dbio.1998.9103. [DOI] [PubMed] [Google Scholar]
- 25.Sato N, Leopold PL, Crystal RG. Induction of the hair growth phase in postnatal mice by localized transient expression of Sonic hedgehog. J Clin Invest. 1999;104:855–864. doi: 10.1172/JCI7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paladini RD, Saleh J, Qian C, et al. Modulation of hair growth with small molecule agonists of the hedgehog signaling pathway. J Invest Dermatol. 2005;125:638–646. doi: 10.1111/j.0022-202X.2005.23867.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang LC, Liu Z-Y, Gambardella L, et al. Regular articles: Conditional disruption of hedgehog signaling pathway defines its critical role in hair development and regeneration. J Invest Dermatol. 2000;114:901–908. doi: 10.1046/j.1523-1747.2000.00951.x. [DOI] [PubMed] [Google Scholar]
- Lear J, Guminski A, Gutzmer R et al. A phase 2, randomized, double-blind study of sonidegib (LDE225) in patients with advnaced basal cell carcinoma: the BOLT 18-month analysis. Presented at the 24th European Academy of Dermatology and Venereology Congress; October 7–11, 2015; Copenhagen, Denmark. [Google Scholar]
- 29.Teperino R, Amann S, Bayer M, et al. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell. 2012;151:414–426. doi: 10.1016/j.cell.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 30.Mathias SD, Chren MM, Crosby RD, et al. Reliability and validity of the Advanced Basal Cell Carcinoma Index (aBCCdex) Br J Dermatol. 2015;173:713–719. doi: 10.1111/bjd.13877. [DOI] [PubMed] [Google Scholar]
- 31.Abdulla AJ, Jones PW, Pearce VR. Leg cramps in the elderly: Prevalence, drug and disease associations. Int J Clin Pract. 1999;53:494–496. [PubMed] [Google Scholar]
- 32.Bertolasi L, De Grandis D, Bongiovanni LG, et al. The influence of muscular lengthening on cramps. Ann Neurol. 1993;33:176–180. doi: 10.1002/ana.410330207. [DOI] [PubMed] [Google Scholar]
- 33.Pohl C, Happe J, Klockgether T. Cooling improves the writing performance of patients with writer’s cramp. Mov Disord. 2002;17:1341–1344. doi: 10.1002/mds.10251. [DOI] [PubMed] [Google Scholar]
- 34.Siegal T. Muscle cramps in the cancer patient: Causes and treatment. J Pain Symptom Manage. 1991;6:84–91. doi: 10.1016/0885-3924(91)90522-6. [DOI] [PubMed] [Google Scholar]
- 35.Blyton F, Chuter V, Walter KEL, et al. Non-drug therapies for lower limb muscle cramps. Cochrane Database Syst Rev. 2012;1:CD008496. doi: 10.1002/14651858.CD008496.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ally MS, Tang JY, Lindgren J, et al. Effect of calcium channel blockade on vismodegib-induced muscle cramps. JAMA Dermatol. 2015;151:1132–1134. doi: 10.1001/jamadermatol.2015.1937. [DOI] [PubMed] [Google Scholar]
- 37.Voon W-C, Sheu S-H. Diltiazem for nocturnal leg cramps. Age Ageing. 2001;30:91–92. doi: 10.1093/ageing/30.1.91. [DOI] [PubMed] [Google Scholar]
- 38.Baltodano N, Gallo BV, Weidler DJ. Verapamil vs quinine in recumbent nocturnal leg cramps in the elderly. Arch Intern Med. 1988;148:1969–1970. [PubMed] [Google Scholar]
- 39.Serrao M, Rossi P, Cardinali P, et al. Gabapentin treatment for muscle cramps: An open-label trial. Clin Neuropharmacol. 2000;23:45–49. doi: 10.1097/00002826-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Sebo P, Cerutti B, Haller DM. Effect of magnesium therapy on nocturnal leg cramps: A systematic review of randomized controlled trials with meta-analysis using simulations. Fam Pract. 2014;31:7–19. doi: 10.1093/fampra/cmt065. [DOI] [PubMed] [Google Scholar]
- 41.Prateepavanich P, Kupniratsaikul V, Charoensak T. The relationship between myofascial trigger points of gastrocnemius muscle and nocturnal calf cramps. J Med Assoc Thai. 1999;82:451–459. [PubMed] [Google Scholar]
- 42.Bedlack RS, Pastula DM, Hawes J, et al. Open-label pilot trial of levetiracetam for cramps and spasticity in patients with motor neuron disease. Amyotroph Lateral Scler. 2009;10:210–215. doi: 10.1080/17482960802430773. [DOI] [PubMed] [Google Scholar]
- 43.Chan P, Huang T-Y, Chen Y-J, et al. Randomized, double-blind, placebo-controlled study of the safety and efficacy of vitamin B complex in the treatment of nocturnal leg cramps in elderly patients with hypertension. J Clin Pharmacol. 1998;38:1151–1154. [PubMed] [Google Scholar]
- 44.Young JB, Connolly MJ. Naftidrofuryl treatment for rest cramp. Postgrad Med J. 1993;69:624–626. doi: 10.1136/pgmj.69.814.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weil AJ, Ruoff GE, Nalamachu S, et al. Efficacy and tolerability of cyclobenzaprine extended release for acute muscle spasm: A pooled analysis. Postgrad Med. 2010;122:158–169. doi: 10.3810/pgm.2010.07.2182. [DOI] [PubMed] [Google Scholar]
- 46.Baldinger R, Katzberg HD, Weber M. Treatment for cramps in amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2012;4:CD004157. doi: 10.1002/14651858.CD004157.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.US Food and Drug Administration. Qualaquin (quinine sulfate): New risk evaluation and mitigation strategy - risk of serious hematological reactions. Available at http://www.fda.gov/Safety/MedWatch/SafetyInformation/ SafetyAlertsforHumanMedicalProducts/ucm218424.htm. Accessed April 4, 2016.
- 48.Katzberg HD, Khan AH, So YT. Assessment: symptomatic treatment for muscle cramps (an evidence-based review): Report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology. 2010;74:691–696. doi: 10.1212/WNL.0b013e3181d0ccca. [DOI] [PubMed] [Google Scholar]
- 49.Syrjala KL, Yi JC, Artherholt SB, et al. Measuring musculoskeletal symptoms in cancer survivors who receive hematopoietic cell transplantation. J Cancer Surviv. 2010;4:225–235. doi: 10.1007/s11764-010-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Syrjala KL. MJM (muscle and joint measure). Available at https://http://www.fredhutch.org/content/dam/public/labs-projects/clinical-research/biobehavioral/MJM_updated_1-15-10_Syrjala_et_al_PDF.pdf. Accessed May 2, 2016.
- 51.Feng P, Huang L, Wang H. Taste bud homeostasis in health, disease, and aging. Chem Senses. 2014;39:3–16. doi: 10.1093/chemse/bjt059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rawal S, Hoffman HJ, Bainbridge KE, et al. Prevalence and risk factors of self-reported smell and taste alterations: Results from the 2011-2012 US National Health and Nutrition Examination Survey (NHANES) Chem Senses. 2016;41:69–76. doi: 10.1093/chemse/bjv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hovan AJ, Williams PM, Stevenson-Moore P, et al. A systematic review of dysgeusia induced by cancer therapies. Support Care Cancer. 2010;18:1081–1087. doi: 10.1007/s00520-010-0902-1. [DOI] [PubMed] [Google Scholar]
- 54.Gamper E-M, Zabernigg A, Wintner LM, et al. Coming to your senses: Detecting taste and smell alterations in chemotherapy patients. A systematic review. J Pain Symptom Manage. 2012;44:880–895. doi: 10.1016/j.jpainsymman.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 55.Barlow LA. Progress and renewal in gustation: New insights into taste bud development. Development. 2015;142:3620–3629. doi: 10.1242/dev.120394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brisbois TD, de Kock IH, Watanabe SM, et al. Delta-9-tetrahydrocannabinol may palliate altered chemosensory perception in cancer patients: Results of a randomized, double-blind, placebo-controlled pilot trial. Ann Oncol. 2011;22:2086–2093. doi: 10.1093/annonc/mdq727. [DOI] [PubMed] [Google Scholar]
- 57.Le Moigne M, Saint-Jean M, Jirka A, et al. Dysgeusia and weight loss under treatment with vismodegib: Benefit of nutritional management. Support Care Cancer. 2016;24:1689–1695. doi: 10.1007/s00520-015-2932-1. [DOI] [PubMed] [Google Scholar]
- 58.Schiffman SS, Sattely-Miller EA, Taylor EL, et al. Combination of flavor enhancement and chemosensory education improves nutritional status in older cancer patients. J Nutr Health Aging. 2007;11:439–454. [PubMed] [Google Scholar]
- 59.Heckmann SM, Hujoel P, Habiger S, et al. Zinc gluconate in the treatment of dysgeusia--a randomized clinical trial. J Dent Res. 2005;84:35–38. doi: 10.1177/154405910508400105. [DOI] [PubMed] [Google Scholar]
- 60.Halyard MY, Jatoi A, Sloan JA, et al. Does zinc sulfate prevent therapy-induced taste alterations in head and neck cancer patients? Results of phase III double-blind, placebo-controlled trial from the North Central Cancer Treatment Group (N01C4) Int J Radiat Oncol Biol Phys. 2007;67:1318–1322. doi: 10.1016/j.ijrobp.2006.10.046. [DOI] [PubMed] [Google Scholar]
- 61.Ferguson JS, Hannam S, Toholka R, et al. Hair loss and Hedgehog inhibitors: A class effect? Br J Dermatol. 2015;173:262–264. doi: 10.1111/bjd.13619. [DOI] [PubMed] [Google Scholar]
- 62.Fischer TW, Schmidt S, Strauss B, et al. [Hairdex: A tool for evaluation of disease-specific quality of life in patients with hair diseases] Hautarzt. 2001;52:219–227. doi: 10.1007/s001050051293. [article in German] [DOI] [PubMed] [Google Scholar]
- 63.Tang JY, Mackay-Wiggan JM, Aszterbaum M, et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012;366:2180–2188. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alkeraye S, Maire C, Desmedt E, et al. Persistent alopecia induced by vismodegib. Br J Dermatol. 2015;172:1671–1672. doi: 10.1111/bjd.13630. [DOI] [PubMed] [Google Scholar]
- 65.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 66.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Cancer-related fatigue. Version 1.2016. Available at http://www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf. Accessed February 2, 2016.
- 67.National Cancer Institute. Fatigue for health professionals (PDQ). Available at http://www.cancer.gov/about-cancer/treatment/side-effects/fatigue/fatigue-hp-pdq#section/all. Accessed February 2, 2016.
- 68.Spathis A, Fife K, Blackhall F, et al. Modafinil for the treatment of fatigue in lung cancer: Results of a placebo-controlled, double-blind, randomized trial. J Clin Oncol. 2014;32:1882–1888. doi: 10.1200/JCO.2013.54.4346. [DOI] [PubMed] [Google Scholar]
- 69.Ruddy KJ, Barton D, Loprinzi CL. Laying to rest psychostimulants for cancer-related fatigue? J Clin Oncol. 2014;32:1865–1867. doi: 10.1200/JCO.2014.55.8353. [DOI] [PubMed] [Google Scholar]
- 70.Smets EMA, Garssen B, Bonke B, et al. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 71.Bower JE, Bak K, Berger A, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: An American Society of Clinical Oncology clinical practice guideline adaptation. J Clin Oncol. 2014;32:1840–1850. doi: 10.1200/JCO.2013.53.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Antiemesis. Version 2.2015. Available at http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed February 2, 2016.
- 73.Russell MC, Cowan RG, Harman RM, et al. The hedgehog signaling pathway in the mouse ovary. Biol Reprod. 2007;77:226–236. doi: 10.1095/biolreprod.106.053629. [DOI] [PubMed] [Google Scholar]
- 74.Spicer LJ, Sudo S, Aad PY, et al. The hedgehog-patched signaling pathway and function in the mammalian ovary: A novel role for hedgehog proteins in stimulating proliferation and steroidogenesis of theca cells. Reproduction. 2009;138:329–339. doi: 10.1530/REP-08-0317. [DOI] [PubMed] [Google Scholar]
- Sekulic A, Weiss GJ, Solomon JA et al. Amenorrhea or irregular menses in patients treated with vismodegib. Presented at the 2014 Winter Clinical Dermatology Conference; January 17–22, 2014; Kona Coast, HI. [Google Scholar]
- 76.Basch E, Reeve BB, Mitchell SA, et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) J Natl Cancer Inst. 2014;106:dju244. doi: 10.1093/jnci/dju244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunstfeld R, Hauschild A, Zloty D et al. MIKIE: A randomized, double-blind, regimen-controlled, phase II, multicenter study to assess the efficacy and safety of two different vismodegib regimens in patients with multiple basal cell carcinomas. Presented at the 50th Annual Meeting of the American Society of Clinical Oncology; May 30–June 3, 2014; Chicago, IL. [Google Scholar]
- Mortier L, Saiag P, Lecca MT et al. A phase II study to assess vismodegib in the neoadjuvant treatment of locally advanced basal cell carcinoma (laBCC): The Vismodegib Neoadjuvant (VISMONEO) study. Presented at the 50th Annual Meeting of the American Society of Clinical Oncology; May 30–June 3, 2014; Chicago, IL. [Google Scholar]
- Chang ALS, Lewis K, Arron ST et al. Safety and efficacy of vismodegib in elderly patients: analysis of the ERIVANCE BCC and expanded access studies. Presented at the Third Annual World Cutaneous Malignancies Congress; October 29–31, 2014; San Francisco, CA. [Google Scholar]