This review focuses on the management of immune-related adverse events (irAEs) after treatment with anti-programmed death-1 antibodies as monotherapy or in combination in patients with advanced melanoma. A better understanding of the management of irAEs will help ensure the safe and appropriate use of these agents in melanoma and other tumor types.
Keywords: Programmed cell death 1 receptor, Nivolumab, Pembrolizumab, Drug-related side effects and adverse reactions, Melanoma
Abstract
Immune checkpoint inhibitors have emerged as a mainstay of melanoma therapy and are playing an increasingly important role in the treatment of other tumor types. The clinical benefit afforded by these treatments can be accompanied by a unique spectrum of adverse events, called immune-related adverse events (irAEs), which reflect the drug’s immune-based mechanism of action. IrAEs typically originate in the skin, gastrointestinal tract, liver, and endocrine system, although other organ systems may also be affected. This article provides an overview of irAEs associated with anti-programmed death-1 (anti-PD-1) antibodies (nivolumab and pembrolizumab) as monotherapy or in combination with anti-cytotoxic T-lymphocyte antigen-4 inhibition (ipilimumab), followed by a discussion of irAEs of special clinical interest based on the potential for morbidity, frequent steroid use, and inpatient admission. We review clinical trial data and provide recommendations on how to manage irAEs associated with anti-PD-1 agents based on clinical experience and established management guidelines. We further illustrate the practical considerations of managing irAEs by presenting three cases of immune-related toxicity in melanoma patients treated with nivolumab or pembrolizumab. A better understanding of the identification and management of irAEs will help inform health care providers about the risks associated with anti-PD-1 treatment, to ensure the safe and appropriate use of these important new treatments.
Implications for Practice:
Immune checkpoint inhibitors have demonstrated significant clinical benefit in advanced melanoma and other tumor types. These treatments are associated with immune-related adverse events (irAEs), which most commonly affect the skin and gastrointestinal tract, and, to a lesser extent, the liver, endocrine system, and other organs. This review focuses on the management of irAEs after treatment with anti-programmed death-1 (anti-PD-1) antibodies (nivolumab or pembrolizumab) as monotherapy or in combination with anti-cytotoxic T-lymphocyte antigen-4 inhibition (ipilimumab) in patients with advanced melanoma. A better understanding of the management of irAEs will help ensure the safe and appropriate use of anti-PD-1 agents in melanoma and other tumor types.
Introduction
Antibodies that target key immune checkpoints, such as cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed death-1 (PD-1), have emerged as clinically effective treatment options for melanoma and other tumor types, including non-small-cell lung cancer and renal cell carcinoma. Ipilimumab, a fully human IgG1 monoclonal antibody against CTLA-4, was the first therapy to demonstrate an overall survival (OS) benefit in a randomized, controlled, phase 3 study of patients with advanced melanoma [1] and the first immune checkpoint inhibitor to be approved by the Food and Drug Administration (FDA). In 2014, the anti-PD-1 antibodies nivolumab and pembrolizumab were added to the list of FDA-approved agents for advanced melanoma and have shown benefit in randomized phase 3 studies. Nivolumab demonstrated improved OS versus dacarbazine in patients with BRAF wild-type, previously untreated, advanced melanoma (phase 3 study CheckMate 066) [2] and a higher objective response rate (ORR) than chemotherapy in patients who had progressed after ipilimumab, or ipilimumab and a BRAF inhibitor if BRAF mutation-positive (phase 3 study CheckMate 037) [3]. Pembrolizumab prolonged progression-free survival (PFS) and improved OS versus ipilimumab alone in patients with advanced melanoma (phase 3 study KEYNOTE-006) [4]. In October 2015, the combination of nivolumab plus ipilimumab was added to the treatment armamentarium for advanced melanoma in the U.S. based on improved ORR and PFS versus ipilimumab in previously untreated patients with BRAF wild-type melanoma (randomized phase 2 study CheckMate 069) [5]. In January 2016, the FDA expanded the indication for nivolumab plus ipilimumab to include patients with BRAF-mutated melanoma based on improved PFS versus ipilimumab alone in previously untreated patients (phase 3 study CheckMate 067, which included patients with BRAF wild-type melanoma and those with BRAF-mutated melanoma) [6].
Given the availability of anti-PD-1 agents as treatment options for advanced melanoma, non-small-cell lung cancer, and renal cell carcinoma and the role they are expected to play in other tumor types, it is important to educate health care providers on the safe and appropriate use of these important new treatments to ensure maximum clinical benefit for patients. This article provides a general overview of the safety profile of immune checkpoint inhibitors, followed by a more detailed discussion of adverse events (AEs) of special clinical interest. We provide recommendations on how to manage AEs associated with anti-PD-1 agents based on established treatment guidelines and our clinical experience, illustrated by three case studies of patients with advanced melanoma.
General Safety Profile Associated With Immune Checkpoint Inhibitors
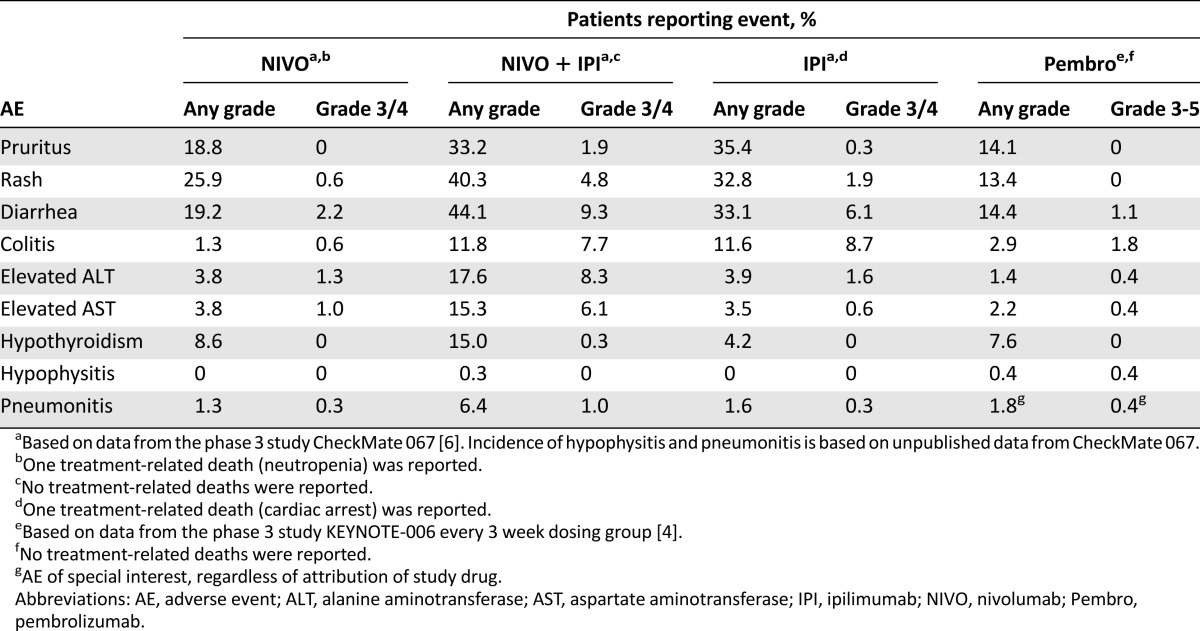
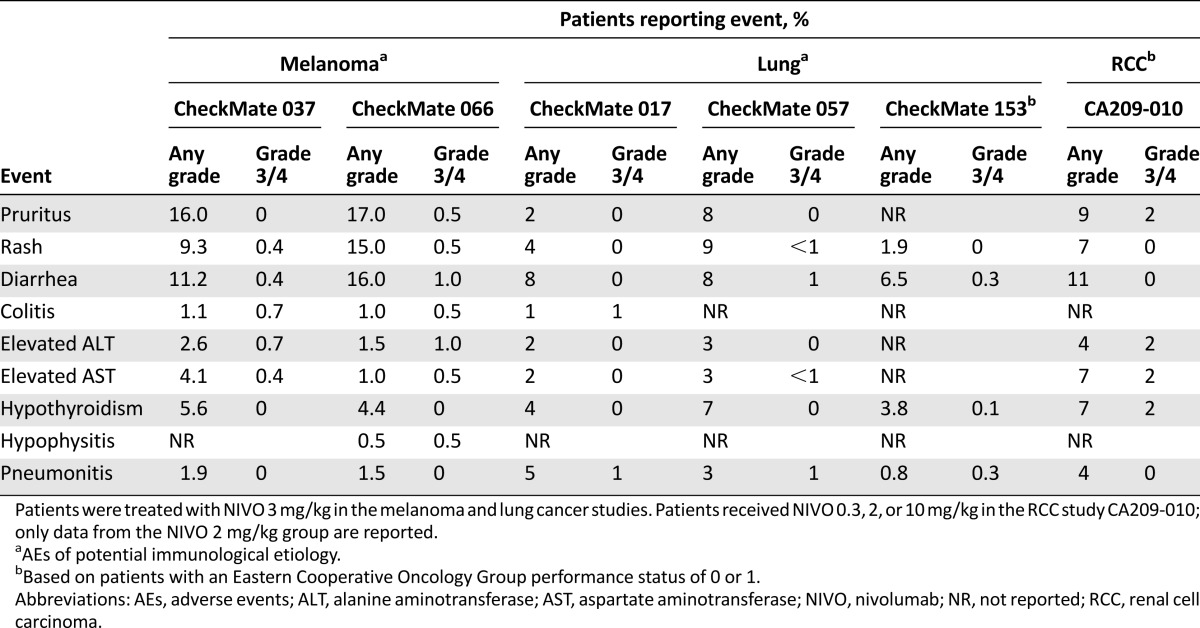
Immune-related adverse events (irAEs) reflect the immune-based mechanism of action of checkpoint inhibitors. The most common irAEs associated with immune checkpoint inhibition affect the skin and gastrointestinal (GI) tract, although the liver, endocrine system, and other organs may also be affected to a lesser extent [7–9]. The rates of high-grade (grade 3/4 for nivolumab alone, ipilimumab alone, or nivolumab plus ipilimumab combination; grades 3–5 for pembrolizumab) treatment-related AEs associated with anti-PD-1 agents are generally lower than those observed with anti-CTLA-4 treatment, with a similar incidence reported with nivolumab and pembrolizumab treatment (Table 1) [4, 6]. The rates of high-grade, treatment-related AEs are higher when anti-PD-1 antibodies are combined with anti-CTLA-4 treatment, compared with anti-PD-1 or anti-CTLA-4 monotherapy (Table 1) [4, 6]. Similar rates of treatment-related grade 3/4 AEs with anti-PD-1 therapy have been reported across tumor types (Table 2) [2, 3, 10–13]. The majority of grade 3/4 irAEs occur during the first 12–14 weeks of treatment, with characteristic timing [14]. In the nivolumab plus ipilimumab combination arm of the randomized phase 2 study CheckMate 069, skin irAEs appeared with a median time of onset within the first ∼2 weeks of treatment, followed by GI irAEs at ∼7 weeks, and endocrine and hepatic events between ∼9 and 12 weeks, respectively [14]. The majority (∼80%) of these events, with the exception of endocrinopathies, resolved within a median of 5 weeks from onset with the use of immune-modulating medication [14]. In the following sections, we discuss irAEs of special clinical interest based on the potential for morbidity, frequent steroid use, and inpatient admission.
Table 1.
Incidence of treatment-related AEs of interest associated with immune checkpoint inhibitors
Table 2.
Incidence of treatment-related AEs of interest in major trials of nivolumab monotherapy in melanoma, lung cancer, and renal cell carcinoma
Gastrointestinal irAEs
GI irAEs are of particular interest to the medical community because they are associated with a high incidence of treatment-related grade 3/4 AEs relative to other organ systems (Table 1) and may lead to inpatient admission due to diarrhea, obstruction, perforation, or toxic megacolon. GI irAEs that are commonly reported with anti-PD-1 treatment include colitis, diarrhea, and enteritis. Gastritis or esophagitis has also been seen. According to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE; version 4.03), colitis refers to abdominal pain, bloody stools, or peritoneal signs, whereas diarrhea is based on stool frequency alone [15]. Enteritis is associated with symptoms similar to colitis, but the inflammation involves the small bowel. Although these terms are separately recorded in clinical trials as per CTCAE, clinically, they present along a similar spectrum. In the phase 3 studies CheckMate 067 and KEYNOTE-006, the incidence of high-grade colitis and diarrhea combined was 2.8% with nivolumab alone, 2.9% with pembrolizumab alone, and ∼17% with the nivolumab plus ipilimumab combination (Table 1) [4, 6].
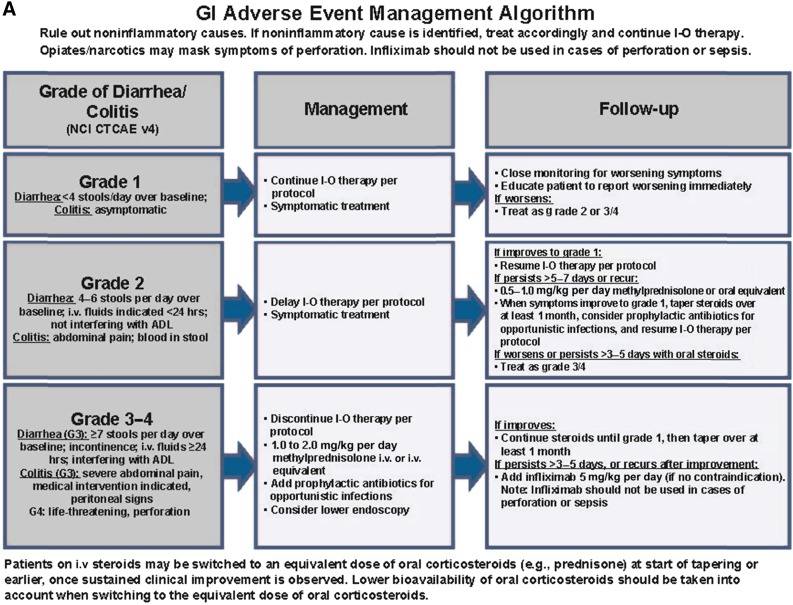
Guidelines are available on how to appropriately manage GI AEs associated with immune checkpoint inhibitors (Fig. 1A). Close monitoring and prompt treatment of early symptoms can reduce the risk of more severe toxicity, such as GI perforation. Noninflammatory causes of symptoms, such as infection with Clostridium difficile or other pathogens, should be ruled out. Colonoscopy and biopsy should be considered if the diagnosis is unclear or in the case of chronic grade 2 AEs (4–6 stools per day over baseline, abdominal pain, blood in stool). Most cases respond to treatment with systemic corticosteroids; loperamide can also be helpful. Patients should be closely monitored and encouraged to report worsening symptoms immediately. Systemic corticosteroids are required in the case of grade 3/4 AEs (≥7 stools per day over baseline, incontinence, severe abdominal pain, and life-threatening perforation) and should be strongly considered if grade 2 AEs persist in spite of supportive care. Oral steroids starting at 1–2 mg/kg per day of prednisone can be used, but for patients requiring hospitalization, who are nil per os (nothing by mouth), or who have significant comorbidities, intravenous methylprednisolone should be used for 1–2 days before beginning an oral taper of prednisone. Waxing and waning of symptoms is common, and several courses of systemic corticosteroids over no less than 30 days may be required. If symptoms improve with steroid treatment, steroids should be continued until grade 1 or 0 toxicity is reached, followed by a taper over at least 30 days. In steroid-refractory cases, after 72 hours, the tumor necrosis factor-α (TNF-α) blocking agent infliximab (5 mg/kg once every 2 weeks) may be used, but not in patients with GI perforation or sepsis. Treatment with infliximab can dramatically improve GI AEs, with occasional alleviation of symptoms within 24 hours.
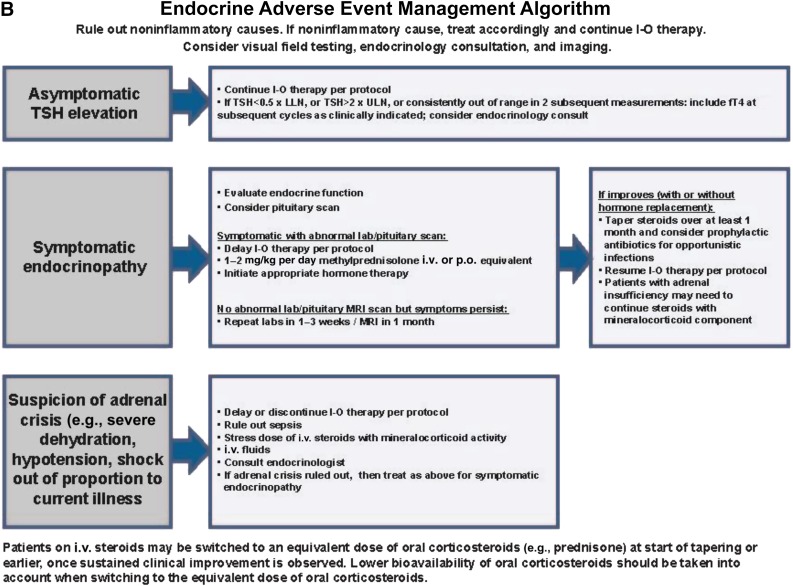
Figure 1.
Management algorithms for GI (A), endocrine (B), hepatic (C), and pulmonary (D) irAEs.
Abbreviations: ADL, activities of daily living; ALT, alanine aminotransferase; AST, aspartate aminotransferase; b.i.d., twice daily; CTCAE, Common Terminology Criteria for Adverse Events; fT4, free T4; G3, grade 3; GI, gastrointestinal; ID, infectious disease; I-O, immuno-oncology; IVIG, i.v. immunoglobulin; LFT, liver function test; LLN, lower limit of normal; MRI, magnetic resonance imaging; NCI, National Cancer Institute; p.o., orally; T. bili, total bilirubin; TSH, thyroid-stimulating hormone; ULN, upper limit of normal; v4, version 4.
Treatment with infliximab can dramatically improve GI AEs, with occasional alleviation of symptoms within 24 hours.
Endocrine irAEs
Endocrine irAEs are also seen following treatment with immune checkpoint inhibitors, and are generally of grade 1 and 2, but they are of special clinical interest because they can be difficult to diagnose. Nonspecific complaints, such as fatigue, nausea, amenorrhea, erectile dysfunction, hypotension, hyponatremia, hypoglycemia, and eosinophilia may reflect endocrine dysfunction. Low rates (<1%) of high-grade hypothyroidism or hypophysitis were reported in CheckMate 067 and KEYNOTE-006 (Table 1) [4, 6].
Guidelines are available on how to appropriately manage endocrine AEs following immune checkpoint inhibition (Fig. 1B). Laboratory testing for adrenocorticotropic hormone (ACTH), cortisol, T4, thyroid-stimulating hormone, and testosterone in males, may help identify endocrine dysfunction. If hypophysitis is suspected, especially with a headache or visual symptoms, consider a magnetic resonance imaging (MRI) scan of the brain with pituitary cuts and visual field testing. Treatment with immune checkpoint inhibition may continue with low-grade endocrine toxicity, although patients should be closely monitored. If adrenal crisis is suspected based on severe dehydration, hypotension, and/or shock out of proportion to the current illness, administer stress-dose steroids in an inpatient setting, delay or discontinue treatment with anti-PD-1 therapy, and consider hormone-replacement therapy. Hypothyroidism can also be preceded by the signs and symptoms of hyperthyroidism over a period of days to weeks, which mimics the clinical presentation of Hashimoto’s thyroiditis.
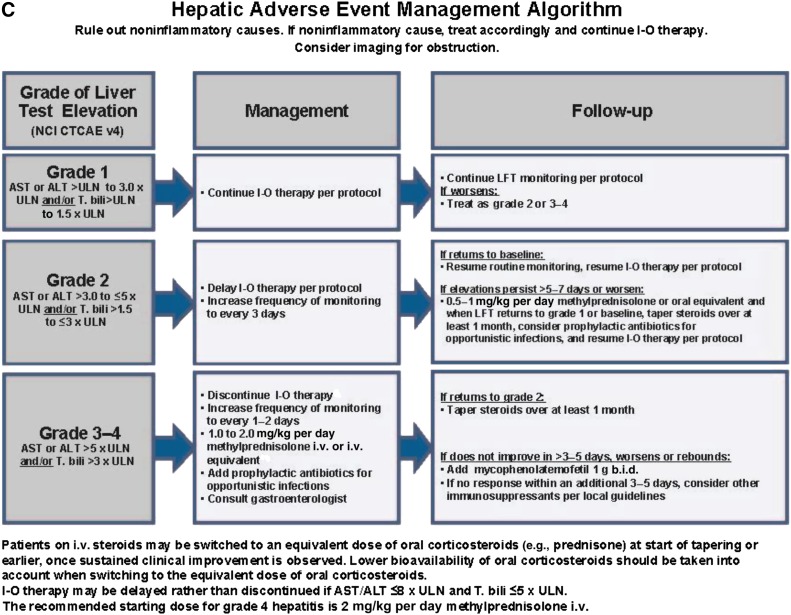
Hepatic irAEs
Hepatotoxicity can be observed following treatment with immune checkpoint inhibition and usually presents as an asymptomatic increase in alanine aminotransferase (ALT), aspartate aminotransferase (AST), and/or total bilirubin. In phase 3 studies CheckMate 067 and KEYNOTE-006, the incidence of high-grade ALT and AST combined was 2.3% with nivolumab alone, 0.8% with pembrolizumab alone, and 14.4% with nivolumab plus ipilimumab combination (Table 1) [4, 6].
Guidelines are available on how to appropriately manage hepatic AEs following immune checkpoint inhibition (Fig. 1C). One should perform a standard workup to ensure that elevations in liver function tests (LFTs) are not because of noninflammatory causes, such as progressive liver disease, viral hepatitis, sepsis, or concurrent medications. Liver imaging tests and biopsy should be considered, and in some cases biopsies have revealed diffuse lymphocytic infiltrates consistent with immune-related hepatitis. For grade 2 hepatic AEs (ALT or AST > 3.0 to ≤5 × upper limit of normal [ULN] and/or total bilirubin > 1.5 to ≤ 3 × ULN), delay treatment and increase the frequency of monitoring to every 2–3 days. If LFTs return to baseline, resume treatment and routine monitoring. If elevations in LFTs persist for >5–7 days or if they worsen, administer intravenous methylprednisolone at a dose of 0.5–1.0 mg/kg per day. Steroids may be tapered over at least 30 days when LFTs return to grade 1 or baseline, and then treatment with anti-PD-1 therapy may resume. For grade 3/4 hepatic AEs (ALT or AST > 5.0 × ULN and/or total bilirubin > 3 × ULN), discontinue treatment, increase the frequency of monitoring to every 1–2 days, and administer intravenous methylprednisolone at a dose of 1.0–2.0 mg/kg per day. Steroids should be tapered over at least 30 days if elevations in LFTs return to grade 2. If LFT elevations do not respond to steroids, administer mycophenolate mofetil orally 1 g twice daily. Infliximab should not be used in this setting because it can cause hepatotoxicity.
Other AEs
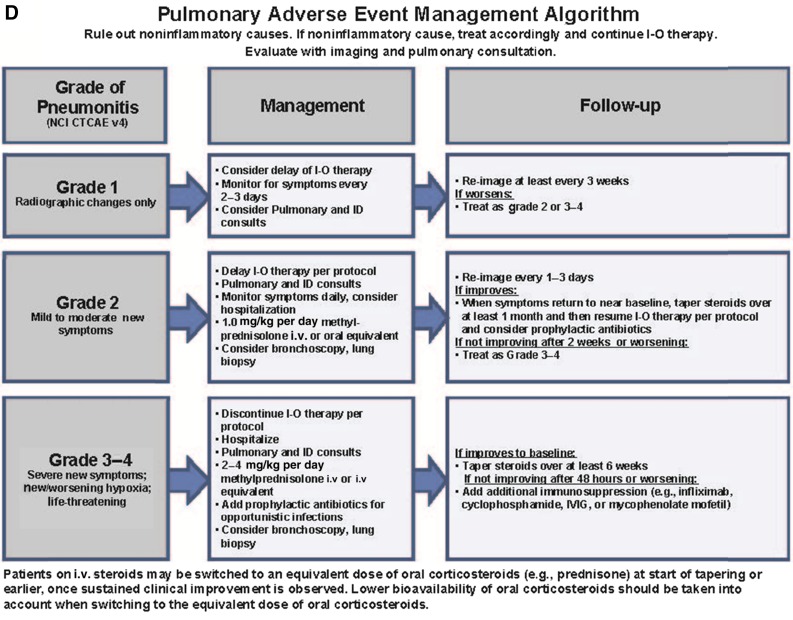
Immune-Related Pneumonitis
Immune-related pneumonitis is a serious AE, particularly in patients with lung cancer who may have poor lung function because of prior smoking [16]. In an early dose-finding study (CA209-003) that evaluated nivolumab monotherapy across multiple tumor types, three treatment-related deaths (1%) due to pneumonitis were reported (two patients with non-small-cell lung cancer and one patient with colorectal cancer) [17]. The rate of grade 3/4 pneumonitis associated with nivolumab treatment was ≤1% in phase 3 non-small-cell lung cancer studies CheckMate 017, CheckMate 057, and CheckMate 153, with no deaths reported due to pneumonitis [10–12]. In the phase 3 melanoma studies CheckMate 067 and KEYNOTE-006, high-grade, immune-related pneumonitis was reported in ≤1% of patients [4, 6], and no deaths were due to pneumonitis. In contrast, symptomatic pneumonitis is quite rare with ipilimumab, and diffuse, grade 3/4 pulmonary toxicity is one of the irAEs that distinguishes anti-PD-1 and anti-CTLA-4 immune checkpoint inhibitors.
Guidelines are available on how to appropriately manage pulmonary immune-related AEs, including pneumonitis, following treatment with immune checkpoint inhibitors (Fig. 1D). Bronchoscopy and lung biopsy should be considered for patients with changes in respiratory status, including symptoms of upper respiratory infection, cough, shortness of breath, or decrease in pulse oximetry below 90% on exertion. For patients with grade 2 mild-to-moderate symptoms or worsening of symptoms from baseline, withhold treatment, monitor the patient daily, and administer corticosteroids (1.0 mg/kg per day intravenous methylprednisolone). If upon reassessment (every 1–3 days) symptoms improve to baseline, taper oral steroids starting at 1 to 2 mg/kg per day of prednisone over at least 30 days before resuming treatment. If no improvement in symptoms is observed after 2 weeks or if symptoms worsen, treat as recommended for grade 3/4 severe events. In severe cases (grade 3/4 symptoms with new or worsening hypoxia or life-threatening symptoms), discontinue treatment, hospitalize the patient with daily monitoring, and administer corticosteroids (2–4 mg/kg per day of intravenous methylprednisolone). If symptoms improve to baseline, patients can take oral steroids, which should be tapered over a period of at least 6 weeks. If symptoms persist or worsen after 2 days, consider the addition of noncorticosteroid immunosuppressive medication, such as infliximab or mycophenolate mofetil.
Immune-Related Pancreatitis
Immune-related pancreatitis has been reported in less than 1% of patients treated with nivolumab or pembrolizumab [8, 9]. Symptoms resemble classic pancreatitis with upper abdominal or back pain. Often radiographic findings such as stranding of the pancreas are not present, but amylase and lipase can be elevated. Although the routine evaluation of amylase and lipase levels in asymptomatic patients is not recommended outside of a clinical trial, symptomatic patients should have their amylase and lipase levels drawn for diagnostic clarification [18]. Treatment of immune-related pancreatitis is based on the severity of symptoms. High-dose corticosteroids starting at 1–2 mg/kg per day of prednisone are recommended in patients with at least moderate symptoms.
Case Studies
In this section, we present three case studies that illustrate unique clinical scenarios and the practical management of irAEs that have occurred following treatment with nivolumab or pembrolizumab. The first case study focuses on a patient with metastatic melanoma who received nivolumab after ipilimumab-induced colitis. The second case study focuses on a patient with metastatic melanoma who had pituitary dysfunction following pembrolizumab treatment. The third case study is a patient with stage IV melanoma who experienced immune-related hepatitis following pembrolizumab treatment.
Case History 1
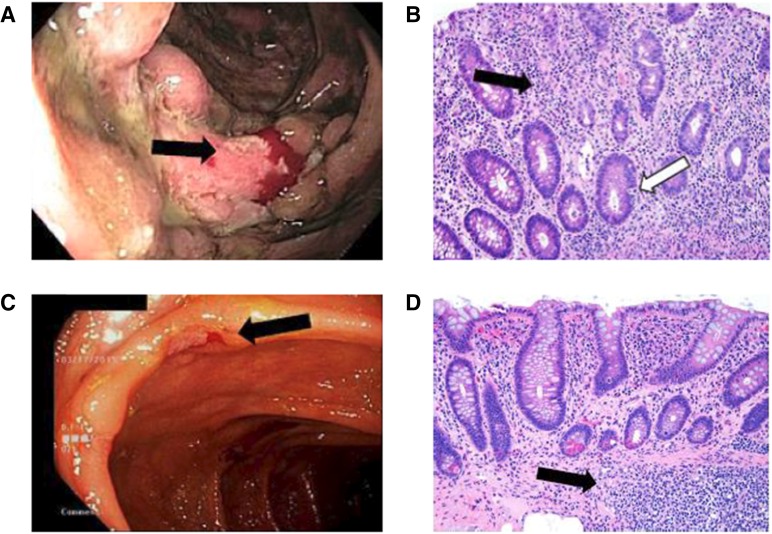
The patient is a 59-year-old man originally diagnosed with superficial spreading melanoma (BRAF V600E/K mutation-negative) from his right posterior shoulder in July 2013 treated with wide excision. He developed a local recurrence in February 2014, and on a subsequent restaging positron emission tomography (PET)-computed tomography (CT), new pulmonary nodules were noted, which were biopsy-proven to be melanoma. Brain MRI was negative for metastasis. Following extensive discussions regarding options, he was treated with ipilimumab from May 2014 to July 2014 (four doses in total). Treatment was complicated by enterocolitis manifested by bloating, mild abdominal pain, and four to five watery stools per day above baseline. CT enterography failed to identify signs of colitis, and infectious workup was negative. He was managed with supportive care and prednisone at a dose of 1 mg/kg by mouth daily. Because of only modest improvement in symptoms, he was hospitalized and given high-dose intravenous (i.v.) steroids and, later, infliximab two times due to refractory colitis. Despite the negative CT enterography, a colonoscopy was performed and demonstrated extensive mucosal ulcerations throughout the large bowel consistent with severe pancolitis (Fig. 2A, 2B). The patient steadily improved, and prednisone was tapered over 8 weeks with no recurrent symptoms.
Figure 2.
Colonoscopy and histopathology results for case history 1. (A): Colonoscopy in August 2014. Discontinuous areas of small and large, deeply cratered ulcerated mucosa are seen throughout entire colon. Mucosa diffusely erythematous with altered vascular pattern, edematous and friable (black arrow). (B): Histopathology (hematoxylin and eosin stain) of right-sided colon (×200). Lamina propria lymphoplasmacytosis and intensely regenerative epithelium (black arrow). Deep crypts contain apoptotic debris (white arrow). (C): Colonoscopy in March 2015. Patchy mild inflammation characterized by pseudopolyps, aphthous ulcers (black arrow), and shallow ulcerations scattered throughout colon. (D): Histopathology (hematoxylin and eosin stain) of cecum (×200) in March 2015. Mild architectural distortion and lamina propria chronic inflammation (similar to quiescent ulcerative colitis). Basal lymphoid aggregate is seen in deep mucosa and submucosa (black arrow).
Initial surveillance imaging after treatment revealed a mixed response. Repeat colonoscopy in October 2014 revealed significantly improved, but persistent, mild colitis. Because of symptomatic progression of disease on his back and right axilla, he underwent palliative resection in November 2014. Subsequent repeat imaging revealed two new brain lesions, which were treated with stereotactic radiosurgery.
Repeat colonoscopy in March 2015 revealed patchy mild inflammation with few scattered ulcerations and mild persistent colitis on biopsy (Fig. 2C, 2D). Because of the need for additional systemic therapy, it was decided to start mesalamine 800 mg twice daily and proceed with nivolumab at 3 mg/kg i.v. every 2 weeks despite the mild residual colitis. After six cycles of nivolumab, repeat imaging demonstrated an excellent partial response and no recurrence of GI symptoms. The patient continued on nivolumab and mesalamine. No repeat colonoscopy has been performed because of the absence of symptoms.
Persistence of symptoms >3 days despite high-dose steroids warrants the consideration of infliximab. Symptoms typically resolve within one to three doses and allow the safe and steady tapering of steroids over time. The safety of anti-PD-1 therapy after resolution or significant improvement in colitis from prior anti-CTLA-4 treatment is unknown. Given the mechanism of action and lower risk of colitis with anti-PD-1 compared with anti-CTLA-4 treatment, agents such as nivolumab may be acceptable treatments after a prior anti-CTLA-4-related side effect such as colitis. This approach needs further study in prospective clinical trials. The effectiveness of anti-inflammatory bowel medications such as mesalamine is unknown, but is also worthy of investigation.
Case History 2
A 59-year-old man with a history of significant depression and stage IIIB cutaneous melanoma underwent a routine surveillance PET scan in December 2014, showing suspicious lesions in his liver and spleen. Biopsy of a liver lesion confirmed NRAS mutant, metastatic melanoma. In October 2014, he began treatment with ipilimumab 3 mg/kg, and he tolerated four doses with only mild pruritus. He continued to feel well, but a PET scan in February 2014 showed progressive disease with increasing size of his hepatic lesions and new subcutaneous lesions. He additionally became more fatigued, which he attributed to becoming particularly depressed by his melanoma progression.
In March 2015, he began treatment with pembrolizumab 2 mg/kg scheduled every 3 weeks. Two weeks after his first infusion with pembrolizumab, he began complaining of even more significantly worsening depression, fatigue, and anxiety. He visited his psychiatrist, who adjusted his psychiatric medications, but his symptoms did not improve. A brain MRI was unremarkable for metastatic disease, and his pituitary appeared normal in size. Basic laboratories including electrolytes and blood counts were normal. Thyroid function testing was normal. A morning cortisol, however, was found to be low at 0.8 μg/dL (lower limit of normal: 5 μg/dL), and ACTH was undetectable. He was diagnosed with secondary adrenal insufficiency, likely due to pituitary dysfunction. After starting treatment with replacement doses of hydrocortisone (20 mg each morning and 10 mg each evening, both by mouth), his energy and depressive symptoms immediately improved. Although his second pembrolizumab dose was delayed, he ultimately resumed pembrolizumab, which has resulted in stabilization of his melanoma—now ongoing for approximately 8 months.
This case illustrates the importance of always considering the possibility of pituitary dysfunction when patients receiving immunotherapy present with fatigue, even though other explanations, such as depression, may seem likely. The rate of pituitary dysfunction with anti-PD-1 agents has been reported <10% in clinical trials [19], but because of variability in assessment as per protocol requirements, the clinical incidence is suspected to be higher. “Fatigue” is one of the most commonly reported symptoms with anti-PD-1 treatment [2, 4]. Although fatigue may be related to many causes such as the underlying cancer and possibly depression, as in our patient’s case, we suggest considering pituitary dysfunction in any patient with this complaint; a correct diagnosis can lead to appropriate treatment and symptomatic relief. Typically, endocrinopathy due to anti-PD-1 agents appears at a median time of ∼10 weeks after treatment initiation [20]. It is possible our patient’s endocrinopathy arose from prior ipilimumab, exacerbated by the more recent initiation of pembrolizumab. After achieving successful symptom relief on stable doses of replacement steroid, we reinitiated pembrolizumab treatment. This demonstrates that patients with this side effect do not need to permanently discontinue anti-PD-1 therapy, particularly early in a treatment course, while experiencing clinical benefit and when on stable hormone doses.
Case History 3
A 48-year-old female was initially diagnosed with stage IIB melanoma of the right gluteal area in March 2007 and was treated with wide excision and right inguinal sentinel lymph node biopsy. In September 2012, the patient was diagnosed with stage IV melanoma with multiple lung metastases, a left axillary lymph node metastasis, and a subcutaneous metastasis of the right abdominal wall, with evidence of BRAF V600E mutation in one of these lesions. Treatment with vemurafenib 960 mg twice daily was initiated, and 4 months later the patient progressed. The patient was then treated with four cycles of ipilimumab 3 mg/kg, but again progressed.
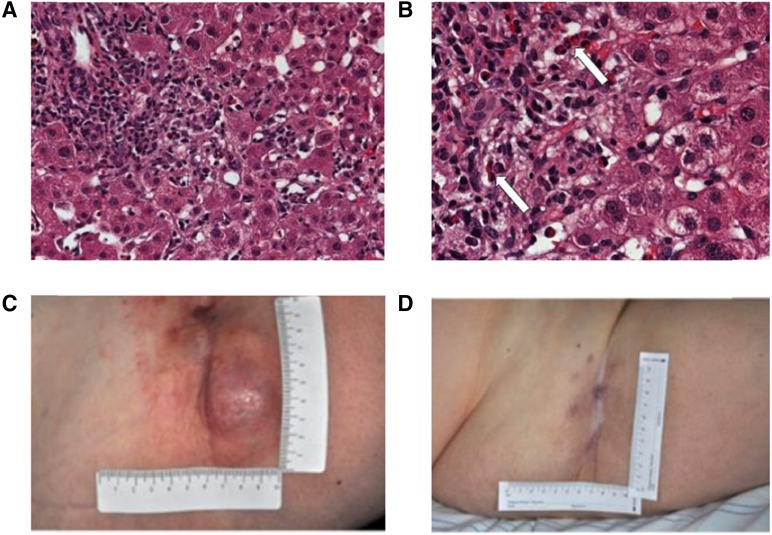
After an initial examination at the Skin Cancer Unit at the University Hospital Essen, the patient was enrolled in the phase 2 study KEYNOTE-002 (pembrolizumab 2 mg/kg versus 10 mg/kg) and received her first dose of study drug in May 2013. One week later, the patient experienced coughing, stinging eyes, transient elevation of γ-glutamyl transpeptidase (γ-GT) (grade 3), total bilirubin (grade 2), and glutamic oxaloacetic transaminase/glutamic pyruvic transaminase (GOT/GPT) (grade 1). C-reactive protein (CRP) was 31 mg/dL (normal <0.5 mg/dL). Hepatitis serology and liver autoantibodies were normal. LFT results and CRP levels were noted to be spontaneously decreasing. A CT scan of the thorax/abdomen showed no evidence of pneumonitis and normal hepatic parenchyma. There was no evidence of melanoma progression. At a follow-up appointment, ∼3 weeks after the patient received a single dose of pembrolizumab, she had recurrent ocular pain, redness, and photophobia. Ophthalmological examination revealed evidence of iritis, and laboratory tests revealed the following: total bilirubin, 3.8 mg/dL (<1.2); GOT, 1,557 U/L (<35); GPT, 1,122 U/L (<35); alkaline phosphatase, 628 U/L (<100); γ-GT, 313 U/L (<35); glutamate dehydrogenase, 57.9 U/L (<5); and CRP, 4.8 mg/dL (<0.5). These AEs were classified as immune-related hepatitis and iritis, and the patient was admitted to the hospital. An ultrasound-guided liver biopsy showed (peri-) portal and lobular hepatitis with abundant eosinophils (Fig. 3A, 3B). Treatment with prednisolone 2 mg/kg and mycophenolate mofetil 1 g daily was initiated. The patient received eye drops to treat the mydriasis and dexamethasone eye drops for inflammation. She was monitored daily, and within 7 days, transaminase levels decreased by half.
Figure 3.
Images for case history 3. (A, B): Liver biopsy pathology of (peri-) portal and lobular hepatitis with abundant eosinophils (white arrows) captured at ×200 and ×400, respectively. (C, D): The patient experienced a partial response following a single dose of pembrolizumab treatment.
The onset of immune-related hepatitis and iritis coincided with a clinical response (Fig. 3C, 3D). Radiological evidence from a CT scan of the thorax/abdomen confirmed that the patient had a partial response according to RECIST v1.1. In October 2013, the patient experienced symmetrical leucotrichia, which started on the face and subsequently extended to the hair on the entire body. Tumor-marker and liver serology tests showed that total bilirubin, S-100, GPT, and lactate dehydrogenase levels continued to drop. In November 2013, the patient continued to have a partial response in target lesions; however, there was evidence of new metastases (subcutaneous and pulmonary), and the patient was removed from the KEYNOTE-002 study. In December 2013, reinduction with ipilimumab 3 mg/kg was recommended, and after four cycles, there was no recurrence of irAEs, and LFTs were normal. As of February 2014, the lung metastases were stable, but tumor burden in the right groin was growing. Complete lymph node dissection of the right groin was performed in March 2014, and as of January 2016, the patient is still alive and in good condition.
This case illustrates that treatment of melanoma with an anti-PD-1 agent requires good communication between patients and providers, as well as close clinical and serological monitoring. Despite the more common development of irAEs after several cycles of therapy, irAEs can occur early in the course of treatment, and early recognition and treatment according to established management guidelines is critical. A single dose of pembrolizumab can lead to rapid, long-lasting remission of melanoma metastases, which is maintained during irAE management despite the use of immunosuppressive therapy.
Summary
Recommendations for treating irAEs come from general clinical consensus and experience because no prospective trials have been conducted to test whether one management strategy is superior to another. Early recognition and treatment are believed to be important in mitigating the severity of irAEs. Because irAEs likely arise from general and organ-specific alterations in immunologic reactivity, immunosuppression for relatively brief periods of 2 weeks to 2 months is often necessary. Prolonged immunosuppression greater than 28 days may predispose patients to opportunistic infections, and prophylaxis against infectious organisms may be appropriate, as per National Comprehensive Cancer Network guidelines. The important lessons to be learned from managing irAEs with nivolumab and pembrolizumab include the need for good communication between providers and patients, the need to recognize and treat high-grade irAEs early, and the need to administer prolonged steroid tapers of not less than 30 days duration when treating high-grade irAEs. It is recommended that steroids be completely tapered, or replacement corticosteroids be started in the case of an endocrinopathy, before considering reinstituting treatment with a PD-1 antibody if an irAE was not felt to be dose limiting. General guidelines for the management of irAEs are provided in Figure 1. In addition, a general approach to toxicity management of irAEs is provided by the Food and Drug Administration Risk Evaluation and Management Strategies [21], which was designed for ipilimumab, but is also applicable to nivolumab and pembrolizumab. Persistent grade 2 irAEs more than 5–7 days should be treated as if they were a high-grade irAE, and initial treatment with i.v. steroids for the first 1 or 2 days is appropriate for patients who require hospitalization or who are felt to have significant comorbidities. Lack of resolution of symptoms of colitis and possibly other high-grade irAEs within 72 hours may require use of the i.v. TNF blocking antibody infliximab, except for cases of hepatotoxicity. Dose reductions of PD-1 antibodies and/or ipilimumab have not been utilized in any trial of those agents and are not recommended after resolution of toxicity. A better understanding of the identification and management of irAEs will help ensure the safe and appropriate use of anti-PD-1 agents.
Early recognition and treatment are believed to be important in mitigating the severity of irAEs. Because irAEs likely arise from general and organ-specific alterations in immunologic reactivity, immunosuppression for relatively brief periods of 2 weeks to 2 months is often necessary.
Acknowledgments
Professional medical writing and editorial assistance, including grammatical and stylistic suggestions, and production assistance were provided by Jennifer DiNieri, Ph.D., and Artur Romanchuk, Ph.D., of StemScientific, an Ashfield Company, funded by Bristol-Myers Squibb. Funding for this work was provided by Bristol-Myers Squibb. Bristol-Myers Squibb did not influence the content of this manuscript. The authors did not receive financial compensation for authoring this manuscript.
Footnotes
For Further Reading: Elisa González-Rodríguez, Delvys Rodríguez-Abreu, on behalf of the Spanish Group for Cancer Immuno-Biotherapy (GETICA). Immune Checkpoint Inhibitors: Review and Management of Endocrine Adverse Events. The Oncologist 2016;21:804–816.
Implications for Practice: Immune checkpoint inhibitors are already part of oncologists’ therapeutic arsenal as effective therapies for otherwise untreatable neoplasias, such as metastatic melanoma or lung cancer. Their use is expected to increase exponentially in the near future as additional agents become available and their approval is extended to different tumor types. Adverse events affecting the endocrine system are among the most frequent and complex toxicities oncologists may face, and some may be life-threatening if not recognized. This study reviews endocrinopathies associated to immune checkpoint inhibitors available to date. Incidence, timing patterns, and clinical presentation are discussed, and practical recommendations for management are proposed.
Author Contributions
Conception/Design: Jeffrey S. Weber, Michael Postow, Christopher D. Lao, Dirk Schadendorf
Collection and/or assembly of data: Jeffrey S. Weber, Michael Postow, Christopher D. Lao, Dirk Schadendorf
Data analysis and interpretation: Jeffrey S. Weber, Michael Postow, Christopher D. Lao, Dirk Schadendorf
Manuscript writing: Jeffrey S. Weber, Michael Postow, Christopher D. Lao, Dirk Schadendorf
Final approval of manuscript: Jeffrey S. Weber, Michael Postow, Christopher D. Lao, Dirk Schadendorf
Disclosures
Jeffrey S. Weber: Bristol-Myers Squibb, Merck, GlaxoSmithKline, Roche, Genentech (C/A, H), Bristol-Myers Squibb, Merck, GlaxoSmithKline, Roche, Genentech, MacroGenics (RF), Celldex, Altor, cCAM (OI); Michael Postow: Bristol-Myers Squibb (C/A), Bristol-Myers Squibb, Merck (H), Bristol-Myers Squibb, Novartis (RF); Dirk Schadendorf: Bristol-Myers Squibb, Merck, Novartis, Roche, Genentech, Amgen (C/A), Bristol-Myers Squibb, Merck, Novartis, Roche, Amgen (RF, H). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 3.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 4.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 5.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yervoy (ipilimumab) [package insert]. Princeton, NJ: Bristol-Myers Squibb Co.; 2013.
- Opdivo (nivolumab) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Co.; 2014.
- Keytruda (pembrolizumab) [prescribing information]. Kenilworth, NJ: Merck & Co., Inc.; 2015.
- 10.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares L, Horn L, Borghaei H et al. Phase III, randomized trial (CheckMate 057) of nivolumab versus docetaxel in advanced non-squamous (non-SQ) cell non-small cell lung cancer (NSCLC). Poster presented at: American Society of Clinical Oncology 2015 Annual Meeting; May 29-June 2, 2015; Chicago, IL. [Google Scholar]
- Bauer TM, McCleod M, Chandler JC et al. A phase IIIb/IV safety trial of nivolumab (NIVO) in patients (pts) with advanced or metastatic non-small cell lung cancer (NSCLC) who have progressed during or after receiving at least one prior systemic regimen. Poster presented at American Society of Clinical Oncology 2015 Annual Meeting; May 29–June 2, 2015; Chicago, IL. [Google Scholar]
- 13.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma: Results of a randomized phase II trial. J Clin Oncol. 2015;33:1430–1437. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, Postow MA, Chesney J et al. Clinical response, PFS and safety in patients with advanced melanoma receiving nivolumab combined with ipilmumuab versus ipilimumab monotherapy in CheckMate 069 study. Poster presented at: American Society of Clinical Oncology 2015 Annual Meeting; May 29-June 2, 2015; Chicago, IL. [Google Scholar]
- 15.U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.03. Available at http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed February 8, 2016.
- 16.Sundar R, Cho BC, Brahmer JR, et al. Nivolumab in NSCLC: Latest evidence and clinical potential. Ther Adv Med Oncol. 2015;7:85–96. doi: 10.1177/1758834014567470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26:2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 20.Weber JS, Antonia SJ, Topalian SL, et al. Safety profile of nivolumab (NIVO) in patients (pts) with advanced melanoma (MEL): A pooled analysis. J Clin Oncol. 2015;33(suppl)(9018a) [Google Scholar]
- 21.Bristol-Myers Squibb Company. Risk Evaluation and Mitigation Strategy (REMS). Initial REMS approval: 03/2011; most recent modification: 02/2012. Available at http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/UCM249435.pdf. Accessed February 8, 2016.