There is level 1 evidence for use of adjuvant chemotherapy in stage IIIC endometrial cancer, although trial results are variable. Chemotherapy is also often recommended for high-risk subsets of stage I disease, although prospective trial data to validate this are lacking. Carboplatin plus paclitaxel is the current standard regimen, based on extrapolation of data from the metastatic setting. Few chemotherapy agents have produced meaningful response rates in the second-line setting.
Keywords: Chemoradiotherapy; Drug therapy, adjuvant; Endometrial cancer; Local neoplasm recurrence; Metastasis, neoplasm
Abstract
Level I evidence exists for use of adjuvant chemotherapy in stage IIIC endometrial cancer (positive lymph nodes), although results of randomized trials have varied. Chemotherapy is also often recommended for high-risk subsets of stage I disease, such as serous carcinomas, although prospective trial data to validate this practice are lacking. Carboplatin plus paclitaxel is the current standard regimen, based on extrapolation of data from the metastatic setting. Several clinical trials have compared adjuvant pelvic radiotherapy alone to a combination of radiotherapy and chemotherapy with mixed results. One of the largest of these trials, Postoperative Radiation Therapy in Endometrial Carcinoma 3 (PORTEC-3), has completed accrual and is awaiting data maturation. Metastatic disease is not curable. For tumors of low-grade endometrioid histology with a prolonged time to recurrence, endocrine therapy with a progestin-based regimen is appropriate. Chemotherapy will be used in most other cases, and the standard first-line regimen is carboplatin and paclitaxel. Few chemotherapy agents have been shown to produce meaningful response rates in the second-line setting. Molecularly targeted therapies such as mTOR inhibitors and antiangiogenic agents including bevacizumab have been studied but their role in the armamentarium remains uncertain.
Implications for Practice:
Following surgical resection and staging for endometrial cancer, adjuvant chemotherapy with carboplatin and paclitaxel can be administered to patients with a high risk for recurrence. This includes patients with stage IIIC disease with positive lymph nodes, and high-risk subsets of stage I disease such as serous carcinomas. In the metastatic setting, endocrine therapy can be considered, particularly for patients with lower-grade disease and a prolonged time to recurrence. Combined therapy with carboplatin and paclitaxel is the standard of care used for front-line chemotherapy. Antiangiogenic agents are clearly active, but how they should be integrated into treatment is not yet determined. Immunotherapy is a promising direction for patients with mismatch repair-deficient or polymerase ε-mutated tumors.
Introduction
In the U.S. in 2016, an estimated 60,050 women were diagnosed with uterine cancer, with an estimated 10,470 deaths related to the disease [1]. Most U.S. patients (67%) present with stage I disease, which is associated with a 95.3% 5-year relative survival [2]. Stage I high-grade endometrioid and serous tumors have a worse prognosis, with 5-year disease-specific survival rates of 86% and 74%, respectively [3]. Treatment for stage I disease has historically been total hysterectomy and bilateral salpingo-oophorectomy with or without adjuvant radiotherapy (RT). There is controversy about which patients should have pelvic and/or para-aortic lymph node dissection. Nodal surgery does not improve survival [4], but nodal involvement, as discussed later in this article, has implications for the use of radiotherapy and chemotherapy. Sentinel lymph node mapping is being evaluated and has a category 3 level of evidence in National Comprehensive Cancer Network (NCCN) guidelines [5]. Patients with metastatic disease have a poor prognosis, with a 5-year survival rate of 17% [1].
Adjuvant Radiotherapy
Both whole pelvic radiotherapy and vaginal brachytherapy are used as adjuvant treatment for early-stage disease. Neither has been shown to improve survival, but they do improve local control [6–9]. The Postoperative Radiation Therapy in Endometrial Carcinoma (PORTEC)-1 trial compared postoperative whole pelvic radiation with observation in patients with stage I disease. Lymph node dissection was not required. Five-year locoregional recurrence rates were significantly improved by radiotherapy: 4% in the radiotherapy group and 14% in the control group (p < .001); however, no difference was seen in 5-year overall survival [10]. PORTEC-2, a noninferiority trial, compared whole pelvic radiation with vaginal brachytherapy in patients with stage I and IIA disease. Again, lymph node dissection was not required. Similar rates of recurrence, disease-free survival, and overall survival were observed in the two arms, with fewer gastrointestinal side effects in the vaginal brachytherapy group [11]. The American Society of Clinical Oncology (ASCO) has recently endorsed the American Society for Radiation Oncology (ASTRO) guidelines regarding use of postoperative radiotherapy, with a few minor modifications [12]; these provide welcome guidance in a confusing and controversial area.
Adjuvant Chemotherapy
Current NCCN guidelines recommend adjuvant chemotherapy for poor-prognosis groups, including patients with stage IIIB or stage IIIC disease of any histology and patients with stages IA (with myometrial invasion), IB, II, or IIIA serous or clear cell carcinoma [5]. They state that chemotherapy can be considered for patients with grade 3 stage II and IIIA disease. However, high-level evidence for the use of chemotherapy exists only for patients with positive lymph nodes, and there are not even strong retrospective data for the use of chemotherapy in early-stage clear cell cancers. Adjuvant progestin therapy has been tested in a number of older trials without showing benefit [8].
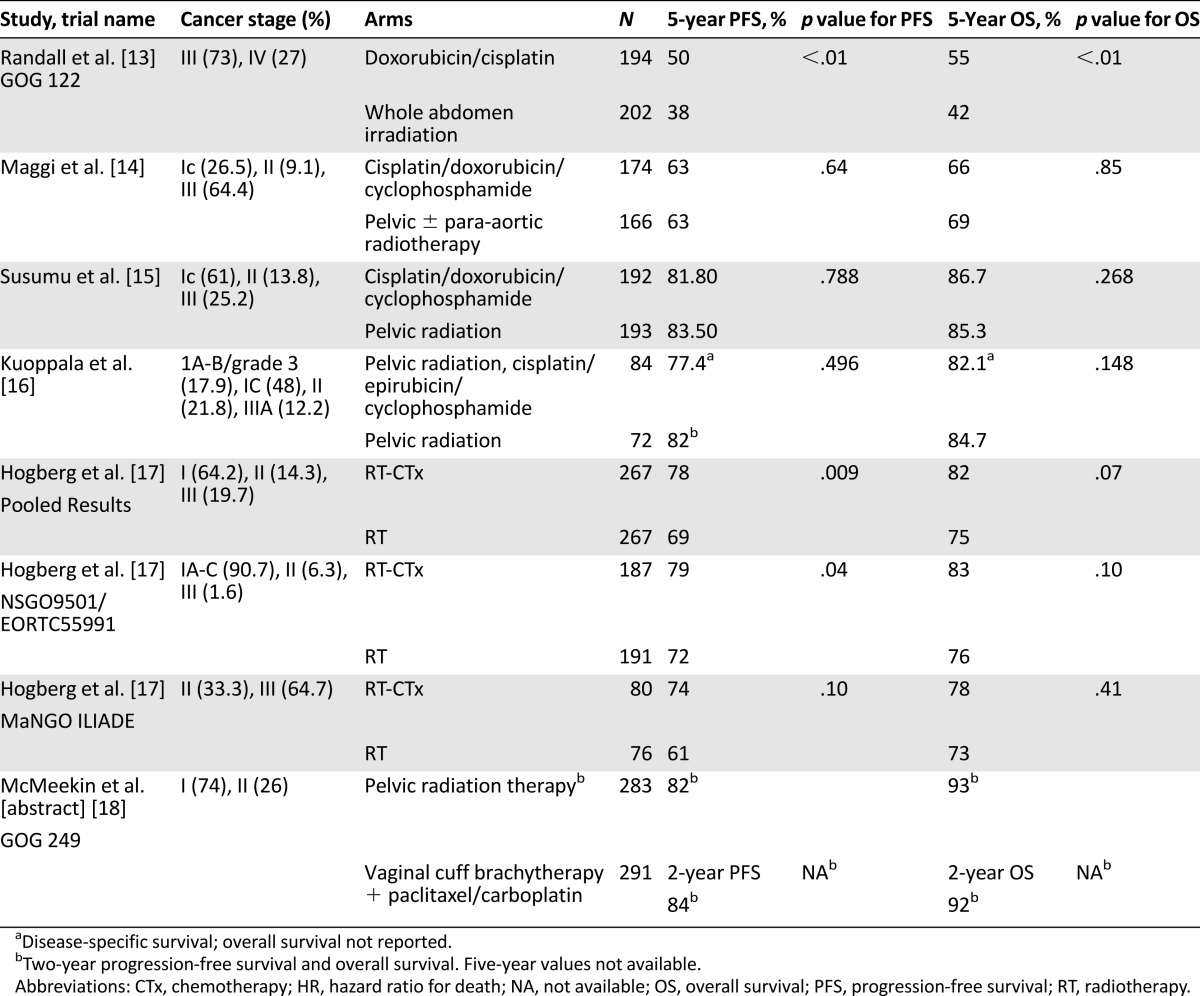
Three published trials have randomized patients to adjuvant radiotherapy alone versus adjuvant chemotherapy alone (Table 1). In the Gynecologic Oncology Group (GOG) 122 trial, 396 patients with stage III or optimally debulked stage IV disease, defined as no single site of residual disease larger than 2 cm, were randomly assigned to whole abdomen irradiation versus doxorubicin and cisplatin. The patients with stage III and stage IV disease both had improved results with chemotherapy, with stage-adjusted hazard ratios (HRs) for progression-free survival (PFS) and overall survival (OS) of 0.71 and 0.68, respectively [13]. These results led to widespread use of adjuvant chemotherapy for U.S. women with stage III endometrial cancer.
Table 1.
Selected adjuvant clinical trials
However, an Italian trial, also published in 2006, randomized 345 patients with stage IC grade 3, stage II grade 3, or stage III endometrial cancer to either external beam pelvic radiation therapy or adjuvant cisplatin, doxorubicin, and cyclophosphamide. There was no significant difference between arms in either PFS or OS [14]. The reason for the difference in outcome between the two trials is not clear [19]. However, greater than 65% of the 290 patients with stage III disease in GOG 122 had stage IIIC disease (positive lymph nodes) compared with only 34% of the stage III patients in the Italian trial; most of the Italian patients had stage IIIA disease. Disease in serosa or adnexae might potentially be more amenable to control by radiation than disease spread to lymph nodes.
A third trial by the Japanese Gynecologic Oncology Group randomized 385 patients to either pelvic radiation therapy or cisplatin/doxorubicin/cyclophosphamide. This trial included mostly patients with early-stage and low-grade disease: stage 1C, 61%; stage II, 13.8%; and stage III, 25.2%. There was no difference in PFS or OS between the radiation and chemotherapy arms [15].
More recent trials have compared pelvic radiotherapy with chemoradiotherapy. The strongest evidence favoring combination therapy comes from pooled results of the Nordic Society of Gynecological Oncology (NSGO)-9501/European Organization for Research and Treatment of Cancer (EORTC)-55991 and Mario Negri Institute (MaNGO) ILIADE-III trials (Table 1), which permitted a variety of stages and chemotherapy regimens. A statistically significant improvement in PFS for combined chemoradiotherapy was seen in the NSGO/EORTC trial and in pooled results of the two trials. Although there was no significant improvement in OS in either individual or pooled results, combination therapy did show an improvement in cancer-specific survival in the NSGO/EORTC trial as well as in the pooled analysis (HR, 0.55; 95% confidence interval [CI], 0.35–0.88; p = .01) [17].
Although most of these trials used four to six cycles of doxorubicin/platinum-based regimens, carboplatin plus paclitaxel is the most commonly used adjuvant therapy based on results in more advanced disease [20]. When chemoradiotherapy is administered, radiation is variably used before chemotherapy, after chemotherapy, or in a “sandwich” fashion, with three cycles of chemotherapy given before radiotherapy and three cycles given after radiotherapy. All approaches appear to be tolerable, although in at least one clinical trial (GOG 184), the cytopenia observed when chemotherapy was given immediately after pelvic radiotherapy necessitated that the trial be amended to require granulocyte-colony stimulating factor during chemotherapy in all patients [21].
A relatively large trial testing chemotherapy in early-stage disease, GOG 249, has completed accrual and been reported in abstract form [18]. A total of 601 participants had either stage I disease of serous or clear cell histology, stage II disease, or stage I “high intermediate risk,” defined as: (a) age >70 years with one risk factor, (b) age >50 years with 2 risk factors, or (c) age >18 years with 3 risk factors, where risk factors were defined as lymphovascular space involvement, grade 2 or 3, and outer 50% myometrial invasion. Subjects were randomly assigned to either vaginal brachytherapy plus three cycles of carboplatin/paclitaxel or whole pelvic radiotherapy. Relapse-free survival at 24 months was not different between the arms (82% for radiotherapy vs. 84% for brachytherapy plus chemotherapy).
Hypotheses for the lack of benefit from chemotherapy in GOG 249 include that three cycles of chemotherapy are inadequate, or that certain biologic subsets are not sensitive to chemotherapy. For example, data suggest that polymerase ε (POLE)-mutated endometrial cancers, although often of grade 3 histology, have an excellent prognosis and, therefore, are unlikely to benefit from adjuvant chemotherapy [22]. However, given that POLE-mutated tumors make up a minority of disease (9.6% of endometrial carcinomas) [23], this is not likely to be the entire explanation for the lack of benefit of chemotherapy in GOG 249. Clear cell endometrial cancers raise a separate issue: They have a poor prognosis, somewhat intermediate between the prognosis of endometrioid and serous tumors. They are rare enough that data on chemotherapy sensitivity are scarce, but they may be less chemosensitive than other histologies [24], which could decrease the benefit from adjuvant chemotherapy.
Future Directions in Adjuvant Therapy
Although there have been randomized trials comparing radiotherapy with chemoradiotherapy, there are not yet prospective data comparing adjuvant chemotherapy alone with radiation therapy plus chemotherapy. GOG 258, which completed accrual in July 2014, randomized patients with stage III or IVA endometrial carcinoma to either volume-directed radiation therapy with two doses of concomitant cisplatin followed by four cycles of carboplatin/paclitaxel, or six cycles of carboplatin/paclitaxel with no radiotherapy. Accrual is complete, and final analysis is expected in 2016.
Another trial asking a similar question regarding the benefit of adding radiotherapy to chemotherapy, but in earlier-stage disease, is the Danish Gynaecological Cancer Group (DGCG)-EN2 adjuvant study, which tests brachytherapy alone versus brachytherapy plus six cycles of carboplatin/paclitaxel chemotherapy in node-negative, stage I–II intermediate or high-risk endometrial cancer. Accrual is ongoing.
A third important trial, PORTEC-3, will address the question of which patients with early-stage disease benefit from chemotherapy. PORTEC-3 randomized patients with high-risk stage I, II, and III disease to whole pelvic radiotherapy alone versus whole pelvic radiotherapy with two doses of concomitant cisplatin followed by four cycles of carboplatin/paclitaxel. Accrual was complete in late 2013 and results are expected in 2016 or 2017. Toxicity results were presented at ASCO 2015 and showed that the early increased symptoms with combination therapy diminished at 1 and 2 years [25]. The trans-PORTEC translational research group is focusing on identifying biologic factors to help select patients most appropriate for adjuvant therapy, given their potential benefit. Serous and clear cell tumors are high risk but have made up too small a fraction of most trials for statistically meaningful conclusions about the benefits of adjuvant therapy. Moreover, they have often been grouped together for analysis although they are biologically very different. Hopefully, some of the newer, larger trials will provide new insights regarding benefits of therapy in these histologic subsets.
Advanced/Recurrent Disease
Hormonal Therapy
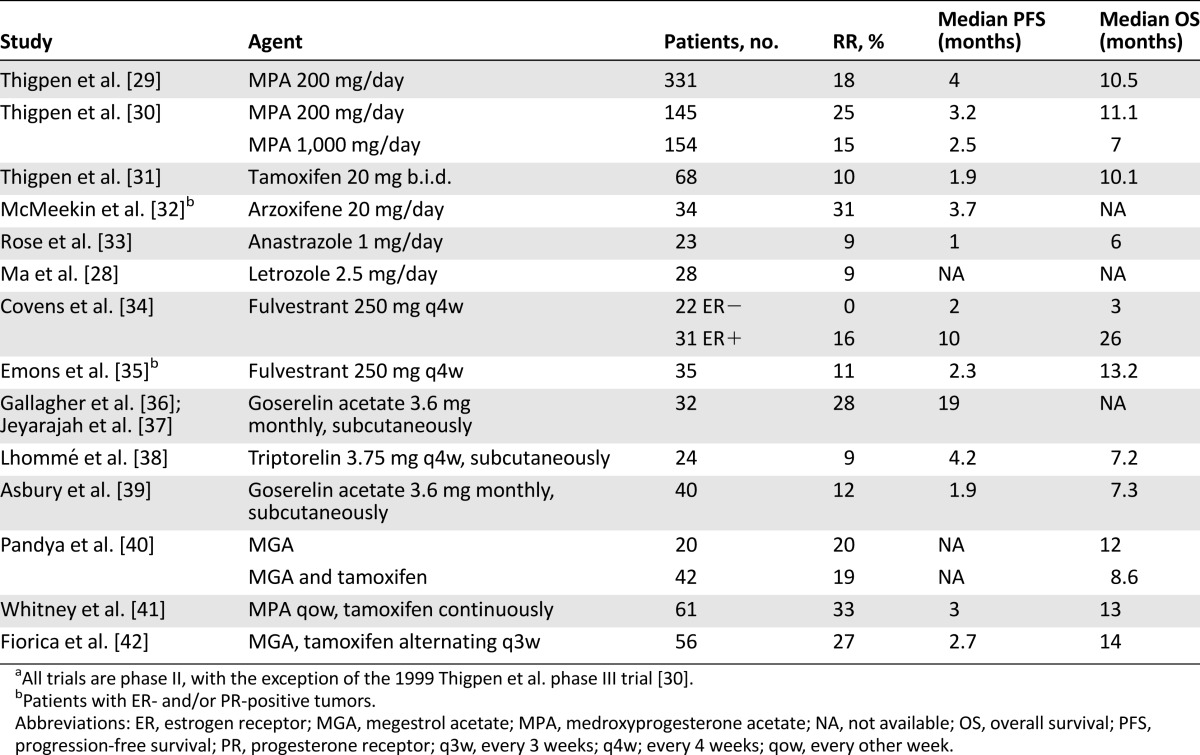
Although hormonal therapy is not recommended in the adjuvant setting, it remains useful in the treatment of metastatic disease, particularly for tumors of low-grade endometrioid histology with a long time to recurrence. The majority of endometrioid carcinomas express estrogen receptor (ER) and progesterone receptor (PR), with less expression in high-grade tumors [26]. Many studies have found that response to hormonal therapy is more common among patients with higher ER and PR expression [27], although one phase II trial found no correlation between hormone receptor expression and response to letrozole [28]. Additionally, a small minority of patients without ER/PR expression has been reported to have a response to progestin-based therapy. Additional predictors of response to hormonal therapy include low histologic grade, prolonged time to relapse, and the location and extent of extrapelvic disease, with low-volume pulmonary disease being classically favorable [26].
Many studies have found that response to hormonal therapy is more common among patients with higher ER and PR expression, although one phase II trial found no correlation between hormone receptor expression and response to letrozole.
Agents studied include progestins, selective estrogen receptor modulators, and aromatase inhibitors (Table 2). Hormonal combinations tested include tamoxifen alternating with either medroxyprogesterone acetate or megestrol acetate. The rationale for alternating therapy is based on data that progestin therapy downregulates expression of PR. Tamoxifen and other estrogenic compounds are known to increase PR expression. It is hypothesized that by combining tamoxifen therapy and progestin therapy, the downregulation of PR can be counteracted and the antitumor activity of progestins increased [41].
Table 2.
Selected hormonal therapy trialsa
Chemotherapy
First-Line Chemotherapy
The majority of recurrent or metastatic endometrial cancers will not be considered likely to respond to hormonal therapy, and will be treated with chemotherapy. The preferred front-line chemotherapy regimen is carboplatin/paclitaxel. Although the randomized phase III trial comparing this regimen with the previous standard of cisplatin/doxorubicin/paclitaxel (GOG 209) has been reported only in abstract form [20], the results of that trial, along with results of a number of nonrandomized phase II trials and the convenience and tolerability of the carboplatin/paclitaxel doublet, have led to its widespread use.
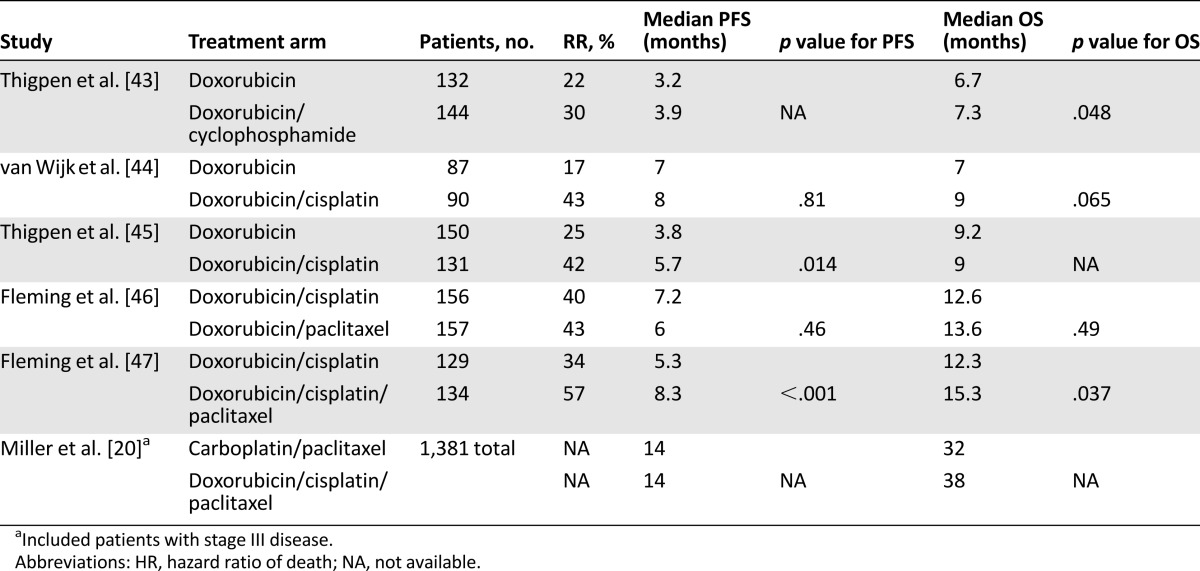
Several single-agent chemotherapies have been tested in the first-line setting, with the best response rates (RRs) demonstrated by platinum agents (RR, 20%–33%), taxanes (RR, 21%–36%), and anthracyclines (RR, 17%–37%) [19]. Randomized phase III trials have compared single-agent doxorubicin with doxorubicin/cyclophosphamide as well as doxorubicin/cisplatin (Table 3). Response rates improved from 17%–25% with single-agent doxorubicin to 33%–43% in the combination therapy arms, but there was no benefit in overall survival [43–45] until the introduction of taxanes. A front-line phase III trial published in 2004 compared doxorubicin/cisplatin with a triplet regimen of paclitaxel/doxorubicin/cisplatin (TAP). The overall response rate was significantly higher with the TAP regimen (RR, 57% vs. 34%), with improvement seen both in progression-free survival (8.3 months vs. 5.3 months) and overall survival (15.3 months vs. 12.5 months; p = .037). However, the TAP regimen requires granulocyte-colony stimulating factor support and it significantly increased the risk for peripheral neuropathy [47]. This led to subsequent phase II trials of first-line, potentially less toxic, doublet regimens, including paclitaxel/cisplatin, carboplatin/paclitaxel, and carboplatin/liposomal doxorubicin, which showed favorable response rates and appeared to have reasonable toxicity profiles [19, 48–50].
Table 3.
Selected phase III front-line chemotherapy trials in advanced/recurrent disease
Therefore, a phase III noninferiority trial, GOG 209, was designed to compare carboplatin/paclitaxel (TC) with TAP in patients with stage III or IV disease. Results have been presented only in abstract form. Progression-free survival was 14 months in both arms, with overall survival of 32 months of the TC arm versus 38 months in the TAP arm (HR, 1.01). Statistically significant grade II or greater toxicities were seen more frequently with TAP, including sensory neuropathy, metabolic derangements, vomiting, diarrhea, thrombocytopenia, and other hematologic toxicities [20]. As a result, TC has become the standard front-line chemotherapy used for recurrent or metastatic endometrial cancer, as well as adjuvant therapy. Regimens using weekly paclitaxel, which have produced a survival benefit in other tumor types such as breast and ovarian cancer, have not yet been compared with every-three-week therapy in endometrial cancer.
Recent efforts have focused on additions to the carboplatin/paclitaxel backbone, and agents of interest have included metformin, temsirolimus, and bevacizumab. Metformin has a number of potential mechanisms of anticancer action, including lowering insulin levels, which is relevant given endometrial cancer is the tumor type most strongly associated with obesity. Based on clinical and preclinical evidence regarding the potential benefit of metformin in endometrial cancer, the GOG/NRG launched an ongoing trial comparing carboplatin/paclitaxel with carboplatin/paclitaxel/metformin (GOG286B).
GOG 86P was a front-line randomized phase II trial (N = 349) in which patients with chemotherapy-naïve metastatic or recurrent disease were assigned to either (a) carboplatin/paclitaxel/bevacizumab followed by maintenance bevacizumab, (b) carboplatin/paclitaxel/temsirolimus followed by maintenance temsirolimus, or (c) carboplatin/ixabepilone/bevacizumab followed by maintenance bevacizumab. As reported in abstract form at ASCO 2015, overall response rates were 60%, 55%, and 53%, respectively. The hazard ratio for PFS, compared with historical control, was not significantly different for any arm, but trended toward improvement with the bevacizumab-containing arms. Overall survival, when compared with historical control, was significantly increased only in the carboplatin/paclitaxel/bevacizumab arm (respective HRs, 0.71, 0.99, and 0.97), although there was an imbalance in baseline characteristics with fewer serous cases in that arm [51]. A second, smaller (N = 108) study, the Multicenter Italian Trials in Ovarian Cancer (MITO) END-2 trial, was also presented at ASCO 2015. This trial enrolled women with one or fewer prior chemotherapy regimens and compared carboplatin/paclitaxel with carboplatin/paclitaxel/bevacizumab. The addition of bevacizumab increased the overall response rate from 54% to 72.7%, with an improvement in PFS from 8.7 months to 13.0 months [52].
Second-Line Chemotherapy
Response rates to second-line chemotherapy have historically been quite poor. The majority of the data for second-line chemotherapy come from nonrandomized phase II trials in patients whose prior chemotherapy was in the setting of metastatic disease. Paclitaxel has performed the best, with response rates consistently greater than 20%, although these data predate the use of paclitaxel as a part of first-line treatment [53–56]. Other agents tested but demonstrating limited response rates include oxaliplatin, topotecan, liposomal doxorubicin, etoposide, cyclophosphamide, pemetrexed, gemcitabine, and ifosfamide [57–65] (Table 4).
Table 4.
Selected second-line chemotherapy trials
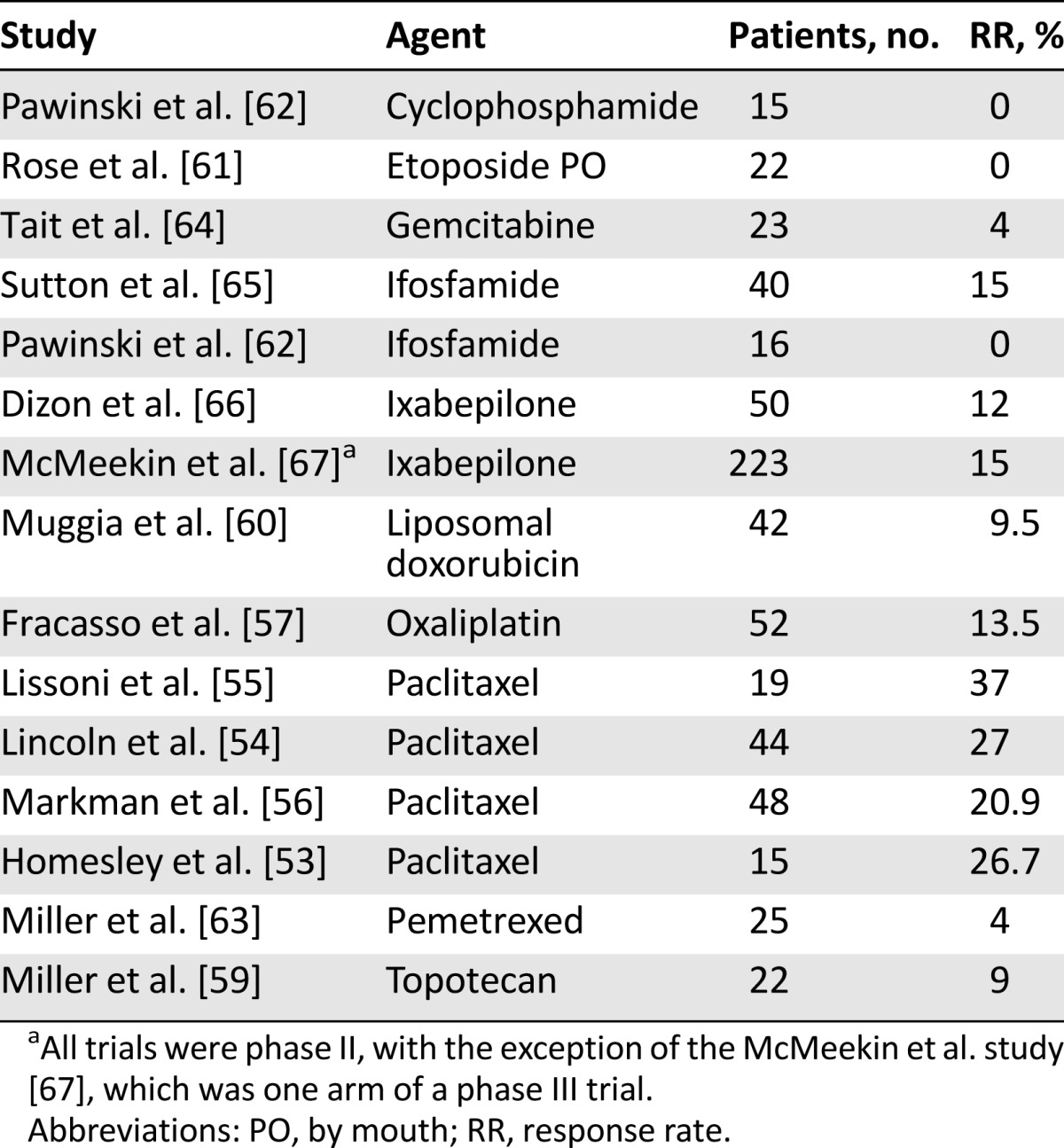
There are very few randomized trials in the second-line setting. Ixabepilone, a synthetic analog of epothilone B, was considered promising enough in the phase II setting [66] to be tested against a control arm of doxorubicin or paclitaxel in a phase III study of patients who had received one prior chemotherapy regimen. For approximately half of the subjects, the prior chemotherapy had been given in the neoadjuvant or adjuvant setting. Patients in the standard therapy arm who had previously been treated with paclitaxel were assigned to doxorubicin, and of the 248 patients in the control arm, 69% received doxorubicin. Overall response rates were similar in the two arms (15.2% for ixabepilone vs. 15.7% for the control arm), but the trial closed early because of a statistically significant difference in OS favoring the control (10.9 months for ixabepilone vs. 12.3 months for the control arm; p = .04) [67].
“Platinum Sensitivity”
As discussed, an increasing number of patients are receiving adjuvant chemotherapy. This raises the question as to whether the same regimen used in the adjuvant setting should be used at time of relapse. A multicenter, retrospective cohort study evaluated the applicability of the concept of “platinum sensitivity” to endometrial cancer. As the length of platinum-free interval (generally the time since adjuvant chemotherapy) increased, PFS and OS with subsequent platinum-based therapy increased. The response rate for patients with a platinum-free interval less than 6 months was 25%, whereas for those with a platinum-free interval of longer than 24 months, it was 65% [68].
Histology and Chemotherapy Response
Although serous tumors have a very different biology from grade 1 and 2 endometrioid tumors, they do not appear to have different response rates to standard cytotoxic chemotherapy regimens. In a review of randomized front-line GOG trials using regimens containing paclitaxel, doxorubicin, and/or cisplatin for recurrent or metastatic disease, endometrioid histology (N = 622) was associated with a response rate of 44%, a median PFS of 6.4 months, and a median OS of 13 months; serous histology (N = 216) was associated with a response rate of 44%, PFS of 6.3 months, and OS of 11 months; and clear cell histology (N = 44) was associated with a response rate of 32%, PFS of 3.2 months, and OS of 8 months [24].
Molecularly Targeted Therapy
In 2013, the Cancer Genome Atlas project published data characterizing DNA, RNA, and protein expression in 343 endometrial tumors of endometrioid, serous, or mixed histology. Four categories of endometrial cancer were described: POLE ultramutated, microsatellite instability hypermutated, copy-number low, and copy-number high. Serous tumors and approximately 25% of high-grade endometrioid tumors were found to have frequent TP53 mutations, extensive copy number alterations, few methylation changes, and low ER and PR expression. In contrast, the majority of endometrioid tumors were found to have few copy number alterations but did have frequent mutations in PTEN, PIK3CA, CTNNB1, ARID1A, and KRAS [22].
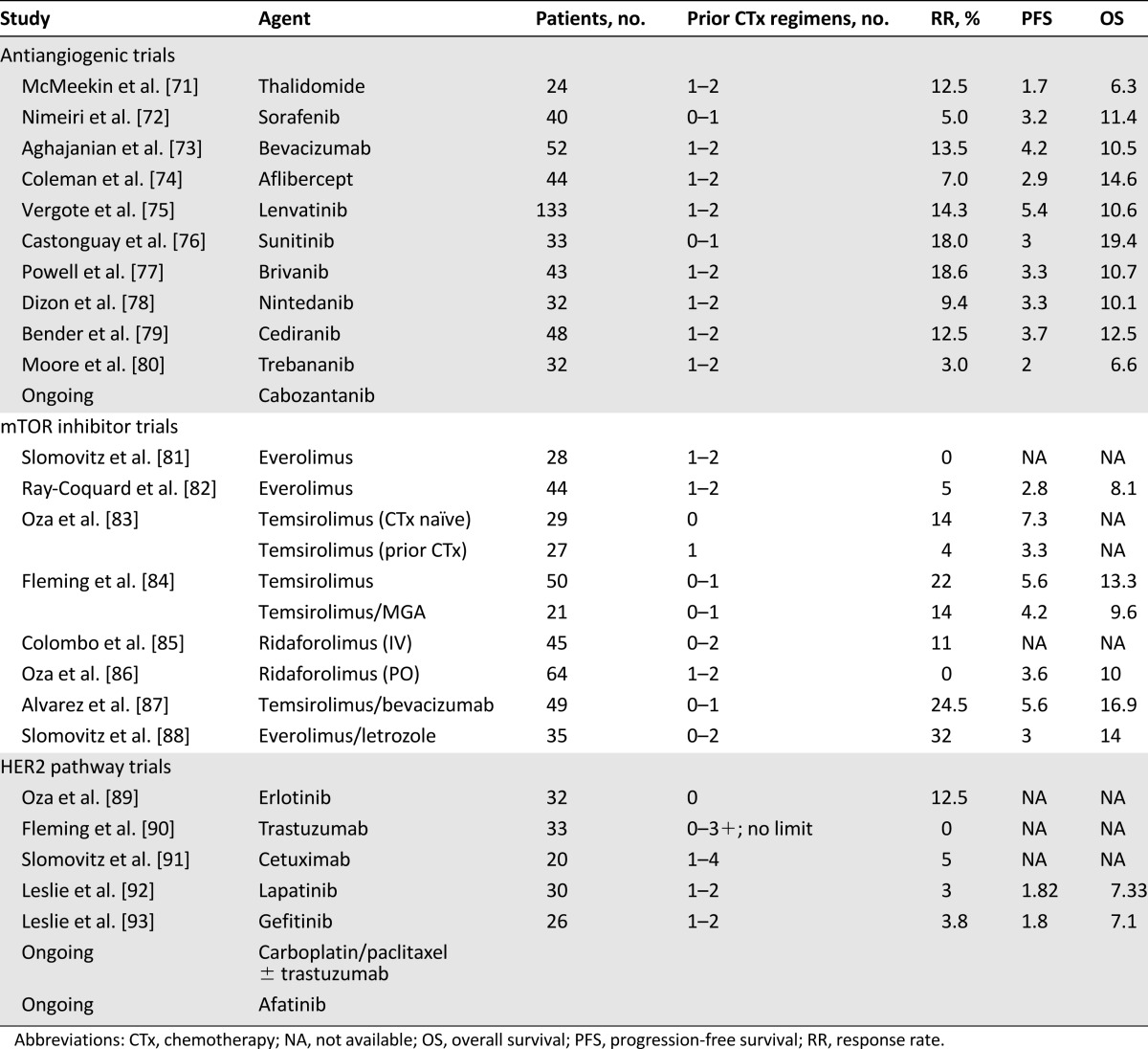
PTEN is a tumor suppressor gene that negatively regulates PI3KCA. PI3KCA activates AKT, causing increased activity of mTOR [69]. The PI3K pathway is targeted by several drug classes, including rapamycin analogs, pan-PI3K inhibitors, PI3K isoform-specific inhibitors, dual PI3K/mTOR catalytic inhibitors, mTOR-specific catalytic inhibitors, and AKT inhibitors [70]. In endometrial cancer, the agents best studied thus far have been rapamycin-analog mTOR inhibitors (Table 5). In general, they appear to have modest activity in chemotherapy-naïve patients. For example, temsirolimus produced a 14% RR in chemotherapy-naïve patients, although only a 4% RR in patients with prior chemotherapy [83]. Many trials of single-agent mTOR inhibitors report occasional patients with prolonged benefit. Several studies have shown no association between tumor PTEN alterations or PI3KCA mutations and clinical benefit from mTOR inhibitors. One report did describe a significant increase in RR and PFS for patients with an activating AKT mutation treated with temsirolimus, suggesting this population, although small, may derive particular benefit from treatment with mTOR inhibitors [94].
Table 5.
Selected phase II trials of biological agents
Several studies have shown no association between tumor PTEN alterations or PI3KCA mutations and clinical benefit from mTOR inhibitors. One report did describe a significant increase in RR and PFS for patients with an activating AKT mutation treated with temsirolimus, suggesting this population, although small, may derive particular benefit from treatment with mTOR inhibitors.
There has been interest in the combination of mTOR inhibitors with hormonal therapy. Upregulation of the PI3K/Akt/mTOR pathway has been associated with resistance to hormonal therapy in endometrial cancer cell lines [95, 96], and the combination of the mTOR inhibitor everolimus plus an aromatase inhibitor, exemestane, has been U.S. Food and Drug Administration (FDA)-approved for use in breast cancer [97]. A trial testing the combination of temsirolimus with megestrol acetate and tamoxifen closed early because of an excess number of venous thromboses, with a 12.5% rate of deep vein thrombosis, pulmonary embolism, or stroke, compared with the 5% reported rate of venous thrombosis with megestrol acetate alone [84]. However, a subsequent study of the combination of everolimus and letrozole was more promising, with an overall response rate of 32%. No patients discontinued therapy because of significant toxicity [88]. The authors noted that the strongest predictor of nonresponse was serous histology. They also observed that patients receiving metformin had an increased response rate to therapy. Whether this represents chance, a direct antitumor, or treatment-sensitizing effect of metformin, or is a surrogate for biologic sensitivity to mTOR inhibition (which causes hyperglycemia) is not clear. There is an ongoing phase II trial of the triplet combination of metformin, everolimus, and letrozole. In addition, there is an ongoing randomized phase II trial, GOG 3007, comparing the combination of everolimus and letrozole with hormonal therapy with tamoxifen and medroxy-progesterone acetate.
Antiangiogenic agents have consistently produced a modest response rate in endometrial cancer (Table 5). A phase II trial of bevacizumab demonstrated an overall response rate of 13.5%, with PFS and OS of 4.2 and 10.5 months, respectively [73]. Although no antiangiogenics or other newer molecularly targeted agents are FDA approved for the therapy of endometrial cancer, bevacizumab currently carries a category IIa recommendation for endometrial cancer by National Comprehensive Cancer Network guidelines [5]. A phase II trial of the combination of bevacizumab and temsirolimus demonstrated a response rate of 24.5%; however, toxicities, including gastrointestinal-vaginal fistulas, intestinal perforations, and venous thromboembolism, were considered unacceptable [87].
HER2 expression has been detected in 17% of serous and 16% of clear cell carcinomas [98]. Therapies such as trastuzumab targeting the HER2 pathway have also been studied [89, 90, 92, 93]. Unfortunately, response rates in these single-agent phase II studies have been disappointing (Table 4). There is an ongoing trial of carboplatin/paclitaxel with or without trastuzumab in patients with serous endometrial cancer with HER2 overexpression or amplification [99].
Future Directions
Poly(ADP-ribose) polymerase (PARP) is involved in the repair of DNA single-strand breaks. PARP inhibitors are effective when used in tumors with a deficiency in the repair of double-strand DNA breaks, such as those with BRCA1 or BRCA2 mutations. The PARP inhibitor olaparib has recently been FDA approved for use in BRCA mutation carriers with ovarian cancer who have already been treated with three or more regimens [100]. Efficacy of PARP inhibition has been seen in endometrial cancer cell lines with a PTEN deficiency, possibly because PTEN plays a role in DNA repair via RAD51 [101]. Mismatch repair-deficient tumors might also theoretically be sensitive to PARP inhibitors. Trials of PARP inhibitors in endometrial cancer are being planned.
There has been recent excitement regarding the FDA approval of immunostimulatory anti PD-1 and PD-L1 inhibitors for a variety of solid tumors. One study evaluated pembrolizumab, an anti-PD-1 antibody, in patients with colorectal cancers, and those with mismatch repair-deficient noncolorectal solid tumors, including two patients with endometrial cancer. Of the two treated patients with mismatch repair-deficient endometrial cancer, both had a response [103]. Both microsatellite instability high and polymerase ε-mutated endometrial cancers have large neoantigen loads and high numbers of tumor-infiltrating lymphocytes, and may be excellent candidates for immunotherapy [102]. There has been one report of a dramatic response to immunotherapy in a POLE-mutated endometrial cancer, but these make up a very small fraction of advanced disease [103, 104].
There is general interest in combining immunotherapy with other treatments to augment antitumor effect. There is recent evidence that mTOR inhibitors have a stimulatory effect on T cells via increased T-cell differentiation, function, and survival of memory T cells. The combination of antiangiogenics and immunotherapy is also promising. For example, bevacizumab is thought to increase dendritic cell maturation, thereby increasing dendritic cell priming of T cells, which may enhance the effect immunotherapy [104].
Conclusion
Adjuvant chemotherapy is used for patients with stage IIIC endometrial cancer, as well as high-risk subsets of stage I and II disease. The preferred adjuvant chemotherapy regimen is carboplatin plus paclitaxel. First-line therapy in the metastatic setting is chemotherapy with carboplatin and paclitaxel, or hormonal therapy for certain patients. Several molecularly targeted therapies have been studied in endometrial cancer including mTOR inhibitors and antiangiogenic agents. Future research is currently centered around PARP inhibitors, immunotherapies such as anti-PD-1 inhibitors, and ways to enhance the efficacy of hormonal therapy.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Author Contributions
Conception/Design: Christine M. Bestvina, Gini F. Fleming
Collection and/or assembly of data: Christine M. Bestvina, Gini F. Fleming
Data analysis and interpretation: Christine M. Bestvina, Gini F. Fleming
Manuscript writing: Christine M. Bestvina, Gini F. Fleming
Final approval of manuscript: Christine M. Bestvina, Gini F. Fleming
Disclosures
The authors indicated no financial relationships.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. Previous version: SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Available at http://seer.cancer.gov/csr/1975_2012/. Accessed October 5, 2015.
- 3.Hamilton CA, Cheung MK, Osann K, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer. 2006;94:642–646. doi: 10.1038/sj.bjc.6603012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: Randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. Uterine neoplasms. Available at http://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf. Accessed November 1, 2015.
- 6.Amant F, Mirza MR, Koskas M, et al. Cancer of the corpus uteri. Int J Gynaecol Obstet. 2015;131(suppl 2):S96–S104. doi: 10.1016/j.ijgo.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Poulsen H, Jacobsen M, Bertelsen K, et al. Adjuvant radiation therapy is not necessary in the management of endometrial carcinoma stage I, low risk cases. Int J Gynecol Cancer. 1996;6:38–43. [Google Scholar]
- 8.Martin-Hirsch PP, Bryant A, Keep SL, et al. Adjuvant progestagens for endometrial cancer. Cochrane Database Syst Rev. 2011;(6):CD001040. doi: 10.1002/14651858.CD001040.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong A, Johnson N, Kitchener HC, et al. Adjuvant radiotherapy for stage I endometrial cancer: An updated Cochrane systematic review and meta-analysis. J Natl Cancer Inst. 2012;104:1625–1634. doi: 10.1093/jnci/djs374. [DOI] [PubMed] [Google Scholar]
- 10.Creutzberg CL, van Putten WL, Koper PC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: Multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000;355:1404–1411. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 11.Nout RA, Smit VT, Putter H, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): An open-label, non-inferiority, randomised trial. Lancet. 2010;375:816–823. doi: 10.1016/S0140-6736(09)62163-2. [DOI] [PubMed] [Google Scholar]
- 12.Meyer LA, Bohlke K, Powell MA, et al. Postoperative radiation therapy for endometrial cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation Oncology Evidence-Based Guideline. J Clin Oncol. 2015;33:2908–2913. doi: 10.1200/JCO.2015.62.5459. [DOI] [PubMed] [Google Scholar]
- 13.Randall ME, Filiaci VL, Muss H, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: A Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36–44. doi: 10.1200/JCO.2004.00.7617. [DOI] [PubMed] [Google Scholar]
- 14.Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: Results of a randomised trial. Br J Cancer. 2006;95:266–271. doi: 10.1038/sj.bjc.6603279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Susumu N, Sagae S, Udagawa Y, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: A Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:226–233. doi: 10.1016/j.ygyno.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 16.Kuoppala T, Mäenpää J, Tomas E, et al. Surgically staged high-risk endometrial cancer: Randomized study of adjuvant radiotherapy alone vs. sequential chemo-radiotherapy. Gynecol Oncol. 2008;110:190–195. doi: 10.1016/j.ygyno.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Hogberg T, Signorelli M, de Oliveira CF, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer--results from two randomised studies. Eur J Cancer. 2010;46:2422–2431. doi: 10.1016/j.ejca.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMeekin DSFV, Aghajanian C, Cho J, et al. Randomized phase III trial of pelvic radiation therapy (PXRT) versus vaginal cuff brachytherapy followed by paclitaxel/carboplatin chemotherapy (VCB/C) in patients with high risk (HR), early stage endometrial cancer (EC): A Gynecologic Oncology Group trial. Gynecol Oncol. 2014;134:438. [Google Scholar]
- 19.Fleming GF. Systemic chemotherapy for uterine carcinoma: Metastatic and adjuvant. J Clin Oncol. 2007;25:2983–2990. doi: 10.1200/JCO.2007.10.8431. [DOI] [PubMed] [Google Scholar]
- 20.Miller D, Filiaci V, Fleming G, et al. Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:771. [Google Scholar]
- 21.Homesley HD, Filiaci V, Gibbons SK, et al. A randomized phase III trial in advanced endometrial carcinoma of surgery and volume directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: A Gynecologic Oncology Group study. Gynecol Oncol. 2009;112:543–552. doi: 10.1016/j.ygyno.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McConechy MK, Talhouk A, Leung S, et al. Endometrial carcinomas with POLE exonuclease domain mutations have a favorable prognosis. Clin Cancer Res. 2016;22:2865–2873. doi: 10.1158/1078-0432.CCR-15-2233. [DOI] [PubMed] [Google Scholar]
- 24.McMeekin DS, Filiaci VL, Thigpen JT, et al. The relationship between histology and outcome in advanced and recurrent endometrial cancer patients participating in first-line chemotherapy trials: A Gynecologic Oncology Group study. Gynecol Oncol. 2007;106:16–22. doi: 10.1016/j.ygyno.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 25.Creutzberg CL, de Boer SM, Putter H, et al. Adjuvant chemotherapy and radiation therapy (RT) versus RT alone for women with high-risk endometrial cancer: Toxicity and quality-of-life results of the randomized PORTEC-3 trial. J Clin Oncol. 2015;33(suppl):abstr 5501. [Google Scholar]
- 26.Mountzios G, Pectasides D, Bournakis E, et al. Developments in the systemic treatment of endometrial cancer. Crit Rev Oncol Hematol. 2011;79:278–292. doi: 10.1016/j.critrevonc.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Singh M, Zaino RJ, Filiaci VJ, et al. Relationship of estrogen and progesterone receptors to clinical outcome in metastatic endometrial carcinoma: A Gynecologic Oncology Group Study. Gynecol Oncol. 2007;106:325–333. doi: 10.1016/j.ygyno.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 28.Ma BB, Oza A, Eisenhauer E, et al. The activity of letrozole in patients with advanced or recurrent endometrial cancer and correlation with biological markers--a study of the National Cancer Institute of Canada Clinical Trials Group. Int J Gynecol Cancer. 2004;14:650–658. doi: 10.1111/j.1048-891X.2004.14419.x. [DOI] [PubMed] [Google Scholar]
- 29.Thigpen JT, Blessing JA, DiSaia P, et al. Oral medroxyprogesterone acetate in advanced or recurrent endometrial carcinoma: Results of therapy and correlation with estrogen and progesterone receptor levels— The Gynecologic Oncology Group experience. In: Baulier E, Iacobelli S, McGuire W, editors. Endocrinology and Malignancy. Pearl River, NY: Parthenon Publishers; 1986. pp. 446–454. [Google Scholar]
- 30.Thigpen JT, Brady MF, Alvarez RD, et al. Oral medroxyprogesterone acetate in the treatment of advanced or recurrent endometrial carcinoma: A dose-response study by the Gynecologic Oncology Group. J Clin Oncol. 1999;17:1736–1744. doi: 10.1200/JCO.1999.17.6.1736. [DOI] [PubMed] [Google Scholar]
- 31.Thigpen T, Brady MF, Homesley HD, et al. Tamoxifen in the treatment of advanced or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. J Clin Oncol. 2001;19:364–367. doi: 10.1200/JCO.2001.19.2.364. [DOI] [PubMed] [Google Scholar]
- 32.McMeekin DS, Gordon A, Fowler J, et al. A phase II trial of arzoxifene, a selective estrogen response modulator, in patients with recurrent or advanced endometrial cancer. Gynecol Oncol. 2003;90:64–69. doi: 10.1016/s0090-8258(03)00203-8. [DOI] [PubMed] [Google Scholar]
- 33.Rose PG, Brunetto VL, VanLe L, et al. A phase II trial of anastrozole in advanced recurrent or persistent endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2000;78:212–216. doi: 10.1006/gyno.2000.5865. [DOI] [PubMed] [Google Scholar]
- 34.Covens AL, Filiaci V, Gersell D, et al. Phase II study of fulvestrant in recurrent/metastatic endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2011;120:185–188. doi: 10.1016/j.ygyno.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 35.Emons G, Günthert A, Thiel FC, et al. Phase II study of fulvestrant 250 mg/month in patients with recurrent or metastatic endometrial cancer: A study of the Arbeitsgemeinschaft Gynäkologische Onkologie. Gynecol Oncol. 2013;129:495–499. doi: 10.1016/j.ygyno.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 36.Gallagher CJ, Oliver RT, Oram DH, et al. A new treatment for endometrial cancer with gonadotrophin releasing-hormone analogue. Br J Obstet Gynaecol. 1991;98:1037–1041. doi: 10.1111/j.1471-0528.1991.tb15343.x. [DOI] [PubMed] [Google Scholar]
- 37.Jeyarajah AR, Gallagher CJ, Blake PR, et al. Long-term follow-up of gonadotrophin-releasing hormone analog treatment for recurrent endometrial cancer. Gynecol Oncol. 1996;63:47–52. doi: 10.1006/gyno.1996.0276. [DOI] [PubMed] [Google Scholar]
- 38.Lhommé C, Vennin P, Callet N, et al. A multicenter phase II study with triptorelin (sustained-release LHRH agonist) in advanced or recurrent endometrial carcinoma: A French Anticancer Federation Study. Gynecol Oncol. 1999;75:187–193. doi: 10.1006/gyno.1999.5538. [DOI] [PubMed] [Google Scholar]
- 39.Asbury RF, Brunetto VL, Lee RB, et al. Goserelin acetate as treatment for recurrent endometrial carcinoma: A Gynecologic Oncology Group study. Am J Clin Oncol. 2002;25:557–560. doi: 10.1097/00000421-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Pandya KJ, Yeap BY, Weiner LM, et al. Megestrol and tamoxifen in patients with advanced endometrial cancer: An Eastern Cooperative Oncology Group Study (E4882) Am J Clin Oncol. 2001;24:43–46. doi: 10.1097/00000421-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Whitney CW, Brunetto VL, Zaino RJ, et al. Phase II study of medroxyprogesterone acetate plus tamoxifen in advanced endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:4–9. doi: 10.1016/j.ygyno.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 42.Fiorica JV, Brunetto VL, Hanjani P, et al. Phase II trial of alternating courses of megestrol acetate and tamoxifen in advanced endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:10–14. doi: 10.1016/j.ygyno.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Thigpen JT, Blessing JA, DiSaia PJ, et al. A randomized comparison of doxorubicin alone versus doxorubicin plus cyclophosphamide in the management of advanced or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. J Clin Oncol. 1994;12:1408–1414. doi: 10.1200/JCO.1994.12.7.1408. [DOI] [PubMed] [Google Scholar]
- 44.van Wijk FH, Aapro MS, Bolis G, et al. Doxorubicin versus doxorubicin and cisplatin in endometrial carcinoma: Definitive results of a randomised study (55872) by the EORTC Gynaecological Cancer Group. Ann Oncol. 2003;14:441–448. doi: 10.1093/annonc/mdg112. [DOI] [PubMed] [Google Scholar]
- 45.Thigpen JT, Brady MF, Homesley HD, et al. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: A Gynecologic Oncology Group study. J Clin Oncol. 2004;22:3902–3908. doi: 10.1200/JCO.2004.02.088. [DOI] [PubMed] [Google Scholar]
- 46.Fleming GF, Filiaci VL, Bentley RC, et al. Phase III randomized trial of doxorubicin + cisplatin versus doxorubicin + 24-h paclitaxel + filgrastim in endometrial carcinoma: A Gynecologic Oncology Group study. Ann Oncol. 2004;15:1173–1178. doi: 10.1093/annonc/mdh316. [DOI] [PubMed] [Google Scholar]
- 47.Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: A Gynecologic Oncology Group Study. J Clin Oncol. 2004;22:2159–2166. doi: 10.1200/JCO.2004.07.184. [DOI] [PubMed] [Google Scholar]
- 48.Dimopoulos MA, Papadimitriou CA, Georgoulias V, et al. Paclitaxel and cisplatin in advanced or recurrent carcinoma of the endometrium: Long-term results of a phase II multicenter study. Gynecol Oncol. 2000;78:52–57. doi: 10.1006/gyno.2000.5827. [DOI] [PubMed] [Google Scholar]
- 49.Hoskins PJ, Swenerton KD, Pike JA, et al. Paclitaxel and carboplatin, alone or with irradiation, in advanced or recurrent endometrial cancer: A phase II study. J Clin Oncol. 2001;19:4048–4053. doi: 10.1200/JCO.2001.19.20.4048. [DOI] [PubMed] [Google Scholar]
- 50.Pignata S, Scambia G, Pisano C, et al. A multicentre phase II study of carboplatin plus pegylated liposomal doxorubicin as first-line chemotherapy for patients with advanced or recurrent endometrial carcinoma: the END-1 study of the MITO (Multicentre Italian Trials in Ovarian Cancer and Gynecologic Malignancies) group. Br J Cancer. 2007;96:1639–1643. doi: 10.1038/sj.bjc.6603787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aghajanian C, Filiaci VL, Dizon DS, et al. A randomized phase II study of paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus and ixabepilone/carboplatin/bevacizumab as initial therapy for measurable stage III or IVA, stage IVB or recurrent endometrial cancer, GOG-86P. J Clin Oncol. 2015;33(suppl):abstr 5500. [Google Scholar]
- 52.Lorusso D, Ferrandina G, Colombo N, et al. Randomized phase II trial of carboplatin-paclitaxel (CP) compared to carboplatin-paclitaxel-bevacizumab (CP-B) in advanced (stage III-IV) or recurrent endometrial cancer: The MITO END-2 trial. J Clin Oncol. 2015;33(suppl):abstr 5502. doi: 10.1016/j.ygyno.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 53.Homesley HD, Meltzer NP, Nieves L, et al. A phase II trial of weekly 1-hour paclitaxel as second-line therapy for endometrial and cervical cancer. Int J Clin Oncol. 2008;13:62–65. doi: 10.1007/s10147-007-0731-5. [DOI] [PubMed] [Google Scholar]
- 54.Lincoln S, Blessing JA, Lee RB, et al. Activity of paclitaxel as second-line chemotherapy in endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2003;88:277–281. doi: 10.1016/s0090-8258(02)00068-9. [DOI] [PubMed] [Google Scholar]
- 55.Lissoni A, Zanetta G, Losa G, et al. Phase II study of paclitaxel as salvage treatment in advanced endometrial cancer. Ann Oncol. 1996;7:861–863. doi: 10.1093/oxfordjournals.annonc.a010768. [DOI] [PubMed] [Google Scholar]
- 56.Markman M, Blessing J, Rubin SC, et al. Phase II trial of weekly paclitaxel (80 mg/m2) in platinum and paclitaxel-resistant ovarian and primary peritoneal cancers: A Gynecologic Oncology Group study. Gynecol Oncol. 2006;101:436–440. doi: 10.1016/j.ygyno.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 57.Fracasso PM, Blessing JA, Molpus KL, et al. Phase II study of oxaliplatin as second-line chemotherapy in endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2006;103:523–526. doi: 10.1016/j.ygyno.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 58.Gupta D, Owers RL, Kim M, et al. A phase II study of weekly topotecan and docetaxel in heavily treated patients with recurrent uterine and ovarian cancers. Gynecol Oncol. 2009;113:327–330. doi: 10.1016/j.ygyno.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller DS, Blessing JA, Lentz SS, et al. A phase II trial of topotecan in patients with advanced, persistent, or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2002;87:247–251. doi: 10.1006/gyno.2002.6804. [DOI] [PubMed] [Google Scholar]
- 60.Muggia FM, Blessing JA, Sorosky J, et al. Phase II trial of the pegylated liposomal doxorubicin in previously treated metastatic endometrial cancer: A Gynecologic Oncology Group study. J Clin Oncol. 2002;20:2360–2364. doi: 10.1200/JCO.2002.08.171. [DOI] [PubMed] [Google Scholar]
- 61.Rose PG, Blessing JA, Lewandowski GS, et al. A phase II trial of prolonged oral etoposide (VP-16) as second-line therapy for advanced and recurrent endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 1996;63:101–104. doi: 10.1006/gyno.1996.0286. [DOI] [PubMed] [Google Scholar]
- 62.Pawinski A, Tumolo S, Hoesel G, et al. Cyclophosphamide or ifosfamide in patients with advanced and/or recurrent endometrial carcinoma: A randomized phase II study of the EORTC Gynecological Cancer Cooperative Group. Eur J Obstet Gynecol Reprod Biol. 1999;86:179–183. doi: 10.1016/s0301-2115(99)00066-4. [DOI] [PubMed] [Google Scholar]
- 63.Miller DS, Blessing JA, Drake RD, et al. A phase II evaluation of pemetrexed (Alimta, LY231514, IND #40061) in the treatment of recurrent or persistent endometrial carcinoma: A phase II study of the Gynecologic Oncology. Gynecol Oncol. 2009;115:443–446. doi: 10.1016/j.ygyno.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 64.Tait DL, Blessing JA, Hoffman JS, et al. A phase II study of gemcitabine (gemzar, LY188011) in the treatment of recurrent or persistent endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2011;121:118–121. doi: 10.1016/j.ygyno.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 65.Sutton GP, Blessing JA, Homesley HD, et al. Phase II study of ifosfamide and mesna in refractory adenocarcinoma of the endometrium. A Gynecologic Oncology Group study. Cancer. 1994;73:1453–1455. doi: 10.1002/1097-0142(19940301)73:5<1453::aid-cncr2820730521>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 66.Dizon DS, Blessing JA, McMeekin DS, et al. Phase II trial of ixabepilone as second-line treatment in advanced endometrial cancer: Gynecologic Oncology Group trial 129-P. J Clin Oncol. 2009;27:3104–3108. doi: 10.1200/JCO.2008.20.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McMeekin S, Dizon D, Barter J, et al. Phase III randomized trial of second-line ixabepilone versus paclitaxel or doxorubicin in women with advanced endometrial cancer. Gynecol Oncol. 2015;138:18–23. doi: 10.1016/j.ygyno.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 68.Nagao S, Nishio S, Michimae H, et al. Applicability of the concept of “platinum sensitivity” to recurrent endometrial cancer: The SGSG-012/GOTIC-004/Intergroup study. Gynecol Oncol. 2013;131:567–573. doi: 10.1016/j.ygyno.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 69.Husseinzadeh N, Husseinzadeh HD. mTOR inhibitors and their clinical application in cervical, endometrial and ovarian cancers: A critical review. Gynecol Oncol. 2014;133:375–381. doi: 10.1016/j.ygyno.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 70.Myers AP. New strategies in endometrial cancer: Targeting the PI3K/mTOR pathway--the devil is in the details. Clin Cancer Res. 2013;19:5264–5274. doi: 10.1158/1078-0432.CCR-13-0615. [DOI] [PubMed] [Google Scholar]
- 71.McMeekin DS, Sill MW, Benbrook D, et al. A phase II trial of thalidomide in patients with refractory endometrial cancer and correlation with angiogenesis biomarkers: A Gynecologic Oncology Group study. Gynecol Oncol. 2007;105:508–516. doi: 10.1016/j.ygyno.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nimeiri HS, Oza AM, Morgan RJ, et al. A phase II study of sorafenib in advanced uterine carcinoma/carcinosarcoma: A trial of the Chicago, PMH, and California Phase II Consortia. Gynecol Oncol. 2010;117:37–40. doi: 10.1016/j.ygyno.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aghajanian C, Sill MW, Darcy KM, et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: A Gynecologic Oncology Group study. J Clin Oncol. 2011;29:2259–2265. doi: 10.1200/JCO.2010.32.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coleman RL, Sill MW, Lankes HA, et al. A phase II evaluation of aflibercept in the treatment of recurrent or persistent endometrial cancer: A Gynecologic Oncology Group study. Gynecol Oncol. 2012;127:538–543. doi: 10.1016/j.ygyno.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vergote I, Teneriello M, Powell MA, et al. A phase II trial of lenvatinib in patients with advanced or recurrent endometrial cancer: Angiopoietin-2 as a predictive marker for clinical outcomes. J Clin Oncol. 2013;31(suppl):abstr 5520. [Google Scholar]
- 76.Castonguay V, Lheureux S, Welch S, et al. A phase II trial of sunitinib in women with metastatic or recurrent endometrial carcinoma: A study of the Princess Margaret, Chicago and California Consortia. Gynecol Oncol. 2014;134:274–280. doi: 10.1016/j.ygyno.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 77.Powell MA, Sill MW, Goodfellow PJ, et al. A phase II trial of brivanib in recurrent or persistent endometrial cancer: An NRG Oncology/Gynecologic Oncology Group Study. Gynecol Oncol. 2014;135:38–43. doi: 10.1016/j.ygyno.2014.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dizon DS, Sill MW, Schilder JM, et al. A phase II evaluation of nintedanib (BIBF-1120) in the treatment of recurrent or persistent endometrial cancer: An NRG Oncology/Gynecologic Oncology Group Study. Gynecol Oncol. 2014;135:441–445. doi: 10.1016/j.ygyno.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bender D, Sill MW, Lankes HA, et al. A phase II evaluation of cediranib in the treatment of recurrent or persistent endometrial cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2015;138:507–512. doi: 10.1016/j.ygyno.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moore KN, Sill MW, Tenney ME, et al. A phase II trial of trebananib (AMG 386; IND#111071), a selective angiopoietin 1/2 neutralizing peptibody, in patients with persistent/recurrent carcinoma of the endometrium: An NRG/Gynecologic Oncology Group trial. Gynecol Oncol. 2015;138:513–518. doi: 10.1016/j.ygyno.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Slomovitz BM, Lu KH, Johnston T, et al. A phase 2 study of the oral mammalian target of rapamycin inhibitor, everolimus, in patients with recurrent endometrial carcinoma. Cancer. 2010;116:5415–5419. doi: 10.1002/cncr.25515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ray-Coquard I, Favier L, Weber B, et al. Everolimus as second- or third-line treatment of advanced endometrial cancer: ENDORAD, a phase II trial of GINECO. Br J Cancer. 2013;108:1771–1777. doi: 10.1038/bjc.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oza AM, Elit L, Tsao MS, et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: A trial of the NCIC Clinical Trials Group. J Clin Oncol. 2011;29:3278–3285. doi: 10.1200/JCO.2010.34.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fleming GF, Filiaci VL, Marzullo B, et al. Temsirolimus with or without megestrol acetate and tamoxifen for endometrial cancer: A Gynecologic Oncology Group study. Gynecol Oncol. 2014;132:585–592. doi: 10.1016/j.ygyno.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Colombo N, McMeekin DS, Schwartz PE, et al. Ridaforolimus as a single agent in advanced endometrial cancer: Results of a single-arm, phase 2 trial. Br J Cancer. 2013;108:1021–1026. doi: 10.1038/bjc.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oza AM, Pignata S, Poveda A, et al. Randomized phase II trial of ridaforolimus in advanced endometrial carcinoma. J Clin Oncol. 2015;33:3576–3582. doi: 10.1200/JCO.2014.58.8871. [DOI] [PubMed] [Google Scholar]
- 87.Alvarez EA, Brady WE, Walker JL, et al. Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2013;129:22–27. doi: 10.1016/j.ygyno.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 88.Slomovitz BM, Jiang Y, Yates MS, et al. Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma. J Clin Oncol. 2015;33:930–936. doi: 10.1200/JCO.2014.58.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oza AM, Eisenhauer EA, Elit L, et al. Phase II study of erlotinib in recurrent or metastatic endometrial cancer: NCIC IND-148. J Clin Oncol. 2008;26:4319–4325. doi: 10.1200/JCO.2007.15.8808. [DOI] [PubMed] [Google Scholar]
- 90.Fleming GF, Sill MW, Darcy KM, et al. Phase II trial of trastuzumab in women with advanced or recurrent, HER2-positive endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2010;116:15–20. doi: 10.1016/j.ygyno.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Slomovitz B, Schmeler K, Miller D, et al. Phase II study of cetuximab (Erbitux) in patients with progressive or recurrent endometrial cancer. Gynecol Oncol. 2010;116:S2–S169. doi: 10.1136/ijgc-2020-001859. [DOI] [PubMed] [Google Scholar]
- 92.Leslie KK, Sill MW, Lankes HA, et al. Lapatinib and potential prognostic value of EGFR mutations in a Gynecologic Oncology Group phase II trial of persistent or recurrent endometrial cancer. Gynecol Oncol. 2012;127:345–350. doi: 10.1016/j.ygyno.2012.07.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leslie KK, Sill MW, Fischer E, et al. A phase II evaluation of gefitinib in the treatment of persistent or recurrent endometrial cancer: A Gynecologic Oncology Group study. Gynecol Oncol. 2013;129:486–494. doi: 10.1016/j.ygyno.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Myers AP, Filiaci VL, Zhang Y, et al. Tumor mutational analysis of GOG248, a phase II study of temsirolimus or temsirolimus and alternating megestrol acetate and tamoxifen for advanced endometrial cancer (EC): An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2016;141:43–48. doi: 10.1016/j.ygyno.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pant A, Lee II, Lu Z, et al. Inhibition of AKT with the orally active allosteric AKT inhibitor, MK-2206, sensitizes endometrial cancer cells to progestin. PLoS One. 2012;7:e41593. doi: 10.1371/journal.pone.0041593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gu C, Zhang Z, Yu Y, et al. Inhibiting the PI3K/Akt pathway reversed progestin resistance in endometrial cancer. Cancer Sci. 2011;102:557–564. doi: 10.1111/j.1349-7006.2010.01829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Konecny GE, Santos L, Winterhoff B, et al. HER2 gene amplification and EGFR expression in a large cohort of surgically staged patients with nonendometrioid (type II) endometrial cancer. Br J Cancer. 2009;100:89–95. doi: 10.1038/sj.bjc.6604814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Clinicaltrials.gov. Evaluation of carboplatin/paclitaxel with and without trastuzumab (Herceptin) in uterine serous cancer. Available at https://clinicaltrials.gov/ct2/show/NCT01367002. Accessed February 12, 2016.
- 100.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 101.Reinbolt R, Hays J. The role of PARP inhibitors in the treatment of gynecologic malignancies. Front Oncol. 2013;3:237. doi: 10.3389/fonc.2013.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Howitt BE, Shukla SA, Sholl LM, et al. Association of polymerase ε-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol. 2015;1:1319–1323. doi: 10.1001/jamaoncol.2015.2151. [DOI] [PubMed] [Google Scholar]
- 103.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]