Recommendations for integrating specialized palliative care (SPC) in clinical care, research, and education are needed. The recommendations in this study should be considered when developing standards for cancer centers of excellence in Germany. Definition and implementation of indicators of SPC integration in Comprehensive Cancer Centers, and evaluation of its effectiveness, are current and future challenges.
Keywords: Delphi technique, Consensus, Palliative care, Integration, Cancer center, Quality indicators
Abstract
Background.
International associations admit that specialized palliative care (SPC) is an obvious component of excellent cancer care. Nevertheless, gaps in integration at the international level have been identified. Recommendations for integrating SPC in clinical care, research, and education are needed, which are subject of the present study.
Materials and Methods.
A Delphi study, with three written Delphi rounds, including a face-to-face-meeting with a multiprofessional expert panel (n = 52) working in SPC in 15 German Comprehensive Cancer Centers (CCCs) funded by the German Cancer Aid was initiated. Initial recommendations are built on evidence-based literature. Consensus was defined in advance with ≥80% agreement based on the question of whether each recommendation was unambiguously formulated, relevant, and realizable for a CCC.
Results.
A total of 38 experts (73.1%) from 15 CCCs performed all three Delphi rounds. Consensus was achieved for 29 of 30 recommendations. High agreement related to having an organizationally and spatially independent palliative care unit (≥6 beds), a mobile multiprofessional SPC team, and cooperation with community-based SPC. Until round 3, an ongoing discussion was registered on hospice volunteers, a chair of palliative care, education in SPC among staff in emergency departments, and integration of SPC in decision-making processes such as tumor boards or consultation hours. Integration of SPC in decision-making processes was not consented by a low-rated feasibility (76.3%) due to staff shortage.
Conclusion.
Recommendations should be considered when developing standards for cancer center of excellence in Germany. Definition and implementation of indicators of integration of SPC in CCCs and evaluation of its effectiveness are current and future challenges.
Implications for Practice:
General and specialized palliative care (SPC) is an integral part of comprehensive cancer care. However, significant diversity concerning the design of SPC in the German Comprehensive Cancer Center (CCC) Network led to the establishment of consensual best practice recommendations for integration of SPC into the clinical structures, processes, research, and education throughout the CCC network. The recommendations contribute to a greater awareness relating to the strategic direction and development of SPC in CCCs. The access to information about SPC and access to offers regarding SPC shall be facilitated by implementing the recommendations in the course of treatment of patients with cancer.
Introduction
In recent years, progress in prevention, early detection, diagnosis, and treatment of cancer has been achieved. This development has improved the overall chance of survival and quality of life for many patients diagnosed with cancer.
Parallel to this development, the American Society of Clinical Oncology recognized “palliative care as a routine part of comprehensive cancer care” [1]. However, at the international level, the extent and depth of integration of palliative care in comprehensive cancer care differ widely [2–5]. Current data reveal heterogeneity with regard to provision of palliative care units, number of beds, consultation services and outpatient clinics, palliative care education, research activities, [3] and integration of specialized palliative care (SPC) into the cancer treatment process, such as participating in interdisciplinary tumor boards [4, 6].
The mode of integration of palliative care may have an impact on the quality of comprehensive cancer care. Hence, it is of interest for all disciplines offering cancer directed therapy within the Comprehensive Cancer Centers (CCCs). In addition, as CCCs can serve as models, they may influence cancer care in general. Therefore, the integration of palliative care into CCCs is one focus of health care policies and funding priorities on national and international level [3, 7–9]. The discussion of defining necessary structures, processes, and outcomes is evolving [9–17]. Besides clinical aspects, the integration of research and education standards for palliative care are deemed equally important [2].
Since 2007, several CCCs in Germany are certified by the German Cancer Society (Deutsche Krebsgesellschaft, DKG; Berlin, Germany, https://www.krebsgesellschaft.de) and funded by the German Cancer Aid (Deutsche Krebshilfe, DKH; Bonn, Germany, http://www.krebshilfe.de/nc/startseite.html) mirroring and adapting international experiences such as the American model of the National Cancer Institute-designated cancer centers. Alongside to this development, a multiprofessional and interdisciplinary working group of palliative care experts in the network of the German CCCs has been established with the aim of strengthening the integration of SPC in clinical processes, research, and education. One core project is to develop a consensus-based best practice model on palliative care in German CCCs.
The aim of this study is the development of consensus-based best practice recommendations for the integration of palliative care in German CCCs according to clinical structures, clinical processes, research, and education based on a prior status analysis [4].
Materials and Methods
In 2014, the Delphi method was applied for development of best practice recommendations. The Delphi technique is a structured approach that provides statements to a group of experts who return their responses for analysis. A number of Delphi rounds in an anonymized and condensed form take place until consensus is reached. The aim of the multiple iterations of the statements is to merge views [18] and to aim at consensus building [19].
Participants
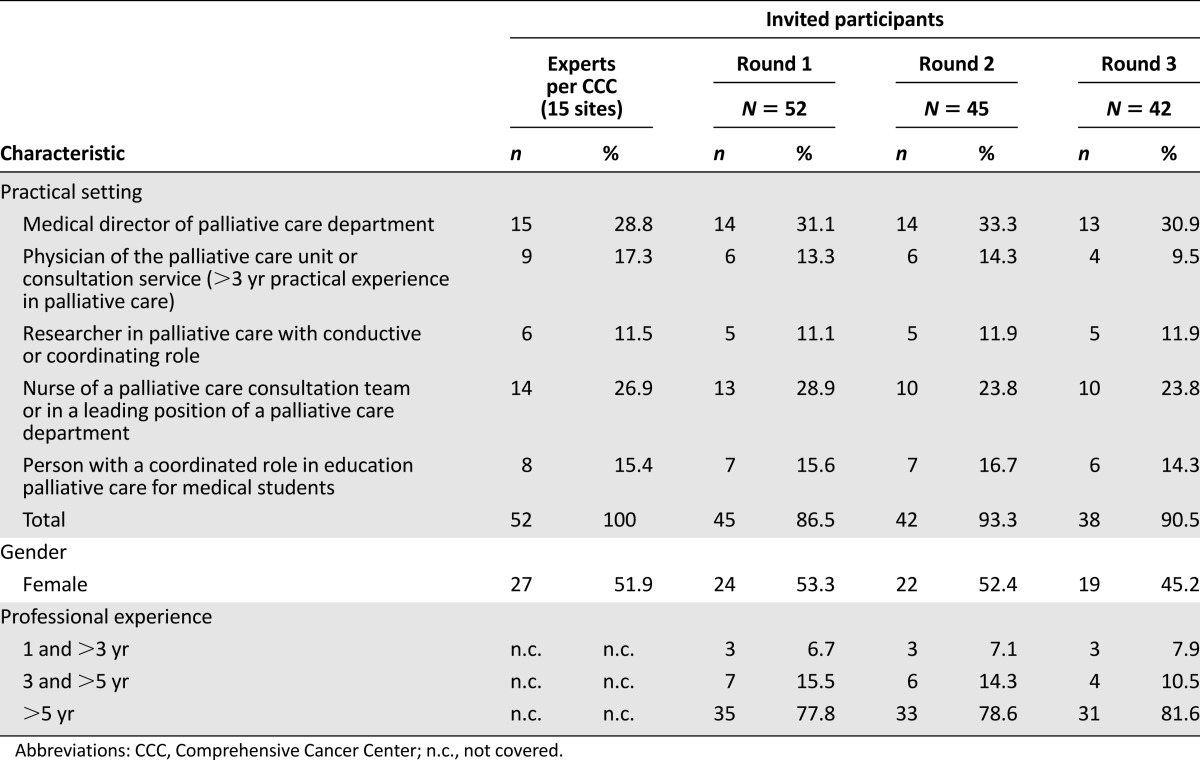
Members of the multidisciplinary palliative care expert panel for this Delphi study [14, 20–22] were defined as individuals with activity related to clinical care, teaching, or research within SPC in a German CCC. All heads of the palliative care departments within the 15 sites of the 14 funded CCCs (one CCC with two sites) were invited via e-mail to name each person of their department who has specific expertise and experience in one of five given practical settings in palliative care (Table 1). Due to heterogeneous structures at each CCC, not all sites were able to name an expert for each field of expertise. In the second and third rounds, only participants with responses in the preceding Delphi round were invited for participation to prevent large variation in replies.
Table 1.
Characteristics of Delphi participants of Comprehensive Cancer Centers
Preparation
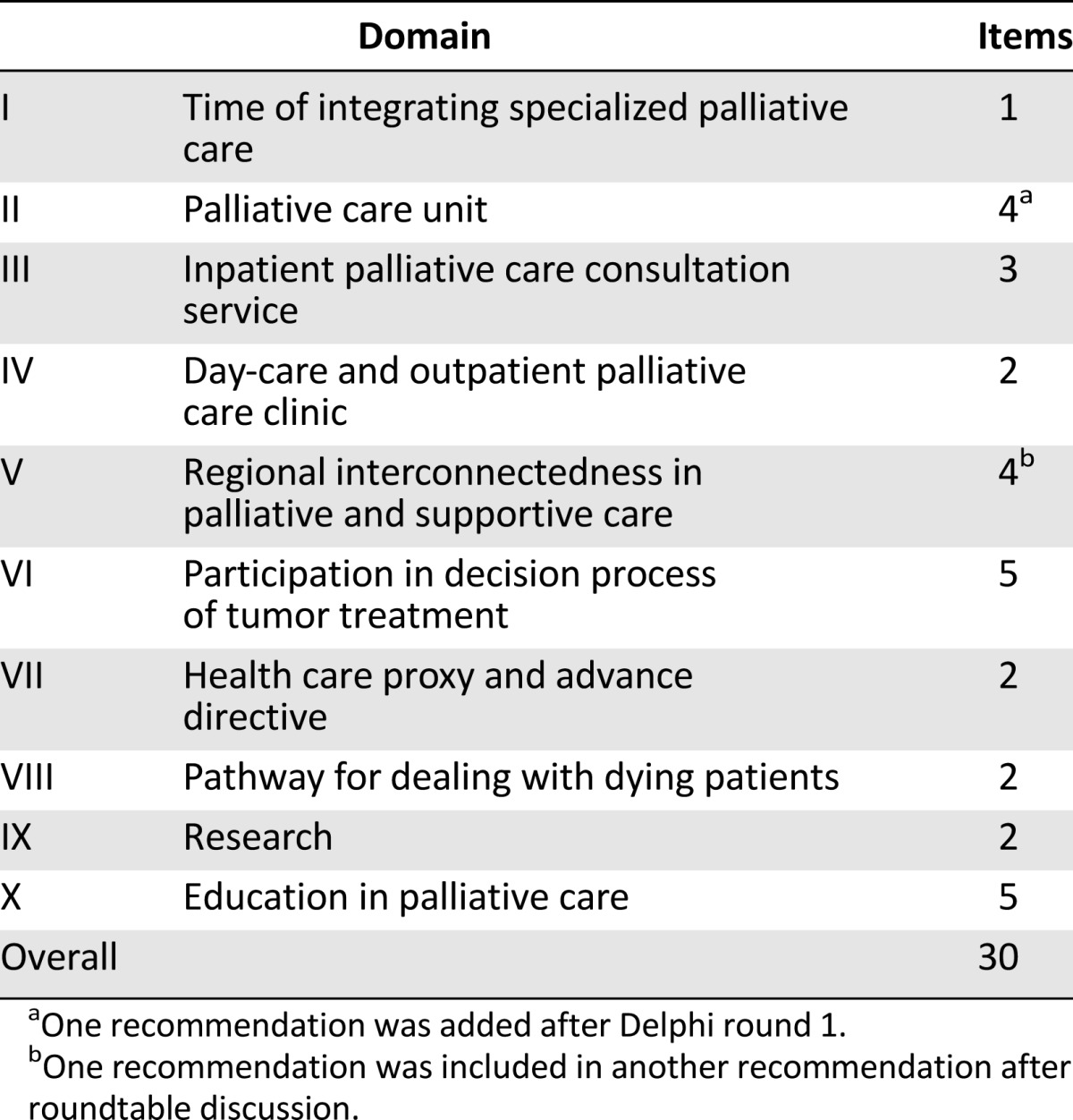
The research team merged 30 recommendations allocated to 10 main topics (Table 2) summarized to the three major categories of (a) clinical structures and processes, (b) research, and (c) education. All recommendations were based on empirical findings from a previous status analysis [4] and the German Level III evidence-based practice guideline for palliative care [23]. This National Guideline Palliative Care for patients with noncurable cancer within the German Guideline Program in Oncology [24] developed and consented 220 recommendations on seven key issues (dyspnea, cancer pain, constipation, depression, communication, dying phase, services structures) by 120 experts in oncology and palliative care from more than 50 medical societies and institutions. The guideline is supported by our project and all recommendations are valid for all CCCs. Some recommendations of the National Guideline were used as an initial resource for this Delphi process and subsequently complemented by new recommendations of themes, which were not addressed in the guideline (e.g., specific structural issues within CCCs, research).
Table 2.
Main topics regarding the Delphi study recommendations
All recommendations include the terms “must” or “should” to determine and emphasize the intensity of recommendation. Each recommendation was evaluated following three criteria:
Wording (w): Recommendation is unambiguously formulated.
Relevance (r): Recommendation is relevant for a CCC.
Feasibility (f): Recommendation is realizable for a CCC.
These criteria were rated by the experts on a 4-point Likert scale (4 = strongly disagree, 3 = rather disagree, 2 = rather agree, 1 = fully agree). High consensus was assumed when the summarized percentages of “rather agree” and “fully agree” were ≥80% and very high consensus was defined as a percentage of ≥80% scoring “fully agree” [14, 25].
Recommendations with moderate or low agreement were adopted for a further Delphi round. A moderate agreement was determined between 60% and 79% of participants scoring “rather agree” or “fully agree”; a low agreement was fixed by <60% (median scoring of 3 or 4). In addition, a free text field was added to each recommendation to allow for additional free responses.
Recommendations were dispatched as an interactive Microsoft Word file form (Microsoft, Redmond, WA, http://www.microsoft.com) via e-mail. Responses to all Delphi rounds were obtained via e-mail or fax.
Pilot Test
A pretest was conducted by two leading physicians from two palliative care institutions of German CCCs. Proposed changes from pilot testing included item and word order, the use of specific terms, and language simplification.
Study Application
Three Delphi rounds were planned. Each round was open for feedback for 4 weeks. The conducting research team sent three reminders to nonrespondents before closing data collection.
In case recommendations reached moderate or low agreement, written comments from the free text fields were used for adaptation. In the upcoming Delphi round, the comments and the adapted recommendations that were not yet consented were fed back to the participants of the previous round. So far, consensus-based recommendations and free text comments were communicated in a separate sheet.
Roundtable
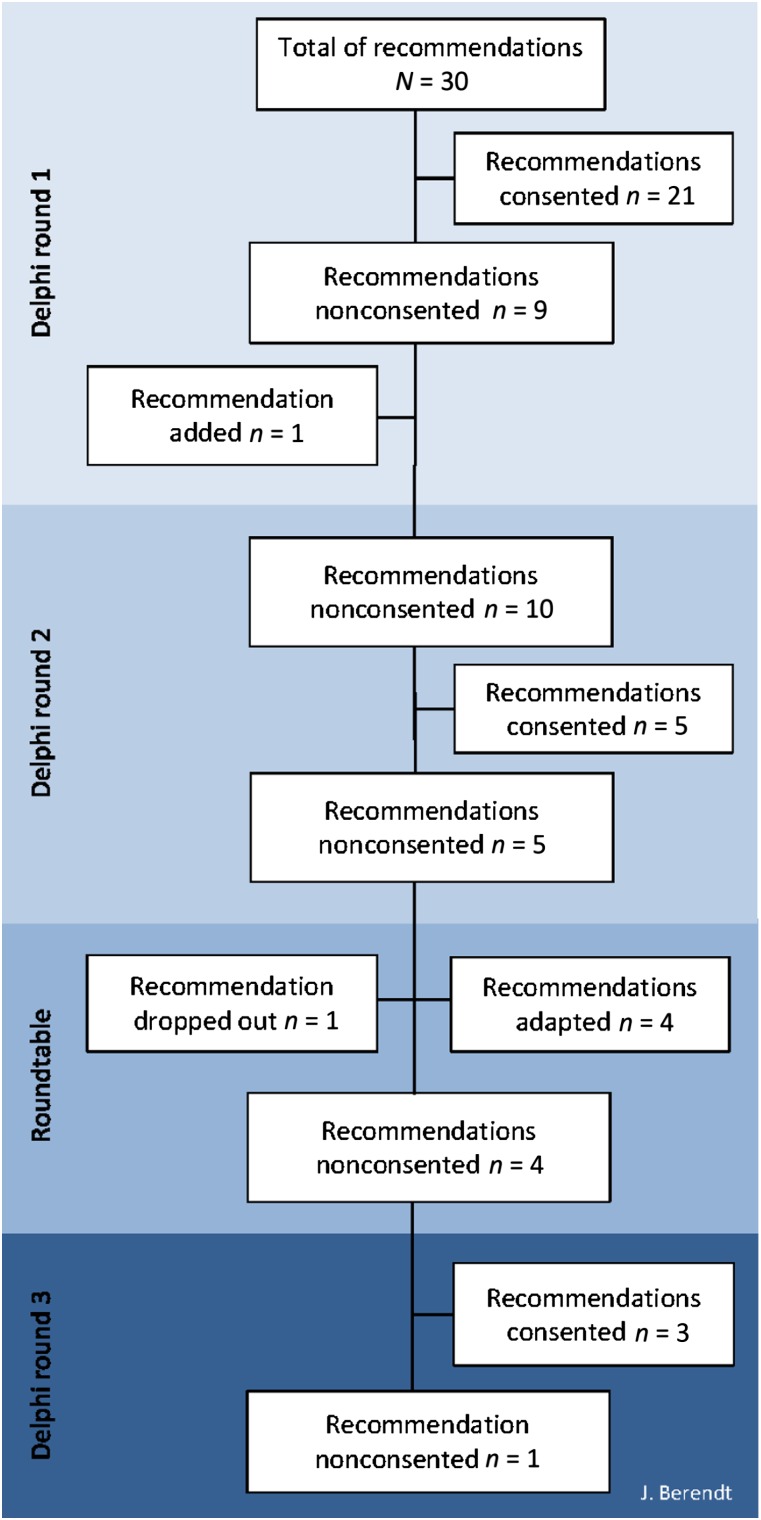
In July 2015, after the second Delphi round and data analysis, an expert meeting with representatives (n = 15) from each CCC site, who were integrated in the previous Delphi rounds, was organized. All open comments collected in Delphi rounds 1 and 2 on the remaining controversial recommendations (n = 5; Fig. 1) without consensus were considered and the recommendations reworded within the expert group. All participants of the expert group agreed to revoke their anonymity for this roundtable. Subsequently, the refined recommendations were given into a third Delphi round to aim for consensus by the whole Delphi group.
Figure 1.
Results of development consensus on recommendations for integrating palliative care into German Comprehensive Cancer Centers.
Analysis and Statistical Method
In all rounds, descriptive data analysis (frequencies, percentage, median and mean value, standard deviation) was performed using statistical software SPSS, version 21.0 (IBM, Armonk, NY, http://www.ibm.com). Written comments were coded with the text analysis software MAXQDA 11.0 (MAXQDA, Berlin, Germany, http://www.maxqda.com).
Results
Delphi Respondents
For the first round, 52 experts were contacted. The total percentage of returned questionnaires during the first round was 86.5% (n = 45). In the second round, 93.3% (n = 42) of the previous 45 respondents completed the questionnaire. After the roundtable discussion, the participation rate for the third Delphi round was 90.5% (n = 38) of the remaining 42 panelists. Altogether, 73.1% performed the entire Delphi process. Among the experts (Table 1), physicians were most strongly represented (40.4%). More than three quarters of all participants have been working in palliative care for more than 5 years.
Delphi Round 1
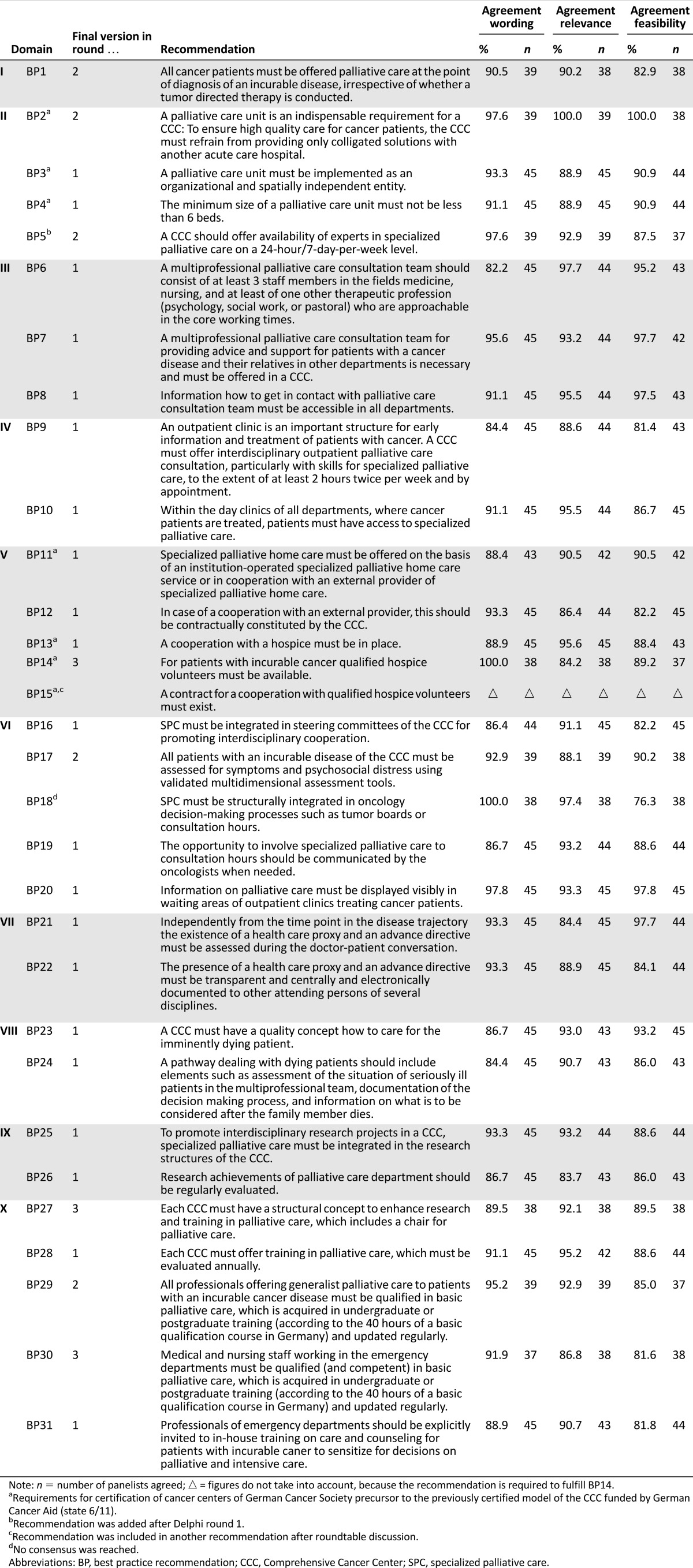
The first Delphi round started in March 2015. At the time, 21 of 30 (70.0%) best practice (BP) recommendations reached consensus for wording, relevance, and feasibility and were therefore accepted unmodified (Fig. 1). High agreement in wording, relevance, and feasibility was found for BP3–4, BP6–13, BP16, BP19–26, BP28, and BP31 (Table 3). Among these were three recommendations with very high agreement (BP8, BP20, BP19). The panelists did not agree with nine recommendations.
Table 3.
Best practice recommendations
The initial recommendation “Within emergency department medical or nursing staff should work with basic knowledge in palliative care” (BP30) achieved no consensus in all three criteria (wording: 79.5%, relevance: 77.3%, feasibility: 61.4%). The main criticism was the words “basic knowledge,” which were perceived as too unspecific. Some experts suggested guaranteeing SPC consultation around the clock instead of basic palliative care qualification acquirement for medical and nursing emergency staff.
Frequently, no consensus was achieved with regard to the recommendations’ feasibility. Less than 80% agreed on feasibility (74.4%) of BP1 on timing of SPC integration. This recommendation was commented 15 times. Respondents criticized that “incurable tumor disease” is defined and understood differently by medical disciplines and that it might currently be difficult for oncologists to address palliative care at an early stage of disease. Furthermore, insufficient staffing in palliative care departments and financial cover was mentioned as a problem to realize the recommendation of BP1. Besides better staffing, strengthening palliative care content in oncologists’ education was named as a solution.
The feasibility of detecting symptoms and psychosocial stress of patients and their relatives through validated multidimensional instruments (BP17) was also rated low (73.8%). The main criticism was the term “incurable” and the inclusion of “relatives.”
Participation in oncology decision-making process such as tumor boards and consultation hours (BP18) was assessed as feasible by 72.7%, again due to a lack of staff and/or funding (10 of 13 comments).
Low agreement was also reached on the presence of a chair for palliative care at a CCC (BP27; wording: 79.5%, relevance: 79.1%, feasibility: 71.4%). One reason for feasibility was funding issues. Another comment regarding relevance included “strengthening of research and teaching is not tied to a chair. It is important that exponentials of palliative medicine represented in executive board in CCC.”
At the beginning, BP29 included only physicians who must be trained in palliative care (basis qualification palliative care, 40 hours). That all physicians who were involved in cancer care must be trained in palliative care was initially seen as not viable (70.5%). In contrast, it was commented that all professionals involved in general palliative care should be trained.
To recommend the support of volunteer workers trained in hospice care (BP14) was not assessed as relevant by all participants (79.5%). Consensus was not reached due to organizational and regional differences between CCCs and its integration of volunteer work. In consideration of these differences, BP15 was registered as nonconsented and adapted for the second round.
Furthermore, one recommendation was formulated and added to the set of recommendations for the second round (BP5).
Delphi Round 2
In May 2015, the second round was performed. The remaining nine recommendations without consent were adapted and one additional recommendation was resent to all respondents from round 1. Subsequently, consensus was achieved for five (BP1–2, BP5, BP17, BP29) of the 10 provided recommendations (Fig. 1).
Of the five nonconsented recommendations, again three recommendations reached a low agreement with respect to feasibility (BP14–15, BP18, BP27, BP30).
The recommendation concerning participation in oncology decision-making processes, such as tumor boards and consultation hours (BP18), resulted in the same consensus as in round 1: staff shortage restricts its feasibility. On the contrary, the wording (95.1%) and the relevance (92.7%) was rated very high. However, considering the high time exposure needed to visit the variety of tumor boards, it was preferred to invest more resources in the education of oncologists and in SPC consultation services.
In round 2, recommendation of basic knowledge for emergency staff (BP30) achieved consensus on wording (82.9%) and relevance (80.5%) but was criticized for its feasibility (74.4%). Experts mentioned individual periodic in-house training courses according to the individual requirements of emergency departments (EDs), the cooperation with an SPC consultation service, and the integration in triage systems of EDs as more effective.
Some participants were concerned about the financial situation of their institution and the possibility of losing the CCC funding if a chair for palliative care (BP27) should be recommended (wording: 85.7%, relevance: 88.1%, feasibility: 75.0%). The wish to replace the term “must” with “should” in the recommendation wording was expressed. In contrast to this, other comments were stated, including, for example:
We only bring evidence-based medicine in palliative care forward by a chair, only a chair offers acquiring meaningful external funding, a chair is needed for setting up an own-research department. Only in this way, the SPC will be taken seriously in academic faculty/the dean’s office (otherwise we are only the supplier/disposer).
Roundtable
In July 2015, the roundtable followed. One of the five controversial recommendations was dropped unanimously (BP15), because its content was included in another of these five controversial recommendations (BP14). In conclusion, four resulting recommendations were provided for a third Delphi round.
Delphi Round 3
The third call took place in July and August 2015. During the third round, four of the five remaining recommendations were consented. One recommendation referring to participation in oncology decision-making processes, such as tumor boards or consultation hours (BP18), found no consensus for feasibility of implementing this recommendation (76.3%), although it was evaluated as highly relevant (97.4%) and clearly formulated (100%). In all three Delphi rounds, staff shortage was specified as the main reason for nonfeasibility.
Simplifying the phrasing of BP14 following the roundtable discussion led to a significantly higher agreement in wording (100%), relevance (84.2%), and feasibility (89.2%).
The modified recommendation BP27 reached consensus by including a parenthesis. In this way, to enhance research and education, a structural concept that includes a chair for palliative care is recommended.
There is agreement that inside the ED, medical and nursing staff must work with a palliative care based qualification (and competence), which is acquired and regularly updated, to be able to recognize the needs for specialized palliative care (BP30). After replacing the word “should” with “must” and by adding “having competence” and “to recognize the need of SPC,” consensus was achieved. The majority (86.8%) considered this recommendation relevant and 81.6% were optimistic about this becoming reality.
Discussion
CCCs should function as institutions that have the highest possible performance standards caring for the most complex cancer patients. The German Cancer Society requests SPC as an essential part of certification features for German cancer centers. Also, for the German Cancer Aid, a high-quality SPC is a mandatory condition for receiving funding as a German CCC. Providing the best possible cancer care must certainly have an enormous impact on the provision of palliative care in these certified centers. The recommendations presented here can serve as guidance for future development in SPC in comprehensive cancer care in Germany and elsewhere. To date, the situation in many CCCs needs much improvement and investment to achieve the standard experts have agreed on, especially in CCCs not certificated by DKG as an oncologic center with its detailed recommendations for palliative care services [26]. Conclusions from the current status quo [4] and the future perspectives arising from the Delphi study emphasize an urgent need for better implementation of SPC into CCCs in Germany. The development of multiprofessional palliative care consultation teams, outpatient clinics, and the integration of SPC in consultation hours of other departments and research projects of CCCs are future goals for German CCCs.
The expert panel of the Delphi study easily found consensus on best practice recommendations for the presence and size of palliative care units. In contrast, international experts do not favor a dedicated number of palliative care beds in hospitals, [2] which is not yet widely established [3]. The main focus lies rather on an inpatient palliative care consultation service and an outpatient palliative care clinic, which were also recommended by German experts for a CCC. However, these are rarely available in Germany compared with availability in the U.S. [3, 4].
Recommendations on day care, health care proxy and advance directives, pathways for dealing with dying patients, and research in a CCC achieved broad consensus quickly. On other issues, in particular concerning feasibility, the process for consensus finding was much more complex or even impossible. The involvement of SPC in tumor boards, for example, has an educational character [4] and aims to detect patients with certain palliative care needs [27]; however, the best practice recommendation on the participation of SPC in tumor boards has not been consented. Whereas this recommendation was rated highly relevant and clearly stated, its feasibility was doubted until the last round. This outcome points at the challenge that the constant participation of SPC in tumor boards as well as other oncology decision-making processes are currently perceived as unsustainable due to large efforts of time and staffing parallel to restricted financial resources of the SPC at CCCs [4]. Nevertheless, international experts stated that the routinely integration of the palliative care team in multidisciplinary tumor boards for patient case discussions is a (minor) quality indicator [2].
The need for the presence of a chair in palliative care at CCCs was discussed controversially throughout the Delphi process. More than half of experts favored the presence of a chair, similarly to international observations [2]. Experts in favor of chairs argue that the foundation of palliative care chairs is central and necessary for fundraising, performing research, and implementing education and that chairs might reinforce the institutional status within the CCCs. This argumentation is supported by the fact that palliative care departments with a chair position have significantly more research grants than departments without a chair [4]. Within an academic environment—and all of the CCCs in Germany are situated at universities—a chair will foster collaboration on “eye level” with the departments for cancer directed treatment. A second argument in favor of palliative care departments with a chair is that the presence of a chair may become a future feature for the approval as a CCC by the German Cancer Aid. Experts who are argue against chairs for palliative care fear the financial burden for medical faculties and universities and, consequently, a reduction of the universities’ financial support for palliative care. Another argument against a chair for palliative care, which was named in status analysis, was to rely on that the subject develops mainly through interdisciplinary networking [4]. In contrast, political and personal weight or visibility must be in place having power on its own. In summary, the expert panel of the Delphi study agreed on “a structural concept to enhance research and education of palliative care which includes a chair of palliative care.”
Consideration to offer palliative care to patients with incurable cancer early in the course of disease is internationally recognized and discussed [28]. Although diverse concepts on early integration are proposed, sufficiently establishment still does not exist in clinical practice. In this study, there was a high acceptance of the wording and relevance of a recommendation on early palliative care from the first Delphi round, but consensus regarding feasibility of early palliative care was lacking until the second round. The approved recommendation underlines mandatory early integration of palliative care but leaves open how this goal can be achieved. More attention should be paid to physicians’ basic palliative care training. With regard to the Delphi findings reported here, the minimal education requirements for all professionals in general palliative care in Germany were set to 40 hours of a basic palliative care course in the second round of the presented Delphi process. This lack of palliative care education underpins international reports that indicate that many oncology fellows or nurses are still insufficiently prepared to provide palliative care to their patients [29–34].
EDs are part of the steering system for patients with urgent needs. Optimal treatment and care following the patients’ needs relies on timely investigations, needs assessment, and first triage in ED. Many patients with advanced chronical diseases or malignancies and their family members could benefit from SPC resources and referrals in EDs [35, 36]. Benefits have been seen in improved patient outcomes [37], patient and family satisfaction [38], and cost savings [39]. In the first two Delphi rounds there were substantial concerns about how SPC could be implemented in the ED practice and routine. Some experts proposed to guarantee SPC consultation around the clock versus basic palliative care qualification for medical and nursing ED staff. Finally, the inclusion of emergency physicians and emergency medical staff into the continuing basic palliative care education was discussed and approved. Despite this debate it is obvious that intercommunication with ED is of special significance for offering adequate cancer and palliative care in a CCC.
Study Limitations
The Delphi technique is an adequate consensus method to improve decision-making in health care [40]. However, it can be argued how powerful the generated recommendations are [20] in terms of implementation into practice. Although more than two thirds of the participants responded during the whole Delphi process, a minor portion from all clinical settings dropped out during the study period, which leads to missing expert opinions. Structural and personal differences between the sites, especially for research and teaching, restrict collecting opinions of different perspectives in relation to the practice settings. Unequal distribution of professional backgrounds might have led to diverging conclusions with regard to the given recommendations. The greatest proportion of physicians is justified by its special comprehensive look to clinical processes, research, and education. However, the high participation in all practical settings and high degree of consensus indicate a representation for recommendations.
Conclusion
The German CCC network connects the cancer centers of excellence that have a leading role in clinical care, education, and research in Germany. Members of the palliative care working group have reached a broad consensus on 29 recommendations of a national best practice for integrating palliative care. These recommendations will be used to optimize the implementation of palliative care into the clinical oncology routine and to strengthen research and teaching activities. Possibly, the consideration of the best practice recommendations and the National Guideline Palliative Care for patients with noncurable cancer into the certification process for Oncologic Centers will compensate for existing gaps in higher-level institutions such as German CCCs funded by the German Cancer Aid. Transfer of positive effects of anchored palliative care into the clinical practice of other national cancer centers is desired.
Acknowledgments
We thank the German Cancer Aid on behalf of the palliative care working group of German CCCs for funding this study (Grant 110696). The funding organization had no influence on design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of the manuscript. The present work was performed in partial fulfillment of the requirements for obtaining the academic degree Dr. rer. biol. hum. by the first author (J.B.).
Author Contributions
Conception/Design: Julia Berendt, Stephanie Stiel, Peter Stachura
Provision of study material or patients: Julia Berendt
Collection and/or assembly of data: Julia Berendt
Data analysis and interpretation: Julia Berendt, Stephanie Stiel, Steffen T. Simon, Peter Stachura, Christoph Ostgathe
Manuscript writing: Julia Berendt, Stephanie Stiel, Steffen T. Simon, Andrea Schmitz, Birgitt van Oorschot, Peter Stachura, Christoph Ostgathe
Final approval of manuscript: Julia Berendt, Stephanie Stiel, Steffen T. Simon, Andrea Schmitz, Birgitt van Oorschot, Peter Stachura, Christoph Ostgathe
Disclosures
The authors indicated no financial relationships.
References
- 1.Ferris FD, Bruera E, Cherny N, et al. Palliative cancer care a decade later: Accomplishments, the need, next steps—from the American Society of Clinical Oncology. J Clin Oncol. 2009;27:3052–3058. doi: 10.1200/JCO.2008.20.1558. [DOI] [PubMed] [Google Scholar]
- 2.Hui D, Bansal S, Strasser F, et al. Indicators of integration of oncology and palliative care programs: An international consensus. Ann Oncol. 2015;26:1953–1959. doi: 10.1093/annonc/mdv269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hui D, Elsayem A, De la Cruz M, et al. Availability and integration of palliative care at US cancer centers. JAMA. 2010;303:1054–1061. doi: 10.1001/jama.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berendt J, Oechsle K, Thomas M, et al. State of integration of palliative care at Comprehensive Cancer Centers funded by German Cancer Aid. Dtsch Med Wochenschr. 2016;141:e16–e23. doi: 10.1055/s-0041-106089. [DOI] [PubMed] [Google Scholar]
- 5.Hui D, Bansal S, Strasser F, et al. Indicators of integration of oncology and palliative care programs: An international consensus. Ann Oncol. 2015;26:1953–1959. doi: 10.1093/annonc/mdv269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keating NL, Landrum MB, Lamont EB, et al. Tumor boards and the quality of cancer care. J Natl Cancer Inst. 2013;105:113–121. doi: 10.1093/jnci/djs502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Center Network (NCCN). NCCN clinical practice guideline in oncology (NCCN guidelines) palliative care version preliminary i.2014. 2014. Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive. Accessed February 3, 2016.
- 8.German Cancer Aid (Deutsche Krebshilfe). Funding priority palliative care - Förderschwerpunkt Palliativmedizin. 2015. Available at http://www.krebshilfe.de/wir-foerdern/ausschreibungen/archiv-ausschreibungen.html#c4482. Accessed February 8, 2016.
- 9.Woitha K, Van Beek K, Ahmed N, et al. Validation of quality indicators for the organization of palliative care: A modified RAND Delphi study in seven European countries (the Europall project) Palliat Med. 2014;28:121–129. doi: 10.1177/0269216313493952. [DOI] [PubMed] [Google Scholar]
- 10.Effendy C, Vissers K, Woitha K, et al. Face-validation of quality indicators for the organization of palliative care in hospitals in Indonesia: A contribution to quality improvement. Support Care Cancer. 2014;22:3301–3310. doi: 10.1007/s00520-014-2343-8. [DOI] [PubMed] [Google Scholar]
- 11.Efstathiou N, Ameen J, Coll AM. Healthcare providers’ priorities for cancer care: A Delphi study in Greece. Eur J Oncol Nurs. 2007;11:141–150. doi: 10.1016/j.ejon.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 12.van Riet Paap J, Vernooij-Dassen M, Dröes RM, et al. Consensus on quality indicators to assess the organisation of palliative cancer and dementia care applicable across national healthcare systems and selected by international experts. BMC Health Serv Res. 2014;14:396. doi: 10.1186/1472-6963-14-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woitha K, Hasselaar J, van Beek K, et al. Testing feasibility and reliability of a set of quality indicators to evaluate the organization of palliative care across Europe: A pilot study in 25 countries. Palliat Med. 2015;29:157–163. doi: 10.1177/0269216314562100. [DOI] [PubMed] [Google Scholar]
- 14.Jünger S, Payne S, Brearley S, et al. Consensus building in palliative care: A Europe-wide delphi study on common understandings and conceptual differences. J Pain Symptom Manage. 2012;44:192–205. doi: 10.1016/j.jpainsymman.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Sasahara T, Kizawa Y, Morita T, et al. Development of a standard for hospital-based palliative care consultation teams using a modified Delphi method. J Pain Symptom Manage. 2009;38:496–504. doi: 10.1016/j.jpainsymman.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 17.Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: A cluster-randomised controlled trial. Lancet. 2014;383:1721–1730. doi: 10.1016/S0140-6736(13)62416-2. [DOI] [PubMed] [Google Scholar]
- 18.Hsu C-CS, Brian A. The delphi technique: Making sense of consensus. PARE. 2007;12:1–8. [Google Scholar]
- 19.von der Gracht HA. Consensus measurement in delphi studies. Review and implications for future quality assurance. TFSC. 2012;79:1525–1536. [Google Scholar]
- 20.Powell C. The delphi technique: Myths and realities. JAN. 2003;41:376–382. doi: 10.1046/j.1365-2648.2003.02537.x. [DOI] [PubMed] [Google Scholar]
- 21.Häder M. Delphi-Befragungen. Ein Arbeitsbuch. Wiesbaden, Germany: Springer VS Verlag für Sozialwissenschaften; 2014. [Google Scholar]
- 22.Domeisen Benedetti F, Ostgathe C, Clark J, et al. International palliative care experts’ view on phenomena indicating the last hours and days of life. Support Care Cancer. 2013;21:1509–1517. doi: 10.1007/s00520-012-1677-3. [DOI] [PubMed] [Google Scholar]
- Leitlinienprogramm Onkologie der Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e.V. (AWMF) DKeVDuDKD. S3-Leitlinie Palliativmedizin für Patienten mit einer nicht heilbaren Krebserkrankung. 2015.
- 24.Association of the Scientific Medical Societies in Germany GCS. German Cancer Aid. German Guideline Program in Oncology (GGPO). 2015. Available at http://leitlinienprogramm-onkologie.de/English-Language.16.0.html. Accessed February 3, 2016.
- 25.van der Steen JT, Radbruch L, Hertogh CM, et al. White paper defining optimal palliative care in older people with dementia: A Delphi study and recommendations from the European Association for Palliative Care. Palliat Med. 2014;28:197–209. doi: 10.1177/0269216313493685. [DOI] [PubMed] [Google Scholar]
- 26.Wesselmann SMH, van Oorschot B. Palliativmedizin in organkrebszentren und onkologischen zentren. Pneumologe. 2012;9:123–129. [Google Scholar]
- 27.Petty JK, Vetto JT. Beyond doughnuts: Tumor board recommendations influence patient care. J Cancer Educ. 2002;17:97–100. doi: 10.1080/08858190209528807. [DOI] [PubMed] [Google Scholar]
- 28.Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: The integration of palliative care into standard oncology care. J Clin Oncol. 2012;30:880–887. doi: 10.1200/JCO.2011.38.5161. [DOI] [PubMed] [Google Scholar]
- 29.Prazak KA, Lester PE, Fazzari M. Evaluation of physician assistant student knowledge and perception of competence in palliative symptom management. J Allied Health. 2014;43:e69–e74. [PubMed] [Google Scholar]
- 30.Chen E, McCann JJ, Lateef OB. Attitudes toward and experiences in end-of-life care education in the intensive care unit: A survey of resident physicians. Am J Hos Palliat Care. 2015;32:738–744. doi: 10.1177/1049909114539038. [DOI] [PubMed] [Google Scholar]
- 31.Lesnock JL, Arnold RM, Meyn LA, et al. Palliative care education in gynecologic oncology: A survey of the fellows. Gynecol Oncol. 2013;130:431–435. doi: 10.1016/j.ygyno.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Buss MK, Lessen DS, Sullivan AM, et al. Hematology/oncology fellows’ training in palliative care: Results of a national survey. Cancer. 2011;117:4304–4311. doi: 10.1002/cncr.25952. [DOI] [PubMed] [Google Scholar]
- 33.Thomas RA, Curley B, Wen S, et al. Palliative care training during fellowship: A national survey of u.S. Hematology and oncology fellows. J Palliat Med. 2015;18:747–751. doi: 10.1089/jpm.2015.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Kindi SG, Zeinah GF, Hassan AA. Palliative care knowledge and attitudes among oncology nurses in Qatar. Am J Hosp Palliat Care. 2014;31:469–474. doi: 10.1177/1049909113489874. [DOI] [PubMed] [Google Scholar]
- 35.Mierendorf SM, Gidvani V. Palliative care in the emergency department. Perm J. 2014;18:77–85. doi: 10.7812/TPP/13-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.George N, Phillips E, Zaurova M, et al. Palliative care screening and assessment in the emergency department: A systematic review. J Pain Symptom Manage. 2016;51:108–19.e2. doi: 10.1016/j.jpainsymman.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Grudzen CR, Stone SC, Morrison RS. The palliative care model for emergency department patients with advanced illness. J Palliat Med. 2011;14:945–950. doi: 10.1089/jpm.2011.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grudzen CR, Richardson LD, Hopper SS, et al. Does palliative care have a future in the emergency department? Discussions with attending emergency physicians. J Pain Symptom Manage. 2012;43:1–9. doi: 10.1016/j.jpainsymman.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Penrod JD, Deb P, Luhrs C, et al. Cost and utilization outcomes of patients receiving hospital-based palliative care consultation. J Palliat Med. 2006;9:855–860. doi: 10.1089/jpm.2006.9.855. [DOI] [PubMed] [Google Scholar]
- 40.Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32:1008–1015. [PubMed] [Google Scholar]