This comprehensive safety analysis of patients enrolled in the PALOMA-3 study placed particular emphasis on neutropenia as the most frequently reported adverse event associated with palbociclib treatment. Palbociclib plus fulvestrant treatment was found to be well-tolerated, and the primary toxicity of asymptomatic neutropenia was effectively managed by dose modification without apparent loss of efficacy.
Keywords: Cyclin-dependent kinase 4, Cyclin-dependent kinase 6, Palbociclib, Neutropenia
Abstract
Background.
Palbociclib enhances endocrine therapy and improves clinical outcomes in hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer (MBC). Because this is a new target, it is clinically important to understand palbociclib’s safety profile to effectively manage toxicity and optimize clinical benefit.
Materials and Methods.
Patients with endocrine-resistant, HR-positive/HER2-negative MBC (n = 521) were randomly assigned 2:1 to receive fulvestrant (500 mg intramuscular injection) with or without goserelin with oral palbociclib (125 mg daily; 3 weeks on/1 week off) or placebo. Safety assessments at baseline and day 1 of each cycle included blood counts on day 15 for the first 2 cycles. Hematologic toxicity was assessed by using laboratory data.
Results.
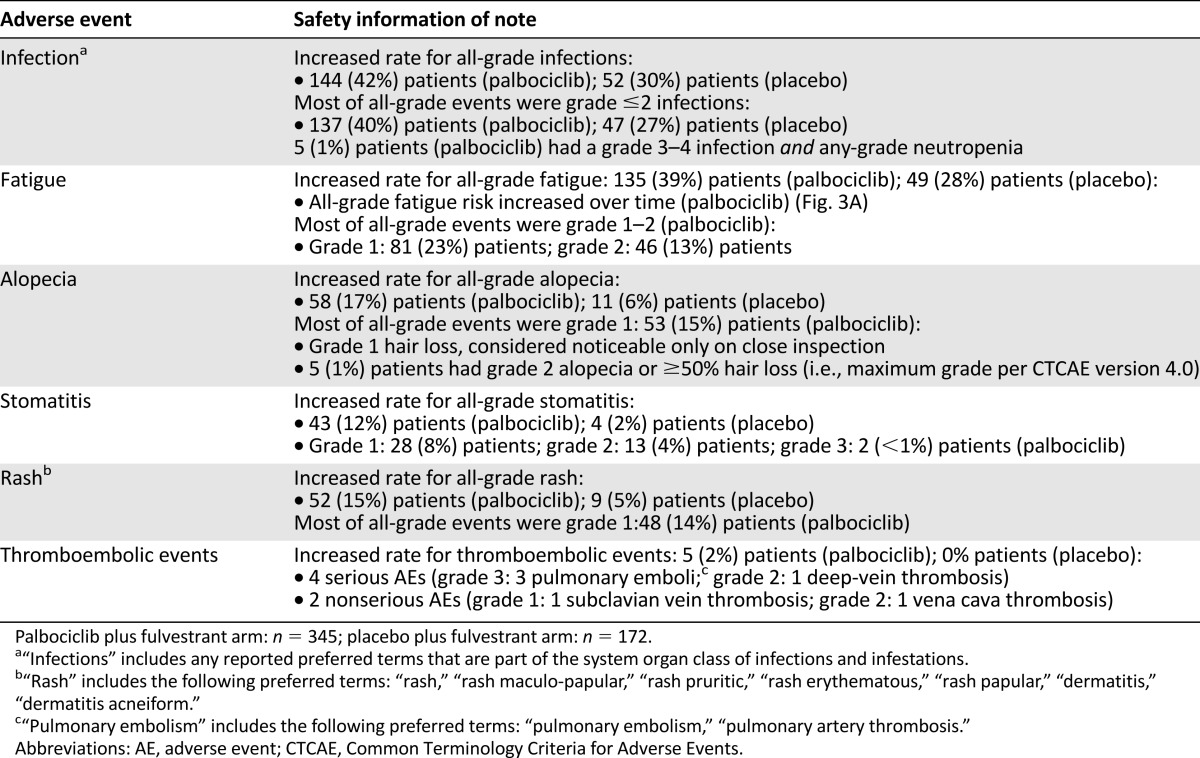
A total of 517 patients were treated (palbociclib, n = 345; placebo, n = 172); median follow-up was 8.9 months. With palbociclib, neutropenia was the most common grade 3 (55%) and 4 (10%) adverse event; median times to onset and duration of grade ≥3 episodes were 16 and 7 days, respectively. Asian ethnicity and below-median neutrophil counts at baseline were significantly associated with an increased chance of developing grade 3–4 neutropenia with palbociclib. Dose modifications for grade 3–4 neutropenia had no adverse effect on progression-free survival. In the palbociclib arm, febrile neutropenia occurred in 3 (<1%) patients. The percentage of grade 1–2 infections was higher than in the placebo arm. Grade 1 stomatitis occurred in 8% of patients.
Conclusion.
Palbociclib plus fulvestrant treatment was well-tolerated, and the primary toxicity of asymptomatic neutropenia was effectively managed by dose modification without apparent loss of efficacy. This study appears at ClinicalTrials.gov, NCT01942135.
Implications for Practice:
Treatment with palbociclib in combination with fulvestrant was generally safe and well-tolerated in patients with hormone receptor (HR)-positive metastatic breast cancer. Consistent with the drug's proposed mechanism of action, palbociclib-related neutropenia differs in its clinical time course, patterns, and consequences from those seen with chemotherapy. Neutropenia can be effectively managed by a dose reduction, interruption, or cycle delay without compromising efficacy. A significant efficacy gain and a favorable safety profile support the consideration of incorporating palbociclib into the routine management of HR-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer.
Introduction
Endocrine therapy is the preferred first-line treatment option for hormone receptor (HR)-positive and human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer (MBC) [1]. However, the fundamental clinical challenge associated with this treatment option is the development of resistance to endocrine therapy [2]. The mechanisms of resistance have yet to be fully elucidated [2]. Among women with disease progression after prior endocrine therapy, treatment options include sequential endocrine-based therapies, as monotherapy or in combination with a targeted therapy (e.g., everolimus for some postmenopausal women), before switching to chemotherapy [1]. Clinical research has focused on enhancing and improving outcomes of endocrine-based therapy to augment disease control, delay the use of chemotherapy, and optimize the length and quality of life [1].
Palbociclib is a first-in-class potent oral inhibitor of cyclin-dependent kinases (CDK) 4/6 and a novel therapeutic option for the treatment of HR-positive/HER2-negative MBC [3]. In the endocrine-resistant setting, the recent global PALOMA-3 trial demonstrated the improved efficacy of palbociclib in combination with fulvestrant over fulvestrant plus placebo in pre-, peri-, and postmenopausal women whose disease had progressed while they were receiving prior endocrine therapy (median progression-free survival [PFS], 9.5 vs. 4.6 months; hazard ratio [HR], 0.46; 95% confidence interval [CI], 0.36–0.59; p = .0001) [4]. The current data therefore suggest that palbociclib enhances endocrine therapy and improves clinical outcomes in both treatment-naïve and endocrine-resistant patients with HR-positive/HER2-negative MBC [3–5].
Because palbociclib is incorporated into treatment paradigms for patients with HR-positive MBC, it is clinically important to understand its safety profile, in order to both effectively manage toxicity and balance risk and benefit. With these goals in mind, a comprehensive safety analysis of patients enrolled in the PALOMA-3 study was undertaken, with particular emphasis given to neutropenia as the most frequently reported adverse event (AE) associated with palbociclib treatment.
Materials and Methods
Patients
Eligible patients had breast cancer and histologic or cytologic confirmation of recurrent local or distant disease progression during or within 12 months of completion of adjuvant endocrine therapy or while receiving or within 1 month after receiving endocrine therapy for MBC. Premenopausal and postmenopausal patients who had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1 and measurable disease, as defined by the Response Evaluation Criteria in Solid Tumors (version 1.1 [6]), or bone-only disease with a lytic lesion were eligible. One prior line of chemotherapy in the advanced setting was allowed, but there was no limit on the number of prior lines of endocrine therapy in the MBC setting. Eligibility criteria and study design details are documented elsewhere [5]. The protocol was approved by an institutional review board/independent ethics committee, and the study was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent before the start of any study procedures.
Study Design
The PALOMA-3 study (NCT01942135), a multicenter, randomized, double-blind, placebo-controlled, phase III trial, was designed to assess the superiority of palbociclib in combination with fulvestrant compared with placebo plus fulvestrant in prolonging PFS in women with HR-positive/HER2-negative MBC and disease progression after prior endocrine therapy. The primary endpoint was PFS, and the secondary endpoints included a comparison of safety between treatment arms [5].
Treatment
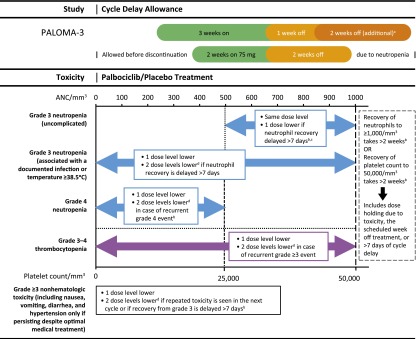
Patients were randomly assigned (2:1) to the palbociclib group (125 mg per day, orally, 3 weeks on and 1 week off) plus fulvestrant (500 mg per month, intramuscularly, on days 1 and 15 of the first 28-day cycle and day 1 of subsequent cycles, with or without goserelin per menopausal status) or the placebo group (placebo plus fulvestrant). If patients experienced a hematologic toxicity, such as grade 3–4 neutropenia, a specific dose modification schema consisting of dose interruption, dose delay, or dose reduction was followed (Fig. 1). Dose modification for fulvestrant was not allowed. Primary prophylactic use of granulocyte colony-stimulating factors (G-CSFs) was not permitted [5]. If neutropenic complications were observed in a cycle in which primary prophylaxis with G-CSFs was not received, secondary prophylaxis was given at the discretion of the investigator, but only if dose modification was not considered a reasonable alternative.
Figure 1.
Palbociclib/placebo dose modification schema for managing treatment-related toxicities. aIf patients still have not returned to grade 2 on day 1 of next cycle. bReduce by 2 dose levels. cIf uncomplicated grade 3 neutropenia recurs in 2 consecutive cycles, after recovery as per retreatment criteria (ANC≥1,000/mm3 and no fever), treatment may restart at the next lower dose level at investigator’s discretion. dIf no further dose reduction is possible (i.e., patient is already receiving 75 mg per day according to schedule 3/1), consider changing the schedule to 75 mg per day, 2 weeks on/2 weeks off, or discontinue palbociclib/placebo and continue with fulvestrant alone.
Abbreviation: ANC, absolute neutrophil count.
Safety Assessment
Adverse events were assessed on the basis of type, incidence, severity, timing, seriousness, and relatedness to study treatment. Severity was graded by using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 [7]. Laboratory safety assessments were performed at baseline and on day 1 of each cycle and included blood counts on day 15 for the first 2 cycles and at end of treatment/withdrawal. Additional blood tests were permitted at the investigator’s discretion as clinically indicated for the purpose of planning treatment, for dose modification, or after AEs.
Statistical Analysis
Descriptive analysis was used to summarize the maximum grade AEs on treatment using terms from the Medical Dictionary for Regulatory Activities (MedDRA). For hematologic toxicities, descriptive analyses of laboratory data were also performed when possible because not all hematologic events may have been reported. The risk difference between the two treatment arms for hematologic events and nonhematologic events of interest was calculated, and the respective 95% CIs were also provided without adjustment for multiplicity. The AE incidence was based on the maximum toxicity grade during the treatment, as reported by investigators. Because many patients can have multiple episodes of an adverse hematologic event, and to further delineate the patterns of neutropenia, some of the analysis was per episode using the laboratory data in aggregate, whereas the risk difference for hematologic events in both arms was considered using the proportion of patients with hematologic events in both arms. Exploratory analyses of PFS were conducted for different subgroups among the patients who received palbociclib plus fulvestrant. The Kaplan-Meier (KM) method was used to estimate the median PFS, and the two-sided log-rank test was performed for the PFS comparisons. Hazard ratios and two-sided 95% confidence intervals were estimated using Cox proportional hazards model. The KM method was also used to estimate the cumulative probability (i.e., cumulative incidence function) of neutropenia and fatigue, separately.
The relationship between baseline characteristics and grade 3–4 neutropenia was examined individually among patients in the palbociclib arm. Univariate analysis was used to assess the individual associations of these risk factors, which are presented as odds ratios with 95% CIs. A multivariate logistic model was run to evaluate the relationship between grade 3 and/or 4 neutropenia versus infection status, treatment, and key baseline factors (simultaneously considered in the model).
Results
Patient Population
Between October 7, 2013, and August 26, 2014, 521 patients were enrolled [5]. Two randomly assigned patients per arm did not receive study treatment. The safety populations (as-treated) comprised 345 patients for the palbociclib arm and 172 patients for the placebo arm. The safety data presented are from the March 16, 2015, cutoff with a median follow-up of 8.9 (interquartile range, 8.7–9.2) months for the intent-to-treat population. More than half (67%) of all patients had ≥2 disease sites, 35% of patients had ≥2 lines of prior therapy in the MBC setting, and 34% of patients had undergone 1 line of prior chemotherapy in the MBC setting.
Overall Safety Profile
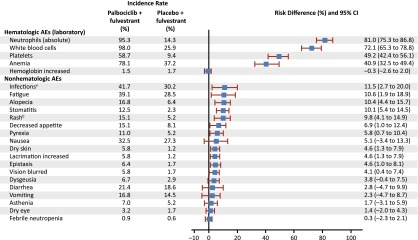
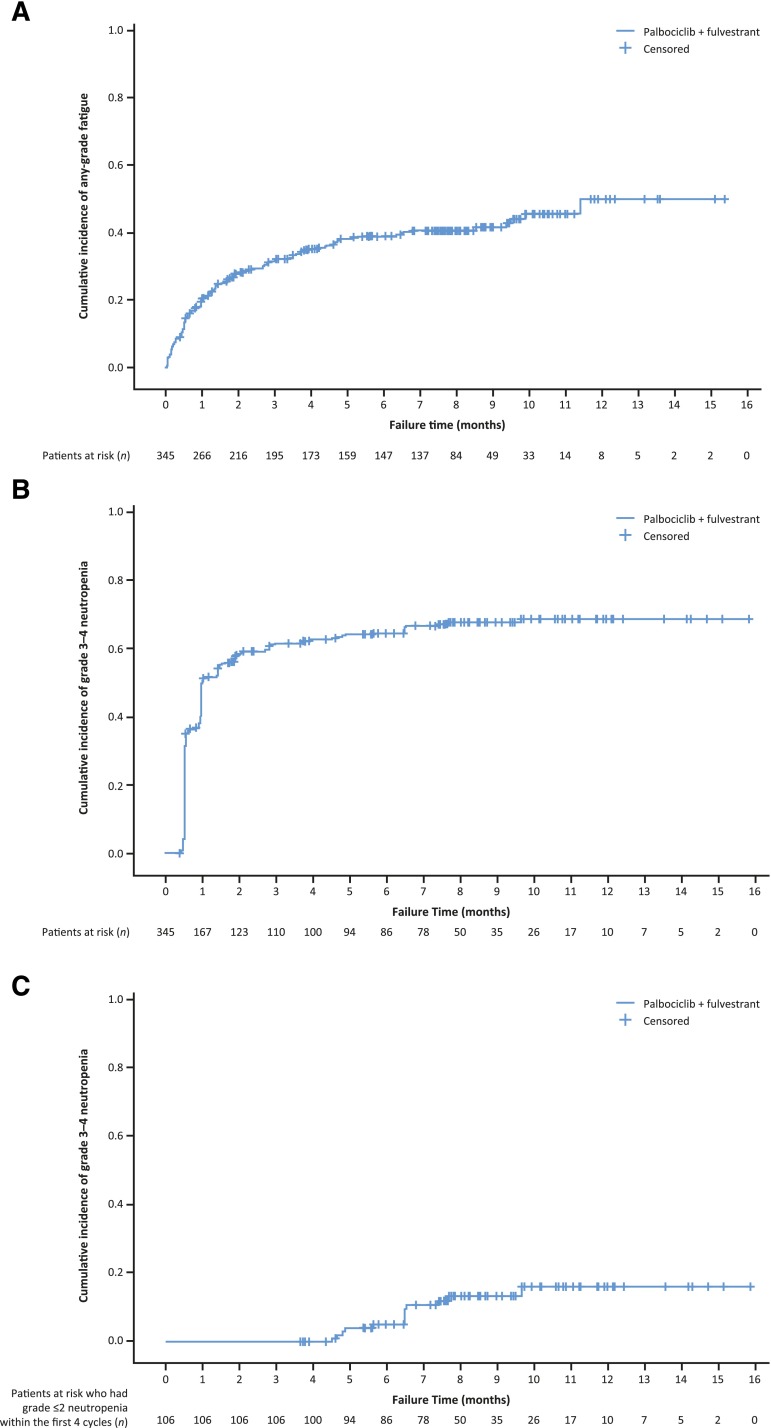
For all cycles, the reported incidence rate of any grade and grade 3–4 AEs was 99% and 73%, respectively, in the palbociclib arm and 90% and 22% in the placebo arm. The risk difference for toxicities was higher for hematologic than for nonhematologic toxicities during the study (Fig. 2). A significant difference (>10%) in the incidence of the following treatment-emergent AEs of any cause (all grades) was reported in the palbociclib arm compared with the placebo arm: neutropenia, leukopenia, anemia, thrombocytopenia, stomatitis, alopecia, and rash (all p < .005), as well as infection and fatigue (both p < .02). AEs of interest are shown in Table 1. Most reported infections were upper respiratory tract infections of likely viral cause. Fatigue in the palbociclib arm was common (all grades, 39%), with 13% and 2% of patients experiencing grade 2 and 3 fatigue, respectively. The cumulative incidence of fatigue over time in the palbociclib arm is shown in Figure 3. Among patients who had stomatitis or alopecia in the palbociclib arm, the severity was predominantly grade 1. Discontinuations due to AEs were similarly low in the palbociclib (4%) and the placebo (2%) arms. In the palbociclib arm, treatment was discontinued for 1 patient each because of the following AEs: grade 4 neutropenia, grade 2 anemia, and grade 2 and 3 thrombocytopenia. Four (1.2%) patients in the palbociclib arm and 3 (1.7%) patients in the placebo arm had grade 5 events (mainly related to disease progression) and after the data cutoff, one case of neutropenic sepsis and multiorgan failure in the context of disease progression was reported.
Figure 2.
Risk difference for AEs (hematologic laboratory and nonhematologic: all cycles, as treated). a“Infections” includes any reported preferred terms that are part of the system organ class of infections and infestations. b“Rash” includes the following preferred terms: “rash,” “rash maculo-papular,” “rash pruritic,” “rash erythematous,” “rash papular,” “dermatitis,” and “dermatitis acneiform.”
Abbreviations: AE, adverse event; CI, confidence interval.
Table 1.
Adverse events of interest in the PALOMA-3 study
Figure 3.
Kaplan-Meier curves showing cumulative incidences of fatigue (all grades) and grade 3‒4 neutropenia. (A): Fatigue in patients treated with palbociclib plus fulvestrant. (B): Grade 3‒4 neutropenia in patients treated with palbociclib plus fulvestrant. (C): Patients treated with palbociclib plus fulvestrant who had grade ≤2 neutropenia within the first 4 cycles and grade 3–4 neutropenia after cycle 4. “Neutropenia” includes the following preferred terms: “neutropenia,” “neutrophil count decreased.” The Kaplan-Meier estimator was used to estimate the cumulative incidence in the plot.
The incidence of all-causality serious AEs (SAEs) was 44 (12.8%) of 345 patients in the palbociclib arm and 30 (17.4%) of 172 patients in the placebo arm. The most frequently reported type of SAE in the palbociclib arm was infections (2.0% vs. 4.1% in the placebo arm), defined as any event that is part of the corresponding MedDRA System Organ Class. Besides infections, no other SAE occurring on study up to 28 days after the last dose of study drug reached an incidence of 2.0% in both arms: In the palbociclib arm, neutropenia and pyrexia were reported in 4 patients each and pulmonary embolism and pleural effusion were reported in 3 patients each; in the placebo arm, pleural effusion and ascites were reported in 3 patients each. Results for SAEs occurring in more than 1 patient are shown in supplemental online Table 1. Thromboembolic events occurred in 2% of patients in the palbociclib arm (4 cases reported as SAEs and 2 cases as AEs) and in no patients in the placebo arm. These events were not considered related to study drug by the investigator; however, the causal role of palbociclib cannot be completely excluded.
Clinical Patterns of Neutropenia, Anemia, and Thrombocytopenia
In the palbociclib arm, grade 3 and 4 hematologic toxicities occurred for neutropenia (55.3% and 9.7%, respectively), leukopenia (41.5% and 1.2%), anemia (2.9% and 0%), and thrombocytopenia (2.1% and 0.9%) per laboratory data. Among the patients who had grade 3‒4 neutropenia, only 3.2% had concurrent grade 3‒4 anemia and 3.2% had concurrent grade 3‒4 thrombocytopenia.
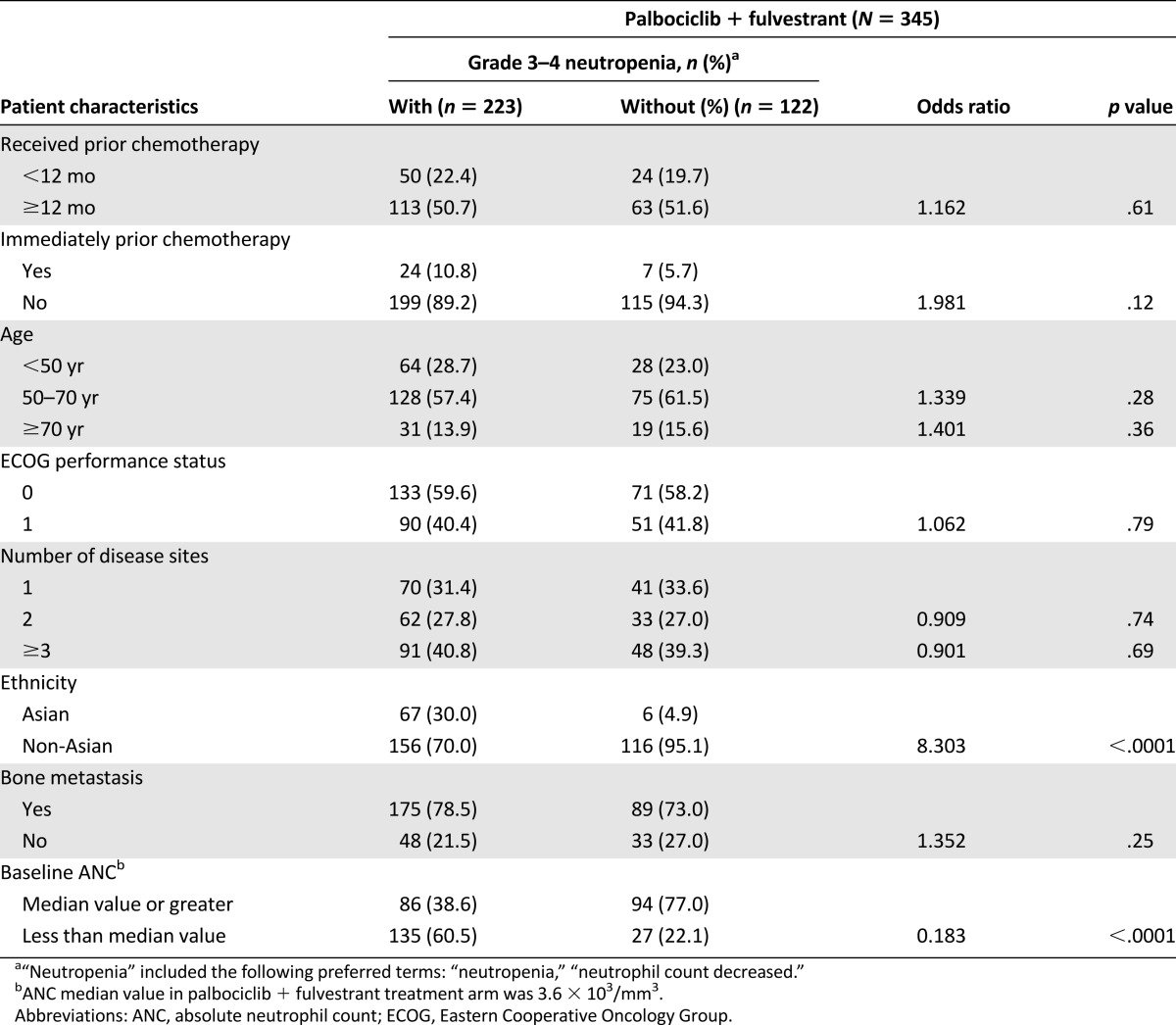
Univariate analysis revealed that Asian ethnicity and a below-median value for absolute neutrophil count (ANC) conferred a significantly increased risk for developing grade 3–4 neutropenia in the palbociclib arm, and this was consistent with the results for grade 3–4 neutropenia in the multivariate analysis (supplemental online Table 2). The percentage of Asian patients who developed grade 3–4 neutropenia was higher than for non-Asians (92% vs. 57%). Prior chemotherapy, age, ECOG performance status, and number of disease sites did not significantly increase the risk for developing grade 3–4 neutropenia (Table 2).
Table 2.
Risk for grade 3 or 4 neutropenia by clinical characteristics (odds ratio): as treated
Median time from the first dose of palbociclib plus fulvestrant to the first appearance of a neutropenia episode of any grade was 15 (range, 13‒140) days, and the onset of the first episode of grade ≥3 neutropenia was 16 (range, 13–293) days (supplemental online Fig. 1A). The median time from the first dose to the lowest ANC on study was 29 (range, 13–334) days. The median time from the first dose to the first occurrence of grade ≥3 anemia or thrombocytopenia was 39.5 (range, 15‒225) and 26.5 (range, 15‒92) days, respectively. The median duration of grade ≥3 episodes of neutropenia, anemia, and thrombocytopenia was 7.0 days for each event, with ranges of 1–98, 1–141, and 1–27 days, respectively (supplemental online Fig. 1B).
Among the 144 patients with a maximum of grade ≤2 (i.e., grade 0, 1, and 2) neutropenia in the first 2 cycles, 25 (17.4%) experienced grade 3 neutropenia beyond cycle 2, whereas 13 (9.8%) of 132 patients with a maximum of grade ≤2 neutropenia in the first 4 cycles experienced grade 3 neutropenia beyond cycle 4, and only 8 (6.3%) of 127 patients with a maximum of grade ≤2 neutropenia in the first 6 cycles experienced grade 3 neutropenia beyond cycle 6. None of the 132 or 127 patients in the palbociclib arm who experienced maximum grade ≤2 neutropenia within the first 4 or 6 cycles, respectively, developed grade 4 neutropenia in subsequent cycles.
Assessment of Dose Modification Strategy and Effect on Efficacy
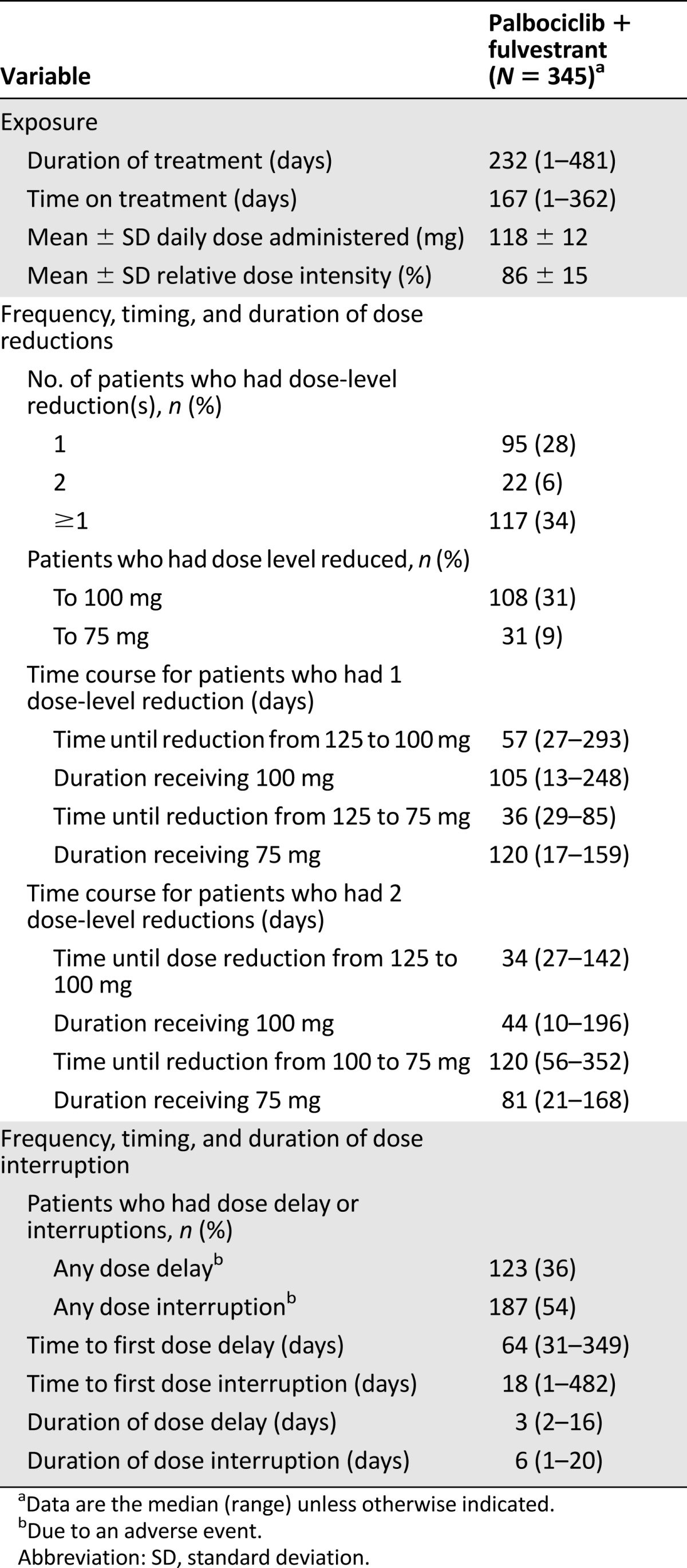
The overall mean relative dose intensity was 86% for palbociclib plus fulvestrant and 98% for placebo plus fulvestrant (supplemental online Fig. 2). For the palbociclib arm, 28% of patients had 1 dose reduction (from 125 to 100 mg or from 125 mg directly to 75 mg) and 6% of patients had 2 dose reductions (Table 3). Among patients who only had 1 dose-level reduction, the median time to the dose reduction was 57.0 days (125 to 100 mg) and 36.0 days (125 to 75 mg; 3 weeks on, 1 week off or 2 weeks on, 2 weeks off). For patients who had 2 dose reductions, the median time to the first dose reduction was 33.5 days (125 to 100 mg) and the median time to the second dose reduction was 119.5 days (100 to 75 mg). Of the 117 (34%) patients in the palbociclib arm who received at least 1 dose reduction, 108 (31%) were treated at the 100-mg dose level and 31 (9%) were treated at the 75-mg dose level. The median duration of a dose interruption or dose delay in the palbociclib arm was 6.0 or 2.5 days, respectively.
Table 3.
Exposure to palbociclib plus fulvestrant and times and durations of dose modifications—dose reductions, dose interruptions, and cycle delays
Dose modification appeared to be effective at reducing the risk for subsequent grade 3‒4 neutropenia. Among the 21 (6%) patients who had a dose reduction owing to grade 4 neutropenia in cycles 1 and 2, only 1 patient developed subsequent grade 4 neutropenia. Among the patients (215 [62%] of 345) who developed grade 3–4 neutropenia in cycles 1 through 6, 56 (26%) had grade 3 neutropenia and 2 (0.9%) had grade 4 neutropenia subsequent to cycle 6. Although the number of repeated grade 3 events was reduced by half, it remained high because there was no mandated dose reduction for repeated grade 3 neutropenia. A dose reduction due to uncomplicated grade 3 neutropenia was implemented if recovery to grade 2 neutropenia was protracted or at the discretion of the investigator if uncomplicated grade 3 neutropenia recurred in 2 consecutive cycles (Fig. 1). The KM plot estimates show that the cumulative incidence of grade 3‒4 neutropenia after receipt of palbociclib plus fulvestrant increased early, predominantly in the first month, and subsequently plateaued (Fig. 3B). For patients who had mild to moderate neutropenia (grade ≤2) within the first 4 cycles of treatment, the risk for grade 3‒4 neutropenia was not substantially increased thereafter; it marginally increased over time up to 9 to 10 months, at which time it plateaued (Fig. 3C).
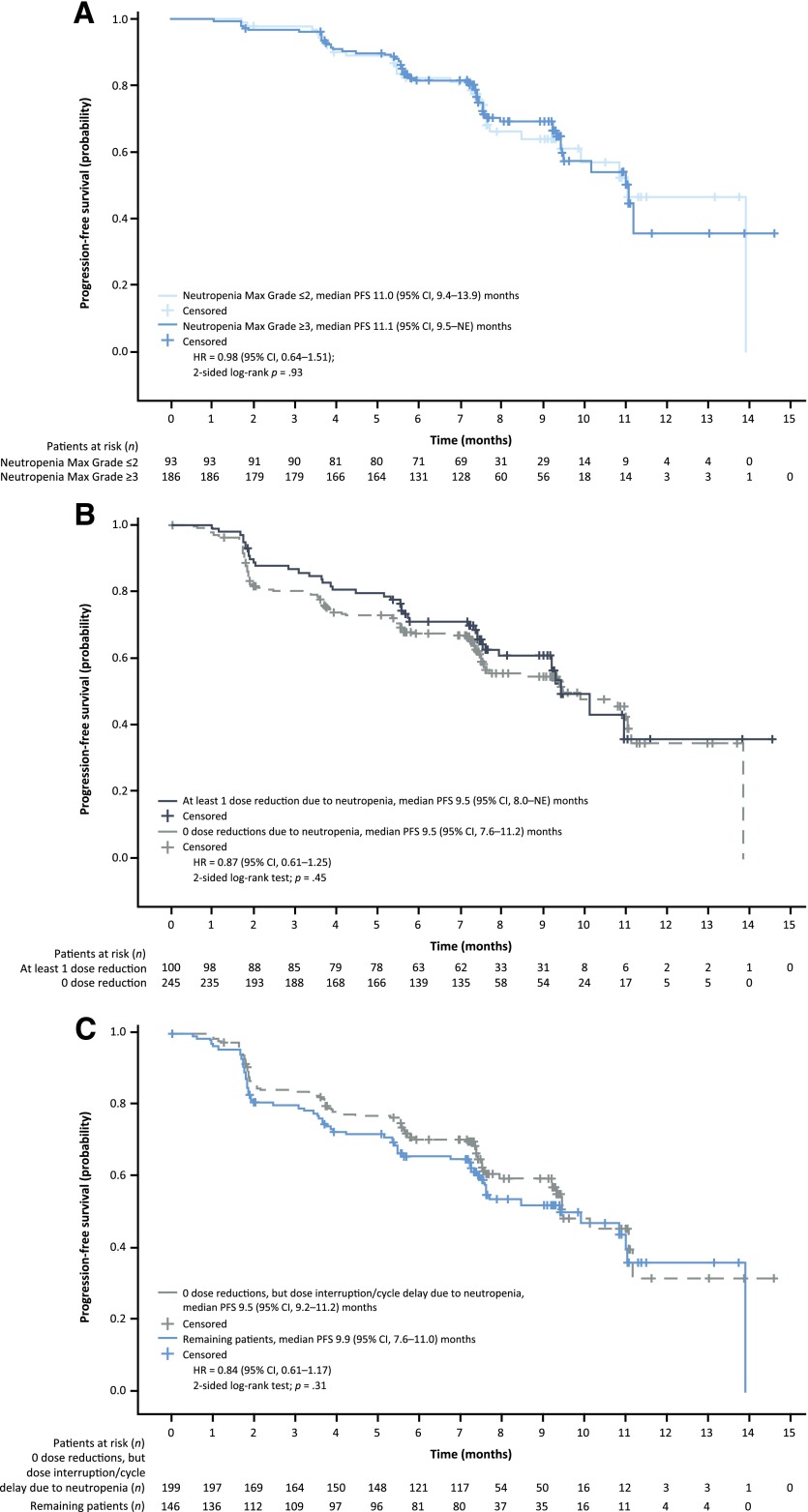
Neutropenia and dose modifications due to neutropenia did not have a detrimental effect on efficacy among patients who had been treated with palbociclib plus fulvestrant for more than 3 cycles. There was no difference in PFS among patients who had grade ≥3 neutropenia versus grade ≤2 neutropenia (median PFS of 11.1 vs 11.0 months, respectively; HR, 0.98 [95% CI, 0.64‒1.51]; 2-sided log-rank test, p = .93; Fig. 4A). PFS was not different among patients in the palbociclib arm who had at least 1 dose reduction because of neutropenia versus patients who had no dose reductions because of neutropenia (median PFS of 9.5 months each; HR, 0.87 [95% CI, 0.61–1.25]; 2-sided log-rank test, p = .45; Fig. 4B). Furthermore, PFS did not differ among palbociclib-treated patients who had at least 1 dose reduction due to any AE versus those patients who had no dose reduction (10.2 vs. 9.5 months, respectively; HR = 0.74 [95% CI, 0.52–1.05)]; 2-sided log-rank test, p = .09; supplemental online Fig. 3). Among palbociclib-treated patients who had no dose reduction but a dose interruption or cycle delay owing to neutropenia, there was no effect on PFS (median PFS of 9.5 vs. 9.9 months; HR, 0.84 [95% CI, 0.61‒1.17]; 2-sided log-rank test, p = .31; Fig. 4C).
Figure 4.
Kaplan-Meier curves of progression-free survival in patients treated with palbociclib plus fulvestrant. (A): Patients treated for more than 3 cycles who had maximum grade ≥3 (n = 186) vs. maximum grade ≤2 neutropenia (n = 93), per investigator assessment. (B): Patients who had at least 1 dose reduction because of neutropenia (n = 100) versus no dose reduction (n = 245). (C): Patients who had no dose reduction but had a dose interruption or cycle delay due to neutropenia (n = 199) versus the remaining patients (n = 146). “Neutropenia” was any event having a preferred term equal to “neutropenia” or “neutrophil decreased.”
Abbreviations: CI, confidence interval; HR, hazard ratio; max, maximum; NE, not estimable; PFS, progression-free survival.
Clinical Outcomes of Neutropenia Associated With Palbociclib Treatment
Although grade 3–4 neutropenia occurred in 221 (65%) of 340 patients in the palbociclib arm, febrile neutropenia was reported in only 3 (0.9%) patients in the palbociclib arm and 2 (0.6%) of 172 patients in the placebo arm. During study treatment, 39 (11%) patients in the palbociclib arm received G-CSF on the basis of the investigator’s judgment.
There was a higher incidence of all-grade infections in the palbociclib arm (42%) than in the placebo arm (30%); however, infections were mainly grade 1‒2 in severity (Table 1). The frequency of grade 3‒4 events was similar between treatment arms (2% and 3%, respectively). Fewer than 2% of patients in the palbociclib arm had concurrent grade 3‒4 neutropenia and grade 3‒4 infections. The multivariate analysis performed to assess the association between grade 3–4 neutropenia and infection showed that infection status was not significantly related to the presence of grade 3‒4 neutropenia (p = .17; supplemental online Table 2) when treatment arm and important baseline characteristics were simultaneously considered in the analysis.
Discussion
The results of this global, randomized, placebo-controlled, phase III study in which palbociclib was combined with fulvestrant to treat patients whose HR-positive HER2-negative breast cancer had progressed while they were receiving prior endocrine therapy confirmed the generally favorable safety profile that was also observed when palbociclib was combined with letrozole as first-line treatment for HR-positive/HER2-negative MBC in a phase II study [3]. The AE incidence was similarly low for nonhematologic events across both treatment arms, and these AEs were mostly mild to moderate in severity. Although low-grade stomatitis (aphthous-type ulcers that can bother patients) occurred more often in the palbociclib arm than the placebo arm, it can be effectively managed by using steroid dental paste. The rate of thromboembolic events in the palbociclib and placebo arms was 2% and 0%, respectively. The overall SAE incidence was also low across both treatment arms. The hematologic AEs identified in the PALOMA-3 study were considered manageable and reversible and were not commonly associated with complications. The rate of treatment discontinuation due to AEs in this study (4%) is much lower than for other treatment options in this setting (i.e., for inhibitors of the phosphatidylinositol 3-kinase /mammalian target of rapamycin pathways) that reached 19% in the Breast Cancer Trials of Oral Everolimus-2 (BOLERO-2) trial [8] and 13% in the Belle-2 study [9]). The improved efficacy, coupled with the favorable safety profile of palbociclib plus fulvestrant, was also reflected in patient-reported outcome data, which demonstrate that patients were able to maintain quality of life during treatment, whereas patients treated with placebo plus fulvestrant experienced a deterioration of their quality of life [10].
Although neutropenia was the most commonly observed grade 3–4 AE, occurring in more than 50% of patients who received palbociclib plus fulvestrant treatment, there was a low rate of associated febrile neutropenia (0.9%) that was not markedly different from that observed in the PALOMA-1 study [3]. This observation confirms prior reports showing that the consequences of myelosuppression experienced during palbociclib treatment are different from those associated with chemotherapy-induced myeloablation [11], which is characterized by a more acute onset of neutropenia [12] and a prolonged suppression of all cell lines. Chemotherapy-induced neutropenia is a well-known cause of complications, such as febrile neutropenia and infections, which may be life-threatening [13, 14]. Febrile neutropenia is not commonly associated with palbociclib, likely owing to the shorter duration and lesser severity of neutropenia compared with chemotherapy [3].This comprehensive safety analysis provides additional evidence that the clinical time course, severity, and pattern of neutropenia are specific to the mechanism of action of palbociclib and do not appear to pose a significant safety risk for patients. Most important, the neutropenia associated with palbociclib treatment was effectively managed within days by dose delay, interruption, or reduction and without routine use of G-CSF, suggesting that mature white blood cells are present in the bone marrow and can rapidly demarginate when drug levels fall.
The clinical patterns of hematologic toxicities associated with palbociclib treatment are the mechanistic result of selective inhibition of CDK 4/6, which has been described in numerous other detailed reports of in vitro and preclinical in vivo experiments [11, 15–17]. This mechanism of action is characterized by a blockage of the G1/S cell cycle transition to induce G1 arrest. Recent published data showed that palbociclib-induced bone marrow suppression follows cell cycle arrest in hematopoietic precursor cells, resulting in quiescence without apoptosis [11, 15, 16], whereas treatment with chemotherapy caused DNA damage and apoptotic cell death in human bone marrow mononuclear cells at clinically relevant concentrations [11, 15, 16]. Moreover, the short-term production of well-differentiated or mature peripheral blood effector cells (i.e., mature neutrophils) was relatively resistant to CDK 4/6 inhibition. Myelosuppressive effects of CDK 4/6 inhibition were rapidly reversible in vivo. In contrast, the destruction of progenitor cells with subsequent development of neutropenia [18] is a major dose-limiting toxicity of chemotherapy [19]. Not only can it delay neutrophil recovery for several weeks, but it often leads to suboptimal treatment because of reduced dose intensity [14].
Preclinical data show that the cell cycle arrest caused by palbociclib plus fulvestrant is transient and reversible in hematopoietic cells [11]. However, cell cycle arrest minimally recovers in the presence of fulvestrant alone during a palbociclib-free phase in breast cancer cells (MCF-7) [11]. The mechanistic difference between the effect of palbociclib on bone marrow versus MCF-7 breast cancer cells supports the current clinical dosing schedule and dose modification strategy by introducing short palbociclib-free periods to enable neutrophil recovery without affecting efficacy. This could also partially explain our finding that PFS was not affected by dose modification (i.e., a dose reduction vs. no dose reduction, or dose interruption vs. dosing without a prolonged break) among patients who experienced grade ≥3 neutropenia compared with those who experienced grade ≤2 neutropenia. Conversely, prospective randomized studies are underway to explore whether an alternative dosing schedule (i.e., shorter palbociclib-free periods or daily dosing at a low dose) may further improve PFS and the tolerability profile of palbociclib [20].
The data presented in this manuscript have important implications for clinical practice. There was no decrease in efficacy despite dose reductions or dose interruptions, suggesting that patients maintained drug levels that were therapeutic during the study. Although many patients can be more susceptible to the myelosuppressive effects of palbociclib, our data suggest that the susceptibility to develop higher-grade neutropenia during treatment can generally be recognized within the first several months of treatment and appropriately tailored dose modifications can be implemented to reduce the likelihood of recurrent episodes of severe neutropenia and/or febrile neutropenia. Therefore, it is important to closely monitor ANC early during treatment with palbociclib so that dose adjustments can be made promptly in patients experiencing grade 4 or prolonged and recurrent grade 3 neutropenia. This is particularly important for patients of Asian ethnicity and patients with a low baseline ANC.
The study showed that the incidence of all-grade infections was greater in the palbociclib arm than the placebo arm. Although the rate of grade ≥3 infections was similar between the palbociclib and placebo arms (2.0% and 2.9%, respectively), it is advisable to alert patients of the potential increased risk for infection.
As with any targeted therapy, adherence to the recommended dose is critical to ensure treatment efficacy as well as safety in individual patients. Patients should be made aware of the palbociclib schedule (3 weeks on/1 week off). With close monitoring of the complete blood count, particularly early on during treatment, dosing can be optimized and ongoing treatment can be administered while minimizing the risk for clinically significant AEs.
Conclusion
Palbociclib has now been shown to be clinically effective in combination with two different endocrine agents in previously untreated and treated patients with HR-positive/HER2-negative MBC. A thorough understanding of the safety profile of this agent is a high-priority consideration as clinicians incorporate this class of drug into their routine clinical practice. Treatment with palbociclib in combination with fulvestrant in the PALOMA-3 study was generally safe and well-tolerated, with neutropenia representing the most common AE associated with its use. Clinical trial experience to date indicates a consistent and therefore predictable safety profile for palbociclib, with neutropenia being predominantly uncomplicated, and with a low associated risk for febrile neutropenia. Palbociclib-induced neutropenia is reversible and can be readily managed by dose delay, dose interruption, or dose modification without affecting efficacy. The data presented here may also be informative for guiding ongoing trials, including those conducted in adjuvant and neoadjuvant treatment settings.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This study was sponsored by Pfizer Inc. Fulvestrant was provided by AstraZeneca. We thank the patients who participated in the PALOMA-3 study, and the investigators, study nurses, and site staff for their support of the trial. We thank Maria Koehler for working on the PALOMA-3 study design and providing guidance on the analysis plan, Kathy Puyana Theall and Carla Giorgetti for their involvement in the conduct of the study, and Xin Huang for the initial concept and design of the statistical analysis used to evaluate the relationship between neutropenia and efficacy. Editorial support was provided by Susan Reinwald, of Complete Healthcare Communications, LLC, and was funded by Pfizer Inc.
Author Contributions
Conception/Design: Sunil Verma, Cynthia Huang Bartlett, Patrick Schnell, Angela M. DeMichele, Sherene Loi, Jungsil Ro, Hiroji Iwata, Nadia Harbeck, Massimo Cristofanilli, Ke Zhang, Alexandra Thiele, Nicholas C. Turner, Hope S. Rugo
Provision of study material or patients: Sunil Verma, Cynthia Huang Bartlett, Angela M. DeMichele, Sherene Loi, Jungsil Ro, Marco Colleoni, Hiroji Iwata, Nadia Harbeck, Massimo Cristofanilli, Alexandra Thiele, Nicholas C. Turner, Hope S. Rugo
Collection and/or assembly of data: Sunil Verma, Cynthia Huang Bartlett, Patrick Schnell, Angela M. DeMichele, Sherene Loi, Jungsil Ro, Hiroji Iwata, Nadia Harbeck, Massimo Cristofanilli, Ke Zhang, Nicholas C. Turner
Data analysis and interpretation: Sunil Verma, Cynthia Huang Bartlett, Patrick Schnell, Angela M. DeMichele, Sherene Loi, Jungsil Ro, Marco Colleoni, Hiroji Iwata, Nadia Harbeck, Massimo Cristofanilli, Ke Zhang, Nicholas C. Turner, Hope S. Rugo
Manuscript writing: Sunil Verma, Cynthia Huang Bartlett, Patrick Schnell, Angela M. DeMichele, Sherene Loi, Jungsil Ro, Hiroji Iwata, Nadia Harbeck, Massimo Cristofanilli, Ke Zhang, Alexandra Thiele, Hope S. Rugo
Final approval of manuscript: Sunil Verma, Cynthia Huang Bartlett, Patrick Schnell, Angela M. DeMichele, Sherene Loi, Jungsil Ro, Marco Colleoni, Hiroji Iwata, Nadia Harbeck, Massimo Cristofanilli, Ke Zhang, Alexandra Thiele, Nicholas C. Turner, Hope S. Rugo
Disclosures
Cynthia Huang Bartlett: Pfizer (E,OI); Angela M. DeMichele: Pfizer, Novartis (C/A), Pfizer, Novartis, Johnson & Johnson, Calithera, Incyte, Genentech (RF); Hiroji Iwata: Daiichi-Sankyo, Chugai (C), Chugai, Eisai, AstraZeneca (H), GlaxoSmithKline, Daiichi-Sankyo, Chugai, Novartis, Pfizer, AstraZeneca, Eli Lilly, Merck Sharp & Dohme (R). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2) Ann Oncol. 2014;25:1871–1888. doi: 10.1093/annonc/mdu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayes EL, Lewis-Wambi JS. Mechanisms of endocrine resistance in breast cancer: An overview of the proposed roles of noncoding RNA. Breast Cancer Res. 2015;17:40. doi: 10.1186/s13058-015-0542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 4.Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 5.Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 6.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. 2009. Available at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed June 14, 2016.
- 8.Beaver JA, Park BH. The BOLERO-2 trial: The addition of everolimus to exemestane in the treatment of postmenopausal hormone receptor-positive advanced breast cancer. Future Oncol. 2012;8:651–657. doi: 10.2217/fon.12.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga J, Im SA, Iwata H et al. PIK3CA status in circulating tumor DNA (ctDNA) predicts efficacy of buparlisib (BUP) plus fulvestrant (FULV) in postmenopausal women with endocrine-resistant HR+/HER2– advanced breast cancer (BC): First results from the randomized, phase III BELLE-2 trial. Presented at: San Antonio Breast Cancer Symposium; December 8–12, 2015; San Antonio, TX. [Google Scholar]
- 10.Harbeck N, Cristofanilli M, Ro J, et al. Double-blind phase 3 trial of fulvestrant with placebo or palbociclib in pre- and post-menopausal women with hormone receptor-positive (HR+ve), HER2-negative (HER2−ve) metastatic breast cancer (MBC) that progressed on prior endocrine therapy (PALOMA 3): Detailed analysis of patient-reported outcomes. Eur J Cancer. 2015;51:S270–S271. [Google Scholar]
- 11.Hu W, Sung T, Jessen B, et al. Mechanistic investigation of bone marrow suppression associated with palbociclib and its differentiation from cytotoxic chemotherapies. Clin Cancer Res. 2016;22:2000–2008. doi: 10.1158/1078-0432.CCR-15-1421. [DOI] [PubMed] [Google Scholar]
- 12.Tofts PS, Chevassut T, Cutajar M, et al. Doubts concerning the recently reported human neutrophil lifespan of 5.4 days. Blood. 2011;117:6050–6052; author reply 6053–6054. doi: 10.1182/blood-2010-10-310532. [DOI] [PubMed] [Google Scholar]
- 13.Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: Risks, consequences, and new directions for its management. Cancer. 2004;100:228–237. doi: 10.1002/cncr.11882. [DOI] [PubMed] [Google Scholar]
- 14.Lyman GH. Chemotherapy dose intensity and quality cancer care. Oncology (Williston Park) 2006;20(suppl 9):16–25. [PubMed] [Google Scholar]
- 15.Roberts PJ, Bisi JE, Strum JC, et al. Multiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer therapy. J Natl Cancer Inst. 2012;104:476–487. doi: 10.1093/jnci/djs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson SM, Torrice CD, Bell JF, et al. Mitigation of hematologic radiation toxicity in mice through pharmacological quiescence induced by CDK4/6 inhibition. J Clin Invest. 2010;120:2528–2536. doi: 10.1172/JCI41402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhatt V, Saleem A. Review: Drug-induced neutropenia--pathophysiology, clinical features, and management. Ann Clin Lab Sci. 2004;34:131–137. [PubMed] [Google Scholar]
- 19.Lalami Y, Paesmans M, Muanza F, et al. Can we predict the duration of chemotherapy-induced neutropenia in febrile neutropenic patients, focusing on regimen-specific risk factors? A retrospective analysis. Ann Oncol. 2006;17:507–514. doi: 10.1093/annonc/mdj092. [DOI] [PubMed] [Google Scholar]
- 20.ClinicalTrials.gov. Study comparing two different schedules of palbociclib plus second line endocrine therapy in women with estrogen receptor positive, HER2 negative advanced/metastatic breast cancer. 2016. Available at https://clinicaltrials.gov/ct2/show/NCT02630693. Accessed April 25, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.