Abstract
When we respond to a stimulus, our decisions are based not only on external stimuli but also on our ongoing performance. If the response deviates from our goals, monitoring and decision-making brain areas interact so that future behavior may change. By taking advantage of natural variation in error salience, as measured by the reaction time taken to correct an error (RTEC), here we argue that an evidence accumulation framework provides a potential underlying mechanism for this variable process of error identification and correction, as evidenced by covariation of frontal monitoring and parietal decision-making processes. We study two early EEG signals linked to monitoring within medial prefrontal cortex – the error-related negativity (ERN) and fronto-central theta activity – and a third EEG signal, the error positivity (Pe), that is thought to share the same parietal substrates as a signal (the P3b) proposed to reflect evidence accumulation. As predicted, our data show that on slow RTEC trials, frontal monitoring resources are less strongly employed, and the latency of the Pe is longer. Critically, the speed of the RTEC also covaries with the magnitude of subsequent neural (inter-trial alpha power) and behavioral (post-error slowing) adjustments following the correction. These results are synthesized to describe a timing diagram for adaptive decision-making after errors, and support a potential evidence accumulation mechanism in which error signaling is followed by rapid behavioral adjustments.
Keywords: error-related negativity, Pe, evidence accumulation, inter-trial alpha power, post-error slowing
Introduction
Activity in parietal and frontal brain regions is critical for adaptive decision-making. Studies in non-human primates suggest a mechanism for decision-making in which information accumulates in parietal (and other) areas until neural activity reaches the threshold level required to make a behavioral response (Mazurek et al. 2003; Gold and Shadlen 2007). Importantly, frontal areas including the medial prefrontal cortex (mPFC) play an important role in this process, monitoring decisions and signaling the need for behavioral adjustments such as the allocation of greater attentional resources (Ito et al. 2003; see Ridderinkhof et al. 2004 and Heekeren et al. 2008 for a review).
Recently the idea has arisen that an evidence accumulation framework may also explain error processing (Murphy et al. 2012; Steinhauser and Yeung 2010; Ullsperger et al. 2010; Wessel et al. 2011; Steinhauser and Yeung 2012). By analogy to the case in which sensory evidence is evaluated, evidence for an erroneous response integrates until a threshold is reached, at which point behavioral corrections can be undertaken. Notably, studies of evidence accumulation in other contexts, such as perceptual decision making, typically take advantage of parametric variation in stimulus salience – e.g. the motion coherence of a moving dot stimulus (Gold & Shadlen, 2007) – to manipulate the amount of sensory evidence. However, the salience of an error cannot be modulated as directly as stimulus salience in perceptual decision making studies. To account for this issue, here we take advantage of natural variation in the reaction time taken to correct the error (RTEC) by dividing instances of error correction into short and long RTEC trials. We hypothesize that the salience of an error correlates inversely with RTEC, an intuition that recent data suggest can be useful: the Pe peak latency, for example, is correlated with the latency at which error awareness is indicated (Murphy et al. 2012).
With this approach, implications of the evidence accumulation hypothesis can also be addressed for other processes necessary for behavioral corrections after errors, including predictions that vary in their degree of certainty. On the one hand, more rapid accumulation of evidence for an error and faster corrections should likely be preceded by stronger correlates of error commission, as indexed by greater response conflict at the time of the initial response (Rodriguez-Fornells et al. 2002). On the other hand, the effect of the speed of evidence accumulation on subsequent processes is potentially unclear. Under one scenario, more rapid accumulation, indicating greater evidence for an error, might be associated with more demand on the cognitive processes responsible for deciding whether to initiate post-error behavioral adjustments. However, the opposite might also be true: because it indicates more confidence about the existence of an error in the context of a time-limited decision (Kiani et al, 2014), more rapid accumulation might place less demand on the decision to initiate such compensations. Specifically, when greater certainty exists that an error was committed (or that the response was correct, for that matter), the decision about whether to engage post-error behavioral adjustments would be easier, compared to the intermediate case in which lesser, more uncertain evidence for an error accumulates more slowly to threshold. Finally, the rapidity of evidence accumulation might have no effect: if such processes are not triggered until evidence for an error reaches a threshold, identical thresholds should trigger the same behavioral adjustments, irrespective of speed.
Of course, a great deal of work has previously investigated the neural correlates of error processing, including electroencephalography (EEG) studies that have identified candidate electrophysiological correlates for many of these processes. When an error occurs, monitoring signals originating in the mPFC are represented in initial signals (both ERN and theta power; e.g. Endrass et al. 2007, 2012; Luu et al. 2004) that may act as one of the sources of information indicating a potential error (Ullsperger et al. 2010). A later EEG signal, the error positivity (Pe), is evoked by errors in comparison with correct responses in parietal areas (Falkenstein et al. 2000; Shalgi et al. 2009; Navarro-Cebrian et al. 2013). This Pe signal has been suggested to share the same neural substrates as the P3b (Ridderinkhof et al. 2009; Ullsperger et al. 2010). Since the P3b has recently been shown to reflect evidence accumulation in decision-making (O’Connell et al. 2012; Kelly et al. 2013), the Pe may also represent the accumulation of evidence for error occurrence (Steinhauser and Yeung 2010, 2012). Finally, cognitive (i.e. inter-trial alpha power) and behavioral (post-error slowing) adjustments are commonly observed after errors. In particular, inter-trial alpha suppression after errors in comparison with correct responses has been considered a measure of post-error modulations in cortical arousal (Carp and Compton 2009; Compton et al. 2011; Navarro-Cebrian et al. 2013); and post-error slowing (Rabbitt 1966), defined as a longer RT in the trial following an error (RTn+1), is often used to measure behavioral adjustments after errors.
Thus, in the current study we take advantage of natural variation in the RTEC as a proxy for parametric variation in the salience of an error, permitting us to evaluate whether the evidence accumulation framework might account for various aspects of error correction. Specifically, as linked by RTEC values, we analyze whether early error-monitoring mechanisms are associated with subsequent variation of Pe latency, and whether later post-error adjustments covary with error-monitoring and evidence accumulation processes. We predicted the following: (1) faster (slower) RTEC will be associated with an increased (decreased) amplitude of early ERN monitoring processes; (1) faster (slower) RTEC will be associated with shorter (longer) Pe latencies; and (3) faster (slower) RTEC will be associated with both increased (reduced) inter-trial alpha power, and reduced (increased) behavioral slowing on the next trial. In testing these predictions via the RTEC, we seek to determine whether previous error-related findings within the literature can be linked to an accumulator model of error correction.
Methods
Participants
Fifteen UC Berkeley students participated in the experiment in exchange for monetary compensation. One participant was excluded for failing to follow task instructions. Two more subjects were rejected from the EEG analysis due to excessive motion and muscle artifacts. Twelve students were included in the final analysis (nine female) ages 18–25 (mean 20.3 ± 2.0). All subjects gave written informed consent in accordance with the Committee for the Protection of Human Subjects at the University of California, San Francisco and University of California, Berkeley. Subjects were right-handed and had normal or corrected-to-normal vision.
Procedure
A total of 100 pictures of male and female faces unknown to our subjects were used in the experiments (Navarro-Cebrian et al. 2013). They were converted to black and white and resized to 113 × 151 pixels using Adobe Photoshop CS4 (Adobe Systems, San Jose, CA). Targets subtended 2.55 degrees of visual angle. Pictures were obtained from one of the authors (ANC) and from the Face Database of the Max-Planck Institute for Biological Cybernetics in Tübingen, Germany (http://faces.kyb.tuebingen.mpg.de; Troje and Bulthoff 1996). The task was presented using Presentation software (Neurobehavioral Systems, Inc.).
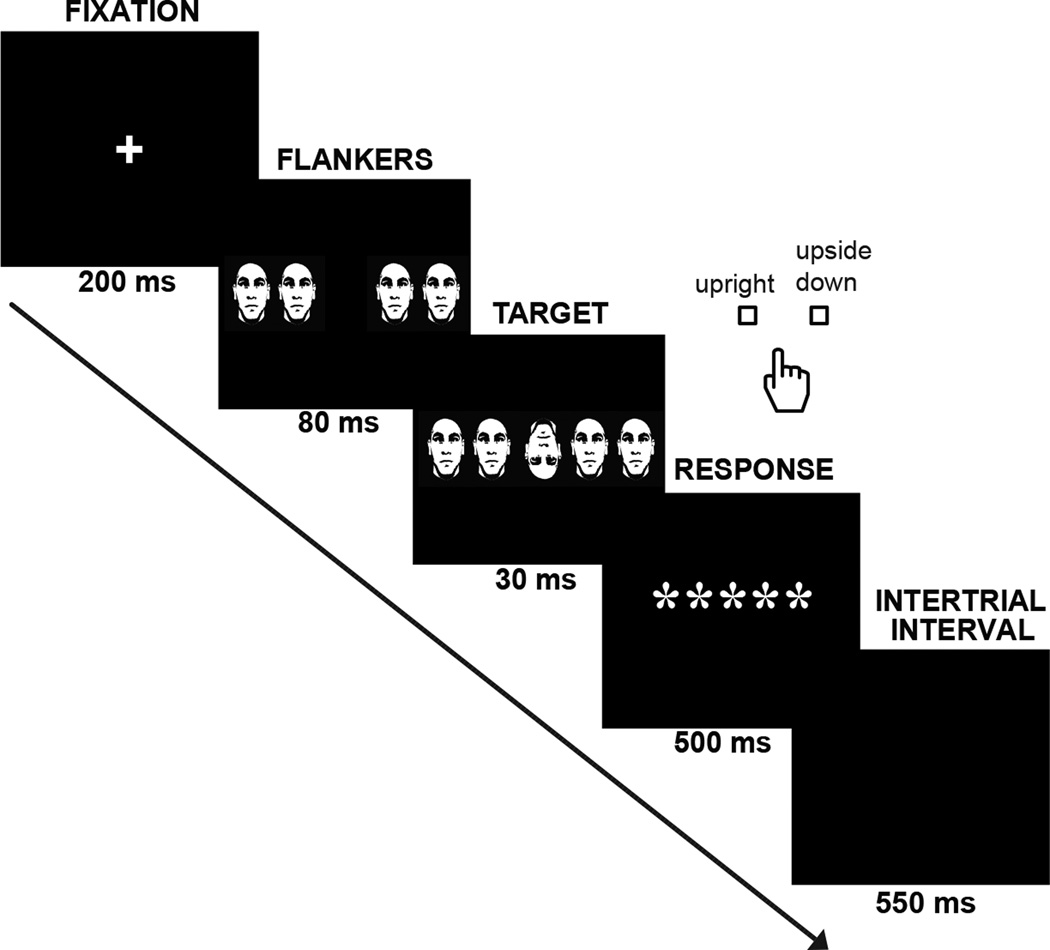
Participants were presented with a previously validated version of the Flanker task (Navarro-Cebrian et al. 2013) designed to produce intermittent errors (Figure 1). All trials contained five high contrast (black and white) human faces, and participants were asked to respond via button press with their right hand (index and middle fingers) whether the target (middle) face was upright or upside down. The trial started with a fixation cross for 200 ms. After the cross disappeared, four flanker faces were shown alone for 80 ms and then together with the target (middle face) for an additional 30 ms. In order to make the task more difficult, the flanker faces could be congruent or incongruent with the target regarding the orientation of the face. After the flanker and target faces disappeared, a group of 5 asterisks was presented in the middle of the screen for 500 ms to indicate when to respond. Importantly, subjects were required to correct themselves as quickly as possible whenever they realized they had made an error by pressing the correct button (giving rise to a second RT denoted as the reaction time taken to correct the error, or RTEC; see below). An additional inter-trial interval of 550 ms preceded the next trial.
Fig. 1. Paradigm.
After a fixation cross, flanker faces (congruent or incongruent with the target) were shown alone for 80 ms and then together with the target for 30 ms more. Participants were instructed to indicate with a button press whether the target was upright or upside down. They were asked to correct themselves as fast as possible if an error was made. An inter-trial interval of 550 ms was presented before the next trial started.
Behavioral analysis
Only those trials in which subjects made an error and corrected themselves were used for analysis. Trials were sorted by the reaction time for the second motor response in each error trial – i.e. the RTEC, as determined by the latency of the correction minus the latency of the initial (erroneous) response. We created 2 groups of trials for which the RTEC values were well-differentiated. Based on the RTEC distribution for each subject, in one group trials with a RTEC equal to or shorter than the 25th percentile within subjects were included; in the other group, trials with an RTEC equal to or greater than the 75th percentile within subjects were included. These quartiles were chosen for analysis in order to maximize potential differences in error salience while retaining an adequate number of trials for comparisons. Additionally, we evaluated possible differences in reaction times in the trials following errors (post-error trials; RTn+1), in order to study the relationship to post-error slowing. We predicted that the uncertainty generated in some trials by reduced evidence for the error should lead to slower reaction times in the trials after the errors (i.e. greater post-error slowing).
EEG procedure
As noted above, two subjects were rejected from the EEG analysis due to excessive motion and muscle artifacts. EEG data were collected from 64 channels at a sampling rate of 512 Hz (Biosemi; http://www.biosemi.com). The Biosemi ActiveTwo system allows low-noise recordings free of interference consisting of a feedback loop between an electrode (CMS) and a passive electrode (DRL- see www.biosemi.com/faq/cms&drl.htm for more details). Vertical and horizontal eye movements were recorded using four electrooculography electrodes placed at the outer canthi of the right and left eyes, and above and below the right eye, aligned with the pupil. EEG data analysis was performed using MATLAB scripts based on functions from the EEGLAB toolbox (Delorme and Makeig 2004). The data were re-referenced offline to an average reference and filtered to remove frequencies > 50 Hz and < 0.3 Hz. Portions of continuous data with excessive artifacts were rejected after visual inspection. In addition, independent components were estimated for individual subjects using the filtered but unsegmented EEG data to identify and remove eye movement and muscular artifacts. ICA decomposition relied on the independent component analysis functions of EEGLAB (“runica” algorithm). 64 ICA components were identified for each subject and IC scalp topographies, time courses, and spectral characteristics were inspected visually to identify and reject components related to blinks and eye-movements (Jung et al., 1998).
Event-related potential analysis
Data were segmented and time locked to the onset of the first motor response for analysis of the ERN and the Pe components. Epochs were created starting 500 ms before the onset of the error and finishing 1000 ms after it. A pre-stimulus time window from −500 ms to the onset of the initial stimulus was used as a baseline. First, we calculated the amplitude of the error-related negativity (ERN), an ERP originating in the mPFC and associated with conflict monitoring and uncertainty (Van Veen and Carter 2002; Navarro-Cebrian et al. 2013). For the ERN component, three frontocentral electrode sites were selected for analysis (Fz, FCz, and Cz). As in our previous research (Navarro-Cebrian et al. 2013), the average minimum amplitude between −20 and 50 ms around the onset of the response was calculated to measure the activity of the ERN component. Next, we analyzed the error-related positivity (Pe) to examine whether the reaction time for the error correction (RTEC) varied with the Pe latency. The Pe latency was defined as the time to the most positive point in the epoch for the average of three centro-parietal electrodes (CP1, CPz and CP2). Statistical analyses were performed in SPSS (Statistical Package for Social Sciences 15.0 for Windows). Comparisons were made using the Fisher least significant difference test (LSD) and T tests.
Time-frequency analysis
Time-frequency calculations were computed using MATLAB scripts based on functions from the EEGLAB toolbox. Specifically, we used the “newtimef” function available for EEGLAB to calculate the event-related changes in spectral power relative to baseline (event-related spectral perturbation; ERSP). The use of the Morlet wavelet decomposition in this function allows power changes to be observed in both the time and frequency domains, in contrast with fast Fourier transform methods that only have resolution in the frequency domain. Wavelet cycles began at 3 and increased with frequency reaching half the number of cycles in the equivalent fast Fourier transform window at its highest frequency. Data were filtered to remove frequencies > 50 Hz and < 0.3 Hz. We were interested in the power of the theta band as a measure of error monitoring (Luu et al. 2004; Debener et al. 2005; Trujillo and Allen 2007; Navarro-Cebrian et al. 2013). To study frontal theta band power, epochs were created starting 2000 ms before the onset of the error and finishing 2000 ms after it. Values from 1000 to 500 ms before the error were used as a baseline to avoid border and edges artifacts. As for the ERPs, RTEC duration was used to evaluate other segments of the trial by separating epochs into two conditions (fast and slow RTEC). Frequencies of 4–7 Hz were used to study the theta band in the FCz channel, as the electrode site FCz is in the most representative location for post-error monitoring activity and has been used in previous research (Cavanagh et al. 2009; Navarro-Cebrian et al. 2013) to measure post-error theta power. Time series were plotted and differences in latency and amplitude of fronto-central theta activity were calculated for the fast and slow RTEC conditions by analyzing the maximum amplitude and the latency at which this maximum occurred from −500 to 500 ms around the error.
In addition to the fronto-central theta power analysis, we calculated whether fronto-central theta phase values were consistent over trials (inter-trial coherence, or ITC) in the FCz channel. ITC was calculated with the “std_itc” function of eeglab. Phase coherence values range from 0 to 1, ‘0’ indicating random phases and ‘1’ indicating perfect phase locking to the response across trials. We compared the ITC values for fast and slow RTEC in the same time window as the ERN and for the theta band (4 to 7 Hz, as above). Since the ERN has been suggested to emerge from the theta activity phase locked to the response (Luu et al. 2004), we predicted that the ITC results would agree with those obtained for the ERN. A greater ITC value at the time of the response would demonstrate more synchronization between activity within the medial prefrontal cortex and the manual response (error).
Lastly, in addition to post-error behavioral changes (post-error slowing), we studied post-error neural adjustments by analyzing the inter-trial interval for differences in alpha band power. Here we evaluate whether alpha power varies with the degree of error salience. Epochs were created starting 2000 ms before stimulus onset and finishing 2000 ms afterward. Values from 1000 to 500 ms before the onset of the stimulus were used as a baseline. The time window from 300 to 500 ms within the inter-trial interval was used for analysis during which decisions and manual responses (corrections) no longer took place. Specifically, although participants could take longer than the 500 ms correction time window to correct themselves, no error correction was detected within the ITI after an additional 300 ms on any trial. Based on previous research into alpha suppression (Carp and Compton 2009), frequencies of 10–14 Hz were included in the analysis and one posterior midline channel location (Pz) was chosen as a representative electrode (Carp and Compton 2009; Navarro-Cebrian et al. 2013). As for the other ERP and time-frequency analyses, two groups were created and included in the analysis based upon the reaction time to the error (fast and slow RTEC).
Results
Behavioral results
Subjects completed an average of 895 trials (SD = 5.45, range = 882–900). They made an average of 148 errors (16.5% of the total number of trials, SD = 40.46, range = 91–211). The average reaction time for error correction (RTEC) was 212 ms (SD = 45, range across subjects = 154–284). In keeping with previous literature, these error correction reaction times were quite rapid, suggesting that the process for movement correction begins very early in the trial (Higgins and Angel 1970; Schmidt and Gordon 1977; Fiehler et al. 2004; Christensen et al. 2008). In contrast, the average RT for the initial erroneous response in the error trials (i.e. the instigating error, or RTE) was 349 ms (SD = 41, range = 266–420), and the average RT for correct trials was 417 ms (SD = 34, range = 358–478). Consistent with other work (Gehring et al. 1993; Ratcliff and Rouder 1998; Pailing et al. 2002; Navarro-Cebrian et al. 2013), the RTE was significantly shorter than the RT for correct responses (t(11) = 7.58, p < 0.0001).
Based on the RTEC, trials were then sorted to create 2 groups: one with trials in which RTEC was equal to or less than the 25th percentile (within subjects), and one with trials in which the RTEC was equal to or greater than the 75th percentile. The average RTEC for the group of trials with the faster error corrections was 126 ms (SD = 43; range across subjects= 64–183 ms) and for the group of trials with the slower error corrections was 326 ms (SD = 69; range across subjects = 243–467 ms). The corresponding RTE for the group of trials with the faster error corrections was 370 ms (SD = 35; range = 326–447 ms) and for the group of trials with the slower error corrections was 322 ms (SD = 54; range = 183–397 ms), both of which remained faster than for correct responses (t(11) = 5.45; p < 0.001 and t(11) = 7.07; p < 0.0001, respectively) and differed significantly from each other (t(11) = 4.1; p = 0.002).
One theory suggests that a single evidence accumulation process begins at stimulus onset (Maylor and Rabbitt 1989; Rabbitt 2002), and that the error correction response reflects not a separate error-related process, but rather the correct outcome of evidence accumulation related to the initial stimulus. Under such a theory, calculating the RTEC from the time of stimulus onset (RTEC-SO), rather than from the time of the initial erroneous response, might equalize average RT values for the fast and slow RTEC trials. In contrast, if the RTEC represents the outcome of a separate (or interacting) error-related process, fast and slow RTEC trials, when locked to the stimulus, should continue to give rise to different RTEC-SO values. To test this idea, we calculated this new stimulus-locked RTEC-SO value relative to the onset of the stimulus for the original fast and slow RTEC groups, rather than relative to the onset of the error. The RTEC-SO remained significantly slower for the slow RTEC condition in comparison with the fast RTEC condition (fast RTEC-SO = 496 ms; slow RTEC-SO = 647ms; t(11) = 10.18 ; p < 0.0001).
Additionally, the combination of congruent and incongruent trials in the current experiment, which was motivated by the need to collect as many trials as possible in each condition, raises the possibility that fast (slow) RTEC trials might consist of a disproportionate number of congruent (incongruent) trials. To rule out this concern, we analyzed the number of each type of trial for each condition. No significant differences were found between the two conditions in the number of congruent (t(11) = 1.39; p = 0.19) and incongruent (t(11) = 0.29; p = 0.78) trials.
ERP results
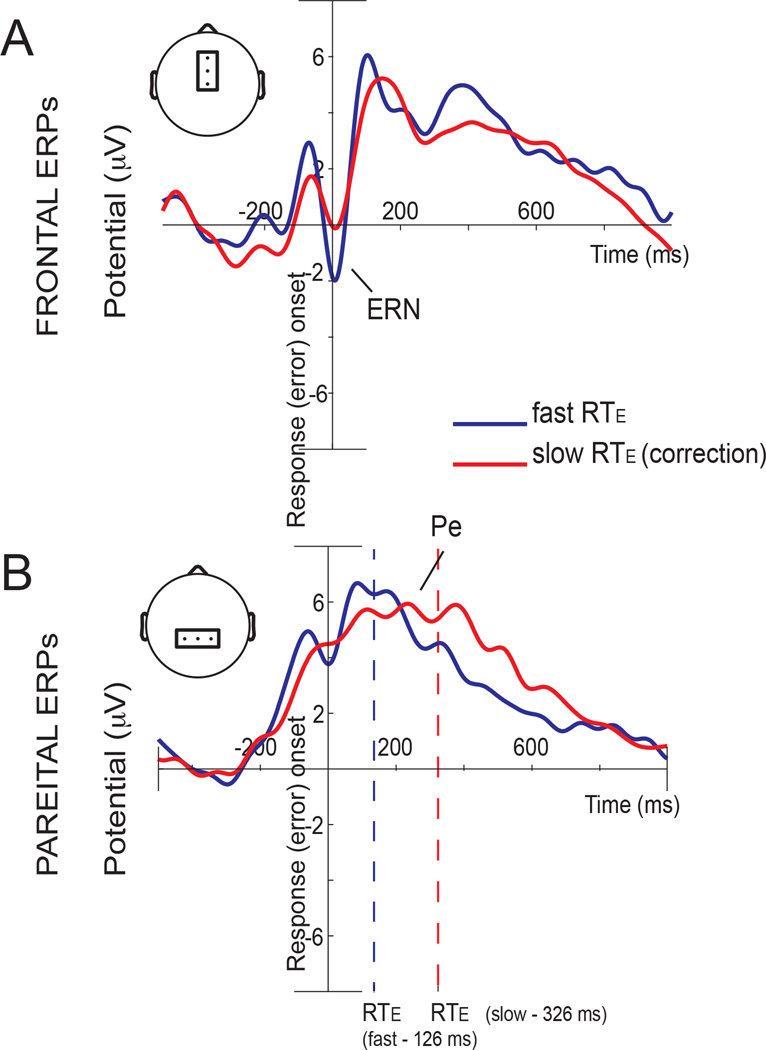
To study whether the ERN or Pe activities were related to the RTEC, we compared the fast RTEC and slow RTEC conditions for the frontal and parietal components of interest (ERN and Pe) evoked by errors – specifically, the amplitude of the ERN and the latency of the Pe. We found significant differences between RTEC conditions (Figure 2): pairwise comparisons (Fisher, LSD) showed that the fast and slow RTEC conditions were significantly different at the time of the ERN (p = 0.027) and the Pe (p = 0.048) signals. This finding was manifest as enhanced ERN activity and shorter Pe latency for the fast RTEC condition.
Fig 2. ERP responses.
The response-locked average in frontal electrodes (Fz, FCz, Cz) is shown in the upper figure and response-locked average in parietal electrodes (CP1, CPz, CP2) is shown in the lower figure. Fast RTEC (error correction) is shown in blue and slow RTEC is shown in red. Panels show a greater ERN amplitude (Figure 2A) and a shorter Pe latency (Figure 2B) for the fast RTEC condition.
Time-frequency results
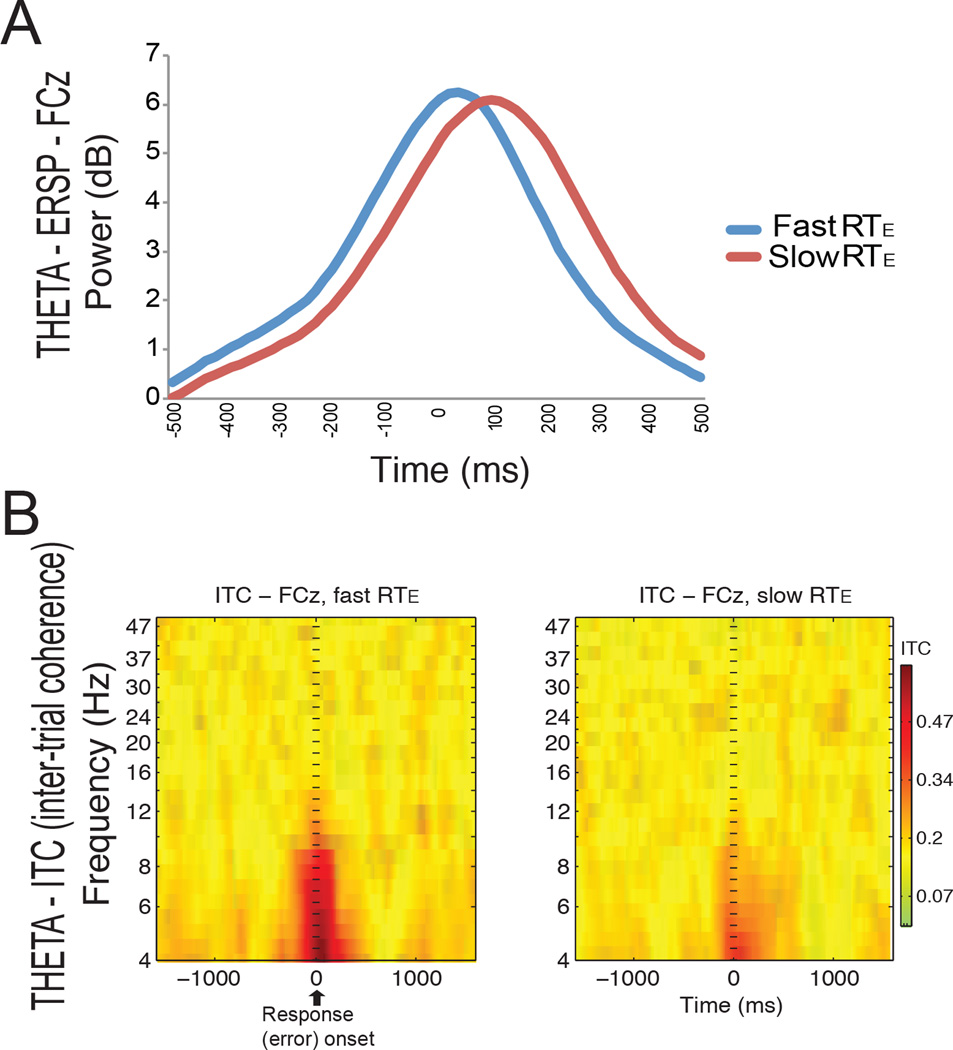
We then analyzed changes in theta power after errors. Statistical analyses demonstrated significant differences in the latency (t(11) = 4.16; p = 0.002), but not in the amplitude (t(11) = 0.61; p = 0.56 (ns)) of the theta power in FCz at the time of the error. Figure 3a shows an earlier peak of the theta power for the fast RTEC (20 ms after the error) compared to the slow RTEC (100 ms after the error). We also found that compared to the slow RTEC condition, the fast RTEC condition showed increased inter-trial coherence (ITC) for theta activity locked to the response across trials (t(11) = 9.93; p = 0.002; Fig 3b).
Fig 3. Event-related changes in theta power.
A. Event-related changes in theta power in the FCz channel. A decreased latency of the theta amplitude for the fast RTEC condition (fast error correction, in blue) can be seen in comparison with the slow RTEC condition (red). B. A greater inter-trial phase coherence (ITC) can be seen in the theta band for the fast RTEC condition (left) compared to the slow RTEC condition (right) at the time of error onset.
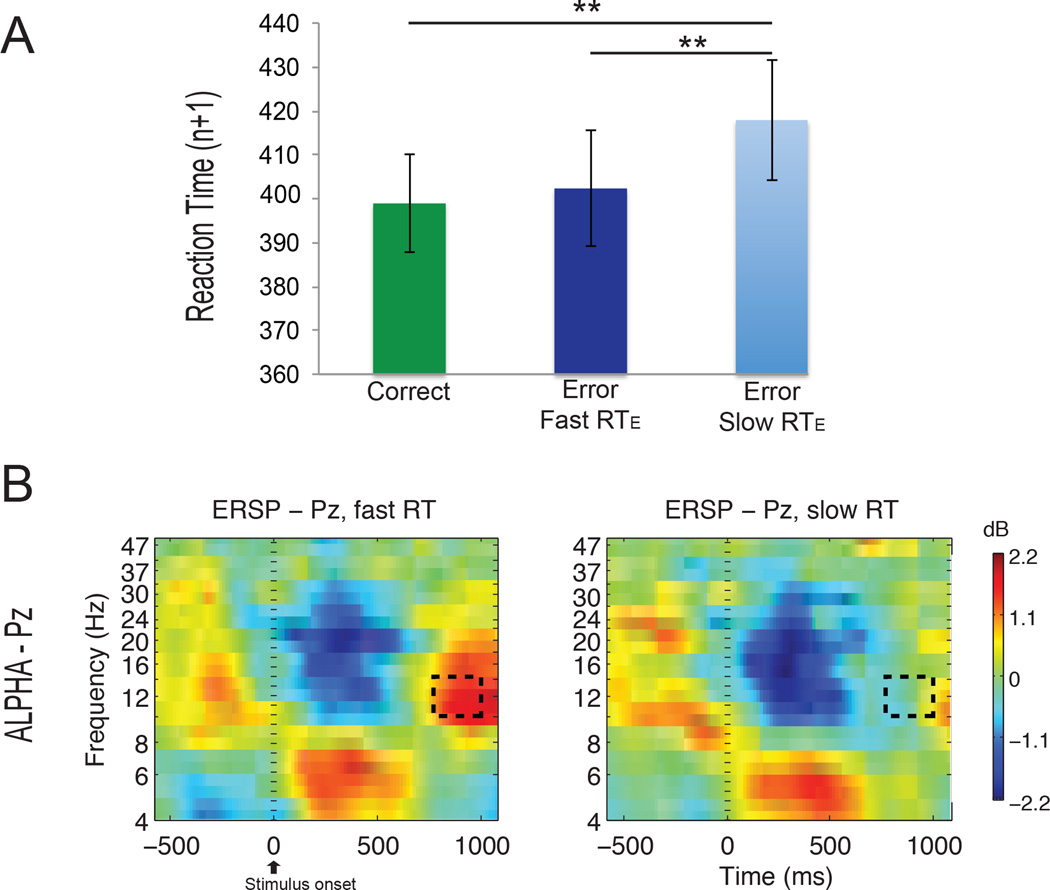
A diminished alpha power after errors, compared with correct responses, has been suggested to be indicative of higher alertness and reflective of neural adjustments (Carp and Compton 2009; Navarro-Cebrian et al. 2013). Time-frequency analyses were performed to test whether there was less post-error alpha power in those cases in which reduced frontal activity in monitoring areas led to greater uncertainty about performance and a slower reaction time to the error. Significant differences in power in the alpha band were found between the fast and slow RTEC in the inter-trial interval (t(11) = 5.21; p = 0.0003; Figure 4 dashed box), indicating less alpha power in the slow RTEC condition.
Fig 4. Post-error adjustments.
A. The reaction time (RTn+1) for trials following correct responses, trials following fast RTEC errors (fast correction), and trials following slow RTEC errors (slow correction), respectively. The plot indicates a greater post-error slowing (RT in the trial following the error) after trials with a slow RTEC error. B. Less alpha power can be seen in the inter-trial interval (dashed rectangle) in the slow RTEC condition (right) compared to the fast RTEC condition (left).
Post-error behavioral adjustments
Finally, we calculated the reaction times in the trials following the errors (post-error trials) to assess differences in post-error slowing (Figure 4). Trials following correct responses (average RTn+1 of 399 ms) did not differ significantly from trials following faster error corrections (average RTn+1 of 402 ms; correct responses versus fast correction trials: t(11) = 0.77, p = 0.46). In contrast, trials following slower error corrections had an average RTn+1 of 418 ms, which was significantly longer than RTs following correct trials (t(11) = 2.75; p = 0.019) and fast correction trials (t(11) = 2.99; p = 0.012). These results indicate increased post-error slowing in the cases in which subjects took a longer time to respond to the error.
Discussion
In order to investigate the role of evidence accumulation in error correction, we took advantage of natural variation in error salience to define fast and slow error correction trials and to evaluate differences in conflict monitoring, decision-making, and post-error adjustments. Previous EEG studies (O’Connell et al. 2012; Kelly et al. 2013) have characterized a centro-parietal electrical signal that increases with incoming evidence and peaks at the time of response execution. This signal shares characteristics with the classical P3b and, based on similarities between the P3b and the Pe (Ridderinkhof et al. 2009; Ullsperger et al. 2010), has led to proposals that the Pe indexes the accumulation of evidence for the existence of an error (Steinhauser and Yeung, 2012). Consistent with this notion, here we found that the amplitude of early monitoring signals was greater, and the latency of the Pe was shorter, when subjects rapidly corrected themselves. Furthermore, we determined that post-error cognitive and behavioral adjustments depended upon this decision-making process and, along with the latency of the Pe, varied with the RTEC. Together these signals define a potential sequence of events that underlies error correction.
Error monitoring processes
The earliest signal in this sequence of EEG events – activity of the mPFC – has long been a focus of attention in EEG studies that analyzed activity in fronto-central electrodes after errors using ERPs (ERN signal – Gehring et al 1993; Endrass et al. 2007, 2012; O’Connell et al. 2007; Shalgi et al. 2009) or time-frequency analysis, specifically in the theta band (Luu et al. 2004; Debener et al. 2005; Trujillo and Allen 2007; Cohen et al. 2008; Cavanagh et al. 2009; Cohen and Cavanagh 2011; Cavanagh et al. 2012). Here we demonstrate that the amplitude of the ERN differs between fast and slow RTEC conditions, suggesting a relationship between this signal and error correction. As in a previous study (Rodriguez-Fornells et al. 2002), our data showed a larger amplitude of the ERN in fast corrections compared with slow corrections, while the latency of this EEG signal was not affected.
Contrary to the results for the ERN amplitude, we did not find differences in the magnitude of the theta power. This discrepancy in our results may be explained by the fact that while the magnitude of the theta power is similar in both conditions (Figure 3 A), a greater amount of the theta activity is phase-locked to the response in the fast RTEC trials. This result agrees with the idea that the ERN component emerges from phase locking of theta band EEG activity (Luu and Tucker 2001). Although early viewpoints argued that ERPs result from an evoked neural response to an event (Hillyard and Picton 1987), recent theories suggest that environmental events induce partial ‘phase resetting’ of ongoing neural activity (Makeig et al. 2002; 2004). Such phase locking has been suggested to correlate with performance (Yamagishi et al. 2008) and to be affected in neurological conditions such as schizophrenia (Ford et al. 2007) and Alzheimer’s disease (Pijnenburg et al. 2004). Consistent with the importance of such synchronization to behavior, intra-area inter-trial phase synchrony (ITC) provided a direct measure of neural synchrony (Makeig et al. 2002) that identified greater phase locking of ongoing theta activity in mPFC for fast versus slow RTEC. Thus, for those trials in which the monitoring activity of the mPFC and the motor response are more synchronized, there is improved error signaling and faster error correction.
Lastly in relation to the ERN results, it is important to note that, supporting previous findings (Pailing et al. 2002), larger ERN amplitudes were preceded by slower RTE, Pailing and colleagues suggested that the magnitude of ERN monitoring activity may reflect individual differences in response control or impulsive behavior. Following that logic, the authors showed that participants with larger mean ERN amplitudes had slower RTE, suggesting a less impulsive response strategy by those individuals. In our data, the fact that larger ERN amplitudes across trials, rather than individuals, are preceded by significantly slower RTE and followed by faster RTEC, further suggests that differences in response control vary significantly from trial to trial within individuals.
The error-related positivity (Pe) and performance decision-making
This initial medial prefrontal signal (ERN) has been hypothesized to relate to the detection of response conflict (Van Veen and Carter 2002), among other theories of medial PFC function after errors (i.e. Holroyd and Coles 2002). If true, greater response conflict should correspond with greater likelihood of an error, and evidence accumulation processes should integrate more rapidly to an error detection threshold when greater response conflict is present (Ullsperger et al. 2010). The error positivity or error-related positivity (Pe) is a parietal electrical signal evoked by errors in comparison with correct responses (Falkenstein et al. 2000; Shalgi et al. 2009). Recent studies (Ridderinkhof et al. 2009; Ullsperger et al. 2010) have argued that this signal is related to the motivational significance of the error in the same way that another well-known parietal ERP, the P3b, is related to the motivational significance of infrequent stimuli (e.g. oddballs). Our results show that the latency of the Pe varies with the RTEC, possibly reflecting the accumulation of evidence for the error that is needed to make a correction. This theory would state that at the time of the Pe, different sources of information signaling the error contribute to integration in parietal areas until the evidence becomes strong enough to make a decision about the performance (Ullsperger et al. 2010). These results support previous data that found a modulation of the Pe amplitude when the level of the decision criterion was varied (Steinhauser and Yeung 2010; Orr and Carrasco 2011).
Interestingly, this link, via the RTEC, between earlier frontal and later parietal signals agrees with previous EEG studies (Philiastides and Sajda 2006; Philiastides et al. 2006) of perceptual decision-making that detected a frontal ERP component occurring approximately 220 ms after the onset of the stimulus (possibly reflecting the typical N2 ERP). This component varied with the level of the perceptual difficulty and predicted the onset of a later component related to evidence accumulation (which could reflect the CPP or typical P3b ERP). Together, these findings suggest that future work might further explore interactions between earlier frontal and later parietal signals as a general mechanism in both the processing of errors (ERN and Pe) and perceptual stimuli (N2 and P3b; Wessel et al. 2012).
More broadly, these data agree with other EEG work supportive of a centro-parietal evidence accumulation mechanism. Recent EEG studies (O’Connell et al. 2012; Kelly et al. 2013), for example, have characterized a brain electrical signal that shares properties described by models of this process in primates and humans (Ratcliff and McKoon 2008). Interestingly, this signal, called the centro-parietal positivity (CPP), shares characteristics with the P3b signal (O’Connell et al. 2012). Although early studies touched on decision-making in relation to the P3b (Smith et al. 1970), the most influential theory, the context-updating model (Donchin 1981), states that the P3b is related to a memory update process. According to this theory, the brain constantly generates hypotheses about the environment, with the P3b signal generated every time that the environment changes and the context representation needs to be updated. However, newer studies (Nieuwenhuis et al. 2001; O’Connell et al. 2012; Kelly et al. 2013) suggest a re-evaluation of previous P3b results to consider this slow EEG potential as a dynamically evolving component rather than the culmination of a unitary event (Kelly et al. 2013).
Post-error adjustments: alpha power and post-error slowing
Once conflict is detected and evidence for an error has reached threshold, not only must a correction be made, but behavioral modifications must also be implemented to avoid the occurrence of further errors in the future. In EEG studies, suppression of the inter-trial interval (ITI) alpha power, for example, is used to measure an increase in cortical arousal (Carp and Compton 2009; Compton et al. 2011, 2013, 2014; van Driel et al. 2012) that may be interpreted as cognitive adjustments occurring after an error is made (Navarro-Cebrian et al. 2013). An increase in alpha power, mainly in posterior areas, often occurs when subjects finish a trial and wait for the next one to start. This alpha in the ITI is considered a sign of task disengagement, and its increase is suppressed when one’s behavior deviates from a goal. In other words, greater alpha suppression after errors suggests higher alertness. In the present study, those trials accompanied by longer RTEC were associated with less alpha power, suggestive of higher alertness following these more uncertain errors. Such findings are consistent with the reduced confidence associated with trials in which evidence accumulates more slowly (Kiani and Shadlen 2009).
In accordance with these results, subjects also slowed down after more uncertain errors (i.e. errors that took longer to correct). Post-error slowing is often defined as longer RTs in trials following errors in comparison to correct responses (Rabbitt 1966). In our study, the fact that RTEC values covaried with activity of mPFC and parietal cortex, as well as the degree of post-error slowing, suggests that signaling in the frontal and parietal cortices may influence these later processes. Interestingly, not all studies have found the same relationship between the ERN and the degree of post-error slowing. In contrast with our finding of an inverse relationship (i.e. faster RTEC is associated with both a greater ERN and lesser post-error slowing), Gehring and colleagues found a greater ERN to be followed by greater post-error slowing (Gehring et al. 1993). One possibility for this discrepancy relates to the nature of the ERN itself. As shown previously, ERN amplitude is increased by perceptually difficult stimuli (Philiastides et al. 2006), possibly reflecting uncertainty at the stimulus phase (Navarro-Cebrian et al. 2013); in contrast, internal fluctuations in monitoring areas at the time of the response may result in a smaller ERN. As a result, including trials following errors related to stimulus uncertainty rather than motor failures might predispose to finding a direct relationship. A second possibility is that the nature of the relationship between ERN amplitude and post-error slowing may be driven by a more proximate signal – i.e. the latency of the Pe. A third possibility has to do with our task design: because the ITI was relatively brief (550ms), slower error correction may simply have impacted subjects’ abilities to attend to the next stimulus. We note that even the slowest of error correction responses had been completed by 300ms into the ITI, and that subsequent alpha power also distinguished these trials. Nonetheless, future work to explicitly vary the duration of the ITI would be helpful.
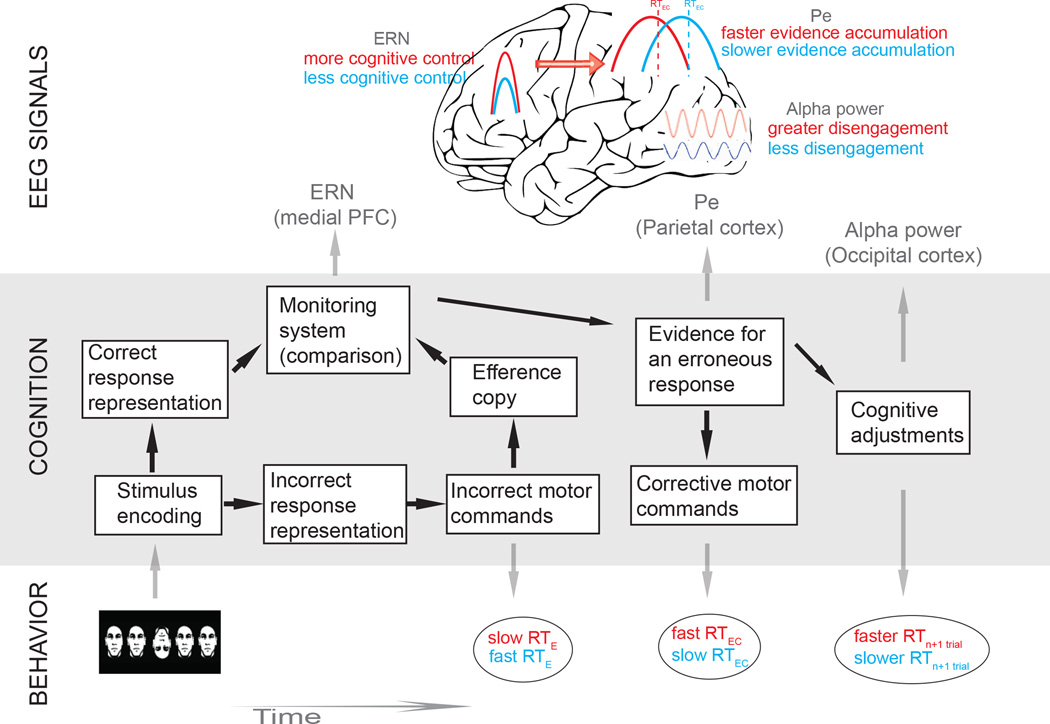
Timing diagram for adaptive decision-making
Taken together, our data link together several findings from previous studies (Pailing et al. 2002; Rodriguez-Fornells et al. 2002; Steinhauser and Yeung 2010; Ullsperger et al. 2010; Wessel et al. 2011; Murphy et al. 2012; Steinhauser and Yeung 2012) under a potential evidence accumulation framework, and provide a hypothetical timing diagram for adaptive decision-making after errors (Figure 5). Specifically, more cognitive control at the time of the selection of the response (slower RTE; Pailing et al. 2002) may lead to a more accurate comparison between the representation of the correct response and the efference copy of the (incorrect) motor commands. As a consequence, a greater mismatch and a larger ERN will be observed in those trials (Pailing et al. 2002). Therefore, stronger error signaling in these responses will lead to more rapid accumulation of evidence for the erroneous response and a faster RTEC.
Fig 5. Timing diagram for adaptive decision-making.
This diagram represents the different cognitive processes (boxes), behavioral signatures (ellipses), and EEG signals that derive from error processing, error correction and post-error adjustments. We hypothesize that after the incorrect motor commands are selected, an efference copy of these commands is generated and compared with the predicted correct response representation. An ERN signal will be elicited when a mismatch is produced, and a greater ERN will lead to more rapid corrections (Rodriguez-Fornells 2002). In addition, the ERN signal will correlate with the speed with which evidence for an error accumulates (Ullsperger et al. 2010) so that a greater ERN signal will lead to a faster evidence accumulation process, as reflected in the latency of the Pe signal (Murphy et al. 2012). Lastly, these differences in cognitive control and decision-making after the initial error will influence post-error cognitive and behavioral adjustments. When participants more easily identify their errors (more cognitive control) and correct them quickly, they are better able to disengage during the ITI (more alpha power), and these errors will have a reduced influence on the next trial (reduced post-error slowing).
It is important to note that the RTEC may occur quickly (average for fast RTEC condition: 126 ms) following the initial erroneous response. These fast corrections, replicating previous studies (e.g. Rodriguez-Fornells et al. 2002), may represent accumulation of evidence for the error if they occur after the comparison of an efference copy of the erroneous motor commands with the correct commands (Figure 5; Higgins and Angel 1970; Coles et al. 2001; Rodriguez-Fornells et al. 2002; Christensen et al. 2008). Moreover, if this comparison between representations of correct and error responses is triggered by the arrival of the efference copy (Coles et al. 2001), it may occur even before the actual motor response (error) takes place. This last possibility may help us to understand both the early latency of the ERN observed in this and previous studies (i.e. Wiersema et al. 2007; Maier and Steinhauser 2013; Navarro-Cebrian et al. 2013) and the fast error-correcting responses (RTEC).
An alternative account would argue that the processes necessary for conscious error-signaling responses and subsequent corrections require more than 150 ms (Rabbitt 2002) and that this type of fast correction may instead represent “delayed correct responses” that are completed after impulsive errors (Maylor and Rabbitt 1989; Rabbitt 2002). Following this idea, our rapid RTEC values may be due to the continuation of the accumulation of the stimulus information without the explicit detection of the initial error; they would therefore not reflect accumulation of evidence for having made an error, and the Pe in the present example would simply be the continuation of the P3b in relation to the processing of the stimulus. This account would be supported by the fact that our task reflects error corrections rather than error signaling (Ullsperger and von Cramon 2006). Our data suggest, however, that this possibility may not explain the early latency found for the ERN, which in our and other work (i.e. Wiersema et al. 2007; Maier and Steinhauser 2013; Navarro-Cebrian et al. 2013) can precede the motor response. An additional complication for this account may come from the results of the RTEC relative to the onset of the stimulus (RTEC-SO). We continue to observe significant differences between the slow and fast RTEC-SO conditions when those reaction times are calculated relative to the onset of the stimulus. One might expect no differences between those two RTEC-SO values if evidence accumulation is related to the processing of the stimulus alone (i.e. irrespective of the erroneous response) in both cases.
Similarly, the argument might be made that fast and slow errors represent qualitatively distinct cognitive processes – specifically, that unlike long values, fast RTEC values reflect a motor plan that has previously been initiated and that is ultimately implemented without awareness of the error. In our previous work using this paradigm (Navarro-Cebrian et al. 2013), we demonstrated that correct and unaware errors are characterized by small ERN amplitudes that differ significantly from the large ERN amplitudes accompanying aware errors. If rapid RTEC values reflected a lack of error awareness, we would expect that ERN amplitudes would be significantly smaller for fast RTEC trials than for slow RTEC trials. However, we find the opposite result, arguing that error awareness cannot explain the difference between these trial types in this data set.
Conclusions
The current data demonstrate how variation in error salience, as indexed by the RTEC, implicates areas previously associated with evidence accumulation in both the evaluation of decisions and the implementation of compensations when those decisions deviate from goals. Importantly, the study of error-related EEG signals within a decision-making context allows us to describe a potential timing diagram for adaptive decision-making when errors are made. Moreover, mechanisms of error correction, beyond the initial detection of an error, may be relevant to therapies that address behavioral change in patient populations. Future work might therefore investigate how this sequence of events differs when the ability to monitor and then alter behavior is impaired.
Acknowledgments
This research was supported by funding from NEI grant EY024554 (A.S.K.), the Alcoholic Beverage Medical Research Foundation / The Foundation for Alcohol Research (A.S.K.), funds from the State of California (A.S.K.) and NINDS Grant NS 2R3721135 and the Nielsen Corporation (R.T.K.). We thank our subjects for their participation in this study.
Bibliography
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Cohen MX, Allen JJ. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. J Neurosci. 2009;29:98–105. doi: 10.1523/JNEUROSCI.4137-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Zambrano-Vazquez L, Allen JJ. Theta lingua franca: a common mid-frontal substrate for action monitoring processes. Psychophysiology. 2012;49:220–238. doi: 10.1111/j.1469-8986.2011.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J, Compton RJ. Alpha power is influenced by performance errors. Psychophysiology. 2009;46:336–343. doi: 10.1111/j.1469-8986.2008.00773.x. [DOI] [PubMed] [Google Scholar]
- Christensen MS, Kristiansen L, Rowe JB, Nielsen JB. Action-blindsight in healthy subjects after transcranial magnetic stimulation. Proc Natl Acad Sci. 2008;105:1353–1357. doi: 10.1073/pnas.0705858105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Cavanagh JF. Single-trial regression elucidates the role of prefrontal theta oscillations in response conflict. Front Psychol. 2011;2:30. doi: 10.3389/fpsyg.2011.00030. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Ridderinkhof KR, Haupt S, Elger CE, Fell J. Medial frontal cortex and response conflict: evidence from human intracranial EEG and medial frontal cortex lesion. Brain Res. 2008;1238:127–142. doi: 10.1016/j.brainres.2008.07.114. 10. [DOI] [PubMed] [Google Scholar]
- Coles MGH, Scheffers MK, Holroyd CB. Why is there an ERN/Ne on correct trials? Response representations, stimulus-related components, and the theory of error-processing. Biol Psychol. 2001;56:173–189. doi: 10.1016/s0301-0511(01)00076-x. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Arnstein D, Freedman G, Dainer-Best J, Liss A. Cognitive control in the intertrial interval: evidence from EEG alpha power. Psychophysiology. 2011;48:583–590. doi: 10.1111/j.1469-8986.2010.01124.x. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Bissey B, Worby-Selim S. Task motivation influences alpha suppression following errors. Psychophysiology. 2014;27:12212. doi: 10.1111/psyp.12212. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Hofheimer J, Kazinka R, Levinson A, Zheutlin A. Alpha suppression following performance errors is correlated with depression, affect, and coping behaviors. Emotion. 2013;13:905–914. doi: 10.1037/a0032739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Donchin E. Presidential address, 1980. Surprise!…Surprise? Psychophysiology. 1981;18:493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Endrass T, Reuter B, Kathmann N. ERP correlates of conscious error recognition: aware and unaware errors in an antisaccade task. Eur J Neurosci. 2007;26:1714–1720. doi: 10.1111/j.1460-9568.2007.05785.x. [DOI] [PubMed] [Google Scholar]
- Endrass T, Klawohn J, Preuss J, Kathmann N. Temporospatial dissociation of Pe subcomponents for perceived and unperceived errors. Front Hum Neurosci. 2012;6:178. doi: 10.3389/fnhum.2012.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biol Psychol. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Fiehler K, Ullsperger M, Von Cramon DY. Neural correlates of error detection and error correction: Is there a common neuroanatomical substrate? Eur J Neurosci. 2004;19:3081–3087. doi: 10.1111/j.0953-816X.2004.03414.x. [DOI] [PubMed] [Google Scholar]
- Ford JM, Krystal JH, Mathalon DH. Neural synchrony in schizophrenia: from networks to new treatments. Schizophr Bull. 2007;33:848–852. doi: 10.1093/schbul/sbm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MG, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Marrett S, Ungerleider LG. The neural systems that mediate human perceptual decision making. Nat Rev Neurosci. 2008;9:467–479. doi: 10.1038/nrn2374. [DOI] [PubMed] [Google Scholar]
- Hester R, Barre N, Murphy K, Silk TJ, Mattingley JB. Human medial frontal cortex activity predicts learning from errors. Cereb Cortex. 2008;18:1933–1940. doi: 10.1093/cercor/bhm219. [DOI] [PubMed] [Google Scholar]
- Hester R, Foxe JJ, Molholm S, Shpaner M, Garavan H. Neural mechanisms involved in error processing: a comparison of errors made with and without awareness. Neuroimage. 2005;27:602–608. doi: 10.1016/j.neuroimage.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Hester R, Madeley J, Murphy K, Mattingley JB. Learning from errors: error-related neural activity predicts improvements in future inhibitory control performance. J Neurosci. 2009;29:7158–7165. doi: 10.1523/JNEUROSCI.4337-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302:120–122. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- Hughes G, Yeung N. Dissociable correlates of response conflict and error awareness in error-related brain activity. Neuropsychologia. 2011;49:405–415. doi: 10.1016/j.neuropsychologia.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JR, Angel RW. Correction of tracking errors without sensory feedback. J Exp Psychol. 1970;84:412–416. doi: 10.1037/h0029275. [DOI] [PubMed] [Google Scholar]
- Jung TP, Humphries C, Lee TW, Makeig S, McKeown MJ, Iragui V, Seinowski TJ. Extended ICA removes artifacts from electroencephalografic recordings. Adv Neural Inf Process Syst. 1998;10:894–900. [Google Scholar]
- Kelly SP, O'Connell RG. Internal and external influences on the rate of sensory evidence accumulation in the human brain. J Neurosci. 2013;33:19434–19441. doi: 10.1523/JNEUROSCI.3355-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani R, Shadlen MN. Representation of confidence associated with a decision by neurons in the parietal cortex. Science. 2009;324:759–764. doi: 10.1126/science.1169405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani R, Corthell L, Shadlen MN. Choice certainty is informed by both evidence and decision time. Neuron. 2014;84:1329–1342. doi: 10.1016/j.neuron.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu P, Tucker DM. Regulating action: alternating activation of midline frontal and motor cortical networks. Clin Neurophysiol. 2001;112:1295–1306. doi: 10.1016/s1388-2457(01)00559-4. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Makeig S. Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clin Neurophysiol. 2004;115:1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Maier ME, Steinhauser M. Updating expected action outcome in the medial frontal cortex involves an evaluation of error type. J Neurosci. 2013;33:15705–15709. doi: 10.1523/JNEUROSCI.2785-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S, Debener S, Onton J, Delorme A. Mining event-related brain dynamics. Trends Cogn Sci. 2004;8:204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, Sejnowski TJ. Dynamic brain sources of visual evoked responses. Science. 2002;295:690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- Maylor EA, Rabbitt PMA. Relation between rate of preparation for and processing of an event requiring a choice response. Quarterly Journal of Experimental Psychology. 1989;41A:47–62. [Google Scholar]
- Mazurek ME, Roitman JD, Ditterich J, Shadlen MN. A role for neural integrators in perceptual decision making. Cereb Cortex. 2003;13:1257–1269. doi: 10.1093/cercor/bhg097. [DOI] [PubMed] [Google Scholar]
- Murphy PR, Robertson IH, Allen D, Hester R, O'Connell RG. An electrophysiological signal that precisely tracks the emergence of error awareness. Front Hum Neurosci. 2012;6:65. doi: 10.3389/fnhum.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Cebrian A, Knight RT, Kayser AS. Error-monitoring and post-error compensations: dissociation between perceptual failures and motor errors with and without awareness. J Neurosci. 2013;33:12375–12383. doi: 10.1523/JNEUROSCI.0447-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychol Bull. 2005;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology. 2001;38:752–760. [PubMed] [Google Scholar]
- O'Connell RG, Dockree PM, Bellgrove MA, Kelly SP, Hester R, Garavan H, Robertson IH, Foxe JJ. The role of cingulate cortex in the detection of errors with and without awareness: a high-density electrical mapping study. Eur J Neurosci. 2007;25:2571–2579. doi: 10.1111/j.1460-9568.2007.05477.x. [DOI] [PubMed] [Google Scholar]
- O'Connell RG, Dockree PM, Kelly SP. A supramodal accumulation-to-bound signal that determines perceptual decisions in humans. Nat Neurosci. 2012;15:1729–1735. doi: 10.1038/nn.3248. [DOI] [PubMed] [Google Scholar]
- Orr JM, Carrasco M. The role of the error positivity in the conscious perception of errors. J Neurosci. 2011;31:5891–5892. doi: 10.1523/JNEUROSCI.0279-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pailing PE, Segalowitz SJ, Dywan J, Davies PL. Error negativity and response control. Psychophysiology. 2002;39:198–206. doi: 10.1017/S0048577202010247. [DOI] [PubMed] [Google Scholar]
- Philiastides MG, Sajda P. Temporal characterization of the neural correlates of perceptual decision making in the human brain. Cereb Cortex. 2006;16:509–518. doi: 10.1093/cercor/bhi130. [DOI] [PubMed] [Google Scholar]
- Philiastides MG, Ratcliff R, Sajda P. Neural representation of task difficulty and decision making during perceptual categorization: a timing diagram. J Neurosci. 2006;26:8965–8975. doi: 10.1523/JNEUROSCI.1655-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijnenburg YA, v d Made Y, van Cappellen van Walsum AM, Knol DL, Scheltens P, Stam CJ. EEG synchronization likelihood in mild cognitive impairment and Alzheimer's disease during a working memory task. Clin Neurophysiol. 2004;115:1332–1339. doi: 10.1016/j.clinph.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Rabbitt PM. Errors and error correction in choice-response tasks. J Exp Psychol. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Rouder J. Modeling response times for two choice decisions. Psychol Sci. 1998;9:347–356. [Google Scholar]
- Rabbitt P. Consciousness is slower than you think. Q J Exp Psychol. 2002;55:1081–1092. doi: 10.1080/02724980244000080. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, McKoon G. The diffusion decision model: theory and data for two-choice decision tasks. Neural Comput. 2008;20:873–922. doi: 10.1162/neco.2008.12-06-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ramautar JR, Wijnen JG. To P(E) or not to P(E): a P3-like ERP component reflecting the processing of response errors. Psychophysiology. 2009;46:531–538. doi: 10.1111/j.1469-8986.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fornells A, Kurzbuch AR, Munte TF. Time course of error detection and correction in humans: neurophysiological evidence. J Neurosci. 2002;22:9990–9996. doi: 10.1523/JNEUROSCI.22-22-09990.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami M, Tsutsui K. The hierarchical organization of decision making in the primate prefrontal cortex. Neurosci Res. 1999;34:79–89. doi: 10.1016/s0168-0102(99)00038-3. [DOI] [PubMed] [Google Scholar]
- Shalgi S, Barkan I, Deouell LY. On the positive side of error processing: error-awareness positivity revisited. Eur J Neurosci. 2009;29:1522–1532. doi: 10.1111/j.1460-9568.2009.06690.x. [DOI] [PubMed] [Google Scholar]
- Schmidt RA, Gordon GB. Errors in motor responding, "rapid" corrections, and false anticipations. J Mot Behav. 1977;9:101–111. doi: 10.1080/00222895.1977.10735099. [DOI] [PubMed] [Google Scholar]
- Smith DB, Donchin E, Cohen L, Starr A. Auditory averaged evoked potentials in man during selective binaural listening. Electroencephalogr Clin Neurophysiol. 1970;28:146–152. doi: 10.1016/0013-4694(70)90182-3. [DOI] [PubMed] [Google Scholar]
- Steinhauser M, Yeung N. Decision processes in human performance monitoring. J Neurosci. 2010;30:15643–15653. doi: 10.1523/JNEUROSCI.1899-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauser M, Yeung N. Error awareness as evidence accumulation: effects of speed-accuracy trade-off on error signaling. Front Hum Neurosci. 2012;6:240. doi: 10.3389/fnhum.2012.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troje NF, Bulthoff HH. Face recognition under varying poses: the role of texture and shape. Vision Res. 1996;36:1761–1771. doi: 10.1016/0042-6989(95)00230-8. [DOI] [PubMed] [Google Scholar]
- Trujillo LT, Allen JJ. Theta EEG dynamics of the error-related negativity. Clin Neurophysiol. 2007;118:645–668. doi: 10.1016/j.clinph.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. How does error correction differ from error signaling? An event-related potential study. Brain Res. 2006;1105:102–109. doi: 10.1016/j.brainres.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Harsay HA, Wessel JR, Ridderinkhof KR. Conscious perception of errors and its relation to the anterior insula. Brain Struct Funct. 2010;214:629–643. doi: 10.1007/s00429-010-0261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Driel J, Ridderinkhof KR, Cohen MX. Not all errors are alike: theta and alpha EEG dynamics relate to differences in error-processing dynamics. J Neurosci. 2012;32:16795–16806. doi: 10.1523/JNEUROSCI.0802-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. J Cogn Neurosci. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Wiersema JR, Van Der Meere JJ, Roeyers H. Developmental changes in error monitoring: an event-related potential study. Neuropsychologia. 2007;45:1649–1657. doi: 10.1016/j.neuropsychologia.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Wessel JR, Danielmeier C, Ullsperger M. Error awareness revisited: accumulation of multimodal evidence from central and autonomic nervous systems. J Cogn Neurosci. 2011;23:3021–3036. doi: 10.1162/jocn.2011.21635. [DOI] [PubMed] [Google Scholar]
- Wessel JR. Error awareness and the error-related negativity: evaluating the first decade of evidence. Front Hum Neurosci. 2012;6:8. doi: 10.3389/fnhum.2012.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi N, Callan DE, Anderson SJ, Kawato M. Attentional changes in pre-stimulus oscillatory activity within early visual cortex are predictive of human visual performance. Brain Res. 2008;1197:115–122. doi: 10.1016/j.brainres.2007.12.063. [DOI] [PubMed] [Google Scholar]
- Yeung N, Cohen JD, Botvinick MM. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]