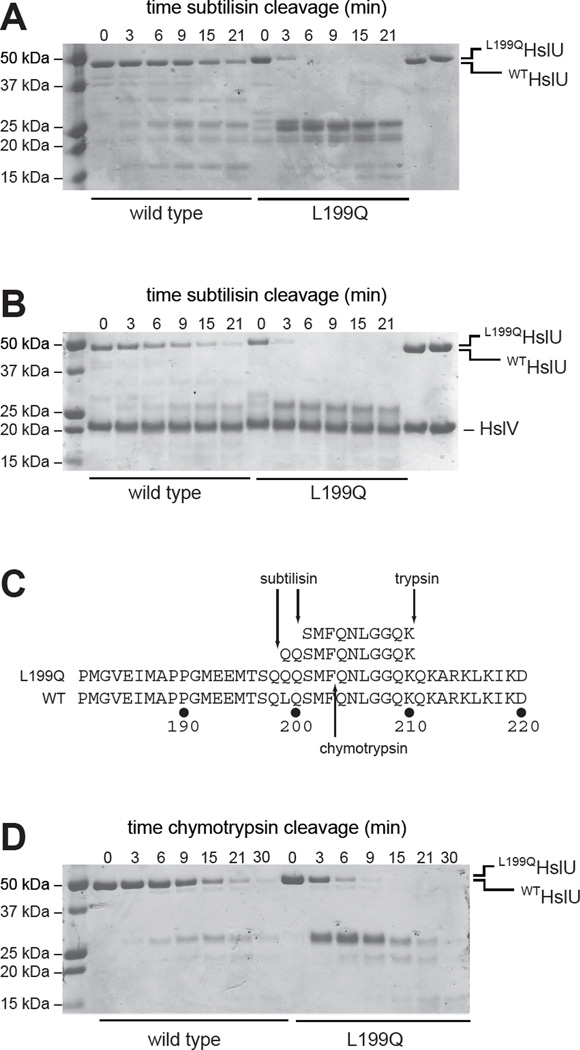
Figure 3. Limited proteolysis.
(A) Kinetics of cleavage of WTHslU and L199QHslU (500 nM each) by subtilisin (11 nM) at 37 °C in the presence of 5 mM ATP, assayed by SDS-PAGE and staining with Coomassie Blue. The rightmost two lanes show the HslU proteins in the absence of subtilisin. (B) Kinetics of cleavage of WTHslUV and L199QHslUV (500 nM HslU; 1000 nM HslV) by subtilisin (11 nM) at 37 °C in the presence of 5 mM ATP. The rightmost two lanes show the HslU and HslV proteins in the absence of subtilisin. (C) Peptide sequences obtained by LC MS/MS following tryptic digestion of the major subtilisin cleavage products of L199QHslU aligned with residues 181–220 of L199QHslU and WTHslU. The major site of chymotryptic cleavage of intact L199QHslU is also shown. (D) Kinetics of cleavage of WTHslU and L199QHslU (500 nM each) by chymotrypsin (60 nM) at 37 °C in the presence of 5 mM ATP.