Abstract
In spinal motoneurons, late spike frequency adaptation (SFA) is defined as the slowing of the firing rate over tens of seconds and can be seen during sustained or intermittent current injection. Although the function of late SFA is not known, it may result in a decrease in force production over time, or muscle fatigue. Because locomotion can persist for long periods of time without fatigue, late SFA was studied using intracellular recordings from adult cat motoneurons during fictive locomotion. Of eight lumbar motoneurons studied, all showed late adaptation during control conditions, but none demonstrated late adaptation during locomotor activity. The most consistent properties that correlated with the presence or absence of late SFA were those related to availability of fast, inactivating sodium channels, particularly action potential rate of rise. Evidence of the reversal of late SFA during locomotion was present for several minutes following locomotor trials, consistent with the suggestion that SFA is modulated through slow metabotropic pathways. The abolition of late adaptation in spinal motoneurons during fictive locomotion is an example of a state-dependent change in the “intrinsic” properties of mammalian motoneurons. This change contributes to increased excitability of motoneurons during locomotion and results in robust firing during sustained locomotion.
Keywords: action potentials, neuromodulation, sodium conductance, walking
Motor behaviors such as locomotion can proceed for long periods of time with little apparent fatigue. On the other hand, with continuous or repetitive stimulation, motoneuron firing rates decrease over time. Spike frequency adaptation (SFA) refers to the slowing of action potential firing rate during supratheshold stimulation. SFA has been well defined in spinal motoneurons, where there is a period of initial adaptation across the first few action potentials (Powers et al. 1999), followed by an “early phase” of SFA with a time constant of around 250 ms (Granit et al. 1963a) and a period of late adaptation occurring over tens of seconds (Kernell and Monster 1982b). Late SFA is present even when an extracellular electrode is used for recording and stimulation, demonstrating that it is not an artifact of electrode penetration, as well as when intermittent stimulation is used, indicating that it might be relevant during phasic motoneuronal activation such as that seen during locomotion (Spielmann et al. 1993). Furthermore, based on the work of Kernell and colleagues (Kernell 1965a; Kernell 1965b; Kernell and Monster 1982a; Kernell and Monster 1982b), it has been suggested that late SFA may be associated with the fatigue seen during a sustained maximal voluntary contraction (Bigland-Ritchie et al. 1986). It is therefore important to understand whether late SFA occurs during locomotion.
Most investigations of spinal motoneurons, including those on SFA, have been carried out in anesthetized, quiescent animals. It is now clear, however, that the “intrinsic” neuronal properties of motoneurons can be modulated in behaviors including locomotion (Brownstone et al. 1992; Krawitz et al. 2001). This modulation is thought to be a mechanism whereby the central nervous system can ensure appropriate motoneuron output for a particular behavior. Given that late SFA could be considered to be counterproductive to the maintenance of sustained locomotor activity, this study was undertaken to examine the hypothesis that late SFA is reduced during fictive locomotion. These studies were performed in the adult decerebrate cat in which fictive locomotor activity was produced by stimulation of the mesencephalic locomotor region (Shik et al. 1966) during intracellular recordings from lumbar spinal motoneurons. We have demonstrated that late SFA is, in fact, abolished during fictive locomotion. Preliminary data have been presented in abstract form (Krawitz and Brownstone 1994; Krawitz et al. 1996).
METHODS
All surgical and experimental protocols were approved by the University of Manitoba Animal Care Committee and performed in accordance with the guidelines of the Canadian Council on Animal Care. The data were obtained from lumbar motoneurons of five cats (2–3 kg). Procedures were as previously described (Brownstone et al. 1992). In brief, animals were anesthetized with oxygenated halothane and nitrous oxide. An adequate surgical level of anesthesia was assured by repeated testing for lack of pedal withdrawal, corneal reflexes, and muscle tone. Branches of the sciatic and femoral nerves bilaterally were dissected free and cut and placed on electrodes for stimulating and recording as previously described. A laminectomy of L4–L7 exposed the lower lumbar spinal cord dorsally. After fixation in a stereotaxic frame and craniectomy, the animal was decerebrated at the precollicular postmamillary level (Shik et al. 1966; Steeves et al. 1975). All tissue rostral to the transection was removed, allowing the anesthesia to be discontinued. The animal was paralyzed with intravenous injections of pancuronium bromide (Pavulon) and artificially ventilated. Norepinephrine was also infused intravenously when necessary to counter hypotension. Electrical stimulation (50- to 220-μA, 0.5- to 1.0-ms rectangular pulses at 10–25 Hz) of the mesencephalic locomotor region (MLR) was used to elicit locomotor activity.
Intracellular recordings from antidromically identified lumbar motoneurons were obtained with glass microelectrodes filled with 2 M potassium citrate (resistance 3–10 MΩ, tip diameter <2 μm) using either a bridge circuit or discontinuous current clamp (Axoclamp 2-A; Axon Instruments, Foster City, CA). Data were recorded on analog tape (Vetter) and digitized using rates of 10 kHz for the intracellular signal.
Firing in control conditions was evoked by intracellular depolarizing current injection delivered at a strength estimated to produce repetitive firing at a frequency that would reasonably match frequencies expected during locomotion. This target was 30–40 Hz, based on previous experiments (Brownstone et al. 1992). Once the strength of the control pulse was determined for a cell, that strength was consistently used for all subsequent depolarizing current injections in the same cell. To mimic the cyclic depolarizations seen during fictive locomotion, we injected repetitive 500-ms depolarizing pulses at a rate of ~1 Hz for 4 min. Four minutes was decided as a suitable duration for comparing frequencies to evaluate late adaptation (Spielmann et al. 1993 used 4 min).
Cell health was monitored by means of depolarizing pulses injected following each 4-min trial of firing (both control and locomotor). A series of single pulses was delivered at 30-s intervals over 5 min. If a cell became inappropriately depolarized (membrane potential more depolarized than −40 mV), action potential amplitude decreased by >10%, or the firing frequency during the 5-min interval between trials failed to recover to baseline level, the cell was rejected from further analysis.
The protocol was varied so that in some cells the locomotor trial preceded the control trial, whereas in others this was reversed. Once trials of control and locomotor firing had each been recorded, the sequence of control and locomotor trials was varied and continued as long as cell health was maintained.
Intracellular recordings were analyzed using software designed for the Spinal Cord Research Centre at the University of Manitoba (see www.scrc.umanitoba.ca/doc/ for details). The maximal rate of rise of the action potential was determined by digitally differentiating the intracellular signal. Action potential and afterhyperpolarization (AHP) amplitude were measured from the voltage threshold, which was defined as the membrane potential at which the rate of rise of the action potential reached 10 V/s (Brownstone et al. 1992). Statistical analysis of these data is not straightforward given the variability in firing rate during fictive locomotion (see Brownstone et al. 1992) and the time it takes to establish fictive locomotion. For example, comparison of the start to the end frequencies may underrepresent the changes seen during long bouts of locomotor activity. Wherever practical, Student’s t-tests have been used, with significance taken as P < 0.05.
RESULTS
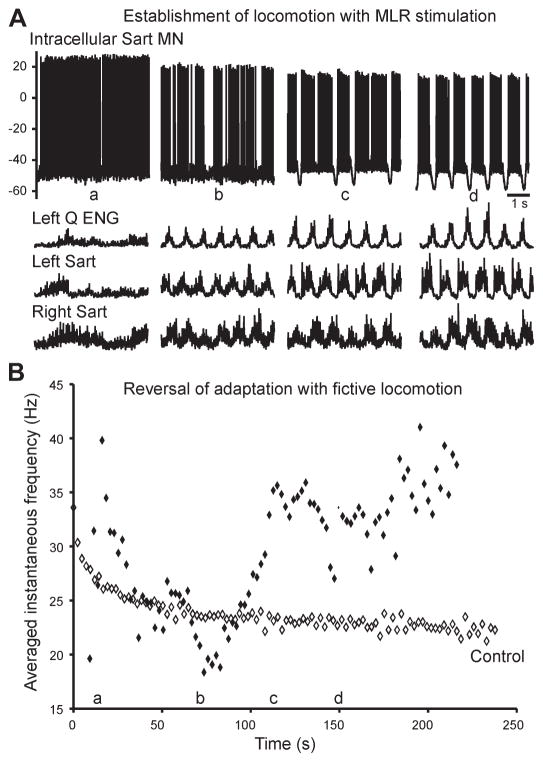
Late SFA was studied using intracellular recordings from eight lumbar motoneurons during well-developed, sustained fictive locomotion. All eight showed an absence of late SFA. In five of eight neurons, the firing frequency initially decreased with the onset of MLR stimulation (over 25 to 90 s) and then increased once rhythmic alternating locomotor activity was established. One of these neurons is shown in Fig. 1, where the top traces illustrate four 5-s periods of activity of a left sartorius motoneuron and the next three traces illustrate the corresponding electroneurogram (ENG) recordings of peripheral nerves (left quadriceps, left sartorius, and right sartorius) extracted from a 220-s trial. It can be seen that this motoneuron initially fired tonically before locomotor activity was established (Fig. 1Aa). During this period of time, the firing rate decreased, as would be expected with SFA (Fig. 1B). This phase was followed by a phase of firing interspersed by silent periods as the ENGs began to show regular periods of activity (Fig. 1Ab). With time, the motoneuron firing became more regular, alternating between phases of membrane depolarization when the cell fired and hyperpolarization when the cell was silent, corresponding to regular phases of the step cycle (Fig. 1A, c and d). During these periods, the firing rate gradually increased and returned to the initial firing rate. In the remaining three of eight motoneurons, the frequency of firing gradually increased from the onset of firing. These data demonstrate that the late SFA which occurred with the onset of motoneuron firing reversed as locomotor activity was established.
Fig. 1.
Development of locomotion is associated with a reversal of late adaptation. A: intracellular recording of a sartorius motoneuron (Sart MN; top) and electroneurograms (ENGs) from left quadriceps (Q) and left and right sartorius. With the onset of mesencephalic locomotor region (MLR) stimulation, tonic activity appeared (a), followed by irregular stepping (b), then more robust stepping with some deletions of the hyperpolarizing component of the locomotor drive potential (c), and, finally, well-developed fictive locomotion (d). The ENG traces demonstrate fictive locomotor activity with the characteristic rhythmic alternation between flexor (sartorius) and extensor (quadriceps) ENG activity as well as between left and right limb activity. B: averaged instantaneous firing frequencies of this motoneuron during the same 220-s trial of locomotion demonstrate the reversal of adaptation as fictive locomotion developed. The letters a–d along the abscissa correspond to those in A, showing where the 5-s extracts fit in the context of the entire trial. At the onset of tonic activity (a), firing gradually increased in frequency, then after ~20 s the average instantaneous frequency began to decrease. This was the onset of adaptation, which continued through the onset of irregular stepping (b). When the locomotion became more robust (c), the adaptation reversed, and this reversal continued throughout the period of fictive locomotion (d).
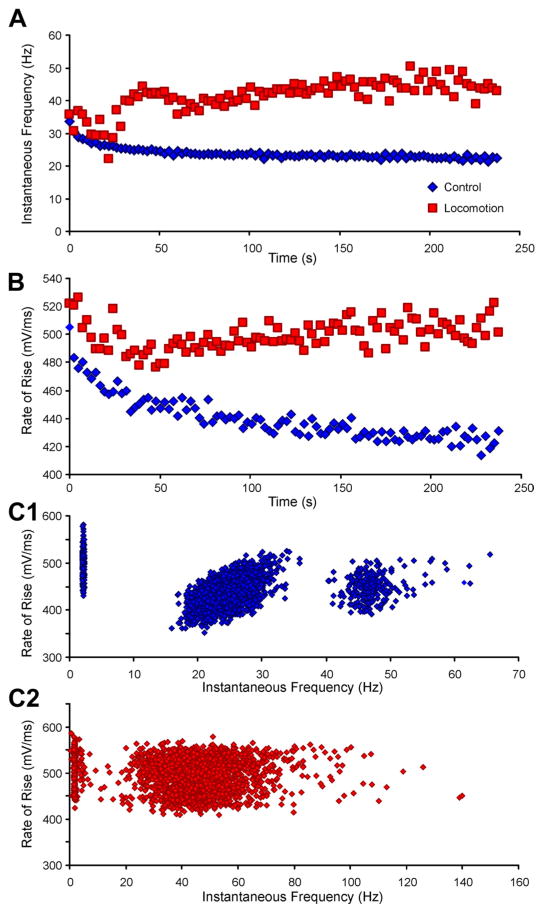
We next used a protocol that allowed examination of motoneuron firing over several minutes in duration during both control and locomotor conditions. In these experiments, firing frequencies during 4-min bouts of locomotion were compared with those during control trials of similar duration, in which the MLR was not stimulated and firing was induced by intracellular injection of repeated steps of depolarizing current. An example is shown in Fig. 2A, in which the mean firing frequency in the control run can be seen to steadily decrease over the run. The mean and standard deviation of the firing rate in the first three steps was 31 ± 10 Hz and in the last three steps was 23 ± 9 Hz (P < 0.0001). However, when the MLR was stimulated, there was an initial decrease in firing rate followed by a reversal of late SFA within the first minute (Fig. 2A). Comparing the first three with the last three steps revealed an insignificant increase from 38 ± 11 to 47 ± 25 Hz (P = 0.12). Six motoneurons were sufficiently stable during these recording periods to allow for quantitative analysis of late SFA. The first and last three steps were compared in both the control and locomotor conditions. In all six neurons, the firing frequencies at the end (mean frequency 33 Hz) of the control runs were lower than at the start (mean frequency 43 Hz; reduction of 10–53%, mean reduction 23%, P < 0.05). During locomotor trials, in three of six neurons (5 trials), the mean firing rate increased from 29 Hz in the first three steps to 42 Hz in the last three steps (19–91% increase, mean increase 47%, P < 0.05). In one of the six neurons, the firing rate increased by 23% in one trial (from 38 to 47 Hz, Fig. 2A) but did not change appreciably during a second 4-min locomotor run (~36 Hz). The firing rates in the remaining two neurons were lower in the last three step cycles than in the first three (one from 71–53 Hz, the other from 36–29 Hz). In the first of these neurons, the rate initially decreased from 71 to 40 Hz, and then as locomotor activity became more established, it increased to 53 Hz, thus demonstrating a reversal of late SFA. During recording of the second of these motoneurons, the locomotor activity was variable; the firing rate increased slightly between 1 and 3 min and then decreased. When data from the nine trials in six neurons were grouped, there was no significant difference in the firing rates at the end of the trials (42 Hz) compared with those at the start (36 Hz; P = 0.24). In summary, in none of the neurons was the characteristic late SFA pattern of a gradual slowing of firing rate observed during fictive locomotion; that is, the late SFA seen in control conditions is absent during locomotion.
Fig. 2.
Late adaptation and action potential accommodation occur in the control condition but are absent throughout a prolonged period of fictive locomotion. A: instantaneous frequencies from a gastrocnemius/soleus motoneuron during control (blue) and fictive locomotor state (red). Typical late adaptation can be seen in the control, but the firing rates did not adapt and in fact increased during locomotion. B: the rate of rise of each action potential is plotted against time, demonstrating the reduction that occurred during control current injection (blue) but the increasing rates of rise during fictive locomotion (red). A decrease in the rate of rise of the action potential (accommodation) was associated with late adaptation in the control condition, but there was no evidence of accommodation during the firing produced by fictive locomotion. C: In control conditions, there was a correlation between spike rate of rise and firing frequency (C1). Note that the lowest firing rates (cluster at left) are the first spikes in each step of current injection and are associated with the highest rates of rise, consistent with a recovery from sodium channel inactivation in the interstimulus interval. The cluster of points above 40 Hz is from the second spike (i.e., first interval) of each current step. The correlation between 15 and 40 Hz is significant (R2 = 0.29; F-test: P < 0.0001). During locomotion (C2), there was no correlation between the rate of rise and the instantaneous firing frequency (R2 = 0.002; F-test: P > 0.05).
Although underlying mechanisms of late SFA and its reversal are difficult to study in in vivo preparations in which pharmacological manipulation is difficult at best, we looked for correlations between changes in late SFA and action potential parameters in an attempt to suggest possible underlying mechanisms by which SFA may be regulated (n = 8). During the control trials, the rates of rise of the action potentials steadily decreased (Fig. 2B), consistent with progressive somatic sodium channel accommodation (Miles et al. 2005). This correlation between accommodation and late SFA during the current injections was consistently seen in our control trials (cf. Richter and Heyde 1975). During locomotion, however, the action potential rate of rise often increased slightly or did not change (Fig. 2B) and in some neurons seemed to roughly parallel the changes in instantaneous firing frequency (compare Fig. 2, A and B). In this neuron, the rate of rise decreased in the control from 490 ± 32 to 428 ± 26 V/s (P < 0.0001), whereas in the locomotor trial, the rate of rise did not significantly change between the first three and last three steps (from 520 ± 33 to 502 ± 36 V/s, P = 0.11). The same six motoneurons and same step cycles (first 3 and last 3) analyzed above for late SFA were included in a quantitative analysis of action potential rate of rise. In the controls, the rate of rise decreased by 4–26% over the 4-min run (mean reduction 15%, P < 0.001). In the locomotor trials, the rate of rise decreased in two neurons (12–30%), remained the same in two neurons (<2% change), and in the remaining two neurons increased in one trial (9 or 17%) and decreased in a second trial (8 or 3%, respectively). Taking the nine trials in six neurons together, there was no significant change in the rate of rise at the end compared with the start of the trials (mean decrease 6%, P = 0.28). The variability in changes in spike rate of rise over time suggests that any relationship between sodium channel availability and late SFA that exists in the control trials would be different in the locomotor trials.
To explore this further, we next analyzed the relationship between spike rate of rise and instantaneous firing frequency. In response to short-duration depolarizing pulses, there was a positive correlation between spike rate of rise and firing frequency (Miles et al. 2005). This was similar to what we found over the 4-min duration of the control trials in these six motoneurons (Fig. 2C 1). This is what would be expected with ongoing sodium channel accommodation: both the firing frequency and the spike rate of rise decrease in parallel. During locomotion, however, there was no correlation between these two parameters; the regression line had a zero slope (Fig. 2C 2). This lack of correlation is consistent with the hypothesis that the firing properties of motoneurons are modulated during locomotion and supports the hypothesis that this modulation affects sodium channel availability.
The amplitude of the AHP, although reduced during fictive locomotion (Brownstone et al. 1992), did not correlate with late SFA in any of the eight motoneurons (data not shown). Therefore, of the parameters studied, the parameters that correlated with late SFA, albeit not in all neurons, were those related to sodium channel availability. This suggests the possibility that during locomotion, slow inactivation of the fast, inactivating sodium conductance may be reduced (Miles et al. 2005).
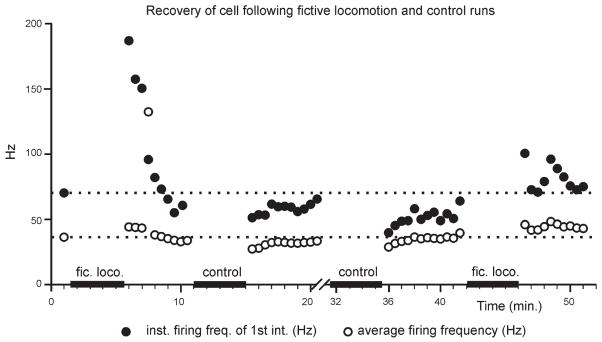
Next, to examine the time course of the events underlying late SFA modulation during locomotion, experiments were done to assess the recovery of motoneurons following trials of firing evoked both by current injection and during locomotion. Both the instantaneous first interval firing frequency and the average firing frequency were plotted in response to rectangular depolarizing pulses (~20 nA, 500-ms duration) given at 30-s intervals over 5 min following each 4-min trial of locomotion or control current injection (Fig. 3). By the end of the 5-min recovery periods subsequent to all locomotor and control trials, the firing frequencies attained pretrial levels, indicating full recovery of the cell after each trial of firing. However, the initial firing frequency following both locomotor trials surpassed the pretrial frequency. In contrast, the firing frequency evoked by the first few pulses following both control trials was decreased before recovering to the pretrial frequency. Such results would be expected if the mechanism underlying late SFA in control conditions was downmodulated during locomotion. Furthermore, the faster firing rates in response to current injection following the locomotor trial would be indicative of a slow recovery from this process, as might be expected if the reduction in SFA were mediated by a slowly conducting, descending neuromodulatory system acting via metabotropic pathways. Such monoaminergic pathways are likely activated during MLR-induced locomotion (see Jordan et al. 2008).
Fig. 3.
Reversal of late spike frequency adaptation (SFA) outlasts the locomotor trial. Rectangular 500-ms depolarizing pulses were injected every 30 s before and after episodes of fictive locomotion or control (intermittent firing produced by current injection to mimic locomotor firing). These pulses produced faster first intervals (●) and higher average firing rates (○) following locomotor episodes, recovering to baseline levels (dotted lines) after several minutes. The recovery of firing rate following each trial is evidence of the stable state and good condition of the motoneuron throughout successive trials.
DISCUSSION
These results demonstrate that late adaptation is eliminated during fictive locomotion. The mechanism that underlies late adaptation is not known but is likely due to either a slow facilitation of outward current(s), a reduction of inward current(s), or a combination of the two. The reversal of late SFA during locomotion could then be mediated by a reversal in either or both of these processes. In the present study, we have examined the same motoneurons in two states, one where there is late SFA (during current injection) and one where late SFA does not occur (during locomotion). The intrinsic properties that appear to be most associated with late adaptation are those that reflect availability of fast, inactivating sodium channels, as evidenced by the changes in rate of rise of the action potentials. We therefore suggest that slow inactivation (on the order of tens of seconds) of sodium channels is one contributing factor to late adaptation.
During locomotion, there is a high degree of variability in motoneuron firing rate, which is consistent with the reduction of the AHP (Person and Kudina 1972; Brownstone et al. 1992; Miles et al. 2005). This variability precludes the quantitative study of the earlier phases of SFA; however, no such early SFA is evident. Although the AHP is classically thought to be responsible for early adaptation (Baldissera et al. 1978; Barrett et al. 1980; Kernell and Sjoholm 1973; Sawczuk et al. 1997), it has recently been shown that slow inactivation of fast, inactivating sodium channels underlies this process (Miles et al. 2005). This is consistent with our findings on late SFA: if sodium channel availability were modulated during locomotion such that slow inactivation was reduced, then both early and late SFA would be reversed.
It has been suggested that late SFA may serve to “optimize force production” by matching motoneuron firing rates to peripheral fatigue mechanisms (Kernell and Monster 1982a). Alternatively, late SFA may represent a central process underlying fatigue (Kernell and Monster 1982a; see Nordstrom et al. 2007 for review), particularly during maximal voluntary contractions (Bigland-Ritchie et al. 1986). If this is the case, then motoneurons would need increasing excitatory synaptic input to maintain muscle force, even after relatively short (minutes) periods of behavior (Kernell and Monster 1982a). This would require an increase in the activity of neurons involved in the central locomotor network. It is not clear what stimulus would lead them to do so, particularly during fictive locomotion. An alternative explanation could be that the nervous system could increase the excitability of motoneurons, for example, by reducing their AHPs over time (Miles et al. 2007; Zagoraiou et al. 2009). Although AHPs are reduced during fictive locomotion (Brownstone et al. 1992; Miles et al. 2007), in the present study we found no relation between AHP amplitudes or durations and late SFA.
There is evidence that sodium channels can be directly modulated (Cantrell et al. 1996; Astman et al. 1998; Cantrell and Catterall 2001; Cantrell et al. 1999), for example, by phosphorylation of the channels, which may slow inactivation (Numann et al. 1991; West et al. 1991; Chen et al. 2006). During fictive locomotion, muscarinic receptors on motoneurons are activated, leading to a reduction in AHP (Miles et al. 2007; Zagoraiou et al. 2009). It is interesting that in hippocampal neurons, muscarinic receptor activation of protein kinase C leads to a reduction of peak sodium current and slowing of sodium channel inactivation (Cantrell et al. 1996). Sodium channel modulation is implicated as a likely mechanism for voltage threshold hyperpolarization associated with locomotion (Dai et al. 2002; Krawitz et al. 2001).
On the other hand, sodium channels have not been found to be directly responsible for late SFA or its reversal during locomotion. In fact, Zeng et al. (2005) demonstrated that there was no change in SFA in rat hypoglossal motoneurons following block of the persistent sodium current by phenytoin. It is therefore prudent to consider the potential role of potassium currents, including an M-current, in this process. For example, in neurons in the medial nucleus of the trapezoid body, it has been shown that activation of the delayed rectifier Kv2.2 current leads to deinactivation of sodium channels and thus higher firing rates (Johnston et al. 2008). It is possible that during locomotion, the known activation of C-bouton synapses on motoneurons (Miles et al. 2007; Zagoraiou et al. 2009) leads to activation of the Kv2.1 channels (cf. Mohapatra and Trimmer 2006) that are present at the postsynaptic membrane (Wilson et al. 2004) and that this in turn leads to a reversal of late SFA, perhaps mediated via an increase in sodium channel availability.
The “intrinsic” properties of mammalian motoneurons change depending on the state of the animal (see also Hultborn 1999), and the present demonstration that late SFA is abolished in spinal motoneurons during locomotion is an example of this. Together, the alterations in spinal motoneuron properties that are known to occur during locomotion (reduced AHP, appearance of plateau properties, hyperpolarization of the voltage threshold, and suppression of late adaptation) all contribute to increased excitability of motoneurons during locomotion and account for the fact that motoneuron firing is more robust than would be predicted based on results obtained with intracellular current injection alone.
Acknowledgments
We thank Drs. Pratip Mitra and Tuan Bui for valuable discussions and assistance with analysis, and Maria Setterbom for work on the figures.
GRANTS
This work was supported by Canadian Institutes of Health Research grants to R. M. Brownstone and L. M. Jordan and a Medical Research Council of Canada Studentship to S. Krawitz.
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- Astman N, Gutnick MJ, Fleidervish IA. Activation of protein kinase C increases neuronal excitability by regulating persistent Na+ current in mouse neocortical slices. J Neurophysiol. 1998;80:1547–1551. doi: 10.1152/jn.1998.80.3.1547. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Gustafsson B, Parmiggiani F. Saturating summation of the afterhyperpolarization conductance in spinal motoneurones: a mechanism for “secondary range” repetitive firing. Brain Res. 1978;146:69–82. doi: 10.1016/0006-8993(78)90218-4. [DOI] [PubMed] [Google Scholar]
- Barrett EF, Barrett JN, Crill WE. Voltage-sensitive outward currents in cat motoneurones. J Physiol. 1980;304:251–276. doi: 10.1113/jphysiol.1980.sp013323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie BR, Dawson NJ, Johansson RS, Lippold OC. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J Physiol. 1986;379:451–459. doi: 10.1113/jphysiol.1986.sp016263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstone RM, Gossard JP, Hultborn H. Voltage-dependent excitation of motoneurones from spinal locomotor centres in the cat. Exp Brain Res. 1994;102:34–44. doi: 10.1007/BF00232436. [DOI] [PubMed] [Google Scholar]
- Brownstone RM, Jordan LM, Kriellaars DJ, Noga BR, Shefchyk S. On the regulation of repetitive firing in lumbar motoneurones during fictive locomotion in the cat. Exp Brain Res. 1992;90:441–445. doi: 10.1007/BF00230927. [DOI] [PubMed] [Google Scholar]
- Cantrell AR, Catterall WA. Neuromodulation of Na+ channels: an unexpected form of cellular plasticity. Nat Rev Neurosci. 2001;2:397–407. doi: 10.1038/35077553. [DOI] [PubMed] [Google Scholar]
- Cantrell AR, Ma JY, Scheuer T, Catterall WA. Muscarinic modulation of sodium current by activation of protein kinase C in rat hippocampal neurons. Neuron. 1996;16:1019–1026. doi: 10.1016/s0896-6273(00)80125-7. [DOI] [PubMed] [Google Scholar]
- Cantrell AR, Smith RD, Goldin AL, Scheuer T, Catterall WA. Dopaminergic modulation of sodium current in hippocampal neurons via cAMP-dependent phosphorylation of specific sites in the sodium channel alpha subunit. J Neurosci. 1997;17:7330–7338. doi: 10.1523/JNEUROSCI.17-19-07330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell AR, Tibbs VC, Westenbroek RE, Scheuer T, Catterall WA. Dopaminergic modulation of voltage-gated Na+ current in rat hippocampal neurons requires anchoring of cAMP-dependent protein kinase. J Neurosci. 1999;19:RC21. doi: 10.1523/JNEUROSCI.19-17-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yu FH, Surmeier DJ, Scheuer T, Catterall WA. Neuromodulation of Na+ channel slow inactivation via cAMP-dependent protein kinase and protein kinase C. Neuron. 2006;49:409–420. doi: 10.1016/j.neuron.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Dai Y, Jones KE, Fedirchuk B, McCrea DA, Jordan LM. A modelling study of locomotion-induced hyperpolarization of voltage threshold in cat lumbar motoneurones. J Physiol. 2002;544:521–536. doi: 10.1113/jphysiol.2002.026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granit R, Kernell D, Shortess GK. The behaviour of mammalian motoneurones during long-lasting orthodromic, antidromic and transmembrane stimulation. J Physiol. 1963a;169:743–754. doi: 10.1113/jphysiol.1963.sp007293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granit R, Kernell D, Shortess GK. Quantitative aspects of repetitive firing of mammalian motoneurons caused by injected currents. J Physiol. 1963b;168:911–931. doi: 10.1113/jphysiol.1963.sp007230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM, Marder E. Modulation of neural networks for behavior. Annu Rev Neurosci. 1991;14:39–57. doi: 10.1146/annurev.ne.14.030191.000351. [DOI] [PubMed] [Google Scholar]
- Hultborn H. Plateau potentials and their role in regulating motoneuronal firing. Prog Brain Res. 1999;123:39–48. doi: 10.1016/s0079-6123(08)62842-3. [DOI] [PubMed] [Google Scholar]
- Johnston J, Griffin SJ, Baker C, Skrzypiec A, Chernova T, Forsythe ID. Initial segment Kv2.2 channels mediate a slow delayed rectifier and maintain high frequency action potential firing in medial nucleus of the trapezoid body neurons. J Physiol. 2008;586:3493–3509. doi: 10.1113/jphysiol.2008.153734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res Rev. 2008;57:183–191. doi: 10.1016/j.brainresrev.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Kernell D. The adaptation and the relation between discharge frequency and current strength of cat lumbosacral motoneurones stimulated by long-lasting injected currents. Acta Physiol Scand. 1965a;65:65–73. doi: 10.1111/j.1748-1716.1965.tb04081.x. [DOI] [PubMed] [Google Scholar]
- Kernell D. The limits of firing frequency in cat lumbosacral motoneurones possessing different time course of afterhyperpolarization. Acta Physiol Scand. 1965b;65:87–100. [Google Scholar]
- Kernell D, Monster AW. Motoneurone properties and motor fatigue. An intracellular study of gastrocnemius motoneurones of the cat. Exp Brain Res. 1982a;46:197–204. doi: 10.1007/BF00237177. [DOI] [PubMed] [Google Scholar]
- Kernell D, Monster AW. Time course and properties of late adaption in spinal motoneurones of the cat. Exp Brain Res. 1982b;46:191–196. doi: 10.1007/BF00237176. [DOI] [PubMed] [Google Scholar]
- Kernell D, Sjoholm H. Repetitive impulse firing: comparisons between neurone models based on “voltage clamp equations” and spinal motoneurones. Acta Physiol Scand. 1973;87:40–56. doi: 10.1111/j.1748-1716.1973.tb05364.x. [DOI] [PubMed] [Google Scholar]
- Krawitz S, Brownstone RM. Late adaptation in cat motoneurones during fictive locomotion (Abstract) Soc Neurosci Abstr. 1994;20:241. [Google Scholar]
- Krawitz S, Brownstone RM, Noga BR, Jordan LM. Can the nervous system overcome a possible central fatigue process—late adaptation? Muscle Nerve Suppl. 1996;4:S52. [Google Scholar]
- Krawitz S, Fedirchuk B, Dai Y, Jordan LM, McCrea DA. State-dependent hyperpolarization of voltage threshold enhances motoneurone excitability during fictive locomotion in the cat. J Physiol. 2001;532:271–281. doi: 10.1111/j.1469-7793.2001.0271g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Dai Y, Brownstone RM. Mechanisms underlying the early phase of spike frequency adaptation in mouse spinal motoneurones. J Physiol. 2005;566:519–532. doi: 10.1113/jphysiol.2005.086033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci USA. 2007;104:2448–24453. doi: 10.1073/pnas.0611134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra DP, Trimmer JS. The Kv2.1 C terminus can autonomously transfer Kv2.1-like phosphorylation-dependent localization, voltage-dependent gating, and muscarinic modulation to diverse Kv channels. J Neurosci. 2006;26:685–695. doi: 10.1523/JNEUROSCI.4620-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom MA, Gorman RB, Laouris Y, Spielmann JM, Stuart DG. Does motoneuron adaptation contribute to muscle fatigue? Muscle Nerve. 2007;35:135–158. doi: 10.1002/mus.20712. [DOI] [PubMed] [Google Scholar]
- Numann R, Catteral WA, Scheuer T. Functional modulation of brain sodium channels by protein kinase C phosphorylation. Science. 1991;254:115–118. doi: 10.1126/science.1656525. [DOI] [PubMed] [Google Scholar]
- Person RS, Kudina LP. Discharge frequency and discharge pattern of human motor units during voluntary contraction of muscle. Electroencephalogr Clin Neurophysiol. 1972;32:471–483. doi: 10.1016/0013-4694(72)90058-2. [DOI] [PubMed] [Google Scholar]
- Powers RK, Sawczuk A, Musick JR, Binder MD. Multiple mechanisms of spike-frequency adaptation in motoneurones. J Physiol (Paris) 1999;93:101–114. doi: 10.1016/s0928-4257(99)80141-7. [DOI] [PubMed] [Google Scholar]
- Richter DW, Heyde F. Accommodative reactions of medullary respiratory neurons of the cat. J Neurophysiol. 1975;38:1172–1180. doi: 10.1152/jn.1975.38.5.1172. [DOI] [PubMed] [Google Scholar]
- Sawczuk A, Powers RK, Binder MD. Contribution of outward currents to spike-frequency adaptation in hypoglossal motoneurons of the rat. J Neurophysiol. 1997;78:2246–2253. doi: 10.1152/jn.1997.78.5.2246. [DOI] [PubMed] [Google Scholar]
- Sawczuk A, Powers RK, Binder MD. Spike frequency adaptation studied in hypoglossal motoneurons of the rat. J Neurophysiol. 1995;73:1799–1810. doi: 10.1152/jn.1995.73.5.1799. [DOI] [PubMed] [Google Scholar]
- Shik ML, Severin FV, Orlovsky GN. Control of walking and running by means of electrical stimulation of the midbrain [in Russian] Biofizika. 1966;11:756–765. [Google Scholar]
- Spielmann JM, Laouris Y, Nordstrom MA, Robinson GA, Reinking RM, Stuart DG. Adaptation of cat motoneurons to sustained and intermittent extracellular activation. J Physiol. 1993;464:75–120. doi: 10.1113/jphysiol.1993.sp019625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves JD, Jordan LM, Lake N. The close proximity of catecholamine-containing cells to the “mesencephalic locomotor region” (MLR) Brain Res. 1975;100:663–670. doi: 10.1016/0006-8993(75)90166-3. [DOI] [PubMed] [Google Scholar]
- West JW, Numann R, Murphy BJ, Scheuer T, Catterall WA. A phosphorylation site in the Na+ channel required for modulation by protein kinase C. Science. 1991;254:866–868. doi: 10.1126/science.1658937. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Rempel J, Brownstone RM. Postnatal development of cholinergic synapses on mouse spinal motoneurons. J Comp Neurol. 2004;474:13–23. doi: 10.1002/cne.20089. [DOI] [PubMed] [Google Scholar]
- Zagoraiou L, Akay T, Martin JF, Brownstone RM, Jessell TM, Miles GB. A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron. 2009;64:645–662. doi: 10.1016/j.neuron.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Powers RK, Newkirk G, Yonkers M, Binder MD. Contribution of persistent sodium currents to spike-frequency adaptation in rat hypoglossal motoneurons. J Neurophysiol. 2005;93:1035–1041. doi: 10.1152/jn.00831.2004. [DOI] [PubMed] [Google Scholar]