Abstract
Since the early 20th century, it has been recognized that motoneurons must fire repetitive trains of action potentials to produce muscle contraction. In 1932, Sir John Eccles, together with Hebbel Hoff, found that action potential spike trains in motor axons were produced by “rhythmic centres”, which were within the motoneurons themselves. Two decades later, Eccles attended a Cold Spring Harbor Symposium in NY, USA entitled “The Neuron”. Two of the many notable presentations at this symposium were juxtaposed: one by Eccles from the University of Otago, Dunedin, NZL, and the other by J. Walter Woodbury and Harry Patton from the University of Washington, Seattle, USA. Both presentations included data obtained using sharp microelectrodes to study the intracellularly recorded potentials of cat motoneurons. In this review, I discuss some of the events leading up to and surrounding this jointly accomplished advance and proceed to discussion of subsequent studies over 5+ decades that have made use of intracellular recordings from motoneurons to study their repetitive firing behavior. This begins with early descriptions of primary and secondary range firing, and continues to the discovery of dendritic persistent inward currents and their relation to plateau potentials, synaptic amplification, and motoneuronal firing. Following a brief description of the possible mechanisms underlying spike frequency adaptation, I discuss the modulation of repetitive firing properties during various motor behaviors. It has become increasingly clear that the central nervous system has exquisite control of the repetitive firing of motoneurons. Eccles’ work laid the foundation for the present-day study of these processes.
Keywords: History, Microelectrode, Motoneuron, Repetitive firing, Spinal cord, Electrophysiology
1. Introduction
In his early work, Sir John Eccles (1903–1997) focused on the study of spinal motoneurons. He began studying activity in motor axons with Sir Charles Sherrington (1857–1952) at the University of Oxford, GBR by means of extracellular recording techniques (Creed et al., 1932). This work led to many key insights into the function of these neurons. Following his move to the University of Otago, NZL, Eccles began to use intracellular recording techniques primarily to study synaptic transmission. His work there and subsequently also laid the foundation for later studies of overall motoneuronal physiology, including repetitive firing (discharge), and this work has continued to the present day. This article begins with a review of Eccles’ contributions to our early understanding of motoneuron physiology, moves on to early studies in repetitive firing behavior by Ragnar Granit (1900–1991), Daniel Kernell, and their colleagues, and then addresses our current understanding of motoneuronal activity during behavior. My intent is not to provide a comprehensive review of motoneuron physiology, which can be found elsewhere (e.g., Rekling et al., 2000; Powers and Binder, 2001). Nor is this article a thorough historical analysis of Eccles’ contributions (for such references, see Curtis and Andersen, 2001; Stuart and Pierce, 2006). Rather, the purpose here is to review a specific aspect of motoneuron physiology: the regulation of repetitive firing. This requires consideration of the state-dependence of motoneuronal properties.
I did not train with Eccles and, indeed, I am two scientific generations removed from him! I trained with Larry Jordan, whose PhD mentor was William Willis. My postdoctoral studies were with Hans Hultborn, who trained with Anders Lundberg. Eccles mentored Willis for his 1962 PhD dissertation research, and Lundberg was a key collaborator of Eccles in the mid-1950s (see Willis, 2006). I met Eccles only once (in 1986 at a meeting on neural plasticity at the University of Washington, Seattle, USA). The historical account that follows is based on my personal interpretation of written work, and discussions with some of the involved parties.
2. Early investigations of the repetitive firing of motor axons (1912–1929)
In the mid-1920s, Alexander Forbes (1882–1965) and James Olmsted (1886–1956) wrote: “ . . . The frequency with which nerve impulses are discharged along the motor neurons in sustained reflex and involuntary contraction of skeletal muscle is a subject about which much has been written and little decided” (p. 17 in Forbes and Olmsted, 1925). They proceeded to discuss the state of the field. It is clear that the relationship between motoneuron firing and muscle contraction was poorly understood at that time. Nevertheless, a preceding German language monograph by Hans Piper (1877–1915) had described human forearm muscle contraction in response to repetitive stimulation of the median nerve, the temperature dependence of the spike component of muscle action potential (AP) firing rate in the turtle, and also the reduction in muscle spike potential firing rate with fatigue (Piper, 1912). According to Forbes and Olmsted (their p. 17), this monograph contains the earliest reference that the rhythm observed in the electromyogram “. . . was that of the impulses in the motor neurons innervating the muscles.”
That repetitive firing is necessary for effective muscular contraction can also be inferred from studies of muscle physiology by John Fulton (1899–1960). Also in the mid-1920s, Fulton wrote: “Broadly speaking, however, the twitch is a physiological abstraction, for single responses seldom occur in the intact animal. Movements are the expression of more or less prolonged tetani” (p. 149 in Fulton, 1926).
In the early 1920s, recording spike potentials directly from motor axons proved to be quite difficult. Forbes and Cattell (1924) attempted such recordings from motor nerves but they were unsuccessful (see also Forbes and Olmsted, 1925). It was Lord Edgar Adrian (1889–1977) and Detlev Bronk (1879–1975) who first recorded spike potentials from individual motor axons. They reported that cat (and human) limb motoneurons discharged at spike rates of ~5–100 Hz in order to effect muscle contraction (Adrian and Bronk, 1929). Furthermore, they stated that the discharge “ . . . varies in frequency according to the general level of excitation in the motor centres” (p. 144 in Adrian and Bronk, 1929).
Derek Denny-Brown (1901–1981) was also successful in recording spike potentials from individual motor units (Denny-Brown, 1929). In studying reflex responses, he found that there was no change in the spike frequency of motor units, but rather there was a stereotypic recruitment of additional motor units. This was the fore-runner to the size principle, which was first postulated by Denny-Brown and Pennybacker (1938) and then demonstrated experimentally by Elwood Henneman (1915–1996) in 1957 (Henneman, 1957; for history, see Henneman and Mendell, 1981).
Denny-Brown (1929) reported that with sudden stretches of the muscle, transient motor unit firing rates as high as 50 Hz were observed. He also recorded initial spike doublets, following which the motor axons discharged “ . . . irregularly and singly at rates which depend upon the strength and rate of afferent stimulus” (p. 272). He noted, however, that there often was no apparent relation between discharge rate and stimulus strength. Denny-Brown also discussed “ . . . a state of central excitation”, which depended on the “ . . . relative amounts of inhibition and excitation summated at that unit” (p. 294). These inputs were recognized to be of both spinal and supraspinal origin.
Thus, by the end of the third decade of the 20th century, it had been established that motoneurons fire repetitively to produce effective muscle contraction, and that the degree of contraction varies with the frequency of discharge. Furthermore, the concept of a general level of excitation of motoneurons and motoneuronal pools was beginning to emerge.
3. Further investigations of the repetitive firing of motor axons (1930–1949)
Recognizing the important work of both Adrian and Denny-Brown, Eccles and Hebbel Hoff (1907–1987) continued the exploration of motoneuronal repetitive firing (Eccles and Hoff, 1932). Recording from motor axons, they studied motoneuron spike discharge induced by reflexes. They found it curious that the discharge of motoneurons had no direct relation to that of the individual afferent stimuli when the stimulation rates were greater than ~10 Hz. The discharge rate varied, however, with the overall intensity of stimulation (rate and strength). This lack of a direct relation implied that there was an intrinsic repetitive firing mechanism in motoneurons, which they explored in further detail. To my knowledge, this 1932 paper is the only one in which Eccles focused primarily on mechanisms underlying repetitive firing in motoneurons. In this paper, the authors foreshadowed much of what was later to be discovered about repetitive firing mechanisms using intracellular recording techniques.
Eccles and Hoff (1932) used the crossed extension reflex to elicit repetitive spike firing in extensor motoneurons while recording extracellulary from individual axons. By evoking antidromic action potentials they altered the observed reflex-evoked pattern of firing. These data led them to define the “rhythmic centre” of the motoneuron, which had an underlying state of excitability that could be modulated. They postulated that the “central excitatory state” (c.e.s.) of this rhythmic center would determine the discharge of the motor units. Further, they reasoned that since an antidromic spike potential could alter the rhythm, and since these spike potentials did not propogate beyond (i.e., central to) motoneurons, the rhythmic center must be the motoneuron, itself: “Each motoneuron has, therefore, an intrinsic rhythmic centre. The rhythm of its discharge is not impressed on it from without, for example, by internuncial neurons next upstream. A rhythmically discharging motoneuron must be subjected to a continuous and more or less uniform bombardment by excitatory impulses from these internuncial neurons. The stimulus of this bombardment activates the rhythmic centre of the motoneuron as evidenced by the discharge so set up. The more intense the bombardment, the faster is the rhythm of this discharge” (p. 105).
In addition, Eccles and Hoff defined the threshold of the c.e.s. to be that point at which rhythmic spike discharge was evoked. This idea was analogous to findings from later current injection experiments, which are discussed below, in which a minimum current needed to evoke repetitive firing was determined.
Eccles and Hoff also showed that an antidromic spike potential elicited during rhythmic firing would lead to a delay in initiation of the next orthodromically activated spike (Fig. 1). That is, the subsequent spike potential did not occur at the predicted time.
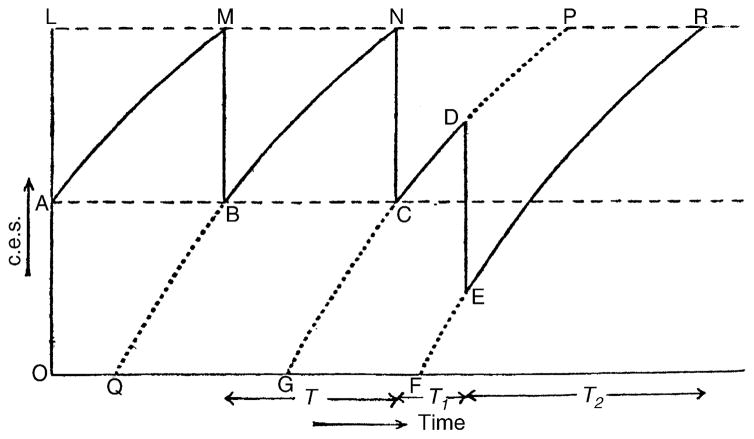
Fig. 1.
A reproduction of Fig. 35 in Eccles and Hoff (1932). The original figure legend read: “Schematic representation of rhythmic discharge and the action of a single antidromic impulse” (p. 507). The associated text read: “The production of a rhythmic reflex discharge according to the conditions postulated is illustrated in Fig. 35, where intensities of c.e.s. are plotted as ordinates against time as absicssae. OF is the line of zero intensity of c.e.s. According to the fourth assumption the intensity of c.e.s., OA, at the point A just after a reflex discharge, equals half the threshold intensity OL. As a result of its uniform production and spontaneous subsidence, c.e.s. is represented as increasing in intensity during normal rhythm along the line AM to again attain threshold at M, where the next reflex discharge is set up. The intensity of c.e.s. falls from M to B, and then increases along the line BN, till at N another reflex discharge is set up, with a consequent fall of c.e.s. to C followed by an increase to P, and so on. By assumption an antidromic impulse acting at a point D, Fig. 35, inactivates an intensity. . . of c.e.s., as shown by the line DE, and c.e.s. then increases along the line ER to regain a threshold intensity at R, where a reflex discharge is set up” (pp. 507–509). The text also provided a mathematical description of these relations and a derivation of the relationship between T2 and T1. Reprinted with permission of the publisher.
Eccles and Hoff interpreted these results as follows. Spike potentials were elicited when the c.e.s. reached a certain threshold (Fig. 1, line LR). Following each spike potential, the c.e.s. decreased by a specific degree, and then slowly increased again due to the maintained excitatory afferent input. The c.e.s. again reached the threshold required to elicit the subsequent spike potential. When an antidromic spike potential was elicited (Fig. 1, point D), the c.e.s. decreased by a standard amount. As the c.e.s. had only partially recovered at the point when the antidromic spike potential was elicited, the associated decrease in c.e.s. would take the neuron even further away from threshold. Therefore, the increase in c.e.s. required to reach spike threshold would take longer (Fig. 1, T2, line ER) than the usual time, T, and the next spike potential would be seen later than its predicted time. Their theory of a c.e.s., its recovery, the reduction of c.e.s. following rhythmic spikes, and the additional reduction following antidromically evoked spike potentials was analogous to the later theory that afterhyperpolarization (AHP) summation can lead to prolongation of the interspike interval. This was proposed several decades later in studies using intracellular recording techniques and/or modelling (see Section 7).
Eccles and Hoff also determined that there was a lower limit to the frequency of repetitive firing in motor axons, and they proceeded to mathematically relate this low frequency to the defined parameters p (rate of production of the c.e.s.) and k (threshold intensity). The parameter p corresponded to what we now understand to be the decay of the AHP. Therefore, this finding also predated by three decades the analogous finding using intracellular microelectrodes. That is, the lower limit of repetitive firing in motoneurons was subsequently shown to relate to AHP duration as measured in an intracellularly recorded, antidromically activated AP (Kernell, 1965a,c).
The concepts that emerged from this single Eccles and Hoff (1932) study foreshadowed by several decades our basic understanding of the mechanisms underlying repetitive firing in motoneurons. Over the next 25 years, however, few such studies were undertaken. There was one study in phrenic motoneurons in which Robert Pitts (1908–1977) concluded that the repetitive firing seen in phrenic motoneurons resulted from the balance between the input they received from inspiratory centers and their recovery of excitability following spike potentials (Pitts, 1943). This paralleled the conclusion of Eccles and Hoff (1932).
4. Intracellular recordings from spinal motoneurons (1949–1957)
The 1949–1952 epoch was a particularly exciting period for neurophysiology. During these years microelectrode techniques were first applied to neurons in the mammalian central nervous system (CNS).
For a history of the iterative invention of intracellular microelectrodes, I draw attention to three letters published in 1983 (Bretag, 1983; Edwards, 1983; Hoyle, 1983). According to these letters, Marshall Barber (1868–1953) used glass micropipettes to first isolate (1902) and then inoculate (1911) single cells (Barber, 1914). Recordings in various animal and plant cells were reported in the early 1920s (Taylor, 1925), and better examples appeared for plant cells in the 1930s (e.g., Osterhout, 1931). Bretag (1983) attributed the first real success in this endeavor for animal cells to Charles Taylor (1885–1946) and Douglas Whitaker (Taylor and Whitaker, 1927). Kenneth Cole (1900–1984) and his colleagues used 2-μm-tip micro-electrodes to record from embryonic myocardial cells in culture (Hogg et al., 1934), and Haruo Kinosita studied Paramecium cells intracellularly (Kinosita, 1936). For excitable nerve cells, Sir Alan Hodgkin (1914–1998) and Sir Andrew Huxley used 40–100 μm diameter glass pipettes to make the first measurements of membrane potential in the squid giant axon (Hodgkin and Huxley, 1939). The same approach was used shortly thereafter by Howard Curtis (1906–1972) and Cole (Curtis and Cole, 1940). In Chicago, USA, Judith Graham Pool (1919–1975) and Ralph Gerard (1900–1974), and Gilbert Ling and Gerard, were the first to report the routine use of KCl-filled fine microelectrodes for the study of the membrane potentials of muscle cells (Graham and Gerard, 1946; Ling and Gerard, 1949). Microelectrodes then became known as “Ling-Gerard” electrodes, although Edwards (1983) proposed the term “Graham-Ling-Gerard” electrodes because Graham (later Graham Pool) was instrumental in the making of these electrodes, which were needed for her PhD thesis work. Having diameters ≤ 1 μm, these electrodes can reasonably be called sharp microelectrodes. Ling perfected this technique and taught it to others, including Karl Frank (1916–1993), Hodgkin, and Walter Woodbury (W. Rall, personal communication).
The above work led inevitably to the study of CNS neurons by the use of intracellular recording techniques. In June 1952 at the Cold Spring Harbor (NY, USA) Symposium “The Neuron”, many of the participating scientists were in the process of forming the basic foundation of modern neuroscience. Five of the attendees went on to receive Nobel Prizes, including Eccles, Hodgkin and Huxley in 1963, Keffer Hartline (1903–1983) in 1967, and Bernard Katz (1911–2002) in 1970. Two consecutive presentations at that meeting reported the use of glass micropipettes for recording intracellular potentials from spinal motoneurons. The first, by Eccles, was entitled “The electrophysiological properties of the motoneuron” (Eccles, 1952) and the second, by Woodbury and Harry Patton (1918–2002), was entitled “Electrical activity of single spinal cord elements (Woodbury and Patton, 1952).” Although Eccles at that time was in the process of moving to the Australian National University, Canberra, AUS, the experiments he reported on had been carried out in Dunedin, NZL, with the initial results reported in the Proceedings of the University of Otaga Medical School (Brock et al., 1951a; Fig. 2).
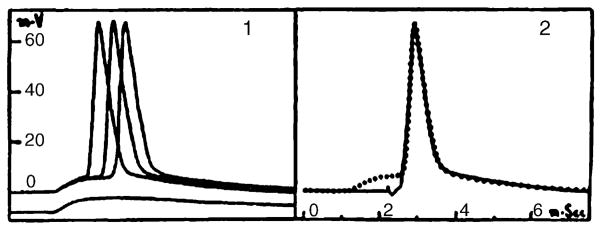
Fig. 2.
A reproduction of Figs. 1 and 2 in Brock et al. (1951a). These are the first known illustrations of an intracellular record from a vertebrate central neuron. The original figure legend read: “Figs. 1 and 2. Intracellular action potentials of motoneurons as described in the text. Potential and time scales common to both figures.” (p. 15). Their Fig. 1 illustrates orthodromically evoked APs evoked by stimulation of group I afferents, with the EPSP shown below. Their Fig. 2 illustrates an antidromically evoked AP, with the dotted line demonstrating a superimposed orthodromic spike. Reprinted with permission of the publisher.
Woodbury and Patton did their work far away from Dunedin in Seattle, USA. They referenced the Brock et al. (1951a,b) report in their Cold Spring Harbor paper. Interestingly, Eccles, himself, did not reference his own report of 1951. Of note, this symposium was also Eccles’ first exposure to the squid axon AP model of Hodgkin and Huxley, which apparently had great impact on him. For example, Wilfred Rall communicated to me “I remember his excited reporting to us when he returned to Dunedin after this meeting. He was so impressed that he included much of H&H in the lectures he presented (and published) in Oxford, only a year later; he made use of prepublication access to the classical (J. Physiol (Lond)) H&H papers. It is clear that he actively associated himself with H&H.”
In addition to the reports referenced below, the following information was obtained in 2005–2006 discussions and correspondence with Dexter Easton, Wilfred Rall, and David Curtis. Having been offered a lectureship by Eccles, Rall moved to Dunedin from Chicago in 1949 (for an autobiographical account, see Rall, 1992). Rall participated in experiments with Eccles, but his thesis work was under the mentorship of Archibald (“Archie”) McIntyre (1913–2002). These experiments involved extracellular recordings “ . . . to explore posttetanic potentiation and to elucidate the reflex effects of different afferent fibers in muscle nerve” (p. 218 in Rall, 1992; see also Brock et al., 1951b; Eccles and Rall, 1950, 1951a,b). Rall, feeling the need to establish his independence, did not participate in the experiments involving intracellular recording. In 1950, Easton arrived at the University of Otaga from Seattle, USA on a postdoctoral Fullbright fellowship. He reported that Woodbury and Patton were developing intracellular recording techniques in Seattle. Woodbury had learned microelectrode techniques directly from Ling and had also worked with Henry Eyring (1901–1981), a renowned physical chemist. Patton traced his scientific lineage to Sherrington, having trained with Theodore Ruch (1906–1983), who had trained with both Sherrington and Fulton (see Stuart et al., 2001). Woodbury and Patton were undoubtedly inspired by Eccles’ earlier work recording extracellular field potentials in the spinal cord. There may have also been rumors that Eccles was pursuing intracellular recording at that time. They therefore enthusiastically pursued this challenge and succeeded, even though this enthusiasm did not persist following their initial report. Rather, they moved on to their primary subsequent interests: Woodbury in neuropharmacology and Patton in the sensorimotor cortex.
There were a number of factors that seemingly inspired Eccles to pursue intracellular recordings as a means to study motoneurons. He had begun to use insulated metal electrodes to record extracellular action and synaptic potentials in motor pools in 1946 (Brooks and Eccles, 1947). He was also very aware of the work of Hodgkin and Huxley as well as Katz’s initial intracellular muscle recordings, which provided a basis on which to consider this technique for recording from motoneurons in vivo. Another factor that may have focused Eccles’ competitive spirit on this technique was Easton’s reports in Dunedin in late 1950 of Woodbury and Patton’s progress in Seattle. Lawrence Brock (1923–1996) was then assigned the task of developing the microelectrodes. The electronic equipment available to Eccles at that time, however, was not suitable for the task at hand, which required the use of high impedance recording microelectrodes. Fortunately, there was a relatively young lecturer in the Department of Physics at that time, John Coombs (1917–1993), whom Eccles recruited to aid in this endeavour. Coombs “was enthusiastic to become involved in neurophysiological research, (and) joined the Dept. of Physiology” (David Curtis, personal communication). Coombs later moved to Canberra with Eccles in late 1952. Coombs based the input stage of a new amplifier on a circuit diagram that had been designed by Jan Tönnies (1902–1970) and given to McIntyre by his former collaborator, David Lloyd (1911–1985) of the Rockefeller Institute, USA. McIntyre already had one built in Dunedin by an electronics technician (W. Rall, personal communication). Eccles initially used McIntyre’s experimental set up (see footnote 4 in Stuart and Pierce, 2006), and Coombs improved on the amplifier design, as well as the design of much of the electronics in the laboratory (see also Curtis and Andersen, 2001; Burke, 2006; Willis, 2006). Curtis communicated the following on Coombs’ contributions: “In Dunedin, in addition to a cathode follower input stage, and direct and capacity-coupled amplifiers, he designed a most versatile electronic stimulating and recording unit based on thermionic ‘valves’ and including a double beam CRT having a linear time base and the facility for sweep expansion. Three electrical pulses were provided, independently controlled for amplitude, duration and timing along the CRT sweep, for stimulating the same or different nerves via RF isolation transmitters. (I continued to use the stimulator portion of one of these in Canberra until 1985 . . .).” Coombs’ general design for microelectrode recording and dual cathode ray tube display is illustrated in Brock et al. (1952). With the new amplifier and the perfection of sharp microelectrodes, Eccles was able to pursue the study of CNS excitatory and inhibitory synapses, which, as can be seen in these pages, was central to his subsequent scientific accomplishments.
Of note, it wasn’t until a few years later that amplifiers were designed so that current could be passed through the recording microelectrode in order to determine the effect of changes in membrane potential on action and synaptic potentials. Eccles and colleagues initially used double-barrelled microelectrodes for this purpose, fabricated by Paul Fatt (Coombs et al., 1953). Tatsunosuke Araki (1926–1985) and Takuzo Otani later described a Wheatstone bridge circuit, with which they were able to pass current through the same, single-barrelled electrode used for recording (Araki and Otani, 1955). Concurrent with this, Coombs and Curtis, the latter as part of his PhD research, had developed a similar bridge circuit for use with single and double electrodes (Coombs et al., 1956, 1959; Curtis and Eccles, 1959). The first single electrode voltage-clamp circuit was not developed for another quarter century (Finkel and Redman, 1983a,b).
It would seem that intracellular recording techniques for the study of mammalian spinal neurons were developed relatively simultaneously (and competitively) in Seattle and Dunedin in the early 1950s. For this reason, both groups are generally given credit for the first use of intracellular microelectrodes in the study of neurons in the vertebrate CNS.
It is interesting to note just how much these two groups described in their reports at the 1952 Cold Spring Harbor Symposium. Eccles described the resting potential of motoneurons (average 70 mV) and recognized that “ . . . the recorded value would be lowered by all defects of the experiment due to injury of the neuron and leakage of current when the micro-electrode fails to self-seal in the membrane” (p. 177 in Eccles, 1952). He proceeded to describe the spike component of the AP (rate of rise at least 500 V/s, overshoot of at least 35 mV, rate of decline of 250 V/s) and the “positive” afterpotential, which was later termed the AHP. He also described some excitatory and inhibitory synaptic responses (Eccles, 1952). Woodbury and Patton recorded from a number of different types of spinal cord neurons at various depths in the spinal cord, including the ventral horn (Woodbury and Patton, 1952). Their specific values differed somewhat from those of Eccles, but they, too, recorded reflex responses in ventral horn neurons as well as antidromic spike potentials (which they differentiated into what were later shown to be the initial segment versus somatodendritic components of the spike; see Frank et al., 1959). Subsequently, Eccles focused on the spike potential and excitatory and inhibitory synaptic potentials in motoneurons (e.g., Coombs et al., 1955a,b,c,d). He did not return to the study of the repetitive firing of motoneurons.
5. Early intracellular investigations of repetitive firing (1958–1975)
Following the demonstration that intracellular recording techniques could be used to study cat spinal motoneurons, this preparation became the favored one in many laboratories for the investigation of motoneuron properties as well as synaptic transmission. Kolmodin and Skoglund (1958) studied repetitive firing during asynchronous synaptic excitation and demonstrated that changing the sensory input (by changing limb position) resulted in changes in the frequency of motoneuronal discharge. They also demonstrated that the voltage threshold for initiation of APs remained constant at any firing rate for any particular motoneuron. When the synaptic excitation increased and the firing rate increased, however, this threshold became more depolarized. There was approximately a linear relationship between threshold and firing rate. They also found that the post-spike AHP was significantly reduced in amplitude with increasing frequency of firing.
With direct impalement of the cell (usually its soma) the motoneuron could be stimulated by using intracellular depolarizing current injection rather than by synaptic excitation. This in turn led to the ability to investigate the autonomous repetitive firing properties of motoneurons. These initial studies were carried out in large part by Daniel Kernell, initially with Granit at the Karolinska Institute in Stockholm, SWE. In particular, two fundamental characteristics of repetitive firing were illustrated: spike frequency adaptation and the relationship between firing frequency and injected current (the f–I relation).
Granit and colleagues found that when subjected to constant intracellular current injection, motoneurons would fire repetitively with a characteristic pattern. The rate of firing would decrease over the first several spikes before reaching a relatively steady state of firing (Granit et al., 1963b). This slowing of the firing rate has subsequently come to be known as spike frequency adaptation (SFA; see Section 7). Granit and colleagues also demonstrated that the firing frequency of motoneurons increased in a linear or bilinear (Fig. 3) fashion with increasing depolarizing current injection (Granit et al., 1963a,b).
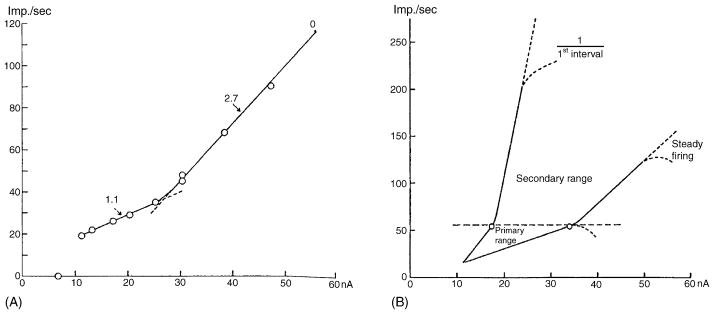
Fig. 3.
A reproduction of Figs. 2 and 8 in Kernell (1965b) demonstrating primary and secondary range firing in a cat motoneuron. The plot in his Fig. 2 is of steady-state firing frequency vs. injected current, and the numbers are the slopes of the primary and secondary range of firing in Hz/nA. His Fig. 8 is a schematic f–I graph demonstrating the primary and secondary ranges for first interval vs. steady-state firing. Reprinted with permission of the publisher.
Fig. 3 shows that in “primary range” firing, motoneurons began to discharge at their lowest rate (found later to be related to the duration of the AP’s AHP; see below), which increased with current up to a mean of 52 Hz (range 30–74 Hz; Kernell, 1965d), with a mean slope of 1.9 Hz/nA (range 0.4–4.5 Hz/nA) (Kernell, 1965a). With increasing depolarizing current injection, the slope of the f–I relation in ~50% of the tested motoneurons increased abruptly to 4.6 Hz/nA (range 4.0–7.5 Hz/nA) (Kernell, 1965b). This second linear phase was termed the “secondary range”. Later, Peter Schwindt described a “tertiary range,” the slope of which could either be higher or lower than that of secondary range firing (Schwindt, 1973).
Kernell suspected that the secondary range was seen only in neurons that were less damaged by microelectrode impalement and had a more hyperpolarized resting membrane potential (Kernell, 1965b). Furthermore, this characteristic relation was seen whether the plot concerned the instantaneous firing frequency represented in the first interspike interval, or the steady-state firing during the later phase of the spike train. With first-interval firing, however, the slopes of both the primary and secondary ranges were greater than in steady-state firing, but the transition frequency from primary to secondary range remained constant (Fig. 3B) (Kernell, 1965b). When studying the first interspike interval, secondary range firing was seen even in those neurons that did not exhibit a secondary range during steady-state firing. Kernell suggested that neurons damaged by impalement could simply not sustain secondary range firing. This study provided an excellent descriptive analysis of repetitive firing. Kernell concluded, however, that it was “ . . . difficult at the present time to give a good theoretical explanation of the facts presented by the above investigations because relatively little is known about the membrane properties of motoneurons and the complicated mechanisms taking part in the production of a repetitive discharge” (p. 83 in Kernell, 1965b).
Kernell also noted consistent changes in the time course of the AHP with changes in firing rate evoked by intracellular current stimulation and suggested that the AHP was a key mechanism controlling firing rate (Kernell, 1965b). He also suggested that the time course of the AHP reflected the “functional state” of the motoneuron. As will be seen below in Sections 8 and 9, the AHP can indeed be modulated during motor behavior.
As mentioned above, secondary range firing was not always present in these early studies, which were carried out in barbiturate-anesthetised cats. Kernell attributed this to possible damage by the impaling microelectrode (Kernell, 1965b). Subsequently, Baldissera and Gustafsson (1971b) found that there was no secondary range firing following acute transection of the spinal cord in pentobarbitone-anesthetised cats. This contrasted with anemically decorticated, pentobarbitone-anesthetised cats in which secondary range firing was present. They suggested that the difference was related to a descending mechanism that decreased the AHP conductance which, when interrupted by spinalization, led to an increase in the AHP. This, in turn, prevented secondary range firing. Their suggestion was in keeping with Kernell’s earlier concept that the AHP reflected the functional state of the motoneuron.
An important question then arose: to what degree does current injected through the microelectrode reflect the synaptic activation of motoneurons? Several investigators subsequently made use of the linear f–I relation to propose that if steady synaptic excitation (or inhibition) was equivalent to the current injected through the microelectrode, then there would simply be a leftward (excitatory effect) or rightward (inhibitory effect) shift of the f–I curve, without a change in its slope. Several such experiments were conducted, and the results were mixed. Granit and colleagues found that segmental excitation and inhibition would summate algebraically with injected current (Granit et al., 1966a,b). This finding was supported by Schwindt and Calvin (1973), who demonstrated that tonic excitation of motoneurons through stimulation of either segmental afferents or the red nucleus led to a shift of the f–I relation in the absence of a change of slope of either the primary or secondary range. They went on to calculate the amount of current provided by the synaptic input based on the extent to which the f–I curve shifted. Their results led the authors to conclude that the mechanisms of action of the two forms of excitation were similar, and that only the “net driving current” was important in the production of repetitive firing. Several deviations from these findings of algebraic summation of injected and synaptically evoked current were found, however. Although Aleksandr Shapovalov (1932–1983) and colleagues showed that the excitation provided to motoneurons by vestibular stimulation added to the injected current, they also demonstrated that similarly evoked hyperpolarizing synaptic current did not summate algebraically, but rather reduced the slope of the f–I relation (Shapovalov et al., 1966). They presented evidence that the descending excitation was operating on the motoneuron’s dendrites whereas the descending inhibition was operating closer to the soma. They did not provide, however, a clear explanation for the non-algebraic summation of inhibitory synaptic current. They may have considered that this reduction in f–I slope was secondary to a decrease in somatic membrane resistance associated with the more proximal location of the inhibitory inputs. In an earlier study, Kernell had found that synaptic current provided by stimulating the ipsilateral brain stem in the region of the red nucleus or by stimulating a peripheral hindlimb nerve, did not necessarily sum algebraically with the injected current (Kernell, 1965d).
Another interesting finding from this era was that secondary range firing was sometimes seen in motoneurons stimulated with combined synaptic and intracellular stimulation when it was not present with intracellular stimulation alone (Granit et al., 1966a,b). These deviations from the primary–secondary range hypothesis must indicate that in some situations, injected current does not necessarily provide the same stimulus to motoneurons as does synaptic current (see Section 6).
After noting that small fluctuations of membrane potential (synaptic “noise”) did little to alter firing rate in the primary range and that large changes in synaptic current often added algebraically with current injected into the motoneuron, Schwindt (1973) postulated that the purpose of the primary range was to maintain “a regular, rhythmic discharge in the face of potentially perturbing influences such as synaptic noise and accommodation” (p. 448). He also postulated that the secondary range occurred “only transiently under sustained synaptic input” (p. 447) and perhaps has “little functional significance” (p. 444).
In summary, by the mid-1970s, some basic repetitive firing properties of spinal motoneurons had been discovered. In particular, such cells were found for the most part to have a bilinear f–I relation, and their AHP was found to be important in the regulation of the interspike interval of spike trains. Furthermore, there were suggestions that although at times synaptic current seemed to have the same effect as current injected through the microelectrode, there were situations when this was not necessarily so. Note that to this point, there had been no consideration of the effects of motoneuron dendrites on repetitive firing.
6. The role of motoneuron dendrites in repetitive firing
On this issue, it is valuable to first briefly examine early thinking about motoneuron dendrites. Shortly after the development of intracellular recording and stimulation techniques, it was noted that in spinal motoneurons the trajectory of the rise and fall of the membrane potential in response to rectangular current injection (Araki and Otani, 1955; Coombs et al., 1956), the strength–latency curve (Frank and Fuortes, 1956), and the decay of EPSPs and IPSPs (Coombs et al., 1956) were all close to having an exponential profile. These effects were related to the membrane time constant. Noting that there were differences in the decay trajectories of EPSPs and IPSPS, and that these decays were not purely exponential, Eccles and colleagues concluded that the slower decays of EPSPs resulted from the “prolonged residuum of excitatory transmitter action” (p. 1050 in Coombs et al., 1956). Rall showed, however, that the change in membrane potential resulting from constant current injection did not follow a single exponential (Rall, 1957). He demonstrated that the transients could be described if one takes into account the cable properties of dendrites. Initially, this interpretation was accepted by Frank and Michelangelo Fuortes (1917–1977) and by Araki and Otani, but not by Eccles (W. Rall, personal communication). Rall’s first full theoretical analysis was published subsequently (Rall, 1959, 1960). He studied cable properties much further, however, and was subsequently involved in two of a landmark series of five papers on synaptic potentials in motoneurons, which were published consecutively (see in their order: Smith et al., 1967; Nelson and Frank, 1967; Burke, 1967; Rall, 1967; Rall et al., 1967). The effects of dendritic cable properties on synaptic potentials were described in these publications. It was only after their publication that Eccles began to appreciate that the properties of dendrites contributed to synaptic integration (W. Rall, personal communication; see also Burke, 2006). Following publication of these papers, increasing consideration was given to the role of the passive cable properties of dendrites in synaptic integration. Nevertheless, dendrites were still thought to behave passively. Therefore, and in light of the experiments of Kernell, Gustafsson, Shapovalov, and Schwindt described above, it was not until much later that dendritic properties were thought to contribute significantly to the repetitive firing properties of motoneurons.
Hultborn and colleagues had noted in the 1970s that following brief stimuli applied to homonymous spindle Ia afferents, motoneurons in decerebrate cats would fire for prolonged periods of time at very steady rates (Hultborn et al., 1975). This firing could be terminated by stimulating a peripheral nerve supplying the antagonist muscle. At that time, they attributed the sustained firing to reverberating activity in excitatory circuits. Shortly thereafter, in two-electrode voltage clamp experiments in anesthetised cats, Schwindt and Wayne Crill described a region of negative slope conductance in the current–voltage relation of motoneurons (Schwindt and Crill, 1977). This current was usually not a net inward current. They speculated at that time that this “persistent inward current” (later called a PIC) could have a role in the amplification of synaptic inputs. Interestingly, they also illustrated sustained firing following depolarizing current injection, although they stated that they did not study this thoroughly. Schwindt and Crill also studied motoneuron membrane properties following administration of penicillin, which led to “spinal seizures,” and found that this current could lead to “self-sustained firing” (Schwindt and Crill, 1980a). That is, in the presence of penicillin, they observed that a short-lasting depolarizing current pulse could produce sustained repetitive firing, which could be terminated by a hyperpolarizing pulse. The PIC was maximal 10–30 mV positive to rest (Schwindt and Crill, 1980c). They concluded that the PIC was generated “on or near the soma” (p. 827 in Schwindt and Crill, 1980b). In addition, they provided evidence that the PICs were mediated by a calcium conductance (Schwindt and Crill, 1980a,b,c). Subsequently, Hultborn and colleagues used intracellular recording techniques to demonstrate that the prolonged firing in response to transient reflex activation was produced intrinsically in the motoneuron by an all-or-none plateau depolarization (Fig. 4; Hounsgaard et al., 1984). They found that they could produce this effect not only by stimulating afferent input but also by stimulating motoneurons with intracellular depolarizing current injection. Similarly, hyperpolarizing current injection could terminate the plateau. When injecting a ramp depolarizing–repolarizing pattern of current injection, they further noted that the frequency–current relation was hysteretic. That is, the firing rate during the repolarizing ramp was faster than at the equivalent current during the depolarizing ramp. Finally, they demonstrated that this “bistable” membrane potential could not be produced following acute spinalization. Injection of 5-hydroxy-tryptophan (a precursor for serotonin), however, led to the re-emergence of this behavior (Hounsgaard et al., 1986).
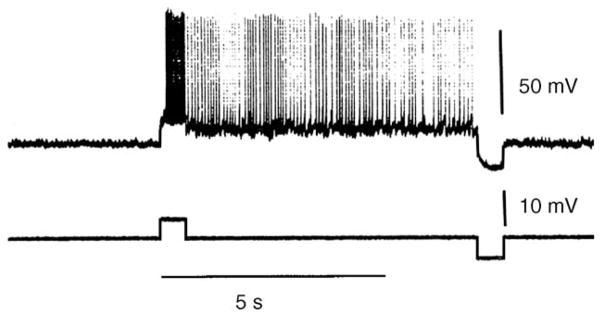
Fig. 4.
A reproduction of Fig. 2A in Hounsgaard et al. (1984) demonstrating sustained firing in a cat motoneuron. The discharge was produced by a short pulse of depolarizing current injection and terminated by a hyperpolarizing pulse. The authors demonstrated that the self-sustained discharge was due to an underlying plateau potential. Reprinted with permission of the publisher.
Given the large number of published reports using intracellular recording and stimulation of motoneurons in the preceding three decades, it is curious that plateau potentials had not been described previously. Recognizing that activity in descending serotonergic pathways was decreased in the commonly used anesthetised cat preparation (Engberg et al., 1968), Hultborn and colleagues attempted to evoke plateau potentials in this preparation, but were unable to do so. Following administration of 5-HTP to enhance serotonergic transmission, or clonidine to mimic noradrenergic transmission, however, plateau potentials could indeed be evoked in anesthetised animals (Conway et al., 1988; Crone et al., 1988; Hounsgaard et al., 1988). The conclusion drawn from these experiments was that monoamines are required for expression of plateau potentials.
Taking the results of Schwindt and Crill and the Hultborn group together, it became clear that there were situations in which the PIC was net inward and the region of negative slope conductance, which was seen as an N-shaped I–V curve, crossed the abscissa with a positive slope at two voltages (Schwindt and Crill, 1977, 1980a,b,c). These two voltages would be stable in two states: at the resting membrane potential and the plateau potential. These situations could occur by the application of drugs (penicillin or potassium channel blockers (Schwindt and Crill, 1980a,b,c)) or neuromodulators (Hounsgaard et al., 1986). That is, plateau potentials and thus PICs in motoneurons are regulated properties.
Jorn Hounsgaard and Ole Kiehn went on to study this phenomenon further in turtle motoneurons. By using an in vitro preparation, they were able to demonstrate conclusively that plateau potentials were mediated by calcium conductances (Hounsgaard and Kiehn, 1985). Furthermore, they demonstrated that these were dihydropyridine-sensitive calcium conductances (Hounsgaard and Kiehn, 1989). That is, plateau potentials in turtle motoneurons are mediated by the L-type (CaV1) family of calcium conductances, which are relatively non-inactivating. Hounsgaard and colleagues later demonstrated in the turtle that activation of metabotropic receptors other than monoaminergic receptors, including metabotropic glutamate receptors and muscarinic receptors, could also lead to activation of plateau potentials (Svirskis and Hounsgaard, 1998). In contrast, in my own laboratory, we have been unable to evoke plateau potentials in mouse motoneurons by activation of muscarinic receptors (Miles and Brownstone, unpublished).
An unresolved issue is where on the motoneuron membrane these calcium conductances are located. Schwindt and Crill (1980b) had suggested originally that they were of somatic or proximal dendritic location. Aron Gutman (1936–1999) reanalyzed the published data of Schwindt and colleagues, presumably using the figures in their papers. In an elegant paper, Gutman (1991) provided extremely convincing evidence that the calcium channels underlying plateau potentials are located in motoneuronal dendrites. Hounsgaard and Kiehn (1993) then showed that in the turtle, differential depolarization of the dendrites could induce plateau potentials, which finding was consistent with Gutman’s argument. After Charles (“C.J.”) Heckman and Robert Lee demonstrated that the synaptic current evoked by group Ia afferent stimulation was enhanced by methoxamine application (which can lead to plateau potentials; Lee & Heckman, 1996), David Bennett together with Hultborn and colleagues demonstrated that the threshold current for activating plateau potentials was much lower when the motoneurons were synaptically activated by Ia afferent stimulation. This finding was consistent with activation of these currents near the site of the synaptic input, i.e., on the dendrites (Bennett et al., 1998). Using somatic voltage clamp of mouse motoneurons in spinal cord slice preparations (Carlin et al., 2000a), we demonstrated delayed inward currents in response to step depolarizations and suggested that the delay was caused by the dendritic location of the conductances (Carlin et al., 2000b; see also Booth and Rinzel, 1995; Booth et al., 1997; Muller and Lux, 1993). In addition, using triangular current injections, we demonstrated that these currents were non-inactivating and dihydropyridine-sensitive and thus equated these conductances with those underlying plateau potentials. Further, we provided immunohistochemical evidence of CaV1.3 channels on the dendrites of mouse motoneurons (Carlin et al., 2000b), a finding that was later replicated in the turtle (Simon et al., 2003). Taken together, these studies clearly demonstrated that PICs originate from dendritic conductances.
Modeling studies have also supported the presence of CaV1-type channels on the dendrites and have led to the suggestion that these channels have relatively low voltages of activation (Booth et al., 1997; Elbasiouny et al., 2005; Bui et al., 2006). It is important to appreciate, however, that given the inaccessibility of these channels to voltage clamp studies (see Carlin et al., 2000b), the biophysical properties of these channels are not known. Although the immunohistochemical data support the presence of CaV1.3 channels on dendritic membranes (Carlin et al., 2000b), this is based on the presence of the α11.3 subunit. The voltage of activation of the channels will depend also on the β and α2δ subunits (Williams et al., 1992; Tomlinson et al., 1993), neither of which have yet been identified and classified in motoneurons. Therefore, caution should be exercised in attributing specific biophysical properties to these channels.
There is recent evidence that PICs include tetrodotoxin(TTX)-sensitive “persistent” sodium currents(Lee & Heckman,2001; Li and Bennett, 2003; for a similar finding on brainstem motoneurons, see Nishimura et al., 1989; Hsiao et al., 1998). Li and Bennett (2003) demonstrated inward currents seen during slow ramp depolarizations that were insensitive to dihydropridines and blocked by TTX. It is known that some NaV subtypes mediate sodium currents with slower time constants of inactivation (Do and Bean, 2004), but the specific subtype of NaV channel which may underlie a Na-PIC is not yet clear. These currents are unlikely to be “persistent” or non-inactivating per se and are more likely to be slowly inactivating (Doand Bean, 2004). Therefore, although the term “PIC” may not be accurate to describe these latter currents, their activation can certainly contribute to the amplification of synaptic input, at least transiently. Furthermore, it has recently been demonstrated that NaV channels can be modulated by protein kinase A (PKA) and protein kinase C (PKC) (Chen et al., 2006). This would give the CNS another mechanism to control the input gain in motoneurons. The contribution of NaV channels to SFA (Section 7) and the apparent modulation of NaV channels during locomotor activity (Section 8) bring to the forefront the apparent importance of these channels in the production of motor behaviors.
In summary, it is likely that at least two types of channels underlie PICs: dendritic CaV1 and NaV channels. The properties of both are modulated by intrinsic signaling pathways in the CNS. PICs amplify synaptic input in both amplitude and time and therefore contribute to the repetitive firing properties of spinal motoneurons. Further details on dendritic PICs have recently been reviewed (Heckman et al., 2003). Here, the focus is next on the effects of these currents on repetitive firing.
It is now clear that motoneurons are indeed “rhythmic centres” (Eccles and Hoff, 1932) and require an appropriate amount of inward current in order to produce an appropriate motor output. Randall Powers and Marc Binder measured the somatic “effective synaptic current” of various synaptic inputs to motoneurons using a modified voltage clamp technique (Powers and Binder, 1995, 2000). They determined that there were conditions in which the synaptic currents they measured were amplified by voltage-gated channels in order to produce the observed rates of repetitive firing. In Timothy Cope’s laboratory, Prather et al. (2001) studied the effects of synaptic input on the f–I relation in motoneurons in the decerebrate cat and demonstrated that there was amplification of two different excitatory inputs. Using computer models, the Kenneth Rose group concluded that PICs are necessary to amplify synaptic input to motoneurons in order to ensure that they fire at the rates needed to accomplish movement (Cushing et al., 2005). This led to the suggestion that the primary role of PICs was to amplify synaptic input. This, in turn, led to this question: do PICs mediate an all-or-none phenomenon leading to plateau potentials, or can they be a variable-gain mechanism for amplifying synaptic input?
Prather et al. (2001) demonstrated that when two inputs to a motoneuron were stimulated together, their amplified current summated linearly. If these inputs are to the same dendrites, and if there is a variable gain, one might expect to see an increase in the slope of the f–I relation when both pathways were stimulated. This was not seen, however, by Prather et al. (2001). Next, Hultborn and colleagues systematically studied the specific question of variable gain (Hultborn et al., 2003). They stimulated the pyramidal tract periodically during ramp current injections and noted that the greater the baseline depolarization, the greater the increase in firing rate. That is, there was no all-or-none jump to an increased firing rate, as one would expect with the initial view of a plateau potential, but rather a graded increase in the amplification of the excitatory synaptic input. The authors interpreted their data as demonstrating that in primary range firing (at the relatively more hyperpolarized potentials during the initial phase of the ramp stimulation), there was little or no activation of the PICs and therefore a fixed increase in firing frequency with stimulation. As the depolarization increased during the ramp, the excitatory input would activate a PIC in the region of the synaptic contacts. The PIC would amplify the input and thus lead to secondary range firing. This increase would be seen as an increase in the change in the firing rate compared to pre-stimulation. The investigators showed that this effect was graded and took this as evidence that there was increasing activation of the PIC with increasing underlying depolarization. That is, the PIC could indeed provide a variable-gain amplification of synaptic excitation. Furthermore, they showed that stimulation of inhibitory inputs (such as recurrent inhibition) only slightly reduced the repetitive firing evoked by a current pulse, but the same stimulation significantly reduced repetitive firing evoked by synaptic excitation. The authors took this as indicating that the pyramidal tract stimulation directly activated PICs (on the dendrites), and the inhibition (distributed throughout the dendritic tree) would thus prevent this activation. In retrospect, this explanation could also be applied to our earlier finding that a small amount of hyperpolarizing current could eliminate motoneuronal repetitive firing during fictive locomotion (Brownstone et al., 1992; see Section 8).
In a recent modeling study, the relationship of PICs to synaptic input was analyzed (Elbasiouny et al., 2006). In agreement with the Hultborn et al. (2003) study, these investigators found that primary range firing was associated with dendritic voltages that were more hyperpolarized than those required to activate PICs. Once PICs were activated, the f–I relation became steeper; i.e., firing moved into the secondary range. At even more depolarized voltages, saturation of PICs led to a flattening of the f–I slope, which the investigators equated with “tertiary range” firing. These authors therefore agreed with the Hultborn et al. (2003) conclusion that PICs provide variable-gain amplification of synaptic input. Interestingly, however, the model also showed that blocking the AHP resulted in PIC activation in an all-or-none fashion (Elbasiouny et al., 2006). It is known that modulation of the AHP results in changes in the slope of the f–I relation (which reflects the gain). Furthermore, it has been shown that the AHP is reduced in motoneurons during fictive locomotion, during which motoneurons often seem to fire in an all-or-none fashion (Brownstone et al., 1992; see Section 8).
The above experimental and modeling studies suggest that the CNS can not only directly modulate PICs but also change the gain of synaptic input to motoneurons by modulating other channels, such as the SK channels producing the AHP (see Section 8).
It bears emphasis that the firing properties discussed above are unlikely to be artifacts of the experimental paradigm. In awake rats, for example, Monica Gorassini, together with Hultborn and colleagues demonstrated that sinusoidal stretches of muscle led to some motor units exhibiting an on–off phenomenon which, based on the activity of all the recorded units, could not be attributed simply to an increase in synaptic drive to the motor pool (Gorassini et al., 1999). Furthermore, phasically firing motor units exhibited more discharge during muscle lengthening than during muscle shortening, as opposed to tonically firing motor units, whose firing rates were modulated seemingly passively by muscle stretch. The authors argued that their results showed that plateau potentials were recruited (and de-recruited) in the phasically firing motoneurons, whereas the tonically firing neurons varied their firing rates in the presence of an already-recruited and sustained plateau potential.
Recent studies have also examined the contribution of PICs to the pathophysiology of motor activity as seen, for example, following spinal cord injury. Bennett and colleagues introduced a model of chronic spinal cord injury, in which the sacral spinal cord was transected in 2-month-old rats (Bennett et al., 1999). Over the subsequent 2 months, these rats developed spastic movements of their tail without the additional problems associated with paraplegia (including bladder and bowel dysfunction). The spinal cord could then be removed and motoneuron properties studied in vitro. In the acutely transected spinal cord, PICs are not present in either cat lumbar motoneurons (Hounsgaard et al., 1988) or rat sacral motoneurons (Bennett et al., 2001). Following chronic injury, however, Bennett et al. (2001) showed that PICs are readily expressed. They attributed this, at least in part, to a developed hypersensitivity of motoneurons to serotonin. Data have also been presented demonstrating that PICs likely play a role in motoneuron firing in humans following spinal cord injury (Gorassini et al., 2004; see also Hornby et al., 2003; Nickolls et al., 2004) and possibly in other conditions, such as cramps (Baldissera et al., 1991). These studies suggest that while the development of spasticity following spinal cord injury is multifactorial (involving, e.g., a variety of interneuronal pathways), the plasticity of motoneuron properties in this pathophysiological process must also be considered.
7. Spike frequency adaptation
Spike frequency adaptation (SFA) is defined as the decrease in firing rate of motoneurons in response to a constant, rectangular-shaped suprathreshold input. It has at least two phases. The first, termed early SFA, is a slowing of firing rate seen over the first few hundred milliseconds (Granit et al., 1963b; Kernell, 1965d). The second phase is termed late SFA. It follows early SFA, and it occurs over many tens of seconds or even minutes (Kernell and Monster, 1982b; Spielmann et al., 1993). Some studies discuss three phases of SFA, with the addition of an “immediate” phase occurring over the first few spikes of a repetitive train (Sawczuk et al., 1995). Although this phenomenology has been known for quite some time, there are still several incompletely answered questions. Is there an advantage to this firing pattern with respect to efficient muscle contraction? What are the biophysical processes underlying these phases of SFA? Are these processes modulatable by neurochemicals found in the spinal cord? Is SFA modulated during behavior? The first three of these questions are addressed in this section, and the fourth will be discussed in Section 8.
The relationship between SFA and muscle contraction was studied by Stein and Parmiggiani (1979). They used extracellular repetitive stimulation of motor nerves and found that the pattern of stimulation most optimal to produce efficient muscle contraction consisted of a short first interspike interval followed by longer subsequent intervals. This led to a more rapid rise in muscle tension, which could then be sustained by the lower stimulation frequencies. This stimulus pattern is similar to the firing pattern that results from rectangular-shaped intracellular stimulation. This similarity, together with previous findings of brief duration first interspike intervals during human motor unit discharge (Norris and Gasteiger, 1955) led the authors to conclude that “ . . . motoneurons appear to be remarkably well designed by evolution and developmental factors to generate optimal patterns for activating the muscles they innervate” (p. 376 in Stein and Parmiggiani, 1979). Although a role for late SFA has not been established, there has been suggestion that it is a contributing factor in muscle fatigue (Kernell and Monster, 1982b; Gandevia, 2001).
Studies on the biophysical processes underlying early SFA were undertaken by Baldissera and Gustafsson (1971a, 1974a,b,c). Cognizant of the earlier finding that post-spike AHPs summated with successive APs (Ito and Oshima, 1962), they showed that the AHP conductance in motoneurons was indeed greater when two APs were elicited at short interval. They used their conductance measurements in a motoneuron model that replicated the early SFA profile (Baldissera et al., 1973; Baldissera and Gustafsson, 1974b). This finding was supported by another modeling study (Kernell, 1968, 1972; Kernell and Sjoholm, 1973). Although the focus continued to be on AHP conductance summation, subsequent investigators considered other processes, particularly in studies on brainstem motoneurons, where the AHP conductance was found to contribute to the early but not later phases of SFA (Sawczuk et al., 1997; Powers et al., 1999; Viana et al., 1993). Studies in neurons of the substantia gelatinosa (Melnick et al., 2004), dorsal root ganglion cells (Blair and Bean, 2003), neocortical neurons (Fleidervish et al., 1996), and hypoglossal motoneurons (Powers et al., 1999) demonstrated that SFA was not necessarily dependent on the AHP and led to the suggestion that the slow inactivation of sodium currents may be a contributing factor.
Our own studies on embryonic stem cell-derived motoneurons found that these neurons in many ways resembled motoneurons (Miles et al., 2004; Soundararajan et al., 2006). These cells demonstrated early SFA, yet were found not to have a measurable AHP conductance (Miles et al., 2004). This prompted us to study SFA in endogenous mouse motoneurons in spinal slices. We found that blocking various AHP conductances, calcium currents, or the M-current did not affect SFA. After measuring the slow inactivation properties of sodium channels, we modeled this property and demonstrated that SFA in the model had the same properties as those of the endogenous motoneurons. This led us to conclude that slow inactivation of the fast inactivating sodium conductance is a key contributing factor to early SFA in mammalian spinal motoneurons (Miles et al., 2005).
The mechanisms underlying late SFA are not clear but it is conceivable that because fast inactivating sodium channels can have many time constants of inactivation, including some quite slow ones, their inactivation may also contributes to late SFA.
It is much more difficult to address the third question regarding whether the processes underlying SFA can be modulated by neuromodulators found in the spinal cord. In light of the above, this question can now be rephrased: can sodium channel inactivation properties be modulated? Although this question has not yet been addressed in motoneurons, a recent study has indeed found that slow inactivation properties of NaV channels can be regulated by PKA and PKC (Chen et al., 2006). Furthermore, since PKA and PKC can be regulated by many neuromodulators, including those found in the spinal cord (e.g., serotonin), it seems reasonable that slow inactivation of sodium conductances, and hence SFA, could be modulated in spinal motoneurons. Furthermore, if the AHP conductance does contribute in any way to SFA, it is clear that this conductance can also be regulated by neuromodulators.
In summary, early and late SFA are fundamental properties of spinal motoneurons. Slow inactivation of fast inactivating sodium channels appears to play an important role in at least early SFA, and it is a property that conceivably can be modulated by the CNS to regulate motoneuron discharge.
8. State-dependence of motoneuron properties: modulation during behavior
Motoneuron firing patterns have been studied in behaving cats (Hoffer et al., 1987) and rats (Eken and Kiehn, 1989; Gorassini et al., 1999, 2000) by use of chronically implanted electrodes to record the ventral-root spike potentials of single motoneurons. Studies have also been undertaken on humans, with an emphasis on recording the discharge of single motor units (e.g., Kiehn and Eken, 1997). In selected instances, the data have suggested that plateau potentials are involved in the observed firing patterns (Eken and Kiehn, 1989; Kiehn and Eken, 1997; Gorassini et al., 1999, 2000; Collins et al., 2001, 2002a,b; Walton et al., 2002). To study the mechanisms (i.e., PICs and their modulation) underlying repetitive firing during behavior, however, it is necessary to use intracellular recording techniques. It would be problematic to accomplish this in intact, behaving animals, of course. Therefore, investigators have relied on fictive locomotion preparations to study motoneuron (and interneuron) activity.
Two mammalian preparations are commonly used to study fictive locomotion. The first is the adult cat, in which locomotor activity is induced either by stimulation of the mesencephalic locomotor region (MLR) (Shik et al., 1966; Jordan et al., 1979; Perrett, 1983) or by systemic administration of DOPA and nialamide (Jankowska et al., 1967). Following administration of neuromuscular blocking drugs to prevent movement, the locomotor pattern can be recorded from multiple peripheral nerves innervating flexor and extensor muscles bilaterally (Jordan et al., 1979). In this preparation, motoneurons can be studied using sharp microelectrodes (Andersson et al., 1978).
The second preparation involves isolation of the spinal cord of either neonatal rats (Kudo and Yamada, 1987; Smith and Feldman, 1987), mice (Nishimaru et al., 2000), or older, “motor functionally mature” mice (Jiang et al., 1999a,b). The latter animals are defined as those at a developmental stage (~P8–P9) where they can bear weight and walk without dragging their abdomens (Jiang et al., 1999a). In such isolated spinal cord preparations, locomotor-like activity can be recorded from the ventral roots. It has been demonstrated in the neonatal rat that activity in the L2 root largely parallels activity in peripheral nerves innervating flexor muscles, and L5 root activity parallels that in extensor-related peripheral nerves (Cowley and Schmidt, 1994b). Application of specific combinations of neurochemicals, e.g., NMDA and serotonin in the neonatal preparations (Cowley and Schmidt, 1994a) or NMDA, serotonin, and dopamine in older mice (Jiang et al., 1999a), leads to locomotor-like ventral-root activity. This involves alternation between the right and left side, and between activity of the L2 and L5 ventral roots. In these preparations, motoneuron activity can be recorded using either sharp microelectrodes or patch-clamp electrodes (Schmidt, 1994). The older animals are needed for the study of properties like PICs because CaV1-type channels do not develop and become functional until after the first post-natal week (Carlin et al., 2000b; Jiang et al., 1999b).
During fictive locomotion induced by stimulation of the MLR in high decerebrate cats, we showed that motoneurons typically fired at relatively high rates (Brownstone et al., 1992). Furthermore, the variance in interspike interval during fictive locomotion, which is a measure of the regularity of firing, was very high. Also, the AHP was reduced in amplitude. The high variance in firing rate during locomotion would be expected with a reduction in AHP (Person and Kudina, 1972; Miles et al., 2005). Another Brownstone et al. (1992) finding was that the reduction in AHP amplitude was associated with a change in the f–I relation: either the slope was increased, or more often it became zero. That is, there was often no relation between spike frequency and the amount of depolarizing current injected through the microelectrode. Similar data were obtained subsequently in the neonatal in vitro rat spinal cord (Schmidt, 1994). These two studies showed that the properties of motoneurons were modulated during this behavior. That is, the repetitive firing characteristics of spinal motoneurons during fictive locomotion differed from those seen in the quiescent animal.
In the same study, we found that small amounts of hyperpolarizing current completely eliminated repetitive firing in motoneurons during fictive locomotion (Brownstone et al., 1992). Cognizant of the then-recent work of Hounsgaard et al. (1988), we postulated that plateau potentials were recruited during fictive locomotion in an all-or-none fashion, and that hyperpolarizing current turned them off, thereby preventing repetitive firing. This prompted a series of experiments with Hultborn in which we demonstrated that the excitatory synaptic input to motoneurons during fictive locomotion activated voltage-dependent channels. As motoneurons were depolarized, they reached a threshold where their input was significantly amplified (Brownstone et al., 1994). We did not conclusively determine whether this voltage-dependence was due to activation of PICs, NMDA receptors, or both (Brownstone et al., 1991, 1994). Nevertheless, these experiments demonstrated quite clearly that motoneurons do not simply respond passively to synaptic current during behavior. In light of the above-mentioned modeling study of Elbasiouny et al. (2006), it seems likely that a reduction of the AHP during fictive locomotion leads to a situation wherein excitatory synaptic input triggers plateau potentials in an all-or-none fashion. This would lead to the high rates of firing seen in locomotor activity and the high variance in interspike interval of the motoneuron AP trains. Furthermore, it would explain why a small amount of hyperpolarizing current eliminates repetitive firing, and why additional depolarizing current does not alter the firing rate.
The AHP and PICs are not the only properties to be modulated during locomotor activity. Further analysis of repetitive firing during MLR-induced fictive locomotion demonstrated that the voltage threshold for initiation of APs is hyperpolarized compared to resting conditions (Krawitz et al., 2001). In a subsequent modeling study, this hyperpolarization was readily accounted for by modulation of the fast inactivating sodium current (Dai et al., 1998).
In light of the above results, combined with evidence that these channels are important in SFA, it is interesting to consider whether SFA is also modulated during fictive locomotion. No evidence of early SFA, which should be seen within a single step cycle, has been found (see Brownstone et al., 1992). It is possible, however, that the high variance in interspike interval could occlude an underlying early SFA process. Given that late SFA is seen even with intermittent stimulation (Spielmann et al., 1993), then if it is present during locomotion, one should record an overall slowing of the firing rate over tens of seconds to minutes. We have presented evidence demonstrating late SFA during a period of time following brainstem stimulation but prior to establishment of locomotor activity (Krawitz et al., 1996). Once alternating locomotor activity began, however, the firing rate increased to the pre-adapted frequency, indicating a “reversal” of late SFA. If late SFA is mediated by a long time constant of inactivation of fast sodium channels (Section 7), then the Krawitz et al. (1996) data, together with the data indicating hyperpolarization of voltage threshold (Krawitz et al., 2001), may indicate modulation of these channels during locomotor activity. Finally, if late SFA is related to fatigue (Kernell and Monster, 1982a; Gandevia, 2001), then modulating (reducing) this property during locomotion may enable the animal to sustain locomotor activity for lengthy periods of time.
In summary, it is clear that the fundamental properties of motoneurons, such as the AHP and voltage threshold, can be modulated during behavior. Although the mechanisms involved in this modulation are far from understood, there have been some recent advances, which are reviewed below.
9. Other possible mechanisms of modulation of motoneuron properties
As presented above, the many conductances important for the production of appropriate rates of repetitive firing in motoneurons during behavior are subject to control by neuromodulators. The pathways by which the CNS modulates the properties of motoneurons during locomotor activity are by no means completely understood. Much of the work to date has focused on monoaminergic regulation of motoneuron properties. Specifically, serotonin and noradrenaline have been studied and shown to modulate PICs (Section 6; see also Lee and Heckman, 1999; Heckman et al., 2003) and voltage threshold (Fedirchuk and Dai, 2004).
We have recently begun to examine the role of muscarinic receptor activation in the regulation of motoneuron discharge (Miles and Brownstone, unpublished). Early on, Conradi and Skoglund (1969) presented immunohistochemical and electronmicroscopic data revealing large terminals on spinal motoneurons. These C-boutons were later shown to be cholinergic (Nagy et al., 1993; Li et al., 1995). They were shown to develop in the early post-natal period in mice (Wilson et al., 2004) and to be in apposition to both post-synaptic type 2 muscarinic (m2) receptors and delayed rectifier KV2.1 channels (Hellström et al., 2003; Muennich and Fyffe, 2004; Wilson et al., 2004).
The functional utility of post-synaptic delayed rectifier channels is not yet clear, although it is reasonable to suggest that this conductance is modulated by muscarinic activation (Wilson et al., 2004). Such modulation has recently been studied in cultured hippocampal neurons and HEK cells, where cholinergic activation has been shown to lead to a leftward shift of the activation curve of the KV2.1 conductance (Mohapatra and Trimmer, 2006). The function of a post-synaptic delayed rectifier channel in motoneurons remains to be investigated, however.
Recently, Robert Fyffe has provided evidence that SK channels are also located at the post-synaptic site of C-boutons (Deng and Fyffe, 2005). This fits with our recent electro-physiological evidence that m2 receptor activation leads to a reduction in the AHP of motoneurons, thus increasing the slope of the f–I relation (Miles and Brownstone, unpublished). In addition, we have evidence that this system is activate during fictive locomotion, thus increasing motoneuronal excitability. It would seem, therefore, that both the descending raphe–spinal serotonergic system and the intraspinal cholinergic system are important for ensuring appropriate motoneuronal output during locomotion.
The existence of the above two rather different systems for regulating motoneuron excitability is interesting to consider. Both develop in the post-natal period (Bregman, 1987; Wilson et al., 2004). The serotonergic system originates in the brainstem raphe nuclei and projects in the ventral spinal cord to motoneurons (Martin et al., 1978), with extensive motoneuronal dendritic innervation (Alvarez et al., 1998). On the other hand, although the neurons of origin of the cholinergic C-boutons have not been definitively demonstrated, it is known that this system is spinal in origin (McLaughlin, 1972). The profound effects of serotonin on motoneurons seem to be mediated primarily by dendritic PICs. That is, there is an increase in the gain of synaptic input in the presence of serotonin. On the other hand, the effect of muscarinic activation is mediated in large part by a reduction in the AHP, which leads to an increase in the f–I slope. In other words, serotonin modulates the input to motoneurons, whereas acetylcholine modulates their output. The combination of the two provides the CNS with exquisite control over motoneuronal excitability.
In summary, there are multiple systems involved in modulating the repetitive firing of motoneurons, and many ion channels are available to mediate this modulation. They include CaV1 channels for plateau potentials and PICs, SK channels for the AHP, and NaV channels, which have properties that control spike voltage threshold and SFA. In addition, KV2.1 channels may also be regulated by acetylcholine. It is likely that other channels involved in the repetitive firing properties of motoneurons are also modulated. Together, these modulatory pathways can lead to complex patterns of firing with response patterns that may differ during different behaviors.
10. Concluding thoughts
Sir John Eccles devoted much time in his career to the study of synaptic integration. The obvious place for him to start was at the end: the study of spinal motoneurons, the final common pathway. His contributions to the field began in his days with Sherrington and continued through his time at the University of Oxford using extracellular recording techniques. In Dunedin, he was instrumental in the development of intracellular recording techniques using sharp microelectrodes. These contributions contributed enormously to the further study of motoneuronal properties in innumerable laboratories around the world. To this day, we are continuing to build upon this early knowledge, which he provided so fully and effectively.
Although it was first demonstrated over seven decades ago that motoneurons have intrinsic “rhythmic centres” for generating repetitive firing (Eccles and Hoff, 1932), and although motoneurons have been studied using intracellular recording over a longer time period than any other type of neuron in the vertebrate CNS, there are still many unanswered questions regarding how the CNS regulates the repetitive firing in these neurons to ensure that there is muscle contraction appropriate for the intended behavior.
Acknowledgments
My work on motoneurons is currently supported by the Canadian Institutes of Health Research and the Nova Scotia Health Research Foundation. In the past, such work has also been supported by the A.L.S. Society of Canada and the Manitoba Health Research Council. The work related to embryonic stem cells is supported by Project A.L.S. I am also supported through a Dalhousie Senior Clinical Research Scholarship. I would like to thank Dexter Easton, Wilfred Rall, and David Curtis for their valuable contributions to the historical content, Hans Hultborn for helpful discussions, Gareth Miles and Jennifer Wilson for their comments on drafts of this manuscript, and Stefan Krueger for assistance in the translation of aspects of Piper’s (1912) monograph.
Abbreviations
- AHP
afterhyperpolarization component of action potential
- AP
action potential
- c.e.s
central excitatory state
- CNS
central nervous system
- EPSP
excitatory post-synaptic potential
- f–I
frequency–current
- IPSP
inhibitory post-synaptic potential
- MLR
mesencephalic locomotor region
- NMDA
N-methyl-D-aspartate
- PIC
persistent inward current
- SFA
spike frequency adaptation
- TTX
tetrodotoxin
References
- Adrian EE, Bronk DW. The discharge of impulses in motor nerve fibres. Part II The frequency of discharge in reflex and voluntary contractions. J Physiol (Lond) 1929;67:119–151. [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Pearson JC, Harrington D, Dewey D, Torbeck L, Fyffe RE. Distribution of 5-hydroxytryptamine-immunoreactive boutons on alpha-motoneurons in the lumbar spinal cord of adult cats. J Comp Neurol. 1998;393:69–83. [PubMed] [Google Scholar]
- Andersson O, Forssberg H, Grillner S, Lindquist M. Phasic gain control of the transmission in cutaneous reflex pathways to motoneurons during ‘fictive’ locomotion. Brain Res. 1978;149:503–507. doi: 10.1016/0006-8993(78)90493-6. [DOI] [PubMed] [Google Scholar]
- Araki T, Otani T. Response of single motoneurons to direct stimulation in toad’s spinal cord. J Neurophysiol. 1955;18:472–485. doi: 10.1152/jn.1955.18.5.472. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Gustafsson B. Regulation of repetitive firing in motoneurons by the afterhyperpolarization conductance. Brain Res. 1971a;30:431–434. doi: 10.1016/0006-8993(71)90096-5. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Gustafsson B. Supraspinal control of the discharge evoked by constant current in the alpha-motoneurons. Brain Res. 1971b;25:642–644. doi: 10.1016/0006-8993(71)90468-9. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Gustafsson B. Afterhyperpolarization conductance time course in lumbar motoneurons of the cat. Acta Physiol Scand. 1974a;91:512–527. doi: 10.1111/j.1748-1716.1974.tb05707.x. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Gustafsson B. Firing behaviour of a neuron model based on the afterhyperpolarization conductance time course and algebraical summation. Adaptation and steady state firing. Acta Physiol Scand. 1974b;92:27–47. doi: 10.1111/j.1748-1716.1974.tb05720.x. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Gustafsson B. Firing behaviour of a neuron model based on the afterhyperpolarization conductance time course. First interval firing. Acta Physiol Scand. 1974c;91:528–544. doi: 10.1111/j.1748-1716.1974.tb05708.x. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Gustafsson B, Parmiggiani F. Adaptation in a simple neuron model compared to that of spinal motoneurons. Brain Res. 1973;52:382–384. doi: 10.1016/0006-8993(73)90676-8. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Cavallari P, Dworzak F. Cramps: a sign of motoneuron ‘bistability’ in a human patient. Neurosci Lett. 1991;133:303–306. doi: 10.1016/0304-3940(91)90594-j. [DOI] [PubMed] [Google Scholar]
- Barber MA. The pipette method in the isolation of single microorganisms and in the inoculation of substances into living cells. Philippine J Sci. 1914;9:307–360. [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998;80:2023–2037. doi: 10.1152/jn.1998.80.4.2023. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Gorassini M, Fouad K, Sanelli L, Han Y, Cheng J. Spasticity in rats with sacral spinal cord injury. J Neurotrauma. 1999;16:69–84. doi: 10.1089/neu.1999.16.69. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol. 2001;86:1955–1971. doi: 10.1152/jn.2001.86.4.1955. [DOI] [PubMed] [Google Scholar]
- Blair NT, Bean BP. Role of tetrodotoxin-resistant Na+ current slow inactivation in adaptation of action potential firing in small-diameter dorsal root ganglion neurons. J Neurosci. 2003;23:10338–10350. doi: 10.1523/JNEUROSCI.23-32-10338.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth V, Rinzel J. A minimal, compartmental model for a dendritic origin of bistability of motoneuron firing patterns. J Comput Neurosci. 1995;2:299–312. doi: 10.1007/BF00961442. [DOI] [PubMed] [Google Scholar]
- Booth V, Rinzel J, Kiehn O. Compartmental model of vertebrate motoneurons for Ca2+-dependent spiking and plateau potentials under pharmacological treatment. J Neurophysiol. 1997;78:3371–3385. doi: 10.1152/jn.1997.78.6.3371. [DOI] [PubMed] [Google Scholar]
- Bregman BS. Development of serotonin immunoreactivity in the rat spinal cord and its plasticity after neonatal spinal cord lesions. Brain Res. 1987;431:245–263. doi: 10.1016/0165-3806(87)90213-6. [DOI] [PubMed] [Google Scholar]
- Bretag A. Who did invent the intracellular microelectrode? Trends Neurosci. 1983;6:365. [Google Scholar]
- Brock LG, Coombs JS, Eccles JC. Action potentials of motoneurons with intracellular electrode. Proc Univ Otago Med Sch. 1951a;29:14–15. [Google Scholar]
- Brock LG, Eccles JC, Rall W. Experimental investigations on the afferent fibres in muscle nerves. Proc Roy Soc Lond B Biol Sci. 1951b;138:453–475. doi: 10.1098/rspb.1951.0035. [DOI] [PubMed] [Google Scholar]
- Brock LG, Coombs JS, Eccles JC. The recording of potentials from motoneurones with an intracellular electrode. J Physiol. 1952;117:431–460. doi: 10.1113/jphysiol.1952.sp004759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CM, Eccles JC. Electrical investigation of the monosynaptic pathway through the spinal cord. J Neurophysiol. 1947;10:251–273. doi: 10.1152/jn.1947.10.4.251. [DOI] [PubMed] [Google Scholar]
- Brownstone RM, Gossard JP, Hultborn H. Voltage-dependency of the motoneruonal locomotor drive potentials during fictive locomotion in the decerebrate cat. J Physiol. 1991;438:216. [Google Scholar]
- Brownstone RM, Jordan LM, Kriellaars DJ, Noga BR, Shefchyk SJ. On the regulation of repetitive firing in lumbar motoneurons during fictive locomotion in the cat. Exp Brain Res. 1992;90:441–455. doi: 10.1007/BF00230927. [DOI] [PubMed] [Google Scholar]
- Brownstone RM, Gossard JP, Hultborn H. Voltage-dependent excitation of motoneurons from spinal locomotor centres in the cat. Exp Brain Res. 1994;102:34–44. doi: 10.1007/BF00232436. [DOI] [PubMed] [Google Scholar]
- Bui TV, Ter-Mikaelian M, Bedrossian D, Rose PK. Computational estimation of the distribution of L-type Ca(2+) channels in motoneurons based on variable threshold of activation of persistent inward currents. J Neurophysiol. 2006;95:225–241. doi: 10.1152/jn.00646.2005. [DOI] [PubMed] [Google Scholar]
- Burke RE. The composite nature of the monosynaptic excitatory postsynaptic potential. J Neurophysiol. 1967;30:1114–1137. doi: 10.1152/jn.1967.30.5.1114. [DOI] [PubMed] [Google Scholar]
- Burke RE. John Eccles’ pioneering role in understanding central synaptic transmission. Prog Neurobiol. 2006;78:173–188. doi: 10.1016/j.pneurobio.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Carlin KP, Jiang Z, Brownstone RM. Characterization of calcium currents in functionally mature mouse spinal motoneurons. Eur J Neurosci. 2000a;12:1624–1634. doi: 10.1046/j.1460-9568.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- Carlin KP, Jones KE, Jiang Z, Jordan LM, Brownstone RM. Dendritic L-type calcium currents in mouse spinal motoneurons: implications for bistability. Eur J Neurosci. 2000b;12:1635–1646. doi: 10.1046/j.1460-9568.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yu FH, Surmeier DJ, Scheuer T, Catterall WA. Neuromodulation of Na+ channel slow inactivation via cAMP-dependent protein kinase and protein kinase C. Neuron. 2006;49:409–420. doi: 10.1016/j.neuron.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Collins DF, Burke D, Gandevia SC. Large involuntary forces consistent with plateau-like behavior of human motoneurons. J Neurosci. 2001;21:4059–4065. doi: 10.1523/JNEUROSCI.21-11-04059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Burke D, Gandevia SC. Sustained contractions produced by plateau-like behaviour in human motoneurons. J Physiol (Lond) 2002a;538:289–301. doi: 10.1113/jphysiol.2001.012825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Gorassini M, Bennett D, Burke D, Gandevia SC. Recent evidence for plateau potentials in human motoneurons. Adv Exp Med Biol. 2002b;508:227–235. doi: 10.1007/978-1-4615-0713-0_28. [DOI] [PubMed] [Google Scholar]
- Conradi S, Skoglund S. Observations on the ultrastructure and distribution of neuronal and glial elements on the motoneuron surface in the lumbosacral spinal cord of the cat during postnatal development. Acta Physiol Scand Suppl. 1969;333:5–52. [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O, Mintz I. Plateau potentials in alpha-motoneurons induced by intravenous injection of L-dopa and clonidine in the spinal cat. J Physiol (Lond) 1988;405:369–384. doi: 10.1113/jphysiol.1988.sp017337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs JS, Eccles JC, Fatt P. The action of the inhibitory synaptic transmitter. Aust J Sci. 1953;16:1–5. [Google Scholar]
- Coombs JS, Eccles JC, Fatt P. The electrical properties of the motoneuron membrane. J Physiol (Lond) 1955a;130:291–325. doi: 10.1113/jphysiol.1955.sp005411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs JS, Eccles JC, Fatt P. Excitatory synaptic action in motoneurons. J Physiol (Lond) 1955b;130:374–395. doi: 10.1113/jphysiol.1955.sp005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs JS, Eccles JC, Fatt P. The inhibitory suppression of reflex discharges from motoneurons. J Physiol (Lond) 1955c;130:396–413. doi: 10.1113/jphysiol.1955.sp005414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs JS, Eccles JC, Fatt P. The specific ionic conductances and the ionic movements across the motoneuronal membrane that produce the inhibitory post-synaptic potential. J Physiol (Lond) 1955d;130:326–374. doi: 10.1113/jphysiol.1955.sp005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs JS, Curtis DR, Eccles JC. Time courses of motoneuronal responses. Nature. 1956;178:1049–1050. doi: 10.1038/1781049a0. [DOI] [PubMed] [Google Scholar]
- Coombs JS, Curtis DR, Eccles JC. The electrical constants of the motoneurone membrane. J Physiol. 1959;145:505–528. doi: 10.1113/jphysiol.1959.sp006158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. A comparison of motor patterns induced by N-methyl-D-aspartate, acetylcholine and serotonin in the in vitro neonatal rat spinal cord. Neurosci Lett. 1994a;171:147–150. doi: 10.1016/0304-3940(94)90626-2. [DOI] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. Some limitations of ventral root recordings for monitoring locomotion in the in vitro neonatal rat spinal cord preparation. Neurosci Lett. 1994b;171:142–146. doi: 10.1016/0304-3940(94)90625-4. [DOI] [PubMed] [Google Scholar]
- Creed RS, Denny-Brown D, Eccles JC, Liddell EGT, Sherrington CS. Reflex Activity of the Spinal Cord. Oxford University Press; Oxford: 1932. [Google Scholar]
- Crone C, Hultborn H, Kiehn O, Mazieres L, Wigstrom H. Maintained changes in motoneuronal excitability by short-lasting synaptic inputs in the decerebrate cat. J Physiol (Lond) 1988;405:321–343. doi: 10.1113/jphysiol.1988.sp017335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DR, Andersen P. John Carew Eccles 1903–1997. Hist Rec Aust Sci. 2001;13:439–473. doi: 10.1071/hr0011340439. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Eccles JC. The time courses of excitatory and inhibitory synaptic actions. J Physiol. 1959;145:529–546. doi: 10.1113/jphysiol.1959.sp006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing S, Bui T, Rose PK. Effect of nonlinear summation of synaptic currents on the input–output properties of spinal motoneurons. J Neurophysiol. 2005;94:3465–3478. doi: 10.1152/jn.00439.2005. [DOI] [PubMed] [Google Scholar]
- Dai Y, Jones KE, Fedirchuk B, Krawitz S, Jordan LM. Modeling the lowering of motoneuron voltage threshold during fictive locomotion. Ann NY Acad Sci. 1998;860:492–495. doi: 10.1111/j.1749-6632.1998.tb09085.x. [DOI] [PubMed] [Google Scholar]
- Deng Z, Fyffe RE. Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2005. Differential distribution of the small conductance Ca2+-activated K+ channel subunit SK3 in alpha-motoneurons in rat lumbar spinal cord; p. 750.1. [Google Scholar]
- Denny-Brown D. On the nature of postural reflexes. Proc Roy Soc B. 1929;104:252–301. [Google Scholar]
- Denny-Brown D, Pennybacker JB. Fibrillation and fasciculation in voluntary muscle. Brain. 1938;61:311–334. [Google Scholar]
- Do MT, Bean BP. Sodium currents in subthalamic nucleus neurons from Nav1.6-null mice. J Neurophysiol. 2004;92:726–733. doi: 10.1152/jn.00186.2004. [DOI] [PubMed] [Google Scholar]
- Eccles JC. Proceedings of the Cold Spring Harbor Symposia on Quantitative Biology, vol. XVII, The Neuron. The Biological Laboratory; Cold Spring Harbor, NY: 1952. The electrophysiological properties of the motoneuron; pp. 175–183. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Hoff HE. The rhythmic discharge of motoneurons. Proc Roy Soc B. 1932;110:483–514. [Google Scholar]
- Eccles JC, Rall W. Post-tetanic potentiation of responses of motoneurons. Nature. 1950;166:465–466. doi: 10.1038/166465a0. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Rall W. Effects induced in a monosynaptic reflex path by its activation. J Neurophysiol. 1951a;14:353–376. doi: 10.1152/jn.1951.14.5.353. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Rall W. Repetitive monosynaptic activation of motoneurons. Proc Roy Soc Lond B Biol Sci. 1951b;138:475–498. doi: 10.1098/rspb.1951.0036. [DOI] [PubMed] [Google Scholar]
- Edwards C. Who invented the intracellular microelectrode? Trends Neurosci. 1983;6:44. [Google Scholar]
- Eken T, Kiehn O. Bistable firing properties of soleus motor units in unrestrained rats. Acta Physiol Scand. 1989;136:383–394. doi: 10.1111/j.1748-1716.1989.tb08679.x. [DOI] [PubMed] [Google Scholar]
- Elbasiouny SM, Bennett DJ, Mushahwar VK. Simulation of dendritic CaV1.3 channels in cat lumbar motoneurons: spatial distribution. J Neurophysiol. 2005;94:3961–3974. doi: 10.1152/jn.00391.2005. [DOI] [PubMed] [Google Scholar]
- Elbasiouny SM, Bennett DJ, Mushahwar VK. Simulation of Ca2+ persistent inward currents in spinal motoneurons: mode of activation and integration of synaptic inputs. J Physiol (Lond) 2006;570:355–374. doi: 10.1113/jphysiol.2005.099119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg I, Lundberg A, Ryall RW. Is the tonic decerebrate inhibition of reflex paths mediated by monoaminergic pathways? Acta Physiol Scand. 1968;72:123–133. doi: 10.1111/j.1748-1716.1968.tb03834.x. [DOI] [PubMed] [Google Scholar]
- Fedirchuk B, Dai Y. Monoamines increase the excitability of spinal neurons in the neonatal rat by hyperpolarizing the threshold for action potential production. J Physiol (Lond) 2004;557:355–361. doi: 10.1113/jphysiol.2004.064022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel AS, Redman S. A shielded microelectrode suitable for single-electrode voltage clamping of neurones in the CNS. J Neurosci Methods. 1983a;9:23–29. doi: 10.1016/0165-0270(83)90105-x. [DOI] [PubMed] [Google Scholar]
- Finkel AS, Redman SJ. The synaptic current evoked in cat spinal motoneurones by impulses in single group 1a axons. J Physiol. 1983b;342:615–632. doi: 10.1113/jphysiol.1983.sp014872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleidervish IA, Friedman A, Gutnick MJ. Slow inactivation of Na+ current and slow cumulative spike adaptation in mouse and guinea-pig neocortical neurons in slices. J Physiol (Lond) 1996;493(Pt 1):83–97. doi: 10.1113/jphysiol.1996.sp021366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes A, Cattell M. Electrical studies in mammalian reflexes. IV The crossed extension reflex. Am J Physiol. 1924;70:140–173. [Google Scholar]
- Forbes A, Olmsted JMD. The frequency of motor nerve impulses in the crossed extension reflex as shown by the alcohol block method. Am J Physiol. 1925;73:17–62. [Google Scholar]
- Frank K, Fuortes MG. Stimulation of spinal motoneurons with intracellular electrodes. J Physiol (Lond) 1956;134:451–470. doi: 10.1113/jphysiol.1956.sp005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank K, Fuortes MG, Nelson PG. Voltage clamp of motoneuron soma. Science. 1959;130:38–39. doi: 10.1126/science.130.3366.38. [DOI] [PubMed] [Google Scholar]
- Fulton JF. Muscular Contraction and the Reflex Control of Movement. Williams & Wilkins; Baltimore: 1926. [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Bennett DJ, Kiehn O, Eken T, Hultborn H. Activation patterns of hindlimb motor units in the awake rat and their relation to motoneuron intrinsic properties. J Neurophysiol. 1999;82:709–717. doi: 10.1152/jn.1999.82.2.709. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Eken T, Bennett DJ, Kiehn O, Hultborn H. Activity of hindlimb motor units during locomotion in the conscious rat. J Neurophysiol. 2000;83:2002–2011. doi: 10.1152/jn.2000.83.4.2002. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain. 2004;127:2247–2258. doi: 10.1093/brain/awh243. [DOI] [PubMed] [Google Scholar]
- Graham J, Gerard RW. Membrane potentials and excitation of impaled single muscle fibers. J Cell Comp Physiol. 1946;28:99–117. doi: 10.1002/jcp.1030280106. [DOI] [PubMed] [Google Scholar]
- Granit R, Kernell D, Shortess GK. The behaviour of mammalian motoneurons during long-lasting orthodromic, antidromic and transmembrane stimulation. J Physiol (Lond) 1963a;169:743–754. doi: 10.1113/jphysiol.1963.sp007293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granit R, Kernell D, Shortess GK. Quantitative aspects of repetitive firing of mammalian motoneurons, caused by injected currents. J Physiol (Lond) 1963b;168:911–931. doi: 10.1113/jphysiol.1963.sp007230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granit R, Kernell D, Lamarre Y. Algebraical summation in synaptic activation of motoneurons firing within the ‘primary range’ to injected currents. J Physiol (Lond) 1966a;187:379–399. doi: 10.1113/jphysiol.1966.sp008097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granit R, Kernell D, Lamarre Y. Synaptic stimulation superimposed on motoneurons firing in the ‘secondary range’ to injected current. J Physiol (Lond) 1966b;187:401–415. doi: 10.1113/jphysiol.1966.sp008098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman AM. Bistability of dendrites. Int J Neural Syst. 1991;1:291–304. doi: 10.1142/s0129065794000104. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci. 2003;26:688–695. doi: 10.1016/j.tins.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Hellström J, Oliveira AL, Meister B, Cullheim S. Large cholinergic nerve terminals on subsets of motoneurons and their relation to muscarinic receptor type 2. J Comp Neurol. 2003;460:476–486. doi: 10.1002/cne.10648. [DOI] [PubMed] [Google Scholar]
- Henneman E. Relation between size of neurons and their susceptibility to discharge. Science. 1957;126:1345–1347. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- Henneman E, Mendell LM. Functional organization of motoneuron pool and its inputs. In: Brooks VB, editor. Handbook of Physiology, The Nervous System, Motor Control. Vol. 1. American Physiological Society; Bethesda, MD: 1981. pp. 423–507. [Google Scholar]
- Hodgkin AL, Huxley AF. Nature. 1939;144:710–711. [Google Scholar]
- Hoffer JA, Sugano N, Loeb GE, Marks WB, O’Donovan MJ, Pratt CA. Cat hindlimb motoneurons during locomotion II Normal activity patterns. J Neurophysiol. 1987;57:530–553. doi: 10.1152/jn.1987.57.2.530. [DOI] [PubMed] [Google Scholar]
- Hogg BM, Goss CM, Cole KS. Potentials in embryo rat heart muscle cultures. Proc Soc Exp Biol Med. 1934;32:304–307. [Google Scholar]
- Hornby TG, Rymer WZ, Benz EN, Schmit BD. Windup of flexion reflexes in chronic human spinal cord injury: a marker for neuronal plateau potentials? J Neurophysiol. 2003;89:416–426. doi: 10.1152/jn.00979.2001. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Ca++ dependent bistability induced by serotonin in spinal motoneurons. Exp Brain Res. 1985;57:422–425. doi: 10.1007/BF00236551. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurons caused by a nifedipine-sensitive calcium plateau potential. J Physiol (Lond) 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Calcium spikes and calcium plateaux evoked by differential polarization in dendrites of turtle motoneurons in vitro. J Physiol (Lond) 1993;468:245–259. doi: 10.1113/jphysiol.1993.sp019769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Intrinsic membrane properties causing a bistable behaviour of alpha-motoneurons. Exp Brain Res. 1984;55:391–394. doi: 10.1007/BF00237290. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Kiehn O. Transmitter-controlled properties of alpha-motoneurons causing long-lasting motor discharge to brief excitatory inputs. Prog Brain Res. 1986;64:39–49. doi: 10.1016/S0079-6123(08)63398-1. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurons in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol (Lond) 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle G. Origins of intracellular microelectrodes. Trends Neurosci. 1983;6:163. [Google Scholar]
- Hsiao CF, Del Negro CA, Trueblood PR, Chandler SH. Ionic basis for serotonin-induced bistable membrane properties in guinea pig trigeminal motoneurons. J Neurophysiol. 1998;79:2847–2856. doi: 10.1152/jn.1998.79.6.2847. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Wigström H, Wängberg B. Prolonged activation of soleus motoneurons following a conditioning train in soleus Ia afferents—a case for a reverberating loop? Neurosci Lett. 1975;1:147–152. doi: 10.1016/0304-3940(75)90030-0. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Denton ME, Wienecke J, Nielsen JB. Variable amplification of synaptic input to cat spinal motoneurons by dendritic persistent inward current. J Physiol (Lond) 2003;552:945–952. doi: 10.1113/jphysiol.2003.050971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Oshima T. Temporal summation of afterhyperpolarization following a motoneuron spike. Nature. 1962;195:910–911. [Google Scholar]
- Jankowska E, Jukes MG, Lund S, Lundberg A. The effect of DOPA on the spinal cord. 6 Half-centre organization of interneurons transmitting effects from the flexor reflex afferents. Acta Physiol Scand. 1967;70:389–402. doi: 10.1111/j.1748-1716.1967.tb03637.x. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Carlin KP, Brownstone RM. An in vitro functionally mature mouse spinal cord preparation for the study of spinal motor networks. Brain Res. 1999a;816:493–499. doi: 10.1016/s0006-8993(98)01199-8. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Rempel J, Li J, Sawchuk MA, Carlin KP, Brownstone RM. Development of L-type calcium channels and a nifedipine-sensitive motor activity in the postnatal mouse spinal cord. Eur J Neurosci. 1999b;11:3481–3487. doi: 10.1046/j.1460-9568.1999.00765.x. [DOI] [PubMed] [Google Scholar]
- Jordan LM, Pratt CA, Menzies JE. Locomotion evoked by brain stem stimulation: occurrence without phasic segmental afferent input. Brain Res. 1979;177:204–207. doi: 10.1016/0006-8993(79)90933-8. [DOI] [PubMed] [Google Scholar]
- Kernell D. The adaptation and the relation between discharge frequency and current strength of cat lumbosacral motoneurons stimulated by long-lasting injected currents. Acta Physiol Scand. 1965a;65:65–73. doi: 10.1111/j.1748-1716.1965.tb04081.x. [DOI] [PubMed] [Google Scholar]
- Kernell D. High-frequency repetitive firing of cat lumbosacral motoneurons stimulated by long-lasting injected currents. Acta Physiol Scand. 1965b;65:74–86. doi: 10.1111/j.1748-1716.1965.tb04081.x. [DOI] [PubMed] [Google Scholar]
- Kernell D. The limits of firing frequency in cat lumbosacral motoneurons possessing different time course afterhyperpolarization. Acta Physiol Scand. 1965c;65:87–100. [Google Scholar]
- Kernell D. Synaptic influence on the repetitive activity elicited in cat lumbosacral motoneurons by long-lasting injected currents. Acta Physiol Scand. 1965d;63:409–410. doi: 10.1111/j.1748-1716.1965.tb04081.x. [DOI] [PubMed] [Google Scholar]
- Kernell D. The repetitive impulse discharge of a simple neuron model compared to that of spinal motoneurons. Brain Res. 1968;11:685–687. doi: 10.1016/0006-8993(68)90157-1. [DOI] [PubMed] [Google Scholar]
- Kernell D. The early phase of adaptation in repetitive impulse discharges of cat spinal motoneurons. Brain Res. 1972;41:184–186. doi: 10.1016/0006-8993(72)90626-9. [DOI] [PubMed] [Google Scholar]
- Kernell D, Monster AW. Motoneuron properties and motor fatigue. An intracellular study of gastrocnemius motoneurons of the cat. Exp Brain Res. 1982a;46:197–204. doi: 10.1007/BF00237177. [DOI] [PubMed] [Google Scholar]
- Kernell D, Monster AW. Time course and properties of late adaptation in spinal motoneurons of the cat. Exp Brain Res. 1982b;46:191–196. doi: 10.1007/BF00237176. [DOI] [PubMed] [Google Scholar]
- Kernell D, Sjoholm H. Repetitive impulse firing: comparisons between neuron models based on ’voltage clamp equations’ and spinal motoneurons. Acta Physiol Scand. 1973;87:40–56. doi: 10.1111/j.1748-1716.1973.tb05364.x. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J Neurophysiol. 1997;78:3061–3068. doi: 10.1152/jn.1997.78.6.3061. [DOI] [PubMed] [Google Scholar]
- Kinosita H. Electric excitation and electric polarization in Paramecium. J Fac Sci Tokyo Imp Univ. 1936;4:155–161. [Google Scholar]
- Kolmodin GM, Skoglund CR. Slow membrane potential changes accompanying excitation and inhibition in spinal moto- and interneurons in the cat during natural activation. Acta Physiol Scand. 1958;44:11–54. doi: 10.1111/j.1748-1716.1958.tb01607.x. [DOI] [PubMed] [Google Scholar]
- Krawitz S, Brownstone RM, Noga BR, Jordan LM. Can the nervous system overcome a possible central fatigue process—late adaptation? Muscle Nerve Suppl. 1996;4:S52. [Google Scholar]
- Krawitz S, Fedirchuk B, Dai Y, Jordan LM, McCrea DA. State-dependent hyperpolarization of voltage threshold enhances motoneuron excitability during fictive locomotion in the cat. J Physiol (Lond) 2001;532:271–281. doi: 10.1111/j.1469-7793.2001.0271g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N, Yamada T. N-methyl-D,L-aspartate-induced locomotor activity in a spinal cord–hindlimb muscles preparation of the newborn rat studied in vitro. Neurosci Lett. 1987;75:43–48. doi: 10.1016/0304-3940(87)90072-3. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Influence of voltage-sensitive dendritic conductances on bistable firing and effective synaptic current in cat spinal motoneurons in vivo. J Neurophysiol. 1996;76:2107–2110. doi: 10.1152/jn.1996.76.3.2107. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Enhancement of bistability in spinal motoneurons in vivo by the noradrenergic alpha1 agonist methoxamine. J Neurophysiol. 1999;81:2164–2174. doi: 10.1152/jn.1999.81.5.2164. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Essential role of a fast persistent inward current in action potential initiation and control of rhythmic firing. J Neurophysiol. 2001;85:472–475. doi: 10.1152/jn.2001.85.1.472. [DOI] [PubMed] [Google Scholar]
- Li W, Ochalski PA, Brimijoin S, Jordan LM, Nagy JI. C-terminals on motoneurons: electron microscope localization of cholinergic markers in adult rats and antibody-induced depletion in neonates. Neuroscience. 1995;65:879–891. doi: 10.1016/0306-4522(94)00511-3. [DOI] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol. 2003;90:857–869. doi: 10.1152/jn.00236.2003. [DOI] [PubMed] [Google Scholar]
- Ling G, Gerard RW. The membrane potential and metabolism of muscle fibers. J Cell Comp Physiol. 1949;34:413–438. doi: 10.1002/jcp.1030340307. [DOI] [PubMed] [Google Scholar]
- Martin RF, Jordan LM, Willis WD. Differential projections of cat medullary raphe neurons demonstrated by retrograde labelling following spinal cord lesions. J Comp Neurol. 1978;182:77–88. doi: 10.1002/cne.901820106. [DOI] [PubMed] [Google Scholar]
- McLaughlin BJ. Propriospinal and supraspinal projections to the motor nuclei in the cat spinal cord. J Comp Neurol. 1972;144:475–500. doi: 10.1002/cne.901440406. [DOI] [PubMed] [Google Scholar]
- Melnick IV, Santos SF, Safronov BV. Mechanism of spike frequency adaptation in substantia gelatinosa neurons of rat. J Physiol (Lond) 2004;559:383–395. doi: 10.1113/jphysiol.2004.066415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Yohn DC, Wichterle H, Jessell TM, Rafuse VF, Brownstone RM. Functional properties of motoneurons derived from mouse embryonic stem cells. J Neurosci. 2004;24:7848–7858. doi: 10.1523/JNEUROSCI.1972-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Dai Y, Brownstone RM. Mechanisms underlying the early phase of spike frequency adaptation in mouse spinal motoneurons. J Physiol (Lond) 2005;566:519–532. doi: 10.1113/jphysiol.2005.086033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra DP, Trimmer JS. The Kv2.1 C terminus can autonomously transfer Kv2.1-like phosphorylation-dependent localization, voltage-dependent gating, and muscarinic modulation to diverse Kv channels. J Neurosci. 2006;26:685–695. doi: 10.1523/JNEUROSCI.4620-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muennich EA, Fyffe RE. Focal aggregation of voltage-gated, Kv2.1 subunit-containing, potassium channels at synaptic sites in rat spinal motoneurons. J Physiol (Lond) 2004;554:673–685. doi: 10.1113/jphysiol.2003.056192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W, Lux HD. Analysis of voltage-dependent membrane currents in spatially extended neurons from point-clamp data. J Neurophysiol. 1993;69:241–247. doi: 10.1152/jn.1993.69.1.241. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Yamamoto T, Jordan LM. Evidence for the cholinergic nature of C-terminals associated with subsurface cisterns in alpha-motoneurons of rat. Synapse. 1993;15:17–32. doi: 10.1002/syn.890150103. [DOI] [PubMed] [Google Scholar]
- Nelson PG, Frank K. Anomalous rectification in cat spinal motoneurons and effect of polarizing currents on excitatory postsynaptic potential. J Neurophysiol. 1967;30:1097–1113. doi: 10.1152/jn.1967.30.5.1097. [DOI] [PubMed] [Google Scholar]
- Nickolls P, Collins DF, Gorman RB, Burke D, Gandevia SC. Forces consistent with plateau-like behaviour of spinal neurons evoked in patients with spinal cord injuries. Brain. 2004;127:660–670. doi: 10.1093/brain/awh073. [DOI] [PubMed] [Google Scholar]
- Nishimaru H, Takizawa H, Kudo N. 5-Hydroxytryptamine-induced locomotor rhythm in the neonatal mouse spinal cord in vitro. Neurosci Lett. 2000;280:187–190. doi: 10.1016/s0304-3940(00)00805-3. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Schwindt PC, Crill WE. Electrical properties of facial motoneurons in brainstem slices from guinea pig. Brain Res. 1989;502:127–142. doi: 10.1016/0006-8993(89)90468-x. [DOI] [PubMed] [Google Scholar]
- Norris FH, Jr, Gasteiger EL. Action potentials of single motor units in normal muscle. Electroencephalogr Clin Neurophysiol Suppl. 1955;7:115–125. doi: 10.1016/0013-4694(55)90064-x. [DOI] [PubMed] [Google Scholar]
- Osterhout WJV. Physiological studies of single plant cells. Biol Rev Camb Philos Soc. 1931;6:369–411. [Google Scholar]
- Perrett C. Neural Origin of Rhythmic Movements. Cambridge University Press; New York: 1983. Centrally generated pattern of motoneuron activity during locomotion in the cat; pp. 405–422. [PubMed] [Google Scholar]
- Person RS, Kudina LP. Discharge frequency and discharge pattern of human motor units during voluntary contraction of muscle. Electroencephalogr Clin Neurophysiol. 1972;32:471–483. doi: 10.1016/0013-4694(72)90058-2. [DOI] [PubMed] [Google Scholar]
- Piper H. Elektrophysiologie menschlicher Muskeln. Verlag von Julius Springer; Berlin: 1912. [Google Scholar]
- Pitts RF. The basis for repetitive activity in phrenic motoneurons. J Neurophysiol. 1943;6:439–454. [Google Scholar]
- Powers RK, Binder MD. Effective synaptic current and motoneuron firing rate modulation. J Neurophysiol. 1995;74:793–801. doi: 10.1152/jn.1995.74.2.793. [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Summation of effective synaptic currents and firing rate modulation in cat spinal motoneurons. J Neurophysiol. 2000;83:483–500. doi: 10.1152/jn.2000.83.1.483. [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Input–output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol. 2001;143:137–263. doi: 10.1007/BFb0115594. [DOI] [PubMed] [Google Scholar]
- Powers RK, Sawczuk A, Musick JR, Binder MD. Multiple mechanisms of spike-frequency adaptation in motoneurons. J Physiol (Paris) 1999;93:101–114. doi: 10.1016/s0928-4257(99)80141-7. [DOI] [PubMed] [Google Scholar]
- Prather JF, Powers RK, Cope TC. Amplification and linear summation of synaptic effects on motoneuron firing rate. J Neurophysiol. 2001;85:43–53. doi: 10.1152/jn.2001.85.1.43. [DOI] [PubMed] [Google Scholar]
- Rall W. Membrane time constant of motoneurons. Science. 1957;126:454. doi: 10.1126/science.126.3271.454. [DOI] [PubMed] [Google Scholar]
- Rall W. Branching dendritic trees and motoneuron membrane resistivity. Exp Neurol. 1959;1:491–527. doi: 10.1016/0014-4886(59)90046-9. [DOI] [PubMed] [Google Scholar]
- Rall W. Membrane potential transients and membrane time constant of motoneurons. Exp Neurol. 1960;2:503–532. doi: 10.1016/0014-4886(60)90029-7. [DOI] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967;30:1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Rall W. Path to biophysical insights about dendrites and synaptic function. In: Samson F, Adelman G, editors. The Neurosciences: Paths of Discovery. II. Birkhäuser; Boston: 1992. pp. 214–238. [Google Scholar]
- Rall W, Burke RE, Nelson PG, Smith TG, Frank K. The dendritic location of synapses and possible mechanisms for the monosynaptic EPSP in motoneurons. J Neurophysiol. 1967;30:1169–1193. doi: 10.1152/jn.1967.30.5.1169. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawczuk A, Powers RK, Binder MD. Spike frequency adaptation studied in hypoglossal motoneurons of the rat. J Neurophysiol. 1995;73:1799–1810. doi: 10.1152/jn.1995.73.5.1799. [DOI] [PubMed] [Google Scholar]
- Sawczuk A, Powers RK, Binder MD. Contribution of outward currents to spike-frequency adaptation in hypoglossal motoneurons of the rat. J Neurophysiol. 1997;78:2246–2253. doi: 10.1152/jn.1997.78.5.2246. [DOI] [PubMed] [Google Scholar]
- Schmidt BJ. Afterhyperpolarization modulation in lumbar motoneurons during locomotor-like rhythmic activity in the neonatal rat spinal cord in vitro. Exp Brain Res. 1994;99:214–222. doi: 10.1007/BF00239588. [DOI] [PubMed] [Google Scholar]
- Schwindt PC. Membrane-potential trajectories underlying motoneuron rhythmic firing at high rates. J Neurophysiol. 1973;36:434–439. doi: 10.1152/jn.1973.36.3.434. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Calvin WH. Equivalence of synaptic and injected current in determining the membrane potential trajectory during motoneuron rhythmic firing. Brain Res. 1973;59:389–394. doi: 10.1016/0006-8993(73)90278-3. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. A persistent negative resistance in cat lumbar motoneurons. Brain Res. 1977;120:173–178. doi: 10.1016/0006-8993(77)90510-8. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Role of a persistent inward current in motoneuron bursting during spinal seizures. J Neurophysiol. 1980a;43:1296–1318. doi: 10.1152/jn.1980.43.5.1296. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Effects of barium on cat spinal motoneurons studied by voltage clamp. J Neurophysiol. 1980b;44:827–846. doi: 10.1152/jn.1980.44.4.827. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Properties of a persistent inward current in normal and TEA-injected motoneurons. J Neurophysiol. 1980c;43:1700–1724. doi: 10.1152/jn.1980.43.6.1700. [DOI] [PubMed] [Google Scholar]
- Shapovalov AI, Kurchavyi GG, Strogonova MP. Synaptic mechanisms of vestibulospinal influences on alpha-motoneurons. Fiziol Zh SSSR. 1966;52:91–100. (Trans.) [PubMed] [Google Scholar]
- Shik ML, Severin FV, Orlovskii GN. Control of walking and running by means of electric stimulation of the midbrain. Biofizika. 1966;11:659–666. [PubMed] [Google Scholar]
- Simon M, Perrier JF, Hounsgaard J. Subcellular distribution of L-type Ca2+ channels responsible for plateau potentials in motoneurons from the lumbar spinal cord of the turtle. Eur J Neurosci. 2003;18:258–266. doi: 10.1046/j.1460-9568.2003.02783.x. [DOI] [PubMed] [Google Scholar]
- Smith JC, Feldman JL. In vitro brainstem–spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J Neurosci Methods. 1987;21:321–333. doi: 10.1016/0165-0270(87)90126-9. [DOI] [PubMed] [Google Scholar]
- Smith T, Wuerker R, Frank K. Membrane impedance changes during synaptic transmission in cat spinal motoneurons. J Neurophysiol. 1967;30:1072–1096. doi: 10.1152/jn.1967.30.5.1072. [DOI] [PubMed] [Google Scholar]
- Soundararajan P, Miles GB, Rubin LL, Brownstone RM, Rafuse VF. Motoneurons derived from embryonic stem cells express transcription factors and develop phenotypes characteristic of medial motor column neurons. J Neurosci. 2006;26:3256–3268. doi: 10.1523/JNEUROSCI.5537-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielmann JM, Laouris Y, Nordstrom MA, Robinson GA, Reinking RM, Stuart DG. Adaptation of cat motoneurons to sustained and intermittent extracellular activation. J Physiol (Lond) 1993;464:75–120. doi: 10.1113/jphysiol.1993.sp019625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RB, Parmiggiani F. Optimal motor patterns for activating mammalian muscle. Brain Res. 1979;175:372–376. doi: 10.1016/0006-8993(79)91019-9. [DOI] [PubMed] [Google Scholar]
- Stuart DG, Pierce PA. The academic lineage of Sir John Carew Eccles (1903–1997) Prog Neurobiol. 2006;78:136–155. doi: 10.1016/j.pneurobio.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Stuart DG, Pierce PA, Callister RJ, Brichta AM, McDonagh JC. Sir Charles Sherrington: humanist, mentor, and movement neuroscientist. In: Latash ML, Zatsiorsky V, editors. Classical Papers in Movement Science. Human Kinetics; Champaign (IL): 2001. pp. 317–374. [Google Scholar]
- Svirskis G, Hounsgaard J. Transmitter regulation of plateau properties in turtle motoneurons. J Neurophysiol. 1998;79:45–50. doi: 10.1152/jn.1998.79.1.45. [DOI] [PubMed] [Google Scholar]
- Taylor CV. Improved micromanipulation apparatus. Univ Calif Publ Zool. 1925;26:443–454. [Google Scholar]
- Taylor CV, Whitaker DM. Potentiometric determinations in the protoplasm and cell-sap of Nitella. Protoplasma. 1927;3:1–6. [Google Scholar]
- Tomlinson WJ, Stea A, Bourinet E, Charnet P, Nargeot J, Snutch TP. Functional properties of a neuronal class C L-type calcium channel. Neuropharmacology. 1993;32:1117–1126. doi: 10.1016/0028-3908(93)90006-o. [DOI] [PubMed] [Google Scholar]
- Viana F, Bayliss DA, Berger AJ. Multiple potassium conductances and their role in action potential repolarization and repetitive firing behavior of neonatal rat hypoglossal motoneurons. J Neurophysiol. 1993;69:2150–2163. doi: 10.1152/jn.1993.69.6.2150. [DOI] [PubMed] [Google Scholar]
- Walton C, Kalmar JM, Cafarelli E. Effect of caffeine on self-sustained firing in human motor units. J Physiol (Lond) 2002;545:671–679. doi: 10.1113/jphysiol.2002.025064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ME, Feldman DH, McCue AF, Brenner R, Velicelebi G, Ellis SB, Harpold MM. Structure and functional expression of alpha 1, alpha 2, and beta subunits of a novel human neuronal calcium channel subtype. Neuron. 1992;8:71–84. doi: 10.1016/0896-6273(92)90109-q. [DOI] [PubMed] [Google Scholar]
- Willis WD. John Eccles’ studies of spinal cord presynaptic inhibition. Prog Neurobiol. 2006;78:189–214. doi: 10.1016/j.pneurobio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Rempel J, Brownstone RM. Postnatal development of cholinergic synapses on mouse spinal motoneurons. J Comp Neurol. 2004;474:13–23. doi: 10.1002/cne.20089. [DOI] [PubMed] [Google Scholar]
- Woodbury JW, Patton HD. Proceedings of the Cold Spring Harbor Symposia on Quantitative Biology, vol. XVII, The Neuron. The Biological Laboratory; Cold Spring Harbor, NY: 1952. Electrical activity of single spinal cord elements; pp. 185–188. [DOI] [PubMed] [Google Scholar]