Abstract
Rationale
Many mechanically ventilated patients with acute respiratory distress syndrome (ARDS) develop pulmonary fibrosis. Stresses induced by mechanical ventilation may explain the development of fibrosis by a number of mechanisms (e.g. damage the alveolar epithelium, biotrauma).
Objectives
To test the hypothesis that mechanical ventilation plays an important role in the pathogenesis of lung fibrosis.
Methods
C57BL/6 mice were randomized into four groups: healthy controls; hydrochloric acid (HCl) aspiration alone; vehicle control solution followed 24 h later by mechanical ventilation (peak inspiratory pressure 22 cmH2O and PEEP 2 cmH2O for 2h); and acid aspiration followed 24h later by mechanical ventilation. The animals were monitored for up to 15 days after acid aspiration. To explore the direct effects of mechanical stress on lung fibrotic formation, human lung epithelial cells (BEAS-2B) were exposed to mechanical stretch for up to 48 h.
Measurement and Main Results
Impaired lung mechanics after mechanical ventilation was associated with increased lung hydroxyproline content, and increased expression of transforming growth factor-β (TGF-β), β-catenin and mesenchymal markers (α-SMA and Vimentin) at both the gene and protein levels. Expression of epithelial markers including cytokeratin-8, E-cadherin and pro-surfactant protein B decreased. Lung histology demonstrated fibrosis formation and potential epithelial-mesenchymal transition (EMT). In vitro direct mechanical stretch of BEAS-2B cells resulted in similar fibrotic and EMT formation.
Conclusions
Mechanical stress induces lung fibrosis, and EMT may play an important role in mediating the ventilator-induced lung fibrosis.
Keywords: Acute lung injury, ventilator-induced lung injury, extracellular matrix
Introduction
Despite major advances in management, the mortality of acute respiratory distress syndrome (ARDS) remains as high as 44% based on observational studies and 36% based on randomized controlled trials (1). Most ARDS patients survive the acute phase, but many go on to die, often with evidence of pulmonary fibrosis (2). Pulmonary fibrosis was found in open-lung biopsies of 53% of ventilated patients who had ARDS for ≥ 5 days (3). Transbronchial lung biopsy revealed that 64% of ARDS patients who were ventilated for an average of 12 days developed pulmonary fibrosis, and their mortality rate was 57% compared to 0% in patients without pulmonary fibrosis (4). The outcome of ARDS patients may be improved by using information from open-lung biopsies to guide anti-fibrotic therapy after 5 days of ARDS (3, 5), thus demonstrating that the clinical progress of lung fibrosis is potentially reversible in ARDS patients, a finding which is different than typical patients with idiopathic pulmonary fibrosis (IPF), in which the disease is usually irreversible (6–8).
The mechanisms underlying the fibrotic phase of ARDS are largely unknown. Some investigations have focused on the underlying immune cell response in the acute phase of ARDS as a key factor in the development of fibrosis (9). Another possibility relates to the role of the alveolar epithelium which has largely been thought of asa passive bystander in the development of chronic pulmonary fibrosis. However, recent studies have suggested that ongoing alveolar epithelial cell apoptosis and retarded wound repair may play an important role in the pathogenesis of pulmonary fibrosis (8, 10–13) mediated through epithelial-mesenchymal transition (EMT) signaling pathways.
Virtually all patients with ARDS receive mechanical ventilation. Regional alveolar overdistension during ventilation can induce excessive mechanical stresses to the extracellular matrix, leading to the development of interstitial edema, extracellular matrix fragmentation (14), epithelial injury, and biotrauma, characterized by neutrophil infiltration and inflammatory cytokine production (14, 15). These mechanisms are also of direct relevance to the development of fibrosis. Therefore we hypothesized that ventilator-induced lung injury may play a role in ARDS-associated fibrosis, characterized by changes in EMT markers, a mechanism that may have previously been overlooked (2–5, 16–18).
To test this hypothesis we used an in vivo mouse model of acid aspiration-induced acute lung injury followed by mechanical ventilation, and employed an in vitro mechanical stretch system on human lung epithelial cells to examine specifically the effects of mechanical stress on the development of fibrosis and EMT.
Materials and methods (see Supplementary Materials for details)
Acid aspiration model and mechanical ventilation
The study protocol was approved by the institutional Animal Care and Use Committee of St. Michael’s Hospital. Male C57BL/6 mice (8 – 12 weeks) were anesthetized with intraperitoneal injection of Ketamine (200 mg/kg) and Xylazine (10 mg/kg), and randomly divided into 3 groups: (1): acid aspiration (HCl, n = 24); (2): vehicle control + mechanical ventilation (MV, n = 24); (3): acid aspiration + mechanical ventilation (HCl+MV, n = 24). Animals received instratracheal instillation of either hydrochloric acid (HCl, pH 1.2, 2 mL/kg) or equal volume of vehicle control solution (PBS, pH 7.4). The animals recovered from anesthesia and were housed in an animal facility with free access to water and food. After 24 h, mice in the MV and HCl+MV groups were anesthetized, intubated, and mechanically ventilated for 2h using FiO2 0.4, PIP 22 cmH2O, PEEP 2 cmH2O, and respiratory rate (RR) 120 breaths/min. The animals were then sent back to the animal facility with free access to water and food and observed for 3 days, 8 days and 15 days after HCl or PBS instillation. Additional healthy mice were sacrificed using an anesthetic overdose (n = 6) and served as a Control group. Upon completion of the experiments the right lung was snap frozen in liquid nitrogen and stored at −80°C for mRNA and protein measurements.
Respiratory mechanics
At the indicated time points 8 mice were anesthesized, paralyzed and tracheotomized for measurement of lung mechanics (FlexiVent rodent ventilator system, Scireq, Montreal, Canada). Lung elastance was assessed using the multifrequency forced oscillation technique and a constant phase model fit (19).
Lung histopathology and immunohistochemistry
The left lung was stained with Masson’s trichrome for identification of collagen. Histological examination of the lungs was performed by a pathologist (FV) blinded to the experimental groups. Lung injury was scored using five grades from 0 to 4 using 9 parameters, including microscopic atelectasis, microscopic emphysema, perivascular edema, alveolar edema, congestion, alveolar hemorrhage, perivascular hemorrhage, alveolar and interstitial polymorphonuclear leukocytes (PMN) infiltration, and hyaline membrane formation (20). Lung fibrosis was quantitatively evaluated on a numerical scale (21).
Fluorescent dual stainings for cytokeratin and α-smooth muscle actin (α-SMA) were performed (22). Briefly, the sections were blocked by a mixture of Fab fragments of goat anti-mouse IgG (Jackson ImmunoResearch) and bovine serum albumin (Sigma Chemicals, St. Louis, MO, USA). Incubation with a mouse monoclonal anti-cytokeratin (Santa Cruz Biotechnology, CA) overnight was followed by goat-anti-mouse Alexa-484 (Invitrogen). After careful washing, a mouse monoclonal anti-α-SMA antibody conjugated with Cy3 (Sigma) was applied. Slides were counterstained by DAPI (Sigma Chemical Co.) if necessary. Slides were analyzed using a confocal scanning laser microscope (Zeiss510, Oberkochen, Germany).
Hydroxyproline measurements
Lung hydroxyproline content (23, 24) was assessed as described in the Supplementary Materials.
Fibrocyte detection
Buffy coat was obtained from whole blood collected using sodium citrate anticoagulant tube. Cells were seeded to 96-well plates (Falcon, BD Biosciences) at 100 μL/well at a density of 1 × 106 cells per well, and stained for the surface marker CD45 (PerCP Rat anti-mouse CD45 antibody, BD Pharmingen) at 5 μg/mL or isotype control antibody (IgG1, BD Biosciences) for 30 min, followed by washes. Cells were permeabilized with0.25% Triton X-100 and then incubated with rabbit anti–mouse collagen-1 antibody (Millipore, Billerica, MA) or IgG isotypecontrol antibody (R&D Systems, Minneapolis, MN) for 30 min and washed, followed with secondary antibody (Goat polyclonal secondary antibody to rabbit IgG, DyLight® 488, Abcam, Cambridge, MA) at 1:20,000 dilution and fixation. Flow cytometry was performed with FACSCanto and FACSDiva software (BD Biosciences). Cells gated for CD45 were analyzed for collagen-1 expression. A matched IgG isotype control was used to set negative control thresholds (25). Fibrocyte numbers are expressed as percent total leukocyte counts.
Cells and mechanical stretch system
Human lung epithelial cells (BEAS-2B, ATCC, Manassas, VA, USA) were seeded into BioFlex Culture 6-well plates (FlexcelI International Corporation, Hillsborough, NC) at a density 5 × 105 cells/well in cDMEM containing 10% FBS for 30 h, and in DMEM containing 1% FBS for 24 h. The cells were then subjected to cyclic stretch for 24 and 48h at a frequency of 30 cycles/min and 30% elongation (FX-4000® FlexcelI International Corporation, Hillsborough, NC).
Reverse transcriptase-polymerase chain reaction (RT-PCR) assay
The mouse lung tissue was homogenized and human BEAS-2B cells were lyzed and total RNA was extracted by using the Isol-RNA Lysis Reagent (5 PRIME, Markham, ON, Canada). The primers used to amplify the conserved regions of the genes of interest in BEAS-2B cells and mouse lung tissue are shown in Tables 1 and 2, respectively. PCR products were resolved on a 1% agarose gel, stained with ethidium bromide, photographed under ultraviolet illumination. The band intensity was quantified by Image J 1.42q software (Scion Corporation, Frederick, MD).
SDS-polyacrylamide gel electrophoresis and Western blotting
Lung tissue was homogenized and BEAS-2B cells were detached in lysis buffer, resolved on 10% SDS-PAGE using a Mini-Protean 3 Electrophoresis Cell (Bio-Rad) at 150 V, 100 mAmp for 1 h, and transferred to polyvinylidene fluoride (PVDF, Bio-Rad) membrane. The membrane was washed and blocked with Tris-buffered saline (TBS) containing 0.1% Tween-20 and 10% nonfat dry milk before overnight incubation with appropriate primary antibodies (i.e., cytokeratin-8 (Santa Cruz Biotechnology, CA), E-Cadherin (Santa Cruz), vimentin (Santa Cruz) and α-SMA (Abcam, Cambridge, UK) in blocking buffer at 4°C. The secondary antibodies were then used with 1:6000goat anti-mouse IgG-Horseradish Peroxidase (HRP) for cytokeratin-8, E-Cadherin, vimentin or 1:6000goat anti-rabbit IgG-HRP for α-SMA (Pierce, Thermo Scientific, Ottawa, ON, Canada) in blocking buffer for 1 hat room temperature. The bands were visualized using enhanced chemiluminescence (Amersham ECL Western Blotting Detection Reagents, GE Healthcare, Baie d’Urfe, QC, Canada). Anti-human or anti-mouse β-actin antibodies (Santa Cruz Biotechnology Inc.) were used as loading controls.
Statistical analysis
Data are reported as mean ± SEM. A two-way analysis of variance for multiple comparisons with group and time factors was conducted followed by a Bonferroni test. A non-parametric test was performed to compare cells with and without stretch in the in vitro cell experiments. GraphPad Prism 5.0 (GraphPad Software Inc, La Jolla, CA) was used for the analysis. Differences were considered statistically significant if p<0.05.
Results
Lung mechanics and hydroxyproline
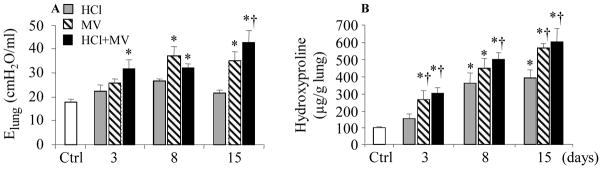
The tidal volume was 11 – 13 mL/kg using pressure controlled ventilation. Lung elastance (Elung) increased at day 3 in the HCl+MV group, and at days 8 and 15 in the MV and HCl+MV groups, compared to Control (Fig. 1A). Hydroxyproline levels in the lung increased at day 15 in the ventilation alone group compared to the HCl alone or control group (Fig. 1B).
Figure 1.
Effects of HCl aspiration and/or mechanical ventilation (MV) on lung compliance and tissue content of hydroxyproline over time. Ctrl = healthy control mice receiving anesthesia but not HCl or MV; Elung = lung elastance. * p<0.05 vs Ctrl; † p<0.05 vs HCl at identical conditions, respectively.
Lung histology analysis
Lung injury and interstitial PMN infiltration were observed at day 3 in all experimental groups, compared to Control (Fig. 2A). The total lung injury score increased in all 3 experimental conditions with the greatest injury seen in the HCl+MV group (Fig. 2B).
Figure 2.
Representative high-power (x40) lung histological features (A) and lung injury score (B). Hematoxylin-eosin (H&E)-stained, formalin-fixed, paraffin-embedded lung tissues from mice, subjected to HCl aspiration alone, high pressure mechanical ventilation alone (MV), and HCL + MV for 15 days. Mice that received anesthesia alone served as controls (Ctrl). Mice in the experimental conditions exhibited evidence of extensive lung injury with interstitial and alveolar edema, hemorrhage, and inflammatory cell infiltration compared with control conditions. n = 9 mice/group. * p<0.05 vs Ctrl; † p<0.05 vs HCl at identical conditions, respectively.
At day 15, the classical histological presentation of lung fibrosis was more evident in the HCl+MV groups compared to the HCl or MV alone group with larger areas of collagen deposition in the alveolar epithelium, associated with thickened alveolar walls and tissue patches (Fig. 3A&B). The increased staining of collagen was associated with more myofibroblasts positive for α-SMA in alveolar walls of the MV and HCl+MV groups (Fig. 3C). Smooth muscle surrounding vascular walls served as an endogenous positive control (arrow in Fig. 3C). In contrast, control histological samples were devoid of the concentrated blue staining regions, indicative of the lack of collagen deposition in healthy lung (Fig. 3D). Lung fibrosis scores were higher in all three experimental conditions at day 3 and further increased over time (Fig. 3E). Lung fibrosis scores were greater in the HCl+MV group than in the other two groups (Fig. 3E).
Figure 3.
Pulmonary fibrosis after challenge with HCl and mechanical ventilation. Mice were challenged with intratracheal HCl, high pressure mechanical ventilation (MV) or HCl and MV (HCl + MV). After 15 days, lung histologic analysis was performed with Masson’s trichrome staining (A, low-power x40 and B, high-power x60) to localize collagen fibers (blue) or α-SMA [(red) C, high-power x60] to identify myofibroblasts. Mice receiving anesthesia alone served as controls (D, low-power at upper panel and high-power at lower panel). Lung fibrosis score (E). n = 9/group. * p<0.05 vs Ctrl; † p<0.05 vs HCl and MV alone.
Fibrotic mediators and EMT markers in vivo mechanical ventilation conditions
The mRNA expression of transforming growth factor β1 (TGF-β1) increased dramatically at day 3 and day 8, and β-catenin mRNA expression elevated throughout the study in the HCl+MV group, indicating a multiplicative effect of the 2 stimuli; levels returned to baseline on day 15 (Fig. 4).
Figure 4.
mRNA expression of TGF-β and β-Catenin after challenge with HCl and mechanical ventilation (MV). Data are reported as relative densitometry of TGF-β or β-catenin over β-actin in bar graphs. * p<0.05 vs Control (Ctrl); † p<0.05 vs HCl or MV alone.
The expression of cytokeratin-8, E-Cadherin and pro-SP-B decreased while expression of α-SMA and vimentin at both mRNA (Fig. 5A) and protein (Fig. 5B) levels increased; these changes were more pronounced in the HCl+MV group compared to HCl or MV alone (Fig. 5).
Figure 5.
EMT markers in lung tissue of mice after challenge with HCl and mechanical ventilation (MV). A. mRNA expression of the epithelial markers cytokeratin-8, E-Cadherin and SP-B, and the mesemchymal marker α-SMA and Vimentin in lung homogenates at indicated time points. B. Protein expression of the EMT markers in lung homogenates. Data are reported as relative densitometry of the EMT markers over β-actin in bar graphs. * p<0.05 vs Control (Ctrl); † p<0.05 vs HCl; ‡ p<0.05 vs MV.
Taken together, our results demonstrate increased fibrotic markers and decreased epithelial markers when the two hits were combined, suggesting an additive effect.
Immunohistochemistry analysis
We further examined whether lung epithelial cells differentiated into myofibroblasts in vivo by investigating colocalization of EMT markers. The dual staining for the lung epithelial cell marker cytokeratin and myofibroblast marker α-SMA demonstrated co-localization in some alveolar cells at day 15 after HCl + MV (Fig. 6A and 6B). These data suggest that EMT may be involved in the mechanism underlying ventilator-induced lung fibrosis. However, in some areas the staining for cytokeratin and α-SMA did not show colocalization, suggesting ongoing α-SMA-positive myofibroblast proliferation (Fig. 6C).
Figure 6.
Confocal microscopic analysis of colocalization of cytokeratin-8 (red fluorescence) with α-SMA-positive myofibroblasts (green) in lung tissue at day 15 after intratracheal instillation of HCl followed 24 h later by high pressure mechanical ventilation (A). Z stack demonstrates colocalization of red and green signals by yellow color corresponding to those in panel A, indicative of EMT (B). α-SMA-positive myofibroblasts without cytokeratin-8 colocalization is present in some area of the lung tissue (C).
Circulating fibrocyte counts
Circulating fibrocytes, defined as CD45 and collagen 1 expressing cells, were not significantly different at day 15 after HCl, MV or HCl and MV (Fig. 7).
Figure 7.

Fibrocyte counts in control mice (Ctrl), and mice 15 days after receiving intratracheal instillation of HCl, mechanical ventilation (MV) or HCl+MV. There are no statistical differences between any groups.
Fibrotic mediators and EMT markers under in vitro mechanical stretch conditions
We examined the contribution of the direct effect of mechanical stretch on EMT using in vitro human lung epithelial BEAS-2B cells. The cells treated with mechanical stretch expressed high mRNA levels of TGF-β1 and β-catenin at 48 h (Fig. 8A), and a progressive decrease in expression of cytokeratin-8, E-cadherin and pro-SPB and an increase in expression of α-SMA and vimentin at mRNA (Fig. 8B) and protein levels (Fig. 8C).
Figure 8.
Expression of fibrotic mediators and EMT markers after mechanical stretch of human lung epithelial cells. BEAS-2B cells were subjected to cyclic stretch for 24 and 48h at a frequency of 30 cycles/min and 30% elongation. A. Representative mRNA expression of TGF-β and β-Catenin in BEAS-2B cells in response to mechanical stretch. Average relative densitometry of TGF-β or β-catenin over β-actin is reported in bar graphs. B. mRNA expression of the epithelial markers cytokeratin-8, E-Cadherin and SP-B, and the mesemchymal marker α-SMA and Vimentin in the BEAS-2B cell lysates after mechanical stretch. C. Protein expression of the EMT markers in lung homogenates. Data are reported as relative densitometry of the EMT markers normalized for β-actin. * p<0.05 vs non-stretch control cells (Ctrl) at identical conditions.
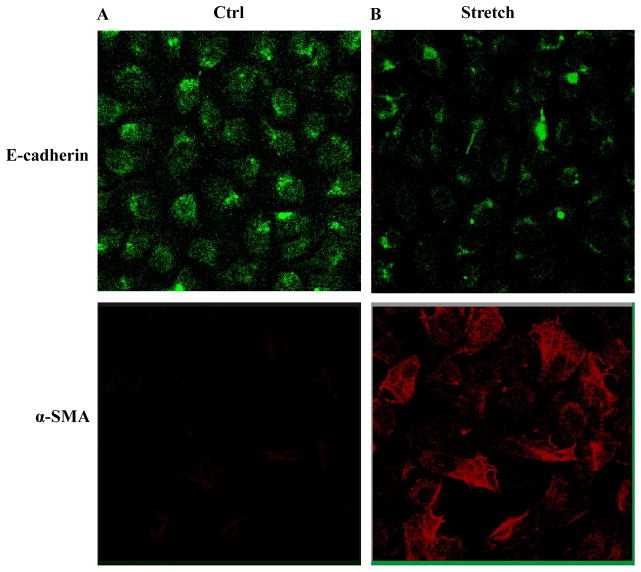
To confirm the EMT phenotype observed in vivo, the lung epithelial cell marker E-cadherin and myofibroblast marker α-SMA were stained in BEAS-2B cells with and without mechanical stretch. There was decreased expression of E-cadherin and increased expression of α-SMA, suggesting an EMT process after direct stretch of the lung epithelial cells (Fig. 9A and 9B).
Figure 9.
Immunofluorescent staining for epithelial and mesenchymal markers in human lung epithelial cells after mechanical stretch. BEAS-2B cells were subjected to cyclic mechanical stretch for 48h at a frequency of 30 cycles/min and a 30% elongation. A. Cells receiving no mechanical stretch served as control (Ctrl). B. Exposure of the cells to mechanical stretch results in a reduction and redistribution of the epithelial marker E-cadherin from intercellular junction areas into the cytoplasm, compared to control. Mesenchymal marker F-actin is enhanced after stretch. Immunofluorescent staining for α-SMA is not detected in the cells under basal conditions but is noticeable after stretch (n = 5 experiments).
Discussion
Pulmonary fibrosis is a common finding in patients with ARDS in the post acute phase; however, the mechanisms mediating the fibrosis are largely unknown. Since the stresses induced by mechanical ventilation can damage the alveolar epithelium (26–28), and can cause biotrauma with release of multiple mediators, we hypothesized that mechanical ventilation plays an important role in initiating/propagating the fibrosis. In the current study, using a mouse model of acute lung injury we demonstrated that mechanical ventilation can induce lung fibrosis that is evident as early as one week after injury. Both our in vivo and in vitro studies suggest that the mechanisms underlying these changes may be epithelial-mesenchymal transition (EMT) and release of pro-fibrotic mediators induced by cell stretch and mechanical ventilation.
We used a two-hit model of acid aspiration and mechanical ventilation which is commonly used and clinically relevant. We found that the animals treated in this way developed increased levels of hydroxyproline, a major component of the protein collagen that is considered to be an early marker and contributor to lung fibrosis (29). We have no data to suggest that there is anything specific about these particular 2 “hits”, and it is possible that any combination of 2 or more hits (e.g. chemical, infectious, mechanical) with mechanical ventilation might have resulted in a similar response. Increased levels of collagen type I in lung tissue have been reported in patients with ARDS (30, 31). In addition, as early as 24 h after diagnosis of ARDS, the N-terminal procollagen peptide-III (N-PCP-III) is elevated in lavage fluid and in serum of patients with ARDS compared to controls (16). N-PCP-III concentrations are significantly higher at 24h and 7 days in ARDS nonsurvivors than in survivors (67% vs 31%) (16).
Damage to alveolar epithelium has been documented in ~90% of ARDS patients who had lung biopsies (18). Inadequate alveolar epithelial repair and remodeling can initiate and/or accelerate fibrosis by several mechanisms: 1) loss of epithelial proliferation, impaired epithelial migration capacity and/or inadequate differentiation of alveolar epithelial cells from their progenitor cells may lead to proliferation of fibroblasts and smooth muscle cells. For example, severe injury and retarded repair of alveolar epithelium in response to hyperoxia has been shown to disturb normal epithelial-fibroblast balance and is sufficient to promote fibrotic proliferation in association with increased collagen production in lung tissue (32); 2) alveolar epithelial cells act as progenitors for fibroblasts via EMT, regulated largely by the extracellular matrix during alveolar epithelial repair and remodeling (10); and 3) lung epithelial cells secrete inflammatory mediators (e.g. TGF-β and Wnt/β-catenin) in response to various stimuli which can contribute to persistent activation of fibrotic mediators (33–35). Taken together, these dysregulated repair mechanisms determine the resolution of ARDS and may lead to lung fibrosis.
We found two major mechanisms associated with the ventilator-induced lung fibrosis in our model. 1) EMT: We observed colocalization of the epithelial marker cytokeratin-8 and the mesenchymal marker α-SMA in lung tissue after mechanical ventilation, and in lung epithelial cells after exposure to mechanical stretch. The decreased expression of cytokeratin-8, E-cadherin, pro-SPB with a simultaneous increase in the expression of α-SMA and vimentin further supports the development of EMT. The increased expression of TGF-β1 and β-catenin may have played a role in the process of EMT (36–38). Recent evidence suggests that the lung epithelium plays a key role in driving the fibrotic response (22). Failure of epithelial repair promotes fibroblast proliferation derived from the epithelium (39) through the EMT process. 2) Lung fibroblast proliferation: In some regions of the lung we found that after mechanical ventilation the expression of α-SMA increased without colocalization with lung epithelial cell protein markers, indicating ongoing α-SMA-positive myofibroblast proliferation. Collagen deposition due to abnormal wound repair and remodeling plays an important role in fibroproliferation (39, 40).
We also examined the possibility that fibrocytes, defined as cells that dually express leukocyte (CD45) and mesenchymal (Collagen I) markers (41, 42), could play a role in development of fibrosis since previous studies have shown that fibrocytes express α-SMA, indicative of myofibroblast differentiation (43) after stimulation with TGF-β. Fibrocytes have also been found to respond to the pro-fibrotic cytokines, IL-4 and IL-13 and differentiated to α-SMA positive cells (44). However, the counts of circulating fibrocytes were not different among the experimental groups.
We and others have previously demonstrated that mechanical stretch of alveolar epithelial type II cells can alter actin filaments associated with increased expression of chemokines and adhesion molecules (45–47). High Vt ventilation in in vivo rat models leads to increased mRNA expression of various extracellular matrix components (48–50). Increased gene expression of procollagen III and fibronectin, fibroblast growth factor, and TGF-β has been observed in lungs ventilated with high airway pressures and high PEEP in vivo and in rat lung parenchymal strips (49, 51–55). Mechanical forces can also result in modification of proteoglycans in healthy rats ventilated with low Vt, which is magnified with higher Vt (48, 56, 57). Mechanical ventilation of healthy mice causes lung injury associated with higher levels of proinflammatory cytokines and fibrin deposition (58). Therefore, a deficiency of lung repair is characterized by persistent and exaggerated inflammation, changes in extracellular matrix architecture and alveolar epithelial damage following mechanical ventilation. Our results extend previous findings by demonstrating that mechanical stress induces decreased expression of lung epithelial markers and increased levels of hydroxyproline content in lung tissue, resulting in potentiation of lung fibrosis.
In a previous study Willis et al reported that rat alveolar epithelial type II cells exposed to TGF-β1 for 6 days demonstrated increased expression of mesenchymal cell markers and a fibroblast-like morphology (36). Studies of in vitro alveolar epithelial cell cultures using cyclic stretch suggest that a 25% elongation corresponds to an 8–12% linear distension of the alveolar epithelium, whereas cyclic stretch at 37–50% elongation corresponds to about 17–22% linear distension, which is relevant to pathophysiological conditions produced by mechanical ventilation (59, 60). We used a 30% elongation for 48 h as a stimulus in BEAS-2B cells. This magnitude of stimulation and timing likely reflects pathological conditions and may explain the rapid increase in mesenchymal markers. We used the BEAS-2B cell line derived from human bronchial epithelium; ideally our findings should be confirmed in primary alveolar epithelial type II cells at multiple magnitudes of mechanical stretch.
Our study focuses on the potential mechanisms of fibrosis in the context of VILI. The direct contribution of each fibrotic marker (TGF-β1 and β-catenin) and possible down-stream signaling pathways are yet to be elucidated. Protective mechanical ventilation is the only treatment that has been shown to decrease mortality in patients with ARDS. To date, this has been ascribed to the acute effects of lung protective strategies on inflammation and lung injury. If our hypothesis that mechanical ventilation is a key contributor to the ALI/ARDS-associated pulmonary fibrosis is correct, this hypothesis would need to be refined. This finding could potentially explain the observation that the separation of the Kaplan-Meier mortality curves observed in studies using lung protective ventilation often do not begin to separate for at least 2 weeks (59–61). For example, in the study examining the use of neuromuscular blockade in patients with ARDS, the mortality curves did not begin to separate until about 18 days, even though the intervention was limited to the first 48 hours (59, 60). In many patients, it will not be possible to provide a ventilatory strategy that is protective for all lung units because of the severity of the underlying disease. In these patients, a therapy that could modulate the fibrotic response could be beneficial. Future mechanistic studies will suggest novel pharmacologic approaches to specifically minimize the pulmonary fibrosis associated with mechanical ventilation, and hopefully lead to improved outcomes in patients who suffer from ARDS.
Acknowledgments
Supported by grants from Ministry of Science of Spain to JV (SAF 2004-06833), FUNCIS (53/04), a specific agreement between Instituto de Salud Carlos III and FUNCIS (EMER07/001) under the ENCYT 2015 framework, Canadian Institutes of Health Research (CIHR) to HZ (MOP77818, MOP69042) and AS (MOP8558), and McLaughlin Foundation to HZ.
The authors thank Rosane Nisenbaum, PhD, Staff Scientist and Senior Biostatistician at the the Keenan Research Centre in the Li Ka Shing Knowledge Institute, St Michael’s Hospital, for her valuable advice on statistical analysis of the study.
References
- 1.Phua J, Badia JR, Adhikari NK, et al. Has mortality from acute respiratory distress syndrome decreased over time?: A systematic review. Am J Respir Crit Care Med. 2009;179(3):220–227. doi: 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]
- 2.Meduri GU, Headley S, Kohler G, et al. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107(4):1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 3.Papazian L, Doddoli C, Chetaille B, et al. A contributive result of open-lung biopsy improves survival in acute respiratory distress syndrome patients. Crit Care Med. 2007;35(3):755–762. doi: 10.1097/01.CCM.0000257325.88144.30. [DOI] [PubMed] [Google Scholar]
- 4.Martin C, Papazian L, Payan MJ, et al. Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome. A study in mechanically ventilated patients. Chest. 1995;107(1):196–200. doi: 10.1378/chest.107.1.196. [DOI] [PubMed] [Google Scholar]
- 5.Meduri GU, Headley AS, Golden E, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1998;280(2):159–165. doi: 10.1001/jama.280.2.159. [DOI] [PubMed] [Google Scholar]
- 6.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161(2 Pt 1):646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 7.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 8.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132(4):1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 9.Lopez AD, Avasarala S, Grewal S, et al. Differential role of the Fas/Fas ligand apoptotic pathway in inflammation and lung fibrosis associated with reovirus 1/L-induced bronchiolitis obliterans organizing pneumonia and acute respiratory distress syndrome. J Immunol. 2009;183(12):8244–8257. doi: 10.4049/jimmunol.0901958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim KK, Kugler MC, Wolters PJ, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103(35):13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuwano K. Epithelial cell apoptosis and lung remodeling. Cell Mol Immunol. 2007;4(6):419–429. [PubMed] [Google Scholar]
- 12.Kuwano K. Involvement of epithelial cell apoptosis in interstitial lung diseases. Intern Med. 2008;47(5):345–353. doi: 10.2169/internalmedicine.47.0713. [DOI] [PubMed] [Google Scholar]
- 13.Willis BC, duBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc Am Thorac Soc. 2006;3(4):377–382. doi: 10.1513/pats.200601-004TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moriondo A, Mukenge S, Negrini D. Transmural pressure in rat initial subpleural lymphatics during spontaneous or mechanical ventilation. Am J Physiol Heart Circ Physiol. 2005;289(1):H263–269. doi: 10.1152/ajpheart.00060.2005. [DOI] [PubMed] [Google Scholar]
- 15.Tremblay L, Valenza F, Ribeiro SP, et al. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997;99(5):944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall RP, Bellingan G, Webb S, et al. Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome. Am J Respir Crit Care Med. 2000;162(5):1783–1788. doi: 10.1164/ajrccm.162.5.2001061. [DOI] [PubMed] [Google Scholar]
- 17.Meduri GU. Late adult respiratory distress syndrome. New Horiz. 1993;1(4):563–577. [PubMed] [Google Scholar]
- 18.Parambil JG, Myers JL, Aubry MC, et al. Causes and prognosis of diffuse alveolar damage diagnosed on surgical lung biopsy. Chest. 2007;132(1):50–57. doi: 10.1378/chest.07-0104. [DOI] [PubMed] [Google Scholar]
- 19.Oostveen E, MacLeod D, Lorino H, et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003;22(6):1026–1041. doi: 10.1183/09031936.03.00089403. [DOI] [PubMed] [Google Scholar]
- 20.Ko SC, Zhang H, Haitsma JJ, et al. Effects of PEEP levels following repeated recruitment maneuvers on ventilator-induced lung injury. Acta Anaesthesiol Scand. 2008;52(4):514–521. doi: 10.1111/j.1399-6576.2008.01581.x. [DOI] [PubMed] [Google Scholar]
- 21.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41(4):467–470. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato M, Hirayama S, Lara-Guerra H, et al. MMP-dependent migration of extrapulmonary myofibroblast progenitors contributing to posttransplant airway fibrosis in the lung. Am J Transplant. 2009;9(5):1027–1036. doi: 10.1111/j.1600-6143.2009.02605.x. [DOI] [PubMed] [Google Scholar]
- 23.Kim KK, Wei Y, Szekeres C, et al. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest. 2009;119(1):213–224. doi: 10.1172/JCI36940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haque S. Inactivation of the N-terminal of ACE reduces bleomycin-induced lung fibrosis. Thorax [Google Scholar]
- 25.Moeller A, Gilpin SE, Ask K, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179(7):588–594. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 26.Hirsch J, Hansen KC, Sapru A, et al. Impact of low and high tidal volumes on the rat alveolar epithelial type II cell proteome. Am J Respir Crit Care Med. 2007;175(10):1006–1013. doi: 10.1164/rccm.200605-621OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oeckler RA, Hubmayr RD. Cell wounding and repair in ventilator injured lungs. Respir Physiol Neurobiol. 2008;163(1–3):44–53. doi: 10.1016/j.resp.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muscedere JG, Mullen JB, Gan K, et al. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med. 1994;149(5):1327–1334. doi: 10.1164/ajrccm.149.5.8173774. [DOI] [PubMed] [Google Scholar]
- 29.Specks U, Nerlich A, Colby TV, et al. Increased expression of type VI collagen in lung fibrosis. Am J Respir Crit Care Med. 1995;151(6):1956–1964. doi: 10.1164/ajrccm.151.6.7767545. [DOI] [PubMed] [Google Scholar]
- 30.Last JA, Siefkin AD, Reiser KM. Type I collagen content is increased in lungs of patients with adult respiratory distress syndrome. Thorax. 1983;38(5):364–368. doi: 10.1136/thx.38.5.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deheinzelin D, Jatene FB, Saldiva PH, et al. Upregulation of collagen messenger RNA expression occurs immediately after lung damage. Chest. 1997;112(5):1184–1188. doi: 10.1378/chest.112.5.1184. [DOI] [PubMed] [Google Scholar]
- 32.Adamson IY, Young L, Bowden DH. Relationship of alveolar epithelial injury and repair to the induction of pulmonary fibrosis. Am J Pathol. 1988;130(2):377–383. [PMC free article] [PubMed] [Google Scholar]
- 33.Borthwick LA, Parker SM, Brougham KA, et al. Epithelial to mesenchymal transition (EMT) and airway remodelling after human lung transplantation. Thorax. 2009;64(9):770–777. doi: 10.1136/thx.2008.104133. [DOI] [PubMed] [Google Scholar]
- 34.Kuwano K, Hagimoto N, Hara N. Molecular mechanisms of pulmonary fibrosis and current treatment. Curr Mol Med. 2001;1(5):551–573. doi: 10.2174/1566524013363401. [DOI] [PubMed] [Google Scholar]
- 35.Budinger GR, Chandel NS. The role of cell suicide or apoptosis in the pathophysiology of acute lung injury. Intensive Care Med. 2001;27(6):1091–1093. doi: 10.1007/s001340100876. [DOI] [PubMed] [Google Scholar]
- 36.Willis BC, Liebler JM, Luby-Phelps K, et al. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166(5):1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwong KY, Literat A, Zhu NL, et al. Expression of transforming growth factor beta (TGF-beta1) in human epithelial alveolar cells: a pro-inflammatory mediator independent pathway. Life Sci. 2004;74(24):2941–2957. doi: 10.1016/j.lfs.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 38.Konigshoff M, Kramer M, Balsara N, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119(4):772–787. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293(3):L525–534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 40.Sheppard D. Transforming growth factor beta: a central modulator of pulmonary and airway inflammation and fibrosis. Proc Am Thorac Soc. 2006;3(5):413–417. doi: 10.1513/pats.200601-008AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bucala R, Spiegel LA, Chesney J, et al. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1(1):71–81. [PMC free article] [PubMed] [Google Scholar]
- 42.Moore BB, Murray L, Das A, et al. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35(2):175–181. doi: 10.1165/rcmb.2005-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abe R, Donnelly SC, Peng T, et al. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166(12):7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 44.Shao DD, Suresh R, Vakil V, et al. Pivotal Advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83(6):1323–1333. doi: 10.1189/jlb.1107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lodyga M, Bai XH, Mourgeon E, et al. Molecular cloning of actin filament-associated protein: a putative adaptor in stretch-induced Src activation. Am J Physiol Lung Cell Mol Physiol. 2002;283(2):L265–274. doi: 10.1152/ajplung.00492.2001. [DOI] [PubMed] [Google Scholar]
- 46.dos Santos CC, Han B, Andrade CF, et al. DNA microarray analysis of gene expression in alveolar epithelial cells in response to TNFalpha, LPS, and cyclic stretch. Physiol Genomics. 2004;19(3):331–342. doi: 10.1152/physiolgenomics.00153.2004. [DOI] [PubMed] [Google Scholar]
- 47.Mourgeon E, Isowa N, Keshavjee S, et al. Mechanical stretch stimulates macrophage inflammatory protein-2 secretion from fetal rat lung cells. Am J Physiol Lung Cell Mol Physiol. 2000;279(4):L699–706. doi: 10.1152/ajplung.2000.279.4.L699. [DOI] [PubMed] [Google Scholar]
- 48.Al-Jamal R, Ludwig MS. Changes in proteoglycans and lung tissue mechanics during excessive mechanical ventilation in rats. Am J Physiol Lung Cell Mol Physiol. 2001;281(5):L1078–1087. doi: 10.1152/ajplung.2001.281.5.L1078. [DOI] [PubMed] [Google Scholar]
- 49.Berg JT, Fu Z, Breen EC, et al. High lung inflation increases mRNA levels of ECM components and growth factors in lung parenchyma. J Appl Physiol. 1997;83(1):120–128. doi: 10.1152/jappl.1997.83.1.120. [DOI] [PubMed] [Google Scholar]
- 50.Foda HD, Rollo EE, Drews M, et al. Ventilator-induced lung injury upregulates and activates gelatinases and EMMPRIN: attenuation by the synthetic matrix metalloproteinase inhibitor, Prinomastat (AG3340) Am J Respir Cell Mol Biol. 2001;25(6):717–724. doi: 10.1165/ajrcmb.25.6.4558f. [DOI] [PubMed] [Google Scholar]
- 51.Caruso P, Meireles SI, Reis LF, et al. Low tidal volume ventilation induces proinflammatory and profibrogenic response in lungs of rats. Intensive Care Med. 2003;29(10):1808–1811. doi: 10.1007/s00134-003-1908-7. [DOI] [PubMed] [Google Scholar]
- 52.de Carvalho ME, Dolhnikoff M, Meireles SI, et al. Effects of overinflation on procollagen type III expression in experimental acute lung injury. Crit Care. 2007;11(1):R23. doi: 10.1186/cc5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farias LL, Faffe DS, Xisto DG, et al. Positive end-expiratory pressure prevents lung mechanical stress caused by recruitment/derecruitment. J Appl Physiol. 2005;98(1):53–61. doi: 10.1152/japplphysiol.00118.2004. [DOI] [PubMed] [Google Scholar]
- 54.Garcia CS, Rocco PR, Facchinetti LD, et al. What increases type III procollagen mRNA levels in lung tissue: stress induced by changes in force or amplitude? Respir Physiol Neurobiol. 2004;144(1):59–70. doi: 10.1016/j.resp.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 55.Parker JC, Breen EC, West JB. High vascular and airway pressures increase interstitial protein mRNA expression in isolated rat lungs. J Appl Physiol. 1997;83(5):1697–1705. doi: 10.1152/jappl.1997.83.5.1697. [DOI] [PubMed] [Google Scholar]
- 56.Mezzano SA, Ruiz-Ortega M, Egido J. Angiotensin II and renal fibrosis. Hypertension. 2001;38(3 Pt 2):635–638. doi: 10.1161/hy09t1.094234. [DOI] [PubMed] [Google Scholar]
- 57.Moriondo A, Pelosi P, Passi A, et al. Proteoglycan fragmentation and respiratory mechanics in mechanically ventilated healthy rats. J Appl Physiol. 2007;103(3):747–756. doi: 10.1152/japplphysiol.00056.2007. [DOI] [PubMed] [Google Scholar]
- 58.Wolthuis EK, Vlaar AP, Choi G, et al. Mechanical ventilation using non-injurious ventilation settings causes lung injury in the absence of pre-existing lung injury in healthy mice. Crit Care. 2009;13(1):R1. doi: 10.1186/cc7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 60.Slutsky AS. Neuromuscular blocking agents in ARDS. N Engl J Med. 2010;363(12):1176–1180. doi: 10.1056/NEJMe1007136. [DOI] [PubMed] [Google Scholar]
- 61.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]