Abstract
Background
Obesity, a major risk factor for cardiometabolic disease, is associated with lower cognitive performance from childhood to senescence, especially on tasks of executive function. In the cardiovascular domain, fat stored viscerally rather than elsewhere in the body carries particularly high risk. It is unknown whether this is also true in case of obesity-cognition relationships. The aim of this study is to assess the cross-sectional relationship between visceral fat (VF) and cognitive performance in a community sample of healthy adolescents.
Methods
In a community-based sample of 983 adolescents (12–18 years old, 480 males), VF was quantified using magnetic resonance imaging, total body fat was measured using a multifrequency bioimpedance and cognitive performance was assessed using a battery of cognitive tests measuring executive function and memory.
Results
We found that larger volumes of VF were associated with lower performance on six measures of executive function (p=0.0001 to 0.02). We also found that the association of VF with executive function was moderated by sex for a subset of measures, such that relationship was present mainly in females and not in males (sex-by-VF interaction: p=0.001 to 0.04). These relationships were independent of the quantity of total body fat and a number of potential confounders, including age, puberty stage and household income.
Conclusions
Our results suggest that the adverse association between obesity and executive function may be attributed to fat stored viscerally and not to fat stored elsewhere in the body. They also suggest that females compared with males may be more sensitive to the potentially detrimental effects of VF on cognition.
Keywords: visceral fat, intra-abdominal fat, obesity, total body fat, executive functioning, adolescence
Introduction
Obesity, the excessive accumulation of body fat, is a major health concern for both children and adults; 33–34% of children and 59–66% of adults in Canada and USA are overweight or obese.1–3 Midlife obesity is associated not only with heightened risk of cardiometabolic disease4 but also with a higher risk of developing dementia later in life.5 In children and adolescents, obesity has been shown to be associated with poorer cognitive performance, especially on tasks of executive functions.6–8 Emerging evidence suggests that the mechanistic pathways underlying these associations may be at play throughout the whole lifespan, and that the negative effects of obesity could accumulate over time, impacting cognitive health. Accordingly, Sabia and collegues9 found that cumulative obesity (assessed by body mass index [BMI] in young adulthood, midlife and late midlife) was associated with poorer cognition in late midlife.
Visceral or intra-abdominal obesity is particularly dangerous in regards to cardiometabolic health. Visceral fat (VF) compared with other body fat exhibits greater lipid turnover. 10 Under postprandial conditions, VF compared with subcutaneous fat shows a higher fat uptake and, under fasting conditions, visceral adipocytes compared with subcutaneous adipocytes are more lipolytically active.11 As a result of these metabolic differences, VF (vs. subcutaneous fat) can contribute more to elevated levels of lipids in plasma.10,11 Moreover, VF drains directly into the portal circulation and the liver, where excess lipids from enlarged VF can contribute to the enhanced de novo synthesis of triacylglycerols in obesity, which are released into the circulation bound in very-low density lipoproteins.11 Thus, given the biological properties and anatomical location, it is not surprising that VF compared with other fat in the body has a strong impact on obesity-related health outcomes, including: dyslipidemia, hypertension, and type 2 diabetes mellitus,10,12,13 all of which have also been associated with increased risk of cognitive decline.14–16
Only a few studies have examined the relationship between VF and cognition in adults. In one such study, Isaac and colleagues17 found a negative association between VF and verbal memory and attention in older adults; the relationship with verbal memory remained after controlling for subcutaneous fat.
While obesity-cognition relationships are suggestive of an underlying role of VF, no studies have examined this relationship in adolescents. This study will utilize magnetic resonance imaging (MRI) of the abdomen to quantify volumes of VF, and examine the cross-sectional relationship between VF and cognition in a community sample of 983 adolescents. We focus on tasks of executive functioning and memory, as poorer performance in both domains of cognition have been associated with higher BMI or VF in adults and/or children.6–9,17 As well, given the known sex differences in VF deposition18 and reported sex differences in VF-cardiometabolic relationships,13,19,20 we will also investigate the interaction between sex and VF on cognitive performance.
Participants and Methods
Participants
The study includes participants from the Saguenay Youth Study (SYS). 21 While the primary focus of SYS is to evaluate the consequences of prenatal exposure to maternal cigarette smoking on adolescents, this study utilizes the SYS cohort to examine the cross-sectional relationship between VF and cognition. Adolescents, aged 12 to 18 (N=983; mean age 15.0 years) were recruited from French-Canadian high schools in the Saguenay—Lac-Saint-Jean region of Quebec, Canada. Details of the recruitment and testing procedures are provided in Pausova et al., 2007.21 In brief, both exposed and non-exposed adolescents, matched on maternal education and school attended, were recruited. Exposure to maternal cigarette smoking was defined retrospectively as smoking one or more cigarettes per day in the second trimester of pregnancy. The SYS uses a family-based design where two or more siblings from the same family are included.
The main exclusion criteria for both exposed and non-exposed adolescents were: (i) premature birth (<35 weeks); (ii) positive history of alcohol abuse during gestation; (iii) positive medical history for meningitis, malignancy, and heart disease requiring heart surgery; (iv) severe mental illness (e.g., autism, schizophrenia) or mental retardation (IQ < 70) and (v) MRI contraindications. The Research Ethics Committee of the Chicoutimi Hospital approved the study protocol. Adolescents and their parents signed informed assent and consent, respectively.
All SYS participants underwent MRI assessment of the abdomen for quantification of VF, multifrequency bioimpedence for the assessment of total body fat, as well as a full neuropsychological battery. Details of all three are described below.
Body Composition and Visceral Fat
Height (0.1 cm precision), weight (0.1 kg precision), BMI (kg/m2) and waist circumference (WC; 1 mm precision) were assessed in all participants. Total body fat (TBF) was measured using a multifrequency bioimpedance (Xitron Technologies, San Diego, CA). Participants were asked to refrain from caffeine, alcohol and vigorous activity for 24 hours before assessment. The volume of VF was assessed using T1-weighted MRI (Phillips 1.0T scanner); a 10-mm axial slice acquired at the level of the umbilicus was segmented into visceral and subcutaneous fat using a previously described method.20
Questionnaires
Parents completed questionnaires regarding socioeconomic status, including household income.21 All adolescents completed the Puberty Development Scale,22 an 8-item, self-report measure of pubertal development based on the Tanner stages. Separate forms were administered to males and females. The PDS yields five categories of pubertal status: (i) prepubertal (ii) beginning pubertal (iii) midpubertal (iv) advanced pubertal and (v) postpubertal. The PDS shows 85–95% accuracy within one Tanner stage to physician ratings.23,24 Further, the PDS is similarly correlated with circulating hormones levels (testosterone and dehydroepiandrosterone in males and females; estradiol in females only) as physical assessments.25
Cognitive Testing
Executive Functioning
Four domains of executive functioning were assessed: (i) Processing Speed, (ii) Working Memory, (iii) Resistance to Interference and (iv) Cognitive Flexibility. Processing Speed was measured using the Ruff 2 & 7 Selective Attention Test 26 and the Symbol Search and Coding subtests from the Wechsler Intelligence Scale for Children-III (WISC-III).27 In the Ruff 2 & 7 Selective Attention Test, participants complete both Automatic and Controlled Detection Trials, in which they must identify the digits 2 and 7 intermixed with letters (Automatic Detection) or other numbers (Controlled Detection). In both conditions, participants are asked to cross out 2s and 7s within a time limit of 15 seconds per block (10 blocks per condition). Speed (total number of targets identified) was calculated for both Automatic and Controlled Detection. Automatic and Controlled Detection Speed have very high test-retest reliability (r>0.90)28 In the Symbol Search subtest of WISC-III, participants are given rows of symbols with a corresponding target symbol, and asked to mark whether or not the target symbol appears in each row; final score was based on the number of correctly answered items in 120 seconds minus incorrect responses. Symbol Search has moderate test-retest reliability (r=0.77).27 In the Coding subtest of WISC-III, participants are asked to transcribe a digit-symbol code; final score was based on the number of correctly transcribed items in 120 seconds. Coding has moderate test-retest reliability (r=0.77).27
Working Memory was assessed using Digit-span subtest from the WISC and the Self-Ordered Pointing Task.29 In the Digit-span subtest, participants are recited a string of digits and asked to repeat the string as heard (Digits Forwards) or in reverse order (Digits Backwards). Digit strings range in length from 2 to 9 digits, with two strings recited per length. Participants are given one point for every correctly repeated string. Digit-span has moderate test-retest reliability (r=0.73).27 In Self-ordered Pointing participants are shown 12 abstract pictures, presented on 12 different pages, with the locations of pictures differing on each page. Participants are asked to point to a different item on each page. Participants complete the task three times, and total errors for all three trials were calculated. Self-ordered pointing has moderate test-retest reliability (r=0.76).30
Resistance to Interference was assessed using the Stroop Color-Word Test.31 The Stroop test consists of three trials (i) reading color names, printed in black ink; (ii) naming colors of Xs printed in green, red or blue; and (iii) naming the color of the ink in which the word is printed (color names are printed in an incongruent color). Participants are given 45 seconds for each trial, scores for each trial were calculated as the number of items correctly named. Stroop Interference was calculated as the difference between true and predicted scores on trial three, where the predicted score is the product of scores on trials one (color names in black ink) and two (Xs in colored ink) divided by the sum of scores on trials one and two. The Stroop Color-Word Test has moderate to high test-retest reliability (r=0.70–0.88).31
Cognitive flexibility was assessed using Verbal Fluency32 consisting of Semantic and Phonemic fluency. In the Semantic condition, participants are asked to say as many (i) animals and (ii) foods and drinks as possible. In the phonemic condition, participants are asked to name as many words as possible starting with the letters (i) S, (ii) F and (iii) A. A one-minute time limit is given for each of the five trials. Total score was calculated as the sum of trials for the semantic and phonemic conditions. Verbal Fluency has moderate test-retest reliability (r>0.70).28
Memory
Visuo-spatial memory was assessed using the Dot-location subtest of the Children’s Memory Scale.33 Participants were shown a 4x4 grid, with a pattern of blue dots placed in eight locations. Participants were then given a blank grid and asked to recreate the array. This procedure was repeated three times. Following an interference trial using red dots, participants were once again asked to recreate the array of blue dots. Short delay recall was calculated based on the first three learning trials and the post-interference trial. Participants were also asked to recreate the array after a delay of 35–40 minutes. Thus three measures of visuo-spatial memory were derived: Learning, Short-delay recall, and Long-delay recall. Learning, Short-delay recall and Long-delay recall all have good test-retest reliability (r=0.81–0.93).33
Verbal memory was assessed using the Stories subtest of the Children’s Memory Scale.33 A short story of 4 to 5 lines was read to participants, and they were asked to recall as many elements as possible both immediately, and after a 30–35 minute delay. Following the second recall trial, participants were also asked a series of questions assessing their recognition for story elements. Thus three measures of verbal memory were derived: immediate recall, delayed recall, and recognition. Immediate recall, delayed recall and recognition all have moderate to good test-retest reliability (r=0.71–0.86).33
Statistical Analyses
The relationship between VF and cognition was assessed using multilevel models to control for correlations of outcomes between siblings. In order to assess the contribution of VF to cognitive performance independently of other fat, each cognitive outcome was modeled at a function of sex, VF and TBF. Sex-by-VF and sex-by-TBF interactions terms were included in the model to assess the moderating impacts of sex on VF and TBF. All analyses were controlled for age, prenatal exposure to maternal cigarette smoking, pubertal stage and household income. In all models, individual participants were nested within families by estimating a random intercept for each family using a variance components matrix and Satterthwaite method of estimating degrees of freedom. For models with significant interactions, simple slopes were examined at one standard deviation above and below the means of both predictors.34
In order to compare VF/TBF-cognition relationships to those observed using traditional anthropomorphic measures, two sets of follow-up analyses were conduced, replacing VF, TBF, and their respective interactions with (1) BMI and a sex-by-BMI interaction and (2) WC and a sex-by-WC interaction. All analyses were controlled for age, prenatal exposure to maternal cigarette smoking, pubertal stage and household income. Model fit of the three sets of models were compared using Hurvich and Tsai’s Criterion (AICc),35 in which smaller values indicate better fit. Likelihood ratio tests were computed to compare model fit.35
Statistical outliers (values greater or less than 3 standard deviations from the mean) were removed. Visceral fat volume, TBF, BMI and WC were log transformed using logarithm with base 10 to normalize their positively skewed distributions. Visuo-spatial memory variables were transformed using a reflected logarithm with base 10 to normalize a negatively skewed distribution. All statistical analyses were carried out using SPSS 20.0 and JMP 9.0, by author DHS.
Results
Participant characteristics can be found in Table 1. While females had higher TBF (p <.0001), and males had higher WC (p <.0001), there were no significant sex differences in BMI (p = 0.56) or VF volume (p = 0.11). But when VF was considered as a ratio of TBF, males had greater relative VF than females (p<.0001). Prevalence of relevant cardiometabolic risk factors for a subset of participants are reported in Supplementary Table 1 and are similar to those reported previously in population-based samples of Caucasian adolescents.36
Table 1.
Demographic Information for Males and Females: Mean, standard deviations and p-values from t-tests
| Males (N=480) | Females (N=503) | p-value | |
|---|---|---|---|
|
Basic Characteristics
| |||
| Age (in months) | 179.28 (21.32) | 181.10 (22.58) | 0.19 |
| Pubertyi | 3.36 (0.86) | 4.08 (0.71) | <0.0001 |
| Stage 1 (% of adolescents) | 2.09% | 0.60% | |
| Stage 2 (% of adolescents) | 12.73% | 1.19% | |
| Stage 3 (% of adolescents) | 38.00% | 14.51% | |
| Stage 4 (% of adolescents) | 41.13% | 56.66% | |
| Stage 5 (% of adolescents) | 6.05% | 27.04% | |
| PEMCS (% Exposed)ii | 44.38% | 51.49% | 0.03 |
| Income (CAN$) | $57,343 ($23,476) | $55,875 ($24,186) | 0.34 |
|
| |||
|
Anthropometry, bioimpedance and MRI of adiposity
| |||
| Height (cm) | 166.95 (10.60) | 159.81 (6.66) | <0.0001 |
| Weight (kg) | 61.46 (17.09) | 56.15 (12.68) | <0.0001 |
| BMI (kg/m2) | 21.79 (4.63) | 21.91 (4.46) | 0.68 |
| Log BMI | 1.33 (0.09) | 1.33 (0.08) | 0.56 |
| WC (cm) | 75.90 (12.41) | 71.46 (9.63) | <0.0001 |
| Log WC | 1.88 (0.06) | 1.85 (0.06) | <0.0001 |
| TBF (kg) | 10.82 (9.06) | 14.82 (8.39) | <0.0001 |
| Log TBF | 0.91 (0.33) | 1.10 (0.25) | <0.0001 |
| VF Volume (cm3) | 23.09 (22.53) | 21.33 (15.16) | 0.15 |
| Log VF Volume (cm3) | 4.22 (0.34) | 4.25 (0.25) | 0.11 |
| Log Relative VFiii | 3.31 (0.22) | 3.15 (0.19) | <0.0001 |
Means (standard deviations) are shown.
PEMCS = Prenatal Exposure to Maternal Cigarette Smoking; BMI=Body Mass Index; WC = Waist Circumference; TBF=Total Body Fat; VF=Visceral Fat,
Puberty stage is based on a an 8-item self report measure of physical development 27.
Analyzed with a χ2-statistic
Relative VF is visceral fat volume divided by total body fat
Visceral Fat
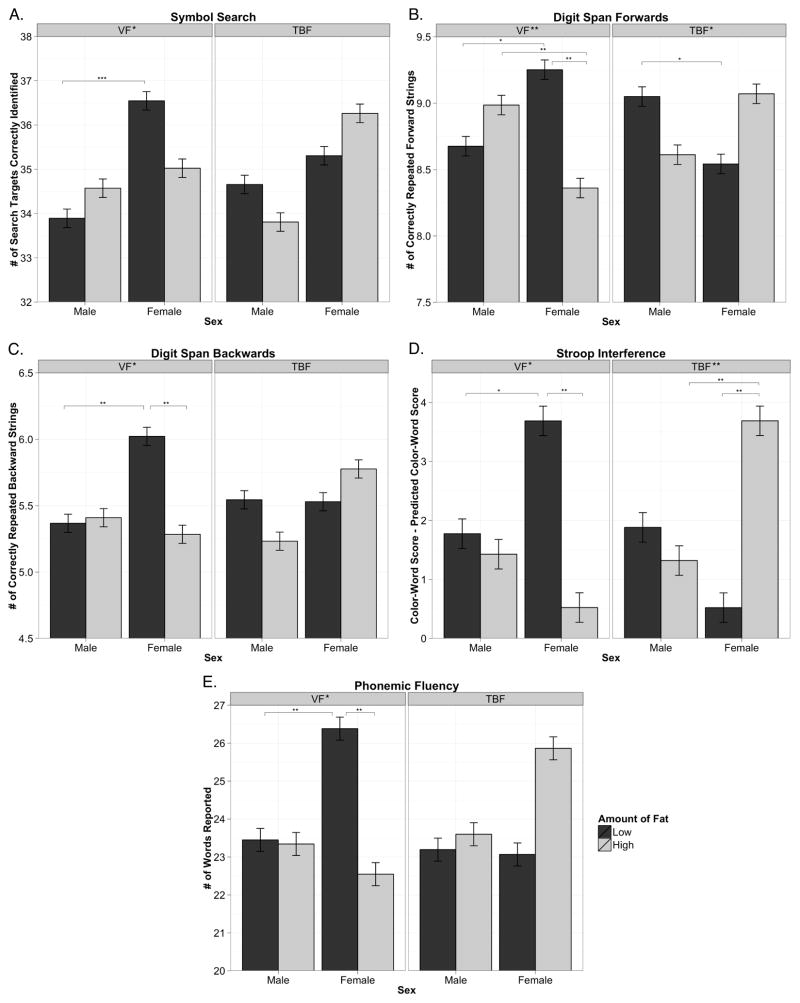
We found that larger volumes of VF were associated with lower performance on six measures of executive functioning (p = 0.0001 to 0.024, Table 2). We also found that sex moderated the relationship between VF and cognition on five measures of executive functioning (p = 0.001 to 0.044, Figure 1). Post hoc analyses of these sex-by-VF interactions revealed that females with low VF performed better than females with high VF on four measures (p = 0.002 to 0.009). There were no differences in performance between males with low and high VF. We found no main effects of VF, nor any sex-by-VF interactions, on any measures of memory. These effects were independent of TBF, and a number of potential confounders including age, prenatal exposure to maternal cigarette smoking, pubertal status and household income. The effects of VF on cognition did not change substantially when physical activity was included as an additional covariate (Supplementary Table 2).
Table 2.
Multilevel Models of Relationship between VF, TBF and Neuropsychological Outcomes: T-statistics for model variables (*p<.05; **p<.01; ***p<.001; ◆ p<.0001).
| Model Variables
|
|||||
|---|---|---|---|---|---|
| VF | TBF | Sex | Sex*VF | Sex*TBF | |
|
Executive Functioning
| |||||
| Processing Speed | |||||
| Automatic Detection Speed | −4.018*** | 1.585 | −1.399 | −0.406 | 0.208 |
| Controlled Detection Speed | −2.495* | 0.131 | 2.833** | 0.540 | −0.204 |
| Symbol Search | −0.746 | 0.088 | 3.636*** | −2.018* | 1.583 |
| Coding | −3.043** | 2.182* | 6.589◆ | −1.222 | 1.444 |
| Working Memory | |||||
| Digit-span Forwards | −1.499 | 0.213 | −0.167 | −3.214** | 2.473* |
| Digit-span Backwards | −1.846 | −0.157 | 1.856 | −2.128* | 1.461 |
| Self-ordered Pointingb | −2.258* | 1.295 | 1.483 | −1.314 | 0.833 |
| Resistance to Interference | |||||
| Stroop Interference | −2.609** | 1.768 | 0.995 | −2.162* | 2.741** |
| Cognitive Flexibility | |||||
| Semantic Fluency | −0.511 | 0.951 | 2.139* | −1.115 | 0.387 |
| Phonemic Fluency | −2.441* | 1.815 | 1.767 | −2.392* | 1.473 |
|
| |||||
|
Memory
| |||||
| Visuo-spatial Memory | |||||
| Dot-location Learning | −1.225 | 1.273 | −2.876** | −0.247 | 0.330 |
| Dot-location Short-delay | −0.843 | 0.659 | −2.861** | −0.210 | 0.385 |
| Dot-location Long-delay | −1.070 | 0.761 | −2.595** | −0.336 | 0.614 |
| Verbal Memory | |||||
| Stories Immediate | −0.214 | 0.891 | −4.138◆ | 0.225 | 0.432 |
| Stories Delayed | −0.576 | 0.924 | −3.853*** | 0.143 | 0.104 |
| Stories Recognition | −1.956 | 1.985* | −3.345*** | −0.331 | 1.604 |
Model outcomes controlled for: age, prenatal exposure to maternal cigarette smoking, pubertal status and household income
Self-ordered Pointing is reverse scored so that higher score indicate better performance.
Abbreviations: VF=Visceral Fat; TBF=Total Body Fat. Sex is positive when females are greater than males.
Figure 1.
The role of sex in moderating the impact of visceral fat (VF) and total body fat (TBF) on cognitive performance for five neuropsychological outcomes. Interactions quantified using continuous variables in multilevel models as detailed in Table 2. For significant interactions, post hoc tests were calculated for simple slopes at one standard deviation above and below the means of both predictors, as graphed here. A. Symbol Search – total number of search targets correctly identified, B. Digit-span Forwards –total number of correctly repeated forward digit strings C. Digit-span Backwards – total number of correctly repeated backward digit strings D. Stroop Interference – performance on color-word incongruent trial (Color-Word Score) controlled for overall speed of naming (Predicted score), see Supplementary Appendix for calculation details. E. Phonemic Fluency – total number of words reported. Low VF/TBF – dark gray, High VF/TBF – light gray, *p<.05, **p<.01, ***p<.001
Total Body Fat
We found that, when controlling for VF, greater TBF was associated with higher performance on one measure of executive functioning (p = 0.029, Table 2). Additionally, sex moderated the effect of TBF on two measures of executive functioning (p = 0.006 to 0.014; see Figure 1 for post hocs). There was a positive main effect of TBF on one measure of verbal memory (p = 0.048), but no sex-by-TBF interactions. The effects of VF on cognition did not change substantially when physical activity was included as an additional covariate (Supplementary Table 2).
Body Mass Index and Waist Circumference
In order to compare VF/TBF-cognition relationships to those observed using traditional anthropomorphic measures, two sets of follow-up analyses were conduced, using BMI (Supplementary Table 3) and WC (Supplementary Table 4) in place of VF and TBF. While there was a negative effect of BMI and WC on four measures of executive functioning (p = 0.001 to 0.032), we observed more significant associations between VF and cognitive variables. As well, while there were five sex-by-VF interactions, there was only one sex-by-BMI interaction (p = 0.041), and no sex-by-WC interactions, suggesting that traditional anthropomorphic measures are not as sensitive to sex differences in fat distribution as VF assessed by MRI.
Using model-fit statistics (AICc), we next compared the three sets of models: (1) VF & TBF (2) BMI and (3) WC (Table 3). Likelihood ratio tests indicate that models based on VF & TBF estimate cognitive performance significantly better than models based on BMI or WC, for all measures except Dot-location Learning and Long-delay (p<0.0001 to 0.0008).
Table 3.
Model Fit (AICc) for three sets of models: (1) Visceral Fat and Total Body Fat (2) Body Mass Index and (3) Waist Circumference
| VF&TBF | BMI | WC | |
|---|---|---|---|
|
Executive Functioning
| |||
| Processing Speed | |||
| Automatic Detection Speed | 8613.81◆◆◇◇ | 8974.67 | 8911.62▽▽ |
| Controlled Detection Speed | 7875.24◆◆◇◇ | 8194.38 | 8139.37▽▽ |
| Symbol Search | 5731.07◆◆◇◇ | 5963.07 | 5910.59▽▽ |
| Coding | 7074.15◆◆◇◇ | 7367.21 | 7314.78▽▽ |
| Working Memory | |||
| Digit-span Forwards | 3775.08◆◆◇◇ | 3922.47 | 3894.45▽▽ |
| Digit-span Backwards | 3717.40◆◆◇◇ | 3859.98 | 3821.49▽▽ |
| Self-ordered Pointing | 4564.00◆◆◇◇ | 4759.36 | 4723.46▽▽ |
| Resistance to Interference | |||
| Stroop Interference | 6046.06◆◆◇◇ | 6319.31 | 6277.93▽▽ |
| Verbal Fluency | |||
| Semantic Fluency | 6658.93◆◆◇◇ | 6934.12 | 6883.11▽▽ |
| Phonemic Fluency | 6360.60◆◆◇◇ | 6619.22 | 6572.32▽▽ |
|
| |||
|
Memory
| |||
| Visuo-spatial Memory | |||
| Dot-location Learning | 663.99 | 669.04 | 666.40 |
| Dot-location Short-delay | 806.74◆◇ | 823.64 | 821.05 |
| Dot-location Long-delay | 148.03 | 133.63 | 135.11 |
| Verbal Memory | |||
| Stories Immediate | 7447.40◆◆◇◇ | 7755.96 | 7703.35▽▽ |
| Stories Delayed | 7450.23◆◆◇◇ | 7761.72 | 7705.53▽▽ |
| Stories Recognition | 4444.01◆◆◇◇ | 4620.77 | 4589.54▽▽ |
VF= Visceral Fat; TBF=Total Body Fat; BMI = Body Mass Index; WC = Waist Circumference
Model fit was estimated using Hurvich and Tsai’s Criterion (AICc).35 Smaller values indicate better fit.
Likelihood ratio tests 35 were computed to assess whether the models with smaller AICc have significantly better fit.
VF&TBF model fits the data better than BMI model, ◆◆p<0001 ◆p<.001
VF&TBF model fits the data better than WC model, ◇ ◇p<.0001 ◇p<.001
WC model fits the data better than BMI model, ▽▽p<.0001
Discussion
Using a large community-based sample, we found evidence for a relationship between VF and cognitive performance in adolescents. In particular, we found a negative association between VF (independent of TBF) and performance on several measures of executive functioning, either as a main effect or an interaction with sex. Further, we found that these negative results were specific to VF; in fact, we found a few positive associations between TBF (independent of VF) and cognitive performance. Further, we found that a subset of these associations were present mainly in females and not in males (Figure 1). Taken together, these results suggest that higher quantities of VF are associated with lower executive functioning as early as adolescence.
Our findings are consistent with previous studies showing a negative relationship between obesity, as indexed by BMI, and cognitive performance in children, particularly on tasks of executive functioning.6–8 These past studies, however, have not distinguished between the impact of visceral and peripheral body-fat on cognition. Further, while past studies control for sex, they have not investigated sex-by-BMI interactions, despite known sex differences in fat deposition.18 We found that, even with the relevant sex interactions, models that included VF and TBF estimated cognition better than models that included only BMI or WC. As VF and TBF influenced cognition in opposite directions, this finding highlights a potential limitation of anthropomorphic measures of obesity.
It is important to highlight that we observed the relationship between VF and cognitive performance in a population of typically developing adolescents. Adolescence is a time of emergence for cardiometabolic disease, with the metabolic syndrome occurring in 4% of all adolescents and 28% of overweight adolescents.20,36 Adolescent cardiometabolic health has been shown to predict cardiovascular health in adulthood,37 and cardiometabolic health in midlife is, in turn, associated with increased risk of dementia.5,38 If elevated VF observed during adolescence remains, VF-cognition relationships observed early on could have cumulative effects, potentially increasing the risk of dementia later in life. Along these lines, Sabia and collegues9 found that cumulative obesity (assessed by BMI three times over approximately 36 years) was associated with decreased cognitive performance in late life.
As this study utilizes an observational design, the reported relationships could reflect one of three causal scenarios: (1) that VF causes executive functioning deficits, (2) that executive functioning deficits cause VF accumulation and (3) that a third factor causes both VF accumulation and executive function deficits. In the first scenario, VF could impact cognitive health via its influence on cardiometabolic health. For example, adipose tissue, in particular VF, secretes pro-inflammatory cytokines into the bloodstream,39 and research in rodents shows that elevated levels of inflammatory cytokines in the periphery result in an exaggerated neuroinflammatory response that impairs cognitive performance.40 In accordance with our observed sex difference, epidemiological evidence suggests that the relationship between VF and cytokines is moderated by sex; such that for every standard deviation increase in VF, women show higher levels of C-reactive protein than men.19 C-reactive protein has also been associated negatively with executive function measures of cognition (including Digit-span Backwards) in obese females, but not males.41 In addition, higher levels of interleukin-6, another biomarker of inflammation, have been found to be associated with a decline in verbal fluency, albeit only in females.42
In the second scenario, lower executive functioning could cause VF accumulation through lower cognitive control of eating impulses and/or food preferences. One study found that lower activity in brain areas underlying executive functioning on a delay-discounting task was associated with increased weight gain in the 1–3 years following participation.43 While it may be the case that poor executive functioning leads to increased VF, understanding this relationship is of equal clinical interest. If poor executive functioning precedes obesity, interventions tailored to increasing executive functioning may improve responses to lifestyle modification methods.
In the third scenario, a third factor could be simultaneously increasing VF deposition and decreasing executive functioning, resulting in the observed negative correlational relationship. Efficient androgen receptors, for example, are associated with increased VF volumes in males.44 As well, males and females with efficient androgen receptors show a more masculine pattern of cortical thickness development across adolescence.45 Thus circulating testosterone, modulated by androgen receptor polymorphisms, might regulate both VF volume and brain morphology underlying executive functioning. As illustrated in Figure 1, females with high VF show no sex advantage in executive functioning, whereas females with low VF do, suggesting that females with high VF show a more masculine pattern of cognitive performance.
Lifestyle factors may also play a role in both VF accumulation and cognitive performance. For example, physical activity is associated with higher cognitive performance in adolescents, even when controlling for cardiorespiratory fitness and body mass index.46 Further, a recent study in adults showed that physical activity, assessed by accelerometers, is negatively associated with VF.47 Thus physical activity may play a role in the observed VF-cognition relationships. Note, however, that including physical activity as a covariate did not substantially change the relationship between VF or TBF and cognitive performance observed in our study. Future studies investigating the impact of interventions involving physical activity will help untangle the putative causal role of physical activity in VF-cognition relationships.
While controlling for VF, we found that TBF contributed positively to two measures of cognitive functioning, and that sex moderated the impact of TBF on two additional measures of cognitive functioning. While past studies have shown that TBF is negatively associated with cognitive performance,6 these studies were not able to consider VF as we were in our analyses. Our results suggest that obesity-associated decrements in cognition may be driven by fat stored viscerally rather than peripherally. As peripheral fat is less metabolically dangerous that VF,11 for a given percentage of body fat, a higher proportion stored peripherally rather than viscerally could mitigate the negative effects of VF. Previous studies show that elevated peripheral fat, when controlled for VF, is associated with lower blood pressure in adolescents,13 lower triglycerides48 and reduced risk of metabolic syndrome in adults.49
While there were a number of strengths to our study, including sample size, use of MRI to measure visceral fat, and availability of multiple measures of cognition, there are a number of limitations that must be considered. First, as previously mentioned, our observation design does not allow for the inference of causality. Future longitudinal and intervention (e.g., diet, physical activity, surgical removal of VF) studies in humans or experimental ones in animal models are necessary to adjudicate between the three causal scenarios described above. Second, due to the number of models included, there is a potential for type-I error inflation in this study. Of the 10 executive functioning tests assessed, only one would be expected to show an effect of VF, or a sex-by-VF interaction by chance alone (20 x 0.05 = 1). We found, however, 11 significant effects (6 main effects and 5 sex-by-VF interactions), suggesting that these findings reflect true relationships rather than type-I error.
In summary, our results suggest a relationship between lower executive functioning and higher visceral fat, independent of total body fat, and that this association is observable as early as adolescence. Further, our results suggest that elevated levels of visceral fat may impact cognition more strongly in females, implying that the physiological mechanisms underlying the relationship between visceral fat may differ by sex. Further research is needed to clarify the nature of these mechanisms.
Supplementary Material
Acknowledgments
Funding
The Saguenay Youth Study project is funded by the Canadian Institutes of Health Research (TP, ZP), Heart and Stroke Foundation of Quebec (ZP), and the Canadian Foundation for Innovation (ZP). DHS is supported by a doctoral grant from the Alzheimer Society of Canada.
We thank all families who took part in the Saguenay Youth Study and the following individuals for their contributions in designing the protocol, acquiring and analyzing the data: psychometricians (Chantale Belleau, Mélanie Drolet, Catherine Harvey, Stéphane Jean, Hélène Simard, Mélanie Tremblay, Patrick Vachon), ÉCOBES team (Nadine Arbour, Julie Auclair, Marie-Ève Blackburn, Marie-Ève Bouchard, Annie Gautier, Annie Houde, Catherine Lavoie), laboratory technicians (Denise Morin and Nadia Mior), nutritionists (Caroline Benoit and Henriette Langlais), MR team (Sylvie Masson, Suzanne Castonguay, Marie-Josée Morin, Caroline Mérette), and cardio nurses (Jessica Blackburn, Mélanie Gagné, Jeannine Landry, Catherine Lavoie, Lisa Pageau, Réjean Savard, France Tremblay, Jacynthe Tremblay). We thank Dr. Jean Mathieu for the medical follow up of participants in who we detected any medically relevant abnormalities. We thank Manon Bernard for designing and managing our online database; Dr. Rosanne Aleong for her assistance in coordinating the project; Dr. Elizabeth Page-Gould and Dr. Melissa Pangelinan for their assistance on statistical analyses and Ms. Katie Goodwin for her assistance in semi-automated delineations of visceral fat. TP, ZP and DHS had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Disclosures
We declare no conflicts of interest.
Supplementary information is available at the journal’s website
Author Contributions
Study concept and design: Paus and Pausova.
Acquisition of data: Leonard, Perron, Richer, Syme, Veillette, Pausova and Paus.
Analysis and interpretation of data: Schwartz, Paus and Pausova.
Drafting of the manuscript: Schwartz
Critical revision of the manuscript for important intellectual content: Paus and Pausova
Obtained funding: Paus and Pausova.
Administrative, technical or material support: Leonard, Perron, Richer, Syme, Veillette, Pausova and Paus.
Study supervision: Paus and Pausova
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Survey CCH. Overweight Canadian children and adolescnets. Vol. 2004. Canada: 2004. [Accessed September 5, 2012]. Available at http://www.statcan.gc.ca. [Google Scholar]
- 3.Survey CCH. Adult obesity in Canada: Measured height and weight. Vol. 2004. Canada: 2004. [Accessed September 5, 2012]. Available at http://www.statcan.gc.ca. [Google Scholar]
- 4.Aronne LJ. Epidemiology, morbidity, and treatment of overweight and obesity. J Clin Psychiat. 2001;62(Suppl 23):13–22. [PubMed] [Google Scholar]
- 5.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330(7504):1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamijo K, Khan NA, Pontifex MB, Scudder MR, Drollette ES, Raine LB, et al. The relation of adiposity to cognitive control and scholastic achievement in preadolescent children. Obesity. 2012;20(12):2406–11. doi: 10.1038/oby.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verdejo-Garcia A, Perez-Exposito M, Schmidt-Rio-Valle J, Fernandez-Serrano MJ, Cruz F, Perez-Garcia M, et al. Selective Alterations Within Executive Functions in Adolescents With Excess Weight. Obesity. 2010;18(8):1572–8. doi: 10.1038/oby.2009.475. [DOI] [PubMed] [Google Scholar]
- 8.Cserjesi R, Molnar D, Luminet O, Lenard L. Is there any relationship between obesity and mental flexibility in children? Appetite. 2007;49(3):675–8. doi: 10.1016/j.appet.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Sabia S, Kivimaki M, Shipley MJ, Marmot MG, Singh-Manoux A. Body mass index over the adult life course and cognition in late midlife: the Whitehall II Cohort Study. Am J Clin Nutr. 2009;89(2):601–7. doi: 10.3945/ajcn.2008.26482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21(6):697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 11.Votruba SB, Jensen MD. Regional fat deposition as a factor in FFA metabolism. Ann Rev Nutr. 2007;27:149–63. doi: 10.1146/annurev.nutr.27.061406.093754. [DOI] [PubMed] [Google Scholar]
- 12.Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism. 1987;36(1):54–9. doi: 10.1016/0026-0495(87)90063-1. [DOI] [PubMed] [Google Scholar]
- 13.Pausova Z, Mahboubi A, Abrahamowicz M, Leonard GT, Perron M, Richer L, et al. Sex differences in the contributions of visceral and total body fat to blood pressure in adolescence. Hypertension. 2012;59(3):572–9. doi: 10.1161/HYPERTENSIONAHA.111.180372. [DOI] [PubMed] [Google Scholar]
- 14.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53(9):1937–42. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 15.Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn. 2009;28(1):75–80. doi: 10.1159/000231980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ. 2001;322(7300):1447–51. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isaac V, Sim S, Zheng H, Zagorodnov V, Tai SE, Chee M. Adverse associations between visceral adiposity, brain structure, and cognitive performance in healthy elderly. Front Ag Neurosci. 2011;3(12):1–8. doi: 10.3389/fnagi.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemieux S, Prud’homme D, Bouchard C, Tremblay A, Despres JP. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am J Clin Nutr. 1993;58(4):463–7. doi: 10.1093/ajcn/58.4.463. [DOI] [PubMed] [Google Scholar]
- 19.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116(11):1234–41. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 20.Syme C, Abrahamowicz M, Leonard GT, Perron M, Pitiot A, Qiu X, et al. Intra-abdominal adiposity and individual components of the metabolic syndrome in adolescence: sex differences and underlying mechanisms. Arch Pediat Adol Med. 2008;162(5):453–61. doi: 10.1001/archpedi.162.5.453. [DOI] [PubMed] [Google Scholar]
- 21.Pausova Z, Paus T, Abrahamowicz M, Almerigi J, Arbour N, Bernard M, et al. Genes, maternal smoking, and the offspring brain and body during adolescence: Design of the Saguenay Youth Study. Hum Brain Mapp. 2007;28(6):502–18. doi: 10.1002/hbm.20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status. J Youth Adolescence. 1988;17(2):117–33. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 23.Schmitz KE, Hovell MF, Nichols JF, et al. A validation study of early adolescents’ pubertal self-assessments. J Early Adolescence. 2004;24(4):357–384. [Google Scholar]
- 24.Brooks-Gunn J, Warren MP, Rosso J, Gargiulo J. Validity of self-report measures of girls’ pubertal status. Child Dev. 1987;58(3):829–841. [PubMed] [Google Scholar]
- 25.Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: correspondence between hormonal and physical development. Child Dev. 2009;80(2):327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruff RM, Allen CC. Ruff 2 and 7 Selective Attention Test professional manual. Odessa, Florida: Psychological Assessment Resources; 1996. [Google Scholar]
- 27.Wechsler D. Wechsler Intelligence Scale for Children. 3. San Antonio, Texas: Psychological Corporation; 1991. [Google Scholar]
- 28.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological tests: Administration, norms, and commentary. 3. New York: Oxford University Press; 2006. [Google Scholar]
- 29.Petrides M, Milner B. Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1982;20(3):249–62. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- 30.Archibald SJ, Kerns KA. Identification and Description of New Tests of Executive Functioning in Children. Child Neuropsychol. 1999;5(2):115–129. [Google Scholar]
- 31.Golden CJ. Stroop Color and Word Test: A manual for clinical and experimental uses. Chicago, Illinois: Stoelting, Co; 1978. [Google Scholar]
- 32.Korkman M, Kirk U, Kemp S. A Development Neuropsychological Assessment Manual. San Antonio, Texas: Psychological Corporation; 1998. [Google Scholar]
- 33.Cohen MJ. Children’s Memory Scale. San Antonio, Texas: Psychological Corporation; 1997. [Google Scholar]
- 34.Aiken LS, West SG, Reno RR. Multiple regression: testing and interpreting interactions. Sage Publications; 1991. [Google Scholar]
- 35.Burnham KP, Anderson DR. Multimodel inference: understanding AIC and BIC in model selection. Sociol Method Res. 2004;33:261–304. [Google Scholar]
- 36.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediat Adol Med. 2003;157(8):821–7. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 37.McGill HC, Jr, McMahan CA. Starting earlier to prevent heart disease. JAMA. 2003;290(17):2320–2. doi: 10.1001/jama.290.17.2320. [DOI] [PubMed] [Google Scholar]
- 38.Kalmijn S, Foley D, White L, Burchfiel CM, Curb JD, Petrovitch H, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arterioscl Throm Vas. 2000;20(10):2255–60. doi: 10.1161/01.atv.20.10.2255. [DOI] [PubMed] [Google Scholar]
- 39.Vohl MC, Sladek R, Robitaille J, Gurd S, Marceau P, Richard D, et al. A survey of genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obesity Res. 2004;12(8):1217–22. doi: 10.1038/oby.2004.153. [DOI] [PubMed] [Google Scholar]
- 40.Barrientos RM, Frank MG, Watkins LR, Maier SF. Aging-related changes in neuroimmune-endocrine function: Implications for hippocampal-dependent cognition. Horm Behav. 2012;62(3):219–27. doi: 10.1016/j.yhbeh.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sweat V, Starr V, Bruehl H, Arentoft A, Tirsi A, Javier E, et al. C-reactive protein is linked to lower cognitive performance in overweight and obese women. Inflammation. 2008;31(3):198–207. doi: 10.1007/s10753-008-9065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gimeno D, Marmot MG, Singh-Manoux A. Inflammatory markers and cognitive function in middle-aged adults: the Whitehall II study. Psychoneuroendocrino. 2008;33(10):1322–34. doi: 10.1016/j.psyneuen.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kishinevsky FI, Cox JE, Murdaugh DL, Stoeckel LE, Cook EW, 3rd, Weller RE. fMRI reactivity on a delay discounting task predicts weight gain in obese women. Appetite. 2012;58(2):582–92. doi: 10.1016/j.appet.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 44.Pausova Z, Abrahamowicz M, Mahboubi A, Syme C, Leonard GT, Perron M, et al. Functional variation in the androgen-receptor gene is associated with visceral adiposity and blood pressure in male adolescents. Hypertension. 2010;55(3):706–14. doi: 10.1161/HYPERTENSIONAHA.109.146720. [DOI] [PubMed] [Google Scholar]
- 45.Raznahan A, Lee Y, Stidd R, Long R, Greenstein D, Clasen L, et al. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. PNAS. 2010;107(39):16988–93. doi: 10.1073/pnas.1006025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruiz JR, Ortega FB, Castillo R, et al. Physical activity, fitness, weight status, and cognitive performance in adolescents. J Pediatr. 2010;157(6):917–922. doi: 10.1016/j.jpeds.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 47.Smith HA, Storti KL, Arena V, et al. Associations between accelerometer-derived physical activity and regional adiposity in young men and women. Obesity. doi: 10.1002/oby.20308. Accepted Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care. 2009;32(6):1068–75. doi: 10.2337/dc08-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demerath EW, Reed D, Rogers N, Sun SS, Lee M, Choh AC, et al. Visceral adiposity and its anatomical distribution as predictors of the metabolic syndrome and cardiometabolic risk factor levels. Am J Clin Nutr. 2008;88(5):1263–71. doi: 10.3945/ajcn.2008.26546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.